Summary
AM-19226 is a pathogenic, non-O1/non-O139 serogroup strain of Vibrio cholerae that uses a Type 3 Secretion System (T3SS) mediated mechanism to colonize host tissues and disrupt homeostasis, causing cholera. Co-culturing the Caco2-BBE human intestinal epithelial cell line with AM-19226 in the presence of bile results in rapid mammalian cell death that requires a functional T3SS. We examined the role of bile, sought to identify the mechanism, and evaluated the contributions of T3SS translocated effectors in in vitro cell death. Our results suggest that Caco2-BBE cytotoxicity does not proceed by apoptotic or necrotic mechanisms, but rather displays characteristics consistent with osmotic lysis. Cell death was preceded by disassembly of epithelial junctions and reorganization of the cortical membrane skeleton, although neither cell death nor cell-cell disruption required VopM or VopF, two effectors known to alter actin dynamics. Using deletion strains, we identified a subset of AM-19226 Vops that are required for host cell death, which were previously assigned roles in protein translocation and colonization, suggesting that they function other than to promote cytotoxicity. The collective results therefore suggest that cooperative Vop activities are required to achieve cytotoxicity in vitro, or alternatively, that translocon pores destabilize the membrane in a bile dependent manner.
Introduction
Although best known as an epidemic disease caused by O1 and O139 serogroup strains of Vibrio cholerae, the diarrheal disease cholera can also result from infection by strains of the more than 200 other defined serogroups, collectively known as non-O1/non-O139 serogroup strains (Sack et al., 2004, Harris et al., 2012). Clinical reports document similar disease symptoms from infection by all serogroups, although non-O1/non-O139 serogroup strains are associated with sporadic outbreaks that occur both temporally and geographically outside of the patterns associated with epidemic disease. In fact, sporadic cholera has been reported worldwide, and the rising incidence of non-O1/non-O139 serogroup associated disease in endemic areas has prompted investigation of the molecular mechanisms responsible for disease (Morris et al., 1981, Bagchi et al., 1993, Ramamurthy et al., 1993, Dalsgaard et al., 1995, Sharma et al., 1998, Dalsgaard et al., 1999, Sack et al., 2003, Faruque et al., 2004, Lee et al., 2007, Chatterjee et al., 2009, Newton et al., 2012, Dutta et al., 2013, Luo et al., 2013).
Despite the similarity of clinical outcomes, there are numerous genetic differences between epidemic associated O1 and O139 serogroup strains, which are largely clonal and conserved in genetic content, and pathogenic non-O1/non-O139 serogroup strains, which have a more diverse genetic repertoire (Bagchi et al., 1993, Dalsgaard et al., 1995, Dalsgaard et al., 1999, Faruque et al., 2003, Faruque et al., 2004, Feng et al., 2008, Chun et al., 2009). Invariably, O1 and O139 serogroup strains colonize the human intestinal epithelium using the toxin co-regulated pilus (TCP), and cause profuse secretory diarrhea as a result of the enzymatic activity of cholera toxin (CT) (Matson et al., 2007, Harris et al., 2012). In contrast, most clinically isolated non-O1/non-O139 strains do not encode TCP or CT, utilizing less well understood mechanisms to colonize host tissues and alter intestinal cell homeostasis. We now know that a subset of non-O1/non-O139 serogroup strains isolated from cholera patients carry genes for a Type 3 Secretion System (T3SS), and studies have shown that the T3SS is required for disease in murine and rabbit animal models of infection (Dziejman et al., 2005, Tam et al., 2007, Rahman et al., 2008, Chatterjee et al., 2009, Tam et al., 2010, Shin et al., 2011, Dutta et al., 2013, Hasan et al., 2013, Luo et al., 2013, Morita et al., 2013, Octavia et al., 2013).
We previously reported that co-culture of T3SS positive strain AM-19226 with the Caco2-BBE intestinal epithelial cell line resulted in rapid eukaryotic cell death (Miller et al., 2012). The phenotype did not require the HlyA hemolysin or the putative thermostable direct-related hemolysin, TRH, but did require a functional T3SS. In other bacterial pathogens, T3SS-mediated in vitro cell death can involve different effector protein activities and pathogen specific mechanisms. For example, Yersinia spp. induce apoptosis in naïve macrophages in vitro, using YopJ and YopP effector activity to interfere with MAPK and NF-κB signaling (Bergsbaken et al., 2009a, Philip et al., 2012). In the case of Salmonella infection, both flagellin and a functional T3SS are required to induce pyroptosis in host cells (Fink et al., 2007). It is also important to note that some pathogens use T3SS translocated proteins to interfere with host pathways in order to prevent cell death and promote colonization and infection. For example, enterohemorrhagic E. coli inhibits apoptosis by translocation of two T3SS effector proteins, NleH and NleD (Wong et al., 2011). We therefore further investigated V. cholerae T3SS-mediated Caco2-BBE cytotoxicity by interrogating the mechanism and identifying T3SS translocated proteins required for cell death in our co-culture model.
Results and Discussion
Role of bile in promoting cytotoxicity
Previous studies determined that in the presence of 0.2% bile, co-culture of V. cholerae strain AM-19226 with the intestinal epithelial cell line Caco2-BBE resulted in ~70–95% cytotoxicity 3h after the start of co-culture, as measured by lactate dehydrogenase (LDH) release (Miller et al., 2012). In contrast, there is no LDH release in the absence of bile, even after six hours of co-culture with V. cholerae. We also identified bile as an inducer of T3SS gene expression for bacteria grown in vitro overnight in LB (Alam et al., 2010).
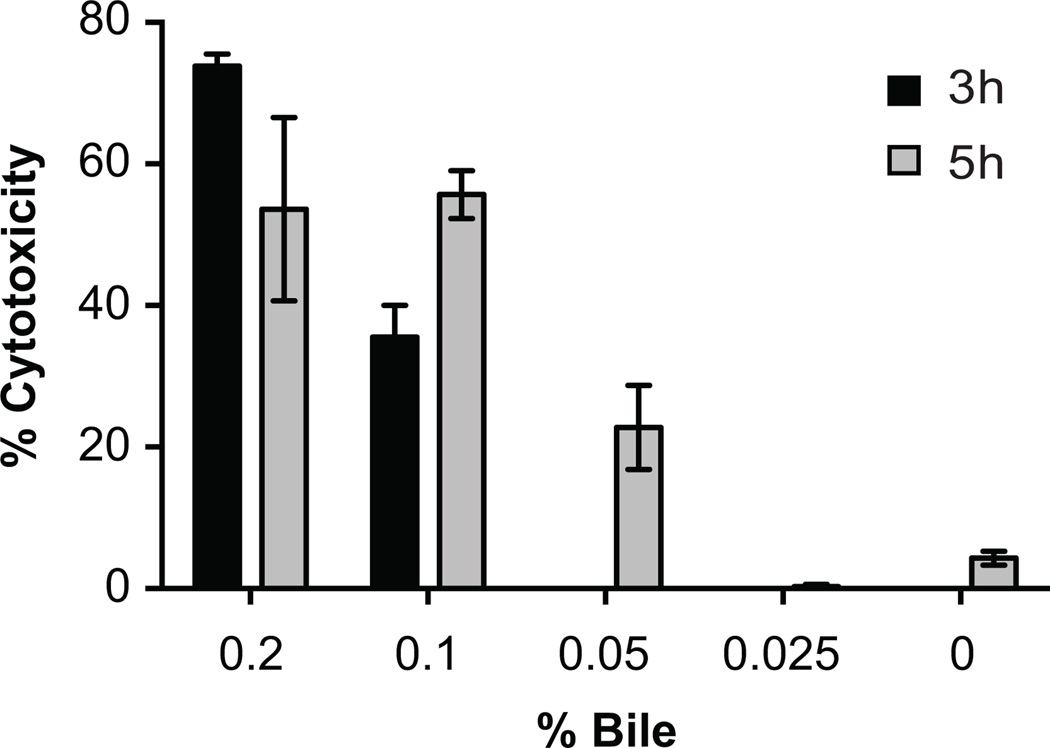
We varied the bile concentration during co-culture from 0.025% to 0.2%, while keeping the AM-19226 multiplicity of infection (MOI) constant at ~10. Compared to the results achieved with 0.2% bile, 0.1% bile results in an overall decrease in cytotoxicity, with 30% cytotoxicity measured after 3h, and 50% measured after 5h (Figure 1). When the bile concentration was further decreased to 0.05%, we observed only 25% cytotoxicity after 5h. 0.025% bile does not result in measurable Caco2-BBE cell death after 5h (Figure 1). The results suggest that 0.2% is the optimal concentration of bile for potentiating the effect of V. cholerae. Increased bile concentrations have been reported to reduce bacterial growth (Provenzano et al., 2000, Hung et al., 2005). We typically recovered similar bacterial counts from the Caco2-BBE co-culture supernatants in the presence and absence of 0.2% bile (e.g. 1.93*107 CFU/mL in the absence of bile vs. 3.18*107 CFU/mL in the presence). We also observed bacterial multiplication in the presence of 0.2% bile during co-culture (15–20 fold increase 3 hpi compared to initial inoculum). We did notice a trend towards ~2-fold increased bacterial adherence in the presence of bile (data not shown), but the relationship between MOI, adherence and cytotoxicity requires further investigation. Having previously demonstrated that cytotoxicity requires both a functional T3SS and bile (Miller et al., 2012) and given the current data, it is possible that a small increase in adherence acts synergistically with T3SS effector protein activity and bile-mediated destabilization of mammalian cell membranes to result in cytotoxicity.
Figure 1. Cytotoxicity occurs in a bile concentration dependent manner.
AM-19226 T3SS WT was grown overnight in LB and used to infect Caco2-BBE cells at an MOI of ~10 in the presence of varying bile concentrations as indicated. After 3h or 5h of co-culture, lactate dehydrogenase (LDH) was measured in the co-culture supernatant as an indicator of membrane permeability. Percent cytotoxicity was calculated relative to a total lysis control sample. Data represent the mean ± standard deviation from one experiment using three individual bacterial colonies. The experiment was repeated twice with similar results.
Since bile is required for T3SS-dependent cytotoxicity and since the bile concentration can modulate the time course and number of Caco2-BBE cells succumbing to cytotoxicity, we reasoned that bile in the co-culture medium might stimulate signaling pathways in the mammalian host cells leading to cell death. Our previous results suggest that bile does not influence T3SS gene expression under co-culture conditions: when strain AM-19226 is grown in standard tissue culture conditions (described in Experimental Procedures), T3SS gene expression levels remain similar whether or not 0.2% bile and/or Caco2-BBE mammalian cells are present (Miller et al., 2012). Alternatively, however, bile might promote bacterial T3SS activity during co-culture. To differentiate among the possibilities, we conducted experiments where AM-19226 inoculating cultures or Caco2-BBE monolayers were pretreated with bile before co-culture, and varied whether bile was present or not at the time of co-culture (Table 1). Earlier experiments determined that stationary phase bacteria grown in LB + bile display maximal T3SS structural gene expression compared to bacteria in exponential phase (or grown in the presence of sodium deoxycholate alone), thus stationary phase cultures were used (Alam et al., 2010). We found that the cytotoxicity phenotype requires the presence of bile specifically during the time of mammalian cell co-culture; if bile was left out of the co-culture media, neither pre-treating Caco2-BBE cells with bile before co-culture, nor growing bacteria overnight in LB+0.4% bile, resulted in cell death. One interpretation is that bile acts on epithelial cells by sensitizing them to bacterial protein activities in a reversible manner, such that the phenotype is revealed only during co-culture with bile. Interestingly, V. parahaemolyticus grown to exponential phase in the absence of bile can cause Caco-2 T3SS2-mediated death, although when bacteria are grown in the presence of bile prior to co-culture, more rapid cell death is observed (Gotoh et al., 2010, Kodama et al., 2010). It is important to note that different in vitro growth conditions promote maximal T3SS gene expression in V. cholerae and V. parahaemolyticus, and that differences in effector protein repertoires likely contribute to the variations in in vitro and in vivo phenotypes observed for the two species (Zhang et al., 2013). For example, maximal T3SS gene expression in V. parahaemolyticus occurs during exponential growth, compared to stationary phase for V. cholerae (Alam et al., 2010, Kodama et al., 2010). Thus, the V. cholerae and V. parahaemolyticus T3SS gene expression networks and response to environmental signals in the presence of mammalian cells are not identical.
Table 1.
Survey of conditions required for Caco2-BBE cytotoxicity.
| AM-19226 Overnight |
Caco2-BBE Pretreatment |
Caco2-BBE/AM-19226 Co-culture |
Caco2-BBE Cytotoxicity? |
|---|---|---|---|
| LB | None | 0.2% bile | Yes |
| LB | None | 0.05% CHAPS | No |
| LB | None | 0.01% Tween-80 | No |
| LB | None | 0.001% Triton X-100 | No |
| LB | None | 0.005% SDS | No |
| LB | None | 0.01% Sodium deoxycholate | No |
| LB | None | 0.04% Sodium cholate | No |
| LB + 0.4% bile | None | No additives | No |
| LB | 0.2% bile 2h | No additives | No |
Bile is a complex mixture of different bile salts, cholesterol, pigments, and fatty acids that collectively have detergent-like properties. Importantly, bile salts are commonly used to promote T3SS activity and activate expression of virulence genes in enteric bacteria, including epidemic V. cholerae strains (Gupta et al., 1997, Gunn, 2000, Begley et al., 2005a, Begley et al., 2005b, Hung et al., 2005, Olive et al., 2007, Gotoh et al., 2010, Yang et al., 2013). To test whether any single bile component could promote the cell death phenotype, we included individual anionic bile salts (sodium deoxycholate, sodium cholate) or other compounds with detergent properties (anionic (SDS), nonionic (Triton X-100, Tween-80) or zwitterionic (CHAPS) detergents), instead of whole bile during Caco2-BBE co-culture with AM-19226. For each reagent, we first determined the highest concentration that did not result in altered Caco2-BBE cell morphology as visualized by microscopy. We then conducted the co-culture experiment with the highest permissible concentration for each compound as listed in Table 1. We did not observe Caco2-BBE cytotoxicity or changes in cellular morphology during co-culture with AM-19226 in the presence of the detergents and bile salts we tested (Table 1). Because we used each component at the highest concentration possible that alone did not cause cytotoxicity, we propose that other components of bile (e.g. cholesterol, fatty acids) or the properties of multiple bile components are required for cytotoxicity.
Investigating apoptosis as the cause of T3SS-dependent cytotoxicity
Results from our earlier studies led to the conclusion that Caco2-BBE cytotoxicity did not require the AM-19226 trh gene product, which in V. parahaemolyticus functions as a pore forming toxin similar to the thermostable direct hemolysin, TDH (Miller et al., 2012). Nonetheless, the rapid, dramatic disruption of monolayers and presence of debris was easily observed by bright field microscopy and cell death measured by lactate dehydrogenase release (Miller et al., 2012). However, previous experiments did not investigate the mechanisms of cell death that may involve apoptotic or necrotic programmed cell death pathways or osmotic cell lysis.
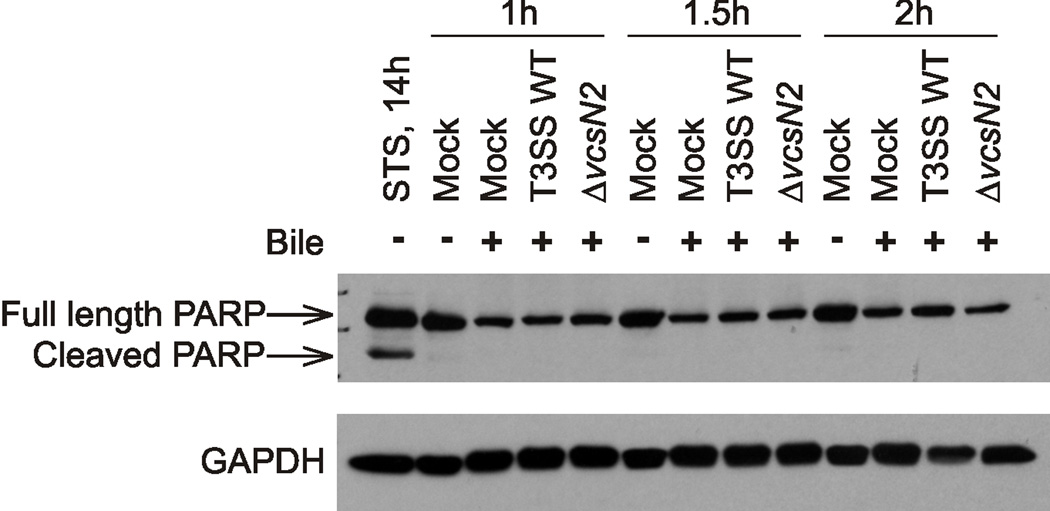
Although we did not observe morphological features consistent with apoptosis (e.g. bright field microscopy did not reveal cytoplasmic shrinkage or membrane blebbing, data not shown), and the rapid progression to loss of membrane integrity is not characteristic of apoptotic cell death, we sought additional evidence against apoptosis as the mechanism of AM-19226 induced intestinal epithelial cell death. Experiments using pan-caspase inhibitors Z-VAD-fmk and Q-VD-OPh, which block caspase activation, did not inhibit cytotoxicity (Table 2). Since Poly- (ADP Ribose) Polymerase (PARP) cleavage is a hallmark of apoptosis, we examined Caco2-BBE cell lysates using Western blot analysis (Krysko et al., 2008). As expected, we detected PARP cleavage after treatment with the apoptosis inducer, staurosporine (Figure 2, STS). However, Caco2-BBE cells co-cultured with AM-19226 T3SS WT, an AM-19226 T3SS-null strain (ΔvcsN2) or mock treated do not exhibit PARP cleavage (Figure 2). Together these results strongly suggest that the observed Caco2-BBE death is not occurring by apoptosis. Interestingly, Tam and coworkers used cell cycle analysis to observe HeLa cells co-cultured with strain AM-19226, and reported a shift of G1 to S phase and an increase in the apoptotic cell population (17%), compared to co-culture with the T3SS-null strain (9%) (Tam et al., 2010). The use of a different cell line, a higher MOI and the absence of bile in their studies may account for the difference in results. However, TUNEL staining identified a much smaller population (<5%) of apoptotic cells in their studies, which is more consistent with our results.
Table 2.
Chemicals and inhibitors that do not block cell death.
| Chemical / Inhibitor | Function |
|---|---|
| Z-VAD-fmk | Pan-caspase inhibitor |
| Z-VD-OPh | Pan-caspase inhibitor |
| Z-YVAD-fmk | Caspase-1 inhibitor |
| Necrostatin-1 | RIP1 kinase inhibitor |
| PJ-34 | PARP inhibitor |
| BAPTA-AM | Intracellular calcium chelator |
| EGTA-AM | Intracellular calcium chelator |
| Glycine | Prevents non-specific ion flux |
| Cyclosporin A | Mitochondrial permeability transition pore inhibitor |
Figure 2. Caco2-BBE PARP is not cleaved during AM-19226 co-culture.
AM-19226 T3SS WT and ΔvcsN2 strains were grown overnight in LB medium and used to infect Caco2-BBE cells at an MOI of ~10 in medium containing 0.2% bile. After 1h, 1.5h, or 2h of co-culture, Caco2-BBE protein lysates were harvested and immunoblotted with anti-poly(ADP-ribose) polymerase (PARP) and anti-GAPDH antibodies. Protein lysates were also harvested from mammalian cells treated with 2µM staurosporine (STS) for 14 hours to induce apoptosis. Experiments were repeated twice and yielded similar results.
Evaluating necrotic mechanisms of cell death and the role of second messengers
T3SS mediated bacterial infections commonly initiate necrotic cell death mechanisms, including pyroptosis and necroptosis (Fink et al., 2007, Bleriot et al., 2016). We tested chemical inhibitors of different programmed necrotic death mechanisms shown in Table 2. Z-VAD-fmk and Z-VD-OPh are pan-caspase inhibitors that block apoptosis as well as pyroptosis, whereas Z-YVAD-fmk specifically blocks activity of caspases that mediate pyroptosis. Glycine can transiently block pyroptosis by preventing non-specific ion fluxes in mammalian cells (Brennan et al., 2000, Bergsbaken et al., 2009b). Since over activated PARP can lead to cell death (Galluzzi et al., 2012), we blocked PARP activation using the inhibitor, PJ-34. Treatment with PJ-34 decreased cytotoxicity (~30%) only at a 100µM concentration, which is ~100-fold greater than concentrations necessary for selective inhibition, suggesting that PARP activation was not the primary cause of cell death and that off target effects could decrease LDH release (data not shown)(Antolin et al., 2012). We also included necrostatin-1, which inhibits RIP1 kinase activity and therefore necroptosis (Galluzzi et al., 2012). None were able to inhibit cytotoxicity. Neither of two intracellular calcium chelators, BAPTA-AM or EGTA-AM, prevented AM-19226 mediated cell death. Since both compounds function in mammalian cells to suppress transient calcium fluxes that can lead to cell death, the results do not support a mechanism that requires calcium mobilization (Tsien, 1981, Zhivotovsky et al., 2011). We also investigated whether mitochondrial damage was involved in cytotoxicity, since evidence suggests that the VopE effector protein can influence mitochondrial dynamics by interacting with Miro GTPase proteins (Suzuki et al., 2014). However, inhibiting the activity of the mitochondrial permeability transition pore using cyclosporin A did not affect cytotoxicity. Together these data suggest that AM-19226 does not induce Caco2-BBE cell death by common programmed necrotic mechanisms.
Investigating osmotic cell lysis as a mechanism of T3SS-dependent cytotoxicity
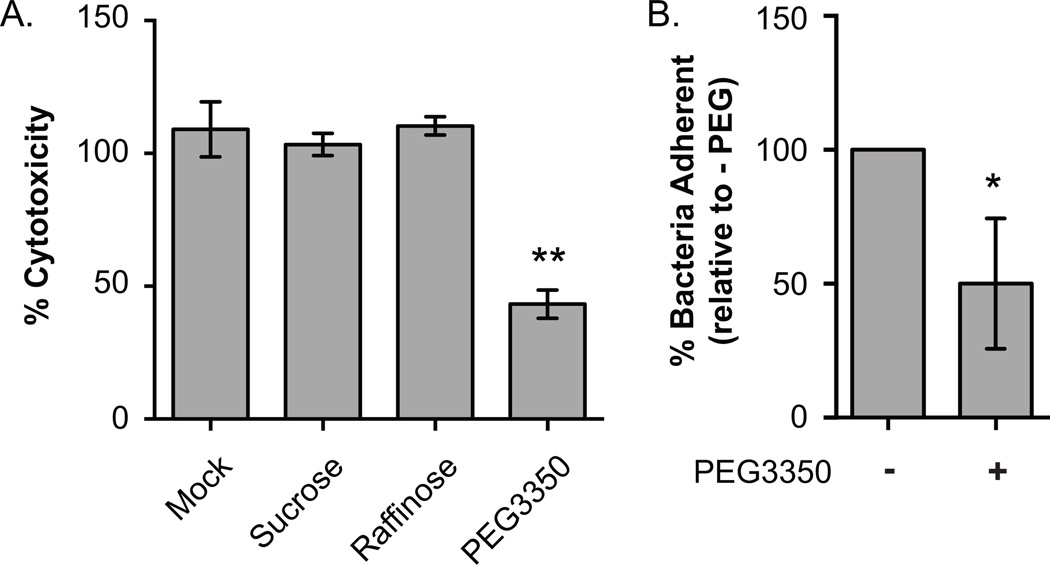
Given that most AM-19226 effector proteins activities are poorly understood, and that bile perturbs mammalian cell membranes and is required for cell death in our model system, we sought to investigate if osmotic lysis contributes to AM-19226 induced intestinal epithelial cell death. Various solutes can be used to attenuate osmotic lysis, and osmoprotectants of known sizes can also decrease water influx into the cell through membrane pores. We therefore added the following molecules to the media during co-culture: sucrose (0.9nm), which also can establish an isotonic environment to prevent water loss across the plasma membrane, raffinose (1.14nm), and PEG3350 (3.80nm), which can also create a hypertonic environment (Clinkenbeard et al., 1991, Fink et al., 2006). Neither sucrose nor raffinose altered cytotoxicity levels, but addition of 30mM PEG3350 decreased cytotoxicity ~2-fold (Figure 3A).
Figure 3.
(A) Pore formation influences cytotoxicity. Three independent colonies of the AM-19226 T3SS WT strain were grown overnight in LB and used to infect Caco2-BBE cells at an MOI of ~10 in the presence of 0.2% bile with or without 30mM sucrose, 30mM raffinose, or 30mM PEG3350. Percent cytotoxicity was calculated at 3h after co-culture began by measuring LDH released into the supernatant. Data represent the mean ± standard deviation from one representative experiment. The experiment was repeated once with similar results. **, P<0.0001 by one-way ANOVA with Dunnett post hoc test. (B) Bacterial adherence in the presence of PEG. AM-19226 T3SS WT was grown overnight in LB and co-cultured with Caco2-BBE and an MOI of ~10 in the presence of 0.2% bile with or without 30mM PEG3350. After 2h, percent of bacteria adherent to Caco2-BBE cells was determined. Data represent the mean ± standard deviation from three independent experiments using four individual bacterial colonies per experiment. * P<0.05 by t-test.
We recovered similar bacterial cell numbers from the co-culture medias at 2 h (7.2×106 CFU/mL for mock vs. 6.6×106 for PEG3350 treated wells), suggesting that PEG3350 does not affect bacterial replication. We next determined whether PEG3350 influences bacterial adherence. We found that the effect of PEG3350 was variable, and the averaged results of 3 experiments suggest that PEG3350 decreased adherence ~2-fold (Figure 3B). While we currently do not understand the precise relationship between adherence, MOI, and cytotoxicity, we do know that ~20–30% of the bacterial population is adherent at 2hrs using an MOI of ~10, and that cytotoxicity remains at ~80% at 3hrs if the MOI drops to ~5. The MOI must be decreased ten-fold to ~1 in order to observe a decrease in cytotoxicity, measured as 2-fold (to ~40% at 3 hrs) (Miller et al., 2016). If the proportion of adherent bacteria at 2hrs, when little replication has occurred, remains similar regardless of MOI, then a 10-fold decrease in adherence is required to elicit the 2-fold decrease in cytotoxicity observed by decreasing the MOI 10-fold to ~1. We therefore propose that in our experiment, a 2-fold decrease in adherence alone is insufficient to affect cytoxicity 2-fold, and suggest that PEG attenuates cytotoxicity due to combined effects on adherence and in a more traditional role as an osmoprotectant.
The phenotype of rapid Caco2-BBE LDH release is consistent with cytotoxicity mediated by pore formation or other mechanisms leading to membrane disruption and osmotic lysis. Currently, none of the identified effector proteins share sequence similarity to pore forming toxins, yet cytotoxicity is T3SS dependent. Examples of T3SS translocon insertion resulting in cell death due to colloid osmotic lysis have been reported for other pathogens, and some translocon components structurally resemble pore-forming toxins (Dacheux et al., 2001, Miki et al., 2010, Barta et al., 2012). However, the precise mechanism of translocon pore-related lysis is difficult to resolve, especially since the internal diameter of the T3SS translocon reportedly ranges from ~1.2–3.5nm, depending on the bacterial species (Galan et al., 2006, Mattei et al., 2011). Effectors that regulate pore formation have been identified in Yersinia and E. coli, and the process is now recognized as complex, involving repair of translocon “pores”, and is likely to be highly regulated (Guignot et al., 2015, Guignot et al., 2016). Our collective results are therefore consistent with a hypothesis that AM-19226 induced cell death likely proceeds by currently undefined molecular mechanisms that shares characteristics with osmotic lysis.
Identifying host cell signaling pathways that respond to bile and T3SS-positive V. cholerae
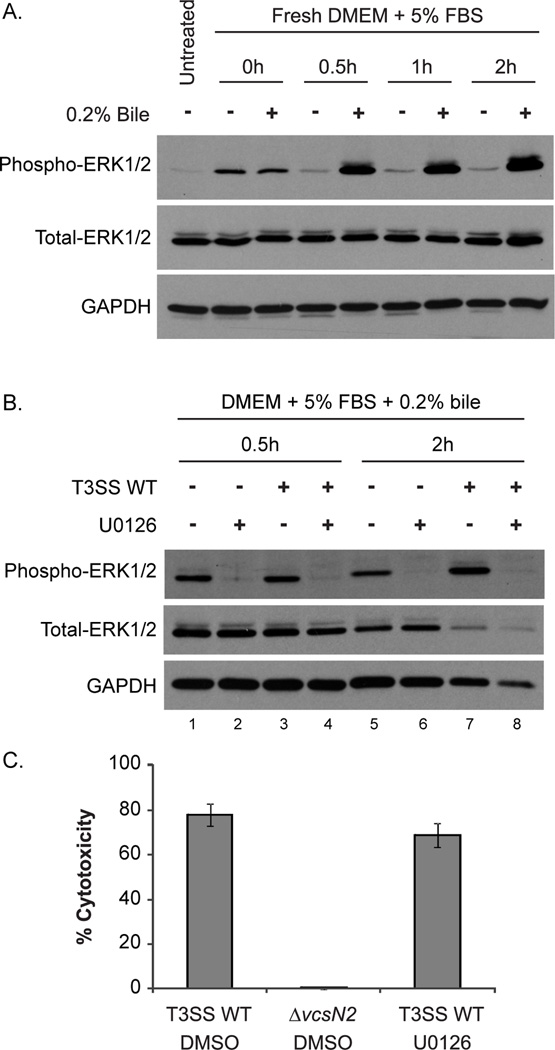
We found several reports in the literature documenting the effects of individual purified bile components in stimulating the MEK/ERK mammalian cell signaling pathway (Qiao et al., 2001, Akare et al., 2005). To our knowledge, however, studies have not examined the impact of whole, crude bile on epithelial cell ERK1/2 signaling. We therefore exposed Caco2-BBE monolayers to 0.2% bile for up to 2h (in the absence of bacteria), harvested the monolayers, and then examined lysates for ERK1/2 phosphorylation (Figure 4A). The untreated sample shows basal levels of ERK1/2 phosphorylation after monolayer growth overnight. Addition of fresh culture medium without bile at 0h results in transient ERK1/2 activation, but addition of medium with bile results in slightly elevated ERK1/2 phosphorylation at 0h that further increases at later time points as compared to untreated cells (Figure 4A). Densitometry calculation quantify a ~15-fold increase in ERK1/2 phosphorylation after a 2h bile treatment, compared to the same time point in the absence of bile. We therefore concluded that crude bile induces ERK1/2 activation in Caco2-BBE cells.
Figure 4.
(A) Bile induces Caco2-BBE ERK1/2 phosphorylation. Caco2-BBE cells were grown for 48h to 80% confluence and protein lysates were harvested from one sample (untreated). Remaining samples were washed with PBS and supplied with fresh DMEM containing 5% FBS with or without 0.2% bile. Protein lysates were harvested 0.5h, 1h, or 2h after media change and probed with antibodies against phosphorylated ERK1/2, total ERK1/2, and GAPDH. The experiment was repeated twice with similar results. (B) U0126 inhibits bile dependent ERK phosphorylation. Caco2-BBE cells were grown as in (A) and were then treated with 10µM U0126 or DMSO in DMEM containing 5% FBS for 1h. Media was removed and replaced with identical medium containing 0.2% bile. Cells were co-cultured with AM-19226 T3SS WT (MOI ~10) grown overnight in LB or were mock infected. Protein lysates were harvested 0.5h or 2h after media change and probed with antibodies against the indicated proteins. The experiment was repeated once with similar results. (C) Blocking ERK1/2 phosphorylation does not inhibit AM-19226 mediated Caco2-BBE cytotoxicity. Caco2-BBE cells were treated with 10µM U0126 or DMSO in DMEM containing 5% FBS for 1h. Media was removed and replaced with identical medium containing 0.2% bile. Caco2-BBE cells were infected with AM-19226 T3SS WT or ΔvcsN2 strains grown overnight in LB medium at an MOI of ~10. After 3h of co-culture, percent cytotoxicity was determined by measuring LDH release in the supernatant. Data shown represents one experiment using five independent colonies per strain. The experiment was repeated once and yielded similar results.
Studies in Saccharomyces cerevisiae show that the AM-19226 effector protein, VopX, may target proteins in the yeast cell wall integrity pathway, which includes protein orthologs to mammalian MEK/ERK family proteins (Qi et al., 2005, Alam et al., 2011, Seward et al., 2015). We reasoned that both bile and bacterial effector proteins could target the MEK/ERK pathway, thus potentiating ERK1/2 over-activation and detrimental consequences. We therefore examined ERK1/2 phosphorylation in the presence of bile, varying whether bacteria were or were not added to the Caco2-BBE cells. Although we observed ERK1/2 phosphorylation at 0.5h and 2h in the presence of bacteria (Figure 4B, lanes 3 and 7), ERK1/2 phosphorylation also occurred in response to bile when no bacteria were present (Figure 4B, compare lane 1 to 3 and lane 5 to 7). Since both bile and bacteria are required for cell death, we could not definitively determine whether V. cholerae alone induced ERK activation that then resulted in cytotoxicity.
However, we recognized the importance of examining whether ERK1/2 phosphorylation (regardless of the stimuli) is important in AM-19226 T3SS-mediated cell death. We therefore blocked the activity of the MEK1/2 kinases that lie upstream of ERK by adding U0126 to the co-culture media, thereby increasing the pool of unphosphorylated, inactive ERK1/2 (Favata et al., 1998, Johnson et al., 2002). We first demonstrated that ERK1/2 phosphorylation was not observed in the presence of U0126 by performing Western blot analysis for ERK1/2 using Caco2-BBE cell lysates from standard co-culture conditions (Figure 4B). To determine if ERK1/2 activation is required for Caco2-BBE cell death, we performed the co-culture assay with bile and AM-19226 in the presence or absence of U0126 and measured LDH release. As shown in Figure 4C, addition of U0126 does not abrogate mammalian cell cytotoxicity, suggesting that ERK1/2 activation does not lead to cell death. Furthermore, we did not observe p38 MAPK or JNK MAPK phosphorylation during Caco2-BBE-V. cholerae co-culture in the presence of bile, suggesting that neither signaling pathway is altered during the time preceding cell death (data not shown). It is therefore plausible that crude bile is acting on the Caco2-BBE membrane in a manner similar to that reported for deoxycholic acid alone, rendering ERK1/2 activation as a marker of cell membrane perturbations rather than a cause of cell death (Akare et al., 2005). Although co-culture conditions differ, in comparison, blocking the activity of upstream kinases MEK1/2 does decrease V. parahaemolyticus T3SS1-dependent cell death (Matlawska-Wasowska et al., 2010, Yang et al., 2011). Our results thus far suggest that although MEK/ERK pathway perturbation occurs during AM-19226/Caco2-BBE co-culture in the presence of bile, blocking MEK/ERK pathway signaling does not prevent the cytotoxic effect of AM-19226.
AM-19226 disrupts Caco2-BBE cell-cell junctions and membrane skeleton organization
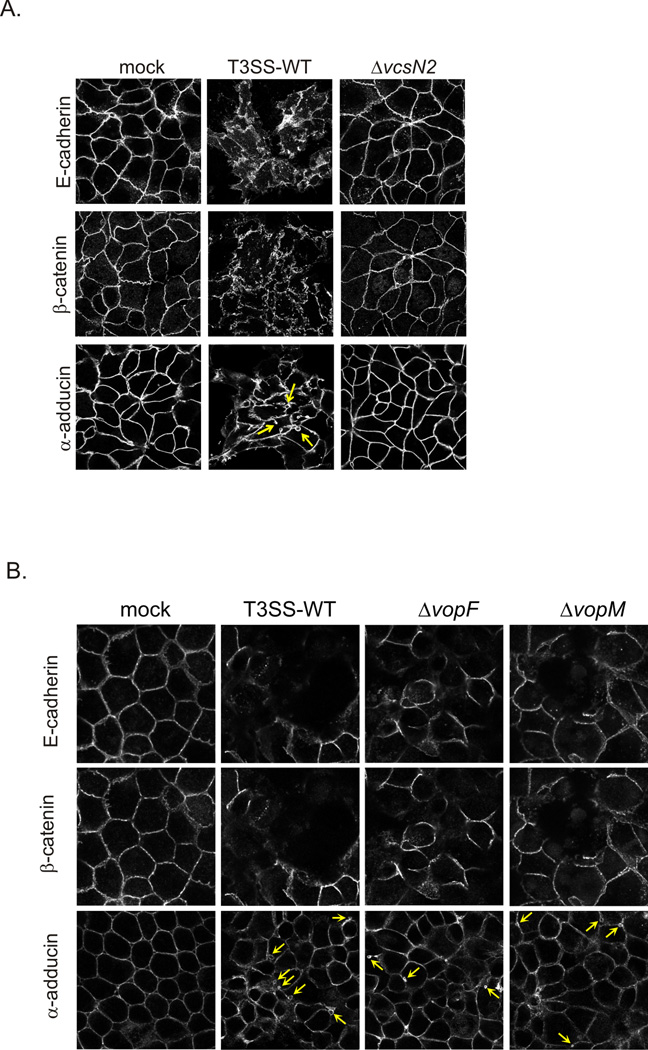
We next examined the response of host cells by determining whether markers for adherens junctions, which are a well-known target of enteric pathogens, were disrupted in the presence of T3SS-positive bacteria (Viswanathan et al., 2009). After 2h of Caco2-BBE/AM-19226 co-culture in the presence of bile, we fixed and stained cells with antibodies against the adherens junction proteins, E-cadherin and β-catenin, and α-adducin, a membrane skeleton protein that is associated with the basolateral plasma membrane of differentiated epithelial cells (Naydenov et al., 2010). Mock treated and AM-19226 ΔvcsN2 (T3SS-null) co-cultured Caco2-BBE cells displayed intact cell-cell junctions as evidenced by continuous staining of E-cadherin and β-catenin at the cell periphery (Figure 5A). In contrast, co-culture with the AM-19226 strain carrying wild-type genes encoding a functional T3SS (T3SS WT) results in disruption of the adherens junctions. Furthermore, T3SS activity induces changes in the distribution of α-adducin, including formation of vesicles indicating increased dynamics of the lateral plasma membrane, as compared to both the mock infected and AM-19226 ΔvcsN2 infected cells.
Figure 5. T3SS activity disrupts Caco2-BBE cell-cell junctions.
(A) AM-19226 T3SS WT or ΔvcsN2 strains were grown overnight in LB medium and were used to infect Caco2-BBE cells at an MOI of ~10 in the presence of 0.2% bile. Two independent colonies and wells were infected for each experiment. After 2h of co-culture, the cells were washed once with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Monolayers were stained with antibodies against E-cadherin, β-catenin, and α-adducin followed by Alexafluor conjugated secondary antibodies. Arrows indicate membrane vesicles. One representative image is shown, and the experiment was repeated three times with similar results. (B) VopM and VopF effectors that alter cytoskeletal dynamics do not contribute to junctional protein rearrangement. The experiment was conducted as described in (A), using AM-19226 T3SS WT, ΔvcsN2 (T3SS-negative), ΔVopM and ΔVopF strains. Images from one representative experiment of four independent experiments are shown.
Because tight junctions are stabilized by the actin cytoskeleton, we investigated whether VopM and VopF, two AM-19226 T3SS effector proteins that modulate host actin, are responsible for the phenotype (Tam et al., 2007, Hiyoshi et al., 2011, Suzuki, 2013). Tam et al. reported a ~50% decrease in transepithelial resistance (TER) and disruption of the tight junction protein, ZO-1 in a polarized T84 intestinal epithelial cell co-culture model (Tam et al., 2010). However, as shown in Figure 5B, co-culture of Caco2-BBE with VopM or VopF deletion strains triggers similar disruption of adherens junctions and vesiculation of the membrane skeleton as compared to cells co-cultured with the isogenic parental strain (T3SS WT). Interestingly, co-culture with AM-19226 ΔvopF resulted in a modest decrease in TER (~30%), suggesting that while VopF is involved in tight junction disruption, other proteins likely contribute to the process or encode redundant functions (Tam et al., 2010). Together, the data suggest that other AM-19226 effector proteins function to remodel host cell adherens and tight junctions via actin reorganization and membranotropic effects. Nonetheless, such changes to the host cell physiology can not only contribute to diarrhea, but also perhaps reveal a niche for increased bacterial colonization (Muza-Moons et al., 2003).
Role of individual effector proteins in cytotoxicity
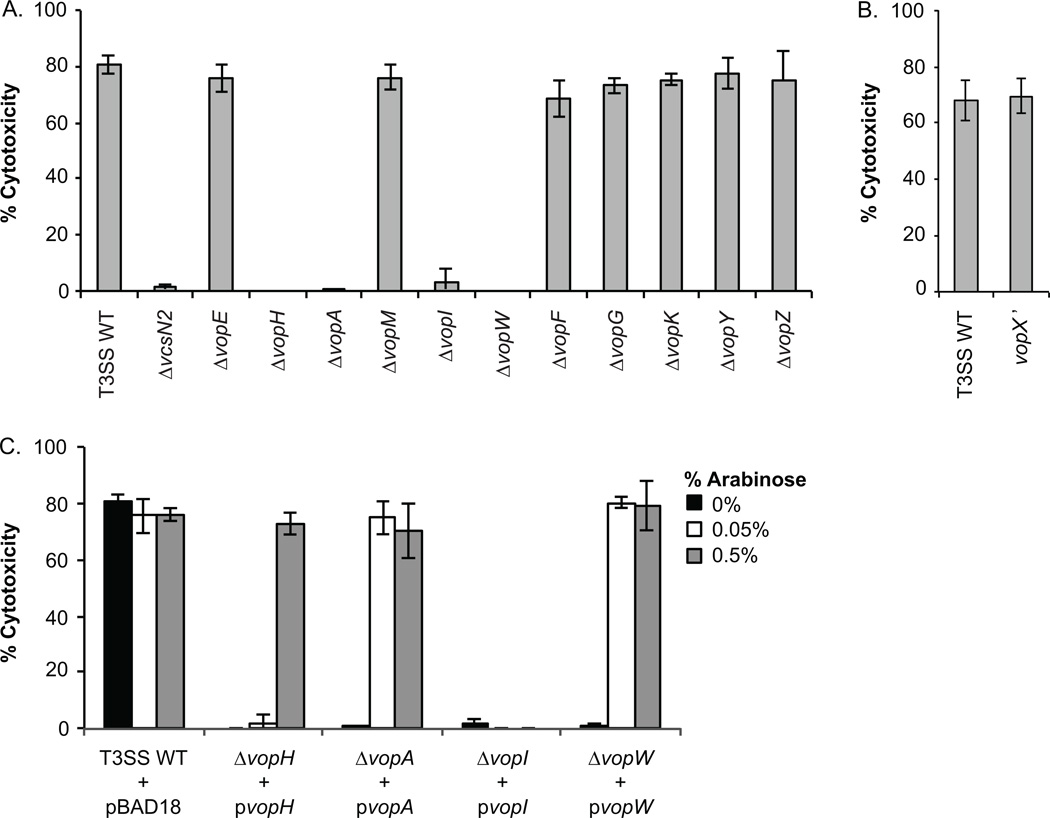
Reasoning that mammalian cell cytotoxicity is due to the action of specific T3SS effector proteins, we constructed individual, in-frame deletions in each 11 known effector proteins in V. cholerae strain AM-19226. Each strain was then assayed for the ability to cause cytotoxicity in our in vitro co-culture assay. As shown in Figure 6A, strains deleted for Vops H/A/I/or W caused less than 5% Caco2-BBE cytotoxicity, similar to a T3SS-null strain (ΔvcsN2), whereas co-culture with strains that did not encode Vops E/M/F/G/K/Y/or Z still caused wild-type levels of cell death (70%-80% cytotoxicity, Figure 6A). Previous studies determined that a VopX deletion strain had altered T3SS gene expression due to regulatory sequences that overlap the vopX coding region, so we constructed and assayed separately an AM-19226 strain carrying a copy of the vopX gene that produces a truncated protein (vopX’) (Miller et al., 2016). Co-culture of Caco2-BBE cells with a strain unable to produce VopX resulted in cytotoxicity similar to the parental strain (Figure 6B), indicating that the VopX effector alone is not responsible for cell death. To complement the null phenotype in the Vops H/A/I/ and W deletion strains, we transformed each effector null strain with a plasmid expressing the appropriate vop gene from an arabinose inducible promoter, and performed the co-culture assay in the presence of 0.2% bile and different concentrations of arabinose (Figure 6C). While we were able to complement the vopH/A/W deletions for cytotoxicity with the respective plasmids (Figure 6C), we were unable to complement the vopI deletion strain (Figure 6C). VopI is encoded within an operon where downstream gene products include structural apparatus components and the VopM and VopW effector proteins (Alam et al., 2010, Chaand et al., 2013). In the current VopI deletion strain, mRNA stability or translational efficiency could be altered, therefore resulting in unanticipated polar effects if structural protein or effector protein levels are critical for function. Based on our results, we conclude that Vops H/A/W are required for Caco2-BBE cytotoxicity. Interestingly, however, Vops H/A/W are also required for translocation of other Vops, suggesting that Vops H/A/W function as part of the T3SS structural or translocation apparatus rather than exerting activities within the host cell to cause cytotoxicity (Chaand et al., 2015). Determination of the role of VopI awaits construction of a truncation mutant, similar to that used to assess VopX function.
Figure 6. Genetic analysis of Vops required for Caco2-BBE cytotoxicity.
AM-19226 strains were grown overnight in LB medium and were used to infect Caco2-BBE cells in the presence 0.2% bile at an MOI of ~10. After 3h of co-culture, percent cytotoxicity was determined by measuring LDH in co-culture supernatants. (A) Co-culture was performed with the parental AM-19226 strain, a strain deleted for vcsN2, strains deleted for individual effector protein genes (Δvop). (B) Co-culture was performed with the parental AM-19226 strain or a vopX truncation mutant strain (vopX’) (C) Co-culture was performed with the parental strain, ΔvopH, ΔvopA, ΔvopI, or ΔvopW carrying pBAD18, pBAD18-vopH, pBAD18-vopA, pBAD18-vopI, or pBAD18-vopW. Arabinose was added to the co-culture medium at the indicated concentrations to induce vop expression from the pBAD18 plasmids. For each panel, data are shown from one experiment using three colonies per strain. Each experiment was repeated twice and produced similar results.
Conclusions
We investigated the conditions that resulted in rapid Caco2-BBE cytotoxicity. Although bile could be acting on the V. cholerae cells to increase expression of T3SS genes, expression of the T3SS structural gene operon, vcsRTCNS2, during co-culture with 0.2% bile is similar to that in the absence of bile (Miller et al., 2012). Alternatively, bile could increase the secretory activity of the T3SS, as has been demonstrated for Shigella. Picking and coworkers presented evidence that sodium deoxycholate increases secretion through the apparatus by recruiting the translocon components, IpaB and IpaD, to the needle tip (Olive et al., 2007, Dickenson et al., 2013). An analogous situation could exist for V. cholerae, where in addition, bile could be acting on the Caco2-BBE cells as a sensitizer or stimulator of signaling pathways targeted by effector protein activity. Bile also likely influences the membrane composition and dynamics of the bacterial or eukaryotic cells, altering fatty acid, lipid and/or cholesterol content, perhaps altering membrane sensitivity or response to T3SS translocon pore formation (Giles et al., 2011). Numerous reports document a role for bile components (specifically deoxycholate) in altering the permeability and structure of epithelial cell junctional barriers, which is consistent with our results demonstrating the bile requirement for cytotoxicity and the disruption and reorganization of junctional proteins during V. cholerae co-culture (Raimondi et al., 2008, Deli, 2009). Interestingly, PEG solutions of various molecular weights have been reported to exert protective effects on intestinal cells damaged by environmental and chemical insults (Bharadwaj et al., 2011, Edelstein et al., 2011).
Based on our current results, we were able to provide convincing data for the role of the T3SS in modulating cytotoxicity although we were unable to identify the mechanism of how T3SS activity or specific effectors lead to cell death. While the targets and functions of most AM-19226 T3SS effector proteins are unknown, both V. cholerae VopF and V. parahaemolyticus VopM homolog, VopV, can reorganize host actin, although it is currently not known whether the proteins encode redundant functions or play separate roles in the host (Tam et al., 2007, Hiyoshi et al., 2011). While our studies were underway, a T3SS2 effector protein was identified in V. parahaemolyticus and named VopZ, distinct from the VopZ effector protein identified earlier in strain AM-19226 (Alam et al., 2011, Zhou et al., 2013). In V. parahaemolyticus, VopZ is critical for host colonization and diarrheal disease in the infant rabbit model, but is not required for adherence (Zhou et al., 2013). Our initial studies indicate that the V. cholerae VopZ homolog, sharing ~68% amino acid similarity with the V. parahaemolyticus protein, may encode different functions, and additional experiments are therefore required to determine what role the protein plays in AM-19226 pathogenicity and cytotoxicity.
Because functional redundancy is a common theme among bacterial effector proteins encoded by a single pathogen, an alternative interpretation allows for multiple AM-19226 T3SS effector proteins to act coordinately to cause mammalian cell death (Galan, 2009). Reports of microvillus blunting and disruption of the intestinal epithelial cell surface in vivo in the infant rabbit model are consistent with our in vitro observations (Shin et al., 2011), and non-apoptotic cell death (e.g. necrosis) is often associated with cell swelling and rupture of the cell membrane leading to release of intracellular contents that is considered pro-inflammatory. Such observations are consistent with reports of inflammation associated with clinical outcomes of non-O1/non-O139 associated disease. We therefore expect the cytotoxicity phenotype will serve as a useful tool to dissect the roles and activities of T3SS-related proteins, and lead to a more complete understanding of how non-epidemic strains can cause gastroenteritis by disrupting host cell homeostasis using novel, cholera-toxin independent mechanisms.
Experimental Procedures
Bacterial strains, growth conditions, and in silico analysis
Strains were maintained and grown under standard conditions and as described. Ampicillin (Amp) and streptomycin (Str) were used at 100µg/ml for Escherichia coli and Vibrio cholerae. Bile (bile bovine B3883; Sigma) was prepared as previously described (Alam et al., 2010). Clone Manager Professional Suite, version 9 (Sci-Ed Software) was used for sequence analysis.
Strain and plasmid construction
Nucleic acid manipulations were performed using standard molecular biology techniques (Sambrook et al., 1989). Non-polar, in-frame deletions of vopE, vopH, vopA, vopM, vopI, vopW, vopF, vopG, vopK, vopY, and vopZ, were constructed in V. cholerae strain AM-19226 ΔhapΔhlyAΔrtxA (Δ3) as previously described (Horton et al., 1989, Donnenberg et al., 1991, Alam et al., 2010, Alam et al., 2011, Chaand et al., 2015). Deletions were confirmed by PCR and Southern blot analyses. pBAD18-vopA was cloned using restriction sites so that the vopA open reading frame is downstream of the arabinose inducible promoter. pBAD18 was converted to a Gateway compatible vector using the Gateway vector conversion system (Invitrogen) to result in pBAD18rfaKM, which was then used in standard Gateway protocols to produce pBAD18-vopH, pBAD18-vopI, and pBAD18-vopW. The vopX’ truncation was constructed by introducing a single nucleotide substitution (A157T) resulting in amino acid change K54X, which introduced a unique BglII site as described (Miller et al., 2016).
Mammalian cell lines and culture conditions
Caco2-BBE cells were maintained in Dulbecco’s modified Eagle medium (4.5 mg/ml glucose, sodium pyruvate, and L-glutamine; Invitrogen) supplemented with 10% FBS (Gemini Bio-Products) at 37°C with 5% CO2. Co-culture of Caco2-BBE and V. cholerae strain AM-19226 was performed in the presence of DMEM supplemented with 5% FBS as previously described, using DMEM containing 1mg/ml glucose (low glucose medium, Invitrogen) for arabinose induction experiments (Miller et al., 2012). Staurosporine was added to Caco2-BBE cells at 2µM for 14h to induce apoptosis.
Lactate dehydrogenase release assay
Percent cytotoxicity was determined as previously described, using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega) as instructed by the manufacturer (Miller et al., 2012). Briefly, Caco2-BBE cells were seeded in 96 well plates, grown ~24h to ~80% confluency, and infected at an MOI ~10 using strains grown overnight at 37°C in LB medium (or LB containing 0.4% bile). To determine bacterial replication following co-culture, co-culture supernatant was serially diluted and plated on LB agar. Bacterial colony forming units were enumerated and compared to the initial bacterial inoculum. Bile and/or arabinose were added to the culture medium as indicated. Detergents and bile salts were added to the co-culture medium at the following concentrations: 0.05% CHAPS, 0.01% Tween-80, 0.001% Triton X-100, 0.005% SDS, 0.01% sodium deoxycholate, 0.04% sodium cholate. For studies using pharmacological inhibitors, Caco2-BBE cells were pretreated for ~1h in DMEM medium lacking bile and the inhibitors were included in the subsequent co-culture media at the following concentrations: 10µM U0126 (VWR Scientific), 100µM necrostatin-1, 50 and 100µM PJ-34 (Santa Cruz Biotechnology), 50µM Z-VAD-fmk, 30µM Z-VD-OPh (MP Biomedicals), 50µM Z-YVAD-fmk (Santa Cruz Biotechnology), 1, 5, and 10µM cyclosporinA (Sigma), 10µM BAPTA-AM (Santa Cruz Biotechnology), 10µM EGTA-AM (gift from Dr. David Yule). Sucrose, raffinose, or polyethylene glycol 3350 (PEG3350) were included in the co-culture medium at 30mM. Glycine was included in the co-culture medium at 5mM and 100mM.
Adherence Assay
Bacterial adherence to Caco2-BBE cells was performed as previously described (Chaand et al., 2015). Briefly, Caco2-BBE cells were seeded on collagen coated coverslips (Neuvitro) in DMEM + 5% FBS for 48h or in 12-well plates. Confluent cell monolayers were washed with PBS and co-cultured with bacteria at an MOI of ~10 in DMEM + 5% FBS + 0.2% bile for 2h. Additionally, bacterial cells were added to wells containing culture medium alone to calculate bacterial growth (output). Following co-culture, wells were washed 3x with PBS. If coverslips were used they were transferred to fresh wells, and washed again in PBS. Caco2-BBE cells were lysed with 0.1% Triton X-100 in PBS for 1h. Serial dilutions were plated to determine the number of adherent bacteria. Percent adherent bacteria = (coverslip-associated bacteria at 2h/output at 2h) × 100.
Western blot analysis of mammalian proteins
3×106 Caco2-BBE cells were seeded into 10cm dishes in DMEM containing 10% FBS, washed the following day (~24h) with PBS and supplied with DMEM containing 5% FBS. Cells were ~80% confluent by the next day (~48h), and were washed with PBS and supplied fresh DMEM containing 5% FBS with or without 0.2% bile. AM-19226 co-culture and a mock co-culture control were performed as described above. At indicated time points mammalian cells were harvested with a cell scraper, washed in PBS, and flash frozen. Mammalian cells were then lysed on ice in RIPA buffer (Tris, EDTA, EGTA, Triton X-100, sodium deoxycholate, SDS, and NaCl) containing mammalian protease and phosphatase inhibitors (Sigma), sonicated for 15s, and cellular debris was pelleted at 13,000 rpm for 10 minutes. Resulting supernatants were electrophoresed on 10% SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with the following antibodies: anti-phosphorylated ERK1/2 (Cell Signaling), anti-total ERK1/2 (Cell Signaling), anti-PARP (Cell Signaling) and anti-GAPDH (Sigma).
Immunofluorescence staining of Caco2-BBE/AM-19226 co-culture
Caco2-BBE cells were seeded on collagen IV coated coverslips (Neuvitro) in 24 well plates. After ~24h, Caco2-BBE cells were infected with AM-19226 strains at an MOI of 10 in the presence of 0.2% bile. Following 2h of co-culture, monolayers were washed with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.1% TritonX-100. Caco2-BBE cells were probed with antibodies against E-cadherin (Invitrogen), β-catenin (Invitrogen), and α-adducin (Santa Cruz Biotechnology) followed by Alexafluor conjugated secondary antibodies. Proteins were visualized on an Olympus confocal microscope.
Statistical analyses
Statistical analyses were conducted using GraphPad Prism with one-way ANOVA followed by Dunnett post hoc test or two-tailed t-test as appropriate.
Acknowledgments
We thank David Yule for sharing reagents, Linda Callahan and the Imaging Core for expert assistance, Michael Elliott, John Frelinger and members of the Dziejman laboratory for helpful discussions. This work was supported by NIH R01 AI0737085 (M.D.), NIH T32 AI007362 (K.A.M), NIH R21 AR062357 (L.A.B.) and NIH R01 DK083968 and DK084953(A.I.I.).
Footnotes
The authors declare no conflicts of interest.
References
- Akare S, Martinez JD. Bile acid induces hydrophobicity-dependent membrane alterations. Biochim Biophys Acta. 2005;1735:59–67. doi: 10.1016/j.bbalip.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Alam A, Miller KA, Chaand M, Butler JS, Dziejman M. Identification of Vibrio cholerae type III secretion system effector proteins. Infect Immun. 2011;79:1728–1740. doi: 10.1128/IAI.01194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A, Tam V, Hamilton E, Dziejman M. vttRA and vttRB Encode ToxR family proteins that mediate bile-induced expression of type three secretion system genes in a non-O1/non-O139 Vibrio cholerae strain. Infect Immun. 2010;78:2554–2570. doi: 10.1128/IAI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolin AA, Jalencas X, Yelamos J, Mestres J. Identification of pim kinases as novel targets for PJ34 with confounding effects in PARP biology. ACS chemical biology. 2012;7:1962–1967. doi: 10.1021/cb300317y. [DOI] [PubMed] [Google Scholar]
- Bagchi K, Echeverria P, Arthur JD, Sethabutr O, Serichantalergs O, Hoge CW. Epidemic of diarrhea caused by Vibrio cholerae non-O1 that produced heat-stable toxin among Khmers in a camp in Thailand. J Clin Microbiol. 1993;31:1315–1317. doi: 10.1128/jcm.31.5.1315-1317.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta ML, Dickenson NE, Patil M, Keightley A, Wyckoff GJ, Picking WD, et al. The structures of coiled-coil domains from type III secretion system translocators reveal homology to pore-forming toxins. J Mol Biol. 2012;417:395–405. doi: 10.1016/j.jmb.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005a;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005b;73:894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Cookson BT. Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation. J Leukoc Biol. 2009a;86:1153–1158. doi: 10.1189/jlb.0309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009b;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S, Vishnubhotla R, Shan S, Chauhan C, Cho M, Glover SC. Higher molecular weight polyethylene glycol increases cell proliferation while improving barrier function in an in vitro colon cancer model. Journal of biomedicine & biotechnology. 2011;2011:587470. doi: 10.1155/2011/587470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleriot C, Lecuit M. The interplay between regulated necrosis and bacterial infection. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- Chaand M, Dziejman M. Vibrio cholerae VttRA and VttRB Regulatory Influences Extend beyond the Type 3 Secretion System Genomic Island. J Bacteriol. 2013;195:2424–2436. doi: 10.1128/JB.02151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaand M, Miller KA, Sofia MK, Schlesener C, Weaver JW, Sood V, Dziejman M. Type 3 Secretion System Island Encoded Proteins Required for Colonization by Non-O1/non-O139 Serogroup V. cholerae. Infect Immun. 2015 doi: 10.1128/IAI.03020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Ghosh K, Raychoudhuri A, Chowdhury G, Bhattacharya MK, Mukhopadhyay AK, et al. Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J Clin Microbiol. 2009;47:1087–1095. doi: 10.1128/JCM.02026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A. 2009;106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinkenbeard KD, Thiessen AE. Mechanism of action of Moraxella bovis hemolysin. Infect Immun. 1991;59:1148–1152. doi: 10.1128/iai.59.3.1148-1152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux D, Goure J, Chabert J, Usson Y, Attree I. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol Microbiol. 2001;40:76–85. doi: 10.1046/j.1365-2958.2001.02368.x. [DOI] [PubMed] [Google Scholar]
- Dalsgaard A, Albert MJ, Taylor DN, Shimada T, Meza R, Serichantalergs O, Echeverria P. Characterization of Vibrio cholerae non-O1 serogroups obtained from an outbreak of diarrhea in Lima, Peru. J Clin Microbiol. 1995;33:2715–2722. doi: 10.1128/jcm.33.10.2715-2722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard A, Forslund A, Bodhidatta L, Serichantalergs O, Pitarangsi C, Pang L, et al. A high proportion of Vibrio cholerae strains isolated from children with diarrhoea in Bangkok, Thailand are multiple antibiotic resistant and belong to heterogenous non-O1, non-O139 O-serotypes. Epidemiol Infect. 1999;122:217–226. doi: 10.1017/s0950268899002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788:892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Dickenson NE, Arizmendi O, Patil MK, Toth RTt, Middaugh CR, Picking WD, Picking WL. N-terminus of IpaB provides a potential anchor to the Shigella type III secretion system tip complex protein IpaD. Biochemistry. 2013;52:8790–8799. doi: 10.1021/bi400755f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Chowdhury G, Pazhani GP, Guin S, Dutta S, Ghosh S, et al. Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerg Infect Dis. 2013;19:464–467. doi: 10.3201/eid1903.121156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci U S A. 2005;102:3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A, Fink D, Musch M, Valuckaite V, Zaborina O, Grubjesic S, et al. Protective effects of nonionic triblock copolymers on bile acid-mediated epithelial barrier disruption. Shock. 2011;36:451–457. doi: 10.1097/SHK.0b013e31822d8de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, Sack DA, et al. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci U S A. 2004;101:2123–2128. doi: 10.1073/pnas.0308485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque SM, Sack DA, Sack RB, Colwell RR, Takeda Y, Nair GB. Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci U S A. 2003;100:1304–1309. doi: 10.1073/pnas.0337468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Feng L, Reeves PR, Lan R, Ren Y, Gao C, Zhou Z, et al. A recalibrated molecular clock and independent origins for the cholera pandemic clones. PLoS One. 2008;3:e4053. doi: 10.1371/journal.pone.0004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DK, Hankins JV, Guan Z, Trent MS. Remodelling of the Vibrio cholerae membrane by incorporation of exogenous fatty acids from host and aquatic environments. Mol Microbiol. 2011;79:716–728. doi: 10.1111/j.1365-2958.2010.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park KS, Dryselius R, et al. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS One. 2010;5:e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignot J, Segura A, Tran Van Nhieu G. The Serine Protease EspC from Enteropathogenic Escherichia coli Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System. PLoS Pathog. 2015;11:e1005013. doi: 10.1371/journal.ppat.1005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignot J, Tran Van Nhieu G. Bacterial Control of Pores Induced by the Type III Secretion System: Mind the Gap. Front Immunol. 2016;7:84. doi: 10.3389/fimmu.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/s1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan NA, Ceccarelli D, Grim CJ, Taviani E, Choi J, Sadique A, et al. Distribution of virulence genes in clinical and environmental Vibrio cholerae strains in Bangladesh. Applied and environmental microbiology. 2013;79:5782–5785. doi: 10.1128/AEM.01113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyoshi H, Kodama T, Saito K, Gotoh K, Matsuda S, Akeda Y, et al. VopV, an F-actin-binding type III secretion effector, is required for Vibrio parahaemolyticus-induced enterotoxicity. Cell Host Microbe. 2011;10:401–409. doi: 10.1016/j.chom.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hung DT, Mekalanos JJ. Bile acids induce cholera toxin expression in Vibrio cholerae in a ToxT-independent manner. Proc Natl Acad Sci U S A. 2005;102:3028–3033. doi: 10.1073/pnas.0409559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kodama T, Gotoh K, Hiyoshi H, Morita M, Izutsu K, Akeda Y, et al. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS One. 2010;5:e8678. doi: 10.1371/journal.pone.0008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Lee YL, Hung PP, Tsai CA, Lin YH, Liu CE, Shi ZY. Clinical characteristics of non-O1/non-O139 Vibrio cholerae isolates and polymerase chain reaction analysis of their virulence factors. J Microbiol Immunol Infect. 2007;40:474–480. [PubMed] [Google Scholar]
- Luo Y, Ye J, Jin D, Ding G, Zhang Z, Mei L, et al. Molecular analysis of non-O1/non-O139 Vibrio cholerae isolated from hospitalised patients in China. BMC Microbiol. 2013;13:52. doi: 10.1186/1471-2180-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlawska-Wasowska K, Finn R, Mustel A, O'Byrne CP, Baird AW, Coffey ET, Boyd A. The Vibrio parahaemolyticus Type III Secretion Systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol. 2010;10:329. doi: 10.1186/1471-2180-10-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007;75:5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei PJ, Faudry E, Job V, Izore T, Attree I, Dessen A. Membrane targeting and pore formation by the type III secretion system translocon. The FEBS journal. 2011;278:414–426. doi: 10.1111/j.1742-4658.2010.07974.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Iguchi M, Akiba K, Hosono M, Sobue T, Danbara H, Okada N. Chromobacterium pathogenicity island 1 type III secretion system is a major virulence determinant for Chromobacterium violaceum-induced cell death in hepatocytes. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07248.x. [DOI] [PubMed] [Google Scholar]
- Miller KA, Hamilton E, Dziejman M. Vcholerae trh is coordinately regulated in vitro with T3SS genes by VttRA/VttRB but does not contribute to Caco2-BBE cell cytotoxicity. Infect Immun. 2012;80:4444–4455. doi: 10.1128/IAI.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Sofia MK, Weaver JW, Seward C, Dziejman M. Regulation by ToxR-like proteins converges on vttRB expression to control T3SS-dependent Caco2-BBE cytotoxicity in V. cholerae. J Bacteriol. 2016 doi: 10.1128/JB.00130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Yamamoto S, Hiyoshi H, Kodama T, Okura M, Arakawa E, et al. Horizontal gene transfer of a genetic island encoding a type III secretion system distributed in Vibrio cholerae. Microbiol Immunol. 2013;57:334–339. doi: 10.1111/1348-0421.12039. [DOI] [PubMed] [Google Scholar]
- Morris JG, Jr, Wilson R, Davis BR, Wachsmuth IK, Riddle CF, Wathen HG, et al. Non-O group 1 Vibrio cholerae gastroenteritis in the United States: clinical, epidemiologic, and laboratory characteristics of sporadic cases. Ann Intern Med. 1981;94:656–658. doi: 10.7326/0003-4819-94-5-656. [DOI] [PubMed] [Google Scholar]
- Muza-Moons MM, Koutsouris A, Hecht G. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun. 2003;71:7069–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell. 2010;21:3506–3517. doi: 10.1091/mbc.E10-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octavia S, Salim A, Kurniawan J, Lam C, Leung Q, Ahsan S, et al. Population structure and evolution of non-O1/non-O139 Vibrio cholerae by multilocus sequence typing. PLoS One. 2013;8:e65342. doi: 10.1371/journal.pone.0065342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun. 2007;75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NH, Brodsky IE. Cell death programs in Yersinia immunity and pathogenesis. Front Cell Infect Microbiol. 2012;2:149. doi: 10.3389/fcimb.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano D, Klose KK. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio chlolerae bile resistance, virulence factor expression and inestinal colonization. Proceedings of the National Academy of Sciences of the United States of America. 2000 doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- Qiao D, Stratagouleas ED, Martinez JD. Activation and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis. 2001;22:35–41. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- Rahman MH, Biswas K, Hossain MA, Sack RB, Mekalanos JJ, Faruque SM. Distribution of genes for virulence and ecological fitness among diverse Vibrio cholerae population in a cholera endemic area: tracking the evolution of pathogenic strains. DNA Cell Biol. 2008;27:347–355. doi: 10.1089/dna.2008.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. American journal of physiology. Gastrointestinal and liver physiology. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- Ramamurthy T, Bag PK, Pal A, Bhattacharya SK, Bhattacharya MK, Shimada T, et al. Virulence patterns of Vibrio cholerae non-O1 strains isolated from hospitalised patients with acute diarrhoea in Calcutta, India. J Med Microbiol. 1993;39:310–317. doi: 10.1099/00222615-39-4-310. [DOI] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Sack RB, Siddique AK, Longini IM, Jr, Nizam A, Yunus M, Islam MS, et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning-A Laboratory Manual. Second. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Seward CH, Manzella A, Alam A, Butler JS, Dziejman M. Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes. Toxins. 2015;7:4099–4110. doi: 10.3390/toxins7104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Thungapathra M, Ghosh A, Mukhopadhyay AK, Basu A, Mitra R, et al. Molecular analysis of non-O1, non-O139 Vibrio cholerae associated with an unusual upsurge in the incidence of cholera-like disease in Calcutta, India. J Clin Microbiol. 1998;36:756–763. doi: 10.1128/jcm.36.3.756-763.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, Waldor MK, Mekalanos JJ. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae. MBio. 2011;2:e00106–e00111. doi: 10.1128/mBio.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Danilchanka O, Mekalanos JJ. Vibrio cholerae T3SS Effector VopE Modulates Mitochondrial Dynamics and Innate Immune Signaling by Targeting Miro GTPases. Cell Host Microbe. 2014;16:581–591. doi: 10.1016/j.chom.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe. 2007;1:95–107. doi: 10.1016/j.chom.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Tam VC, Suzuki M, Coughlin M, Saslowsky D, Biswas K, Lencer WI, et al. Functional analysis of VopF activity required for colonization in Vibrio cholerae. MBio. 2010;1 doi: 10.1128/mBio.00289-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol. 2011;80:1420–1438. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Liu Z, Hughes C, Stern AM, Wang H, Zhong Z, et al. Bile salt-induced intermolecular disulfide bond formation activates Vibrio cholerae virulence. Proc Natl Acad Sci U S A. 2013;110:2348–2353. doi: 10.1073/pnas.1218039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Lee NK, Lee NY, Lee JW, Park SJ. Cell death mediated by Vibrio parahaemolyticus type III secretion system 1 is dependent on ERK1/2 MAPK, but independent of caspases. J Microbiol Biotechnol. 2011;21:903–913. doi: 10.4014/jmb.1104.04044. [DOI] [PubMed] [Google Scholar]
- Zhang L, Orth K. Virulence determinants for Vibrio parahaemolyticus infection. Curr Opin Microbiol. 2013;16:70–77. doi: 10.1016/j.mib.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Zhou X, Gewurz BE, Ritchie JM, Takasaki K, Greenfeld H, Kieff E, et al. A Vibrio parahaemolyticus T3SS effector mediates pathogenesis by independently enabling intestinal colonization and inhibiting TAK1 activation. Cell Rep. 2013;3:1690–1702. doi: 10.1016/j.celrep.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]