Abstract
Albuminuria may be a biomarker of generalized (i.e., microvascular and macrovascular) endothelial dysfunction. According to this concept, endothelial dysfunction of the renal microcirculation causes albuminuria by increasing glomerular capillary wall permeability and intraglomerular pressure, the latter eventually leading to glomerular capillary dropout (rarefaction) and further increases in intraglomerular pressure. However, direct evidence for an association between capillary rarefaction and albuminuria is lacking. Therefore, we examined the cross-sectional association between the recruitment of capillaries after arterial occlusion (capillary density during postocclusive peak reactive hyperemia) and during venous occlusion (venous congestion), as assessed with skin capillaroscopy, and albuminuria in 741 participants of the Maastricht Study, including 211 participants with type 2 diabetes. Overall, 57 participants had albuminuria, which was defined as a urinary albumin excretion ≥30 mg/24 h. After adjustment for potential confounders, participants in the lowest tertile of skin capillary recruitment during postocclusive peak reactive hyperemia had an odds ratio for albuminuria of 2.27 (95% confidence interval, 1.07 to 4.80) compared with those in the highest tertile. Similarly, a comparison between the lowest and the highest tertiles of capillary recruitment during venous congestion yielded an odds ratio of 2.89 (95% confidence interval, 1.27 to 6.61) for participants in the lowest tertile. In conclusion, lower capillary density of the skin microcirculation independently associated with albuminuria, providing direct support for a role of capillary rarefaction in the pathogenesis of albuminuria.
Keywords: chronic kidney disease, diabetic nephropathy, microalbuminuria, endothelium
Albuminuria is strongly associated with cardiovascular disease risk.1 A leading hypothesis to explain this link is that albuminuria is a biomarker of generalized (i.e., microvascular and macrovascular) endothelial dysfunction.2,3 According to this concept, endothelial dysfunction of renal arterioles and capillaries (i.e., the renal microcirculation) causes albuminuria by increasing glomerular capillary wall permeability and increasing intraglomerular pressure,3 the latter eventually leading to glomerular capillary dropout (rarefaction) and further increases in intraglomerular pressure.4 Concomitantly, endothelial dysfunction in coronary and carotid arteries (i.e., the macrocirculation) leads to atherothrombotic cardiovascular disease.2,3
Indeed, studies using flow-mediated dilation of the brachial,5–9 femoral,10 and left anterior descending coronary artery11 have provided strong direct evidence for the presence of endothelial dysfunction in the macrocirculation of individuals with albuminuria.
In contrast, evidence for endothelial dysfunction in the microcirculation of individuals with albuminuria is primarily indirect, because it derives from studies using plasma biomarkers,12–17 the transcapillary escape rate of albumin,18–21 strain-gauge plethysmography after forearm ischemia,22 and laser Doppler flowmetry after either iontophoresis of acetylcholine and sodium nitroprusside23 or arterial occlusion.24 In addition, evidence for an association between capillary rarefaction and albuminuria is confined to a relatively small study, which showed the frequent co-occurrence of both in individuals with hypertension.25
In view of these considerations, we examined in a population–based cohort study the hypothesis that capillary rare-faction is associated with albuminuria. To do this, we used skin capillaroscopy, because capillary rarefaction in the kidney cannot be studied noninvasively in humans. Skin capillaroscopy is a noninvasive technique that allows direct visualization of capillary density in skin by measuring the recruitment of capillaries in response to arterial and venous occlusion, which are thought to be measures of functional and structural capillary density.26
Results
Characteristics of the Study Population
For this study on the basis of the first dataset of the Maastricht Study (n=866), four participants with type 1 diabetes were excluded. In the remaining 862 participants, qualitatively satisfactory data on skin capillaroscopy were available in 818 participants. In another 12 participants, the 24-hour urine collections either were collected erroneously (<20 or >28 hours) or were not handed in at all. Of the remaining 806 participants, we additionally excluded participants with missing data on waist circumference (n=3), smoking behavior (n=14), alcohol consumption (n=17), total cholesterol-to-HDL cholesterol ratio (n=8), triglycerides (n=7), eGFR (n=15), office BP (n=2), and/or prior cardiovascular disease status (n=32). These missing data were not mutually exclusive. The study population, therefore, consisted of 741 participants.
Table 1 shows the clinical characteristics of the study population stratified according to tertiles of the percentage recruitment during postocclusive peak reactive hyperemia. Tertile 1 indicates the tertile with the highest level of capillary recruitment.
Table 1.
Clinical characteristics of the study population according to tertiles of the percentage recruitment during postocclusive peak reactive hyperemia
| Characteristic | Tertiles of the Percentage Recruitment during Postocclusive Peak Reactive Hyperemia | ||
|---|---|---|---|
| Tertile 1 (High), n=247 | Tertile 2 (Middle), n=247 | Tertile 3 (Low), n=247 | |
| Recruitment during postocclusive peak reactive hyperemia, % | 73.7 [55.0–186.7] | 38.9 [27.0–54.9] | 16.3 [0.0–26.9] |
| Demographics | |||
| Age, yr | 59.2±8.5 | 60.0±8.6 | 60.0±8.5 |
| Men | 119 (48.2) | 143 (57.9) | 150 (60.7) |
| Educational level | |||
| Low | 33 (13.4) | 37 (15.0) | 50 (20.2) |
| Middle | 95 (38.5) | 101 (40.9) | 110 (44.5) |
| High | 119 (48.2) | 109 (44.1) | 87 (35.2) |
| Prior cardiovascular disease | 50 (20.2) | 36 (14.6) | 48 (19.4) |
| Lifestyle variables | |||
| Smoking behavior | |||
| Never a smoker | 80 (32.4) | 78 (31.6) | 72 (29.1) |
| Former smoker | 128 (51.8) | 132 (53.4) | 136 (55.1) |
| Current smoker | 39 (15.8) | 37 (15.0) | 39 (15.8) |
| Alcohol consumption | |||
| None | 34 (13.8) | 42 (17.0) | 47 (19.0) |
| Low: women ≤7 glasses per wk; men ≤14 glasses per wk | 126 (51.0) | 135 (54.7) | 131 (53.0) |
| High: women >7 glasses per wk; men >14 glasses per wk | 87 (35.2) | 70 (28.3) | 69 (27.9) |
| Metabolic variables | |||
| Body mass index categories | |||
| Normal weight, <25 kg/m2 | 81 (32.8) | 86 (34.8) | 67 (27.1) |
| Overweight, 25–30 kg/m2 | 117 (47.4) | 106 (42.9) | 122 (49.4) |
| Obesity, ≥30 kg/m2 | 49 (19.8) | 55 (22.3) | 58 (23.5) |
| Waist circumference, cm | |||
| Men | 100.9±11.7 | 101.3±11.6 | 103.2±11.5 |
| Women | 89.5±12.1 | 91.5±13.7 | 92.4±14.3 |
| Waist-to-hip ratio | |||
| Men | 1.00±0.07 | 1.00±0.06 | 1.01±0.07 |
| Women | 0.87±0.07 | 0.88±0.07 | 0.89±0.08 |
| Office systolic BP, mmHg | 136.1±18.2 | 135.9±18.7 | 140.0±19.9 |
| Office diastolic BP, mmHg | 76.5±10.3 | 76.0±10.8 | 77.8±10.5 |
| 24-h Average ambulatory systolic BP, mmHga | 118.3±11.9 | 119.3±11.9 | 120.9±12.6 |
| 24-h Average ambulatory diastolic BP, mmHga | 73.6±7.4 | 74.3±7.4 | 74.6±7.1 |
| Hypertension | 136 (55.1) | 136 (55.1) | 158 (64.0) |
| Glucose metabolism status | |||
| Normal glucose metabolism | 151 (61.1) | 139 (56.3) | 114 (46.2) |
| Impaired fasting glucose | 10 (4.0) | 11 (4.5) | 19 (7.7) |
| Impaired glucose tolerance | 43 (17.4) | 27 (10.9) | 16 (6.5) |
| Type 2 diabetes | 43 (17.4) | 70 (28.3) | 98 (39.7) |
| Fasting glucose, mmol/L | |||
| Without type 2 diabetes | 5.4±0.6 | 5.4±0.6 | 5.5±0.6 |
| With type 2 diabetes | 7.9±1.7 | 7.6±1.4 | 7.7±1.9 |
| HbA1C, %b | |||
| Without type 2 diabetes | 5.7±0.4 | 5.6±0.4 | 5.6±0.3 |
| With type 2 diabetes | 6.9±0.8 | 6.7±0.7 | 6.9±0.9 |
| Total cholesterol, mmol/L | 5.4±1.2 | 5.2±1.2 | 5.0±1.1 |
| HDL cholesterol, mmol/L | |||
| Men | 1.2±0.5 | 1.1±0.3 | 1.1±0.3 |
| Women | 1.5±0.4 | 1.5±0.4 | 1.5±0.5 |
| LDL cholesterol, mmol/L | 3.4±1.1 | 3.3±1.0 | 3.1±1.0 |
| Triglycerides, mmol/L | 1.20 [0.79–1.75] | 1.26 [0.89–1.78] | 1.25 [0.89–1.79] |
| Total cholesterol-to-HDL cholesterol ratio | 4.2±1.2 | 4.3±1.3 | 4.1±1.2 |
| Kidney function | |||
| eGFR, ml/min per 1.73 m2 | 88.7±14.7 | 87.6±14.5 | 87.8±16.0 |
| Albumin excretion rate, mg/24 h | 8.2 [5.4–11.6] | 7.8 [5.5–11.5] | 7.8 [5.5–15.7] |
| Albumin excretion ≥15 mg/24 h | 34 (13.8) | 37 (15.0) | 64 (25.9) |
| Albumin excretion ≥30 mg/24 h | 12 (4.9) | 13 (5.3) | 32 (13.0) |
| Medication | |||
| Antihypertensive medication | 94 (38.1) | 89 (36.0) | 113 (45.7) |
| Renin-angiotensin system inhibitor | 75 (30.4) | 65 (26.3) | 84 (34.0) |
| Lipid-modifying medication | 87 (35.2) | 78 (31.6) | 106 (42.9) |
Data are presented as n (%), mean±SD, median [interquartile range], or (only for the percentage recruitment during postocclusive peak reactive hyperemia) median [range].
Twenty-four–hour average ambulatory BP measurements were missing in n=76 participants (n=27 for tertile 1, n=23 for tertile 2, and n=26 for tertile 3).
To convert to HbA1c values into millimoles per mole: (10.93× HbA1c [%])−23.5.
In general, participants with the lowest recruitment were more often men, were less educated, suffered more often from hypertension and type 2 diabetes, and were more often treated with lipid-modifying or antihypertensive medication. Clinical characteristics according to tertiles of the percentage recruitment during venous congestion are shown in Supplemental Table 1.
Recruitment of Skin Capillaries and the Presence of Albuminuria
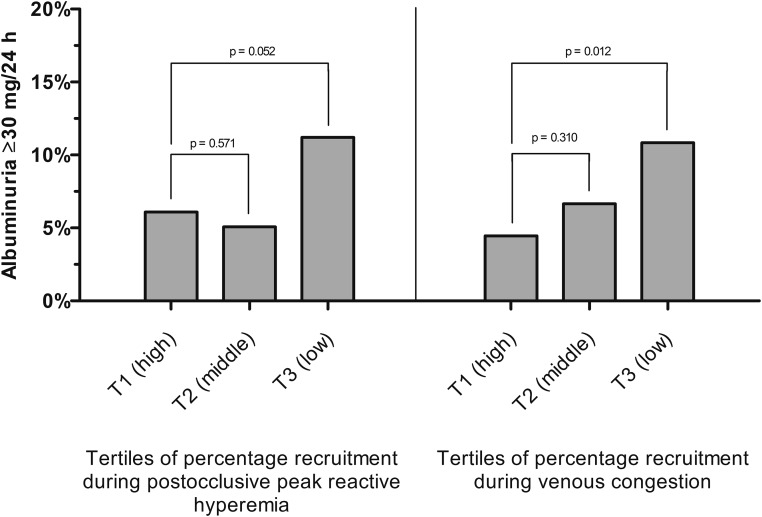
Overall, participants with the lowest recruitment more often had albuminuria (Figure 1).
Figure 1.
Capillary recruitment is associated with albuminuria. Bar charts showing the association between recruitment during postocclusive peak reactive hyperemia (left panel) as well as during venous congestion (right panel) and the presence of albuminuria ≥30 mg/24 h. Tertiles of the percentage recruitment during postocclusive peak reactive hyperemia ranged from 55.0 to 186.7 (tertile 1 [T1]), from 27.0 to 54.9 (tertile 2 [T2]), and from 0.0 to 26.9 (tertile 3 [T3]). Tertiles of the percentage recruitment during venous congestion ranged from 55.8 to 253.3 (T1), from 27.6 to 55.7 (T2), and from −2.9 to 27.5 (T3). Percentages of participants with albuminuria ≥30 mg/24 h per tertile were adjusted for age, sex, and type 2 diabetes (model 2) by marginal standardization. P values were derived from the same models.
After adjustment for potential confounders and compared with participants with the highest percentage recruitment during postocclusive peak reactive hyperemia (reference category), the odds ratio (OR) and 95% confidence interval (95% CI) for albuminuria for participants in the lowest tertile were OR, 2.27 and 95% CI, 1.07 to 4.80 (Table 2, model 3a). After adjustment for potential confounders and compared with participants with the highest percentage recruitment during venous congestion (reference category), the OR (95% CI) for albuminuria for participants in the lowest tertile was 2.89 (95% CI, 1.27 to 6.61) (Table 2, model 3a).
Table 2.
Association between the percentage recruitment during postocclusive peak reactive hyperemia as well as venous congestion and the presence of albuminuria (albumin excretion ≥30 mg/24 h)
| Independent Variable | OR (95% CI) | P Value |
|---|---|---|
| Recruitment during postocclusive peak reactive hyperemia, % | ||
| Model 1 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.09 (0.49 to 2.43) | 0.84 |
| Tertile 3 (low) | 2.92 (1.46 to 5.80) | 0.002 |
| Model 2 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 0.82 (0.36 to 1.88) | 0.57 |
| Tertile 3 (low) | 2.04 (0.99 to 4.19) | 0.05 |
| Model 3a | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 0.95 (0.41 to 2.30) | 0.95 |
| Tertile 3 (low) | 2.27 (1.07 to 4.80) | 0.03 |
| Model 3b | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.25 (0.50 to 3.13) | 0.63 |
| Tertile 3 (low) | 2.19 (0.96 to 5.00) | 0.06 |
| Recruitment during venous congestion, % | ||
| Model 1 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.83 (0.79 to 4.23) | 0.16 |
| Tertile 3 (low) | 3.94 (1.84 to 8.43) | <0.001 |
| Model 2 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.56 (0.66 to 3.67) | 0.31 |
| Tertile 3 (low) | 2.75 (1.25 to 6.06) | 0.01 |
| Model 3a | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.75 (0.72 to 4.26) | 0.74 |
| Tertile 3 (low) | 2.89 (1.27 to 6.61) | 0.01 |
| Model 3b | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.79 (0.69 to 4.65) | 0.23 |
| Tertile 3 (low) | 2.68 (1.10 to 6.52) | 0.03 |
ORs represent the odds of having albuminuria (defined as an albumin excretion ≥30 mg/24 h) in the respective tertile of the percentage recruitment during postocclusive peak reactive hyperemia or venous congestion compared with the odds of having albuminuria in the reference tertile. Model 1 is the unadjusted model. Model 2 is adjusted for age, sex, and type 2 diabetes. Model 3a is model 2 adjusted for waist circumference, total cholesterol-to-HDL cholesterol ratio, triglycerides, use of lipid-modifying drugs, office systolic BP, use of antihypertensive medication, eGFR, prevalent cardiovascular disease, smoking behavior, alcohol consumption, and educational level. Model 3b is model 3a adjusted for 24-hour average ambulatory systolic BP instead of office systolic BP (n=665).
When we replaced office systolic BP with 24-hour average ambulatory systolic BP (n=665), the OR (95% CI) for albuminuria became 2.19 (95% CI, 0.96 to 5.00) for those with the lowest percentage recruitment during postocclusive peak reactive hyperemia (Table 2, model 3b) and 2.68 (95% CI, 1.10 to 6.52) for those with the lowest percentage recruitment during venous congestion (Table 2, model 3b).
When we replaced the percentage change in capillary density with the absolute numbers of capillaries during postocclusive peak reactive hyperemia as well as during venous congestion, the results were similar (Table 3).
Table 3.
Association between the absolute number of capillaries during postocclusive peak reactive hyperemia as well as venous congestion and the presence of albuminuria (albumin excretion ≥30 mg/24 h)
| Independent Variable | OR (95% CI) | P Value |
|---|---|---|
| Postocclusive peak reactive hyperemia, n/mm2 | ||
| Model 1 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 2.51 (1.13 to 5.57) | 0.02 |
| Tertile 3 (low) | 3.01 (1.38 to 6.55) | <0.01 |
| Model 2 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 2.27 (1.00 to 5.15) | 0.05 |
| Tertile 3 (low) | 2.38 (1.07 to 5.31) | 0.03 |
| Model 3a | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 2.63 (1.10 to 6.30) | 0.03 |
| Tertile 3 (low) | 2.61 (1.11 to 6.12) | 0.03 |
| Model 3b | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 2.09 (0.82 to 5.30) | 0.12 |
| Tertile 3 (low) | 2.51 (1.04 to 6.06) | 0.04 |
| Venous congestion, n/mm2 | ||
| Model 1 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.15 (0.55 to 2.41) | 0.72 |
| Tertile 3 (low) | 2.11 (1.08 to 4.13) | 0.03 |
| Model 2 | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 0.95 (0.44 to 2.04) | 0.90 |
| Tertile 3 (low) | 1.72 (0.86 to 3.47) | 0.13 |
| Model 3a | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.12 (0.50 to 2.50) | 0.79 |
| Tertile 3 (low) | 1.76 (0.84 to 3.69) | 0.13 |
| Model 3b | ||
| Tertile 1 (high) | Reference | |
| Tertile 2 (middle) | 1.32 (0.54 to 3.25) | 0.54 |
| Tertile 3 (low) | 2.32 (1.01 to 5.32) | 0.05 |
ORs represent the odds of having albuminuria (defined as an albumin excretion ≥30 mg/24 h) in the respective tertile of the absolute number of capillaries during postocclusive peak reactive hyperemia or venous congestion compared with the odds of having albuminuria in the reference tertile. Model 1 is the unadjusted model. Model 2 is adjusted for age, sex, and type 2 diabetes. Model 3a is model 2 adjusted for waist circumference, total cholesterol-to-HDL cholesterol ratio, triglycerides, use of lipid-modifying drugs, office systolic BP, use of antihypertensive medication, eGFR, prevalent cardiovascular disease, smoking behavior, alcohol consumption, and educational level. Model 3b is model 3a adjusted for 24-hour average ambulatory systolic BP instead of office systolic BP (n=665).
When the variables of models 3a and 3b were added separately to a model adjusted for age and sex, adjustment for type 2 diabetes led to the largest reduction of the OR when the percentage recruitment was the determinant. When the absolute number of capillaries was the determinant, adding additional variables after initial adjustment for age and sex did not materially alter our results (data not shown).
Analyses with interaction terms suggested that the associations between the percentage recruitment during postocclusive peak reactive hyperemia as well as venous congestion and albuminuria were only present in individuals without type 2 diabetes (P value of the interaction term [Pinteraction] <0.10) (Supplemental Table 2), whereas no such interaction was observed for the absolute number of capillaries under both conditions (Pinteraction>0.10) (Supplemental Table 3).
Additional Analyses
First, recruitment of capillaries may be expressed as either the absolute or percentage change in capillary density (Concise Methods). When we replaced the percentage change with absolute change in capillary density, results were not materially altered for either postocclusive peak reactive hyperemia or venous congestion (data not shown). Second, mutual adjustment for the percentage recruitment during venous congestion or the percentage recruitment during postocclusive peak reactive hyperemia suggested multicollinearity (i.e., a strong increase in the standard error of the regression coefficient of the central determinant; data not shown). Third, results were not materially altered when we excluded participants with an albumin excretion >300 mg/24 h; when we replaced office systolic BP with office diastolic BP, office pulse pressure, office mean arterial pressure, presence of hypertension, 24-hour average ambulatory diastolic BP, 24-hour average ambulatory pulse pressure, or 24-hour average ambulatory mean arterial pressure; when we replaced the use of antihypertensive medication with the use of a renin-angiotensin system inhibitor, the use of a diuretic, or their combined use; when we replaced waist circumference with waist-to-hip ratio or body mass index; when we replaced the total cholesterol-to-HDL cholesterol ratio with LDL and HDL; or when we additionally adjusted for hemoglobin A1c (HbA1c) or an inflammation Z score (n=738). Fourth, analyses in the subpopulation with two urine collections (n=666) and the subset of 24-hour urine collections with measured 24-hour urine creatinine excretion within 30% of expected values (n=642) did not indicate nondifferential misclassification caused by biologic variability and inaccurate collection, respectively (data not shown). Fifth, analyses on the basis of quintiles and deciles of the respective capillaroscopy measures were also consistent with a threshold level (Supplemental Tables 4–6). However, these analyses were hampered by a loss of power. Sixth, we defined albuminuria as an albumin excretion ≥15 mg/24 h, in agreement with the fact that an association with (cardiovascular disease) mortality already exists at levels of urinary albumin excretion <30 mg/24 h1 and to explore whether misclassification of albuminuria status occurred with the clinical cutoff value. With this definition, the associations for the entire study population became somewhat weaker (Supplemental Table 7), and there was no statistical interaction with type 2 diabetes (Pinteraction>0.10). Seventh, multivariable linear regression analyses showed no associations between tertiles of the respective capillaroscopy measures and (inverse square root–transformed) continuous urinary albumin excretion (data not shown).
Discussion
The main finding of this population-based study is that lower capillary density was associated with the presence of albuminuria, regardless of whether type 2 diabetes was present. This association was independent of cardiovascular disease risk factors, including 24-hour average ambulatory BP and biomarkers of low-grade inflammation. To the best of our knowledge, this is the first population-based study that provides direct support for a role of capillary rarefaction in the pathogenesis of albuminuria.
An association between capillary rarefaction and albuminuria is in agreement with the Brenner hypothesis4 (i.e., an increase in intraglomerular pressure will lead to glomerular capillary dropout [rarefaction] and further increases in intraglomerular pressure on the one hand and greater permeation of albumin through the glomerular capillary wall on the other hand). Indeed, in individuals with type 2 diabetes, estimated intraglomerular pressure was higher in the presence of albuminuria.27 Additionally, in individuals who underwent a large reduction in renal mass, remaining kidney mass was inversely associated with urinary albumin excretion.28 Furthermore, in a smaller study, capillary rarefaction in the skin microcirculation and albuminuria frequently co-occurred in individuals with hypertension.25 This study extends this knowledge, because it is the first to examine a direct measure of capillary rarefaction in a large population–based sample with adjustment for potential confounders.
A key assumption underlying this study is that skin capillary rarefaction reflects capillary rarefaction of the kidney. Although the skin microcirculation has not been compared directly with that of the kidney, several observations support the view that it is representative for the systemic microcirculation, including the kidney's. First, age-related changes in the skin microcirculation parallel those in the systemic vasculature.29 Second, the microcirculation of the skin and kidney share associations with salt-sensitive hypertension30 and low birth weight.31–34
Both capillary density during venous occlusion and capillary density after arterial occlusion were used as reproducible26,35 estimates of maximal skin capillary density.36 In this study, we could not determine to what extent differences in capillary density were caused by structural (i.e., anatomic) or functional (i.e., nonperfusion) rarefaction. However, the occurrence of multicollinearity after mutual adjustment suggests that both measures assessed the same or at least overlapping construct(s) in this study.
Importantly, misclassification of albuminuria status because of the use of renin-angiotensin system inhibitors may explain why capillary rarefaction in individuals with type 2 diabetes was associated with albuminuria when defined as an albumin excretion ≥15 mg/24 h but not when defined as an albumin excretion ≥30 mg/24 h. Renin-angiotensin system inhibitors reduce urinary albumin excretion by lowering intraglomerular pressure,37,38 an effect that is enhanced by diuretics,39 and an albumin excretion ≥30 mg/24 h is used as an indication for their use, particularly in individuals with type 2 diabetes.40 Hence, individuals previously having had an albumin excretion ≥30 mg/24 h could be classified erroneously as having no albuminuria with this definition, thus obscuring an association with capillary rarefaction. Indeed, the frequent use of renin-angiotensin system inhibitors in individuals with type 2 diabetes and a urinary albumin excretion of 15–30 mg/24 h (64.9% versus 19.5% in individuals with a similar urinary albumin excretion but without type 2 diabetes) supports this explanation. Alternatively, the statistically significant interaction between type 2 diabetes and the percentage recruitment during postocclusive peak reactive hyperemia as well as venous congestion may be attributable to the play of chance given the low number of cases.
A lack of power because of the small variation in urinary albumin excretion with only a few individuals with an albumin excretion ≥30 mg/24 h may explain why the results of this study suggest a threshold level for capillary density and why capillary rarefaction was not associated with continuous urinary albumin excretion. In addition, we measured urinary albumin excretion instead of the permeation of albumin through the glomerular capillary wall, and small increases in permeation may be compensated for by tubular reabsorption.41
A strength of this study is that participants of the Maastricht Study were well characterized, allowing adjustment for an extensive series of potential confounders, including 24-hour average ambulatory BP and low-grade inflammation. In this regard, the use of office BP only could have underestimated any effect of BP on albuminuria and thereby, overestimated the association between capillary rarefaction and albuminuria. However, some of the variables in our models may also be intermediates in the association between capillary rarefaction and albuminuria, possibly leading to overadjustment bias (i.e., the associations reported are conservative).42 For instance, capillary rarefaction may be involved in the pathogenesis of type 2 diabetes, which may subsequently lead to albuminuria via a hyperglycemia-induced increase in glomerular capillary wall permeability.3 Similarly, higher BP may be both a cause and a consequence of capillary rarefaction.43
From a clinical perspective, both capillary rarefaction itself and the resulting increase in intraglomerular pressure may be a target in the management of albuminuria. Indeed, renin-angiotensin system inhibitors, which reduce intraglomerular pressure,37,38 form the mainstay of the current management of albuminuria.40 However, current management is not specifically aimed at regenerating glomeruli. Nonetheless, in a recent study, treatment with a renin-angiotensin system inhibitor led to an increase in kidney vasculature in a rat model of progressive glomerular injury,44 suggesting that capillary rarefaction itself could be a future therapeutic target.
This study had some limitations. First, no direct measure of capillary rarefaction of the kidney was used. However, at present, capillary rarefaction of the kidney, in contrast to capillary rarefaction of skin, cannot be studied noninvasively in humans. Second, because of the logistics of this large–scale population–based study, participants were not asked to come in fasting. However, to minimize the effects of dietary intake on the microcirculation,45–47 participants were asked to have a standardized low–fat breakfast (or lunch) and refrain from caffeine-containing beverages and smoking. In addition, results were not materially altered after adjustment for nonadherence to the dietary and smoking restrictions. Third, the cross-sectional design does not allow us to make strong causal inferences. In addition, a longitudinal design with frequent assessment of urinary albumin excretion and medication use would have avoided misclassification of albuminuria status. Fourth, the study population primarily consisted of white individuals of European descent (99.2%), limiting generalizability to other populations.
In conclusion, lower capillary density of the skin microcirculation was independently associated with the presence of albuminuria, regardless of the presence of type 2 diabetes. Thereby, this is the first population-based study that provides direct support for a role of capillary rarefaction in the pathogenesis of albuminuria.
Concise Methods
The Maastricht Study Population and Design
In this study, we used data from the Maastricht Study, an observational prospective population–based cohort study. The rationale and methodology have been described previously.48 In brief, the study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes mellitus and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals with ages between 40 and 75 years living in the southern part of The Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known type 2 diabetes status for reasons of efficiency. This report includes cross-sectional data from the first 866 participants who completed the baseline survey between November of 2010 and March of 2012. The examinations of each participant were performed within a time window of 3 months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of The Netherlands on the basis of the Health Council’s opinion (Permit 131088–105234-PG) and was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent.
Skin Capillaroscopy
All participants were asked to refrain from smoking and drinking coffee or tea ≥3 hours before the measurements. A light meal (breakfast and/or lunch) low in fat content was allowed before the start of the measurements. Skin capillaroscopy measurements were performed in a quiet, temperature–controlled room (T=24°C) with participants in the supine position as previously described.49
Briefly, capillaries were visualized in the dorsal skin of the distal phalanges of the third and fourth finger of the right hand by use of a digital video microscope (Capiscope; KK Technology, Honiton, United Kingdom) with a system magnification of ×100.49 Capillaries were visualized 4.5 mm proximal to the terminal row of capillaries in the middle of the nailfold. The investigator selected a region of interest of 1-mm2 skin area. Capillary density (mean of two fields) was measured under three conditions. First, baseline capillary density was measured. Baseline capillary density was defined as the number of continuously erythrocyte–perfused capillaries per 1 mm2 skin and was counted for 15 seconds. Second, capillary recruitment during postocclusive peak reactive hyperemia was assessed after 4 minutes of arterial occlusion. Arterial occlusion was applied using a miniature cuff at the base of the investigated finger inflated to suprasystolic pressure (260 mmHg) for 4 minutes. Directly after release of the cuff, all (continuously and intermittently) perfused capillaries were counted for 15 seconds. Third, venous congestion was applied, with the cuff inflated to 60 mmHg for 2 minutes, and all (continuously and intermittently) perfused capillaries were counted for 15 seconds. The number of perfused capillaries was counted in the recorded digital raw data with the use of a semiautomatic procedure (CapiAna)49 by two investigators who were blinded to participants’ clinical status. The intra- and interobserver coefficients of variation for the counting procedure were 2.5% and 5.6%, respectively, as described previously.49
For the primary analyses, we used recruitment during postocclusive peak reactive hyperemia as well as during venous congestion (expressed as the percentage change in capillary density from baseline) and the absolute number of capillaries during postocclusive peak reactive hyperemia as well as during venous congestion (expressed as capillaries per 1 mm2).
Kidney Function
GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation on the basis of both serum creatinine and serum cystatin C (Supplemental Material).50 To assess urinary albumin excretion, participants were requested to collect two 24-hour urine collections (Supplemental Material).
Albuminuria was defined as an albumin excretion ≥30 mg/24 h,51 which is used in clinical practice to guide cardiovascular disease prevention, particularly in individuals with type 2 diabetes.40 In an additional analysis, albuminuria was defined as an albumin excretion ≥15 mg/24 h (the upper level of daily albumin excretion in healthy individuals52) in agreement with the fact that an association with (cardiovascular disease) mortality already exists at levels of urinary albumin excretion <30 mg/24 h1 and to explore whether misclassification of albuminuria status occurred with the clinical cutoff. These definitions were preferably on the basis of the average of two (available in 89.9% of the participants) 24-hour urine collections.
Potential Confounders
We assessed glucose metabolism status, body mass index, waist circumference, hip circumference, office BP, 24-hour average ambulatory BP, fasting glucose, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, medication use, smoking behavior, alcohol consumption, educational level, and prevalent cardiovascular disease as described previously.48,53 Definitions of these potential confounders are provided in Supplemental Material. In addition, we assessed the following plasma biomarkers of inflammation: high–sensitivity C–reactive protein, serum amyloid A, IL-6, IL-8, TNF-α, and soluble intercellular adhesion molecule 1.54
Statistical Analyses
All analyses were performed with IBM SPSS Statistics, Version 22.0 (IBM SPSS, Chicago, IL) unless stated otherwise.
Participants were divided into tertiles of the percentage recruitment during postocclusive peak reactive hyperemia as well as into tertiles of the percentage recruitment during venous congestion, because the association with albuminuria seemed to be nonlinear. Participants with normal glucose tolerance, impaired fasting glucose, and impaired glucose tolerance were combined into one category (participants without type 2 diabetes) because of the small number of participants with impaired fasting glucose and impaired glucose tolerance.
Associations between tertiles of the percentage recruitment during postocclusive peak reactive hyperemia as well as during venous congestion and the presence of albuminuria were evaluated using multivariable logistic regression analyses. Similarly, associations between tertiles of the absolute number of capillaries during postocclusive peak reactive hyperemia as well as during venous congestion and the presence of albuminuria were evaluated. The tertile with the highest recruitment or the highest absolute number of capillaries (tertile 1) was used as reference category. Next, we adjusted for potential confounders as follows: model 1, unadjusted model; model 2, adjusted for age, sex, and type 2 diabetes; model 3a, model 2 adjusted for waist circumference, total cholesterol-to-HDL cholesterol ratio, triglycerides, use of lipid-modifying medication, office systolic BP, use of antihypertensive medication, eGFR, prevalent cardiovascular disease, smoking behavior, alcohol consumption, and educational level; and model 3b, model 3a with replacement of office systolic BP by 24-hour average ambulatory systolic BP.
We used interaction terms to examine whether the associations were modified by the presence or absence of type 2 diabetes. A Pinteraction<0.10 in model 3a was considered to indicate a statistically significant interaction.
Adjusted percentages of participants with albuminuria per tertile of recruitment were derived from the logistic regression models (model 2) with adjustment for age, sex, and type 2 diabetes by marginal standardization55 (calculated with Stata Statistical Software, release 11.2SE; StataCorp., College Station, TX).
Several additional analyses were performed, each starting from the models described above. First, we used the absolute change in capillary density from baseline (expressed as capillaries per 1 mm2) during postocclusive peak reactive hyperemia and during venous congestion to categorize participants. Second, we excluded participants with an albumin excretion >300 mg/24 h (n=8). Third, we replaced office systolic BP with office diastolic BP, office pulse pressure, office mean arterial pressure, presence of hypertension, 24-hour average ambulatory diastolic BP, 24-hour average ambulatory pulse pressure, or 24-hour average ambulatory mean arterial pressure in model 3a, and we replaced the use of antihypertensive medication with the use of a renin-angiotensin system inhibitor, the use of a diuretic, or their combined use. Fourth, we replaced waist circumference with waist-to-hip ratio or body mass index, replaced the total cholesterol-to-HDL cholesterol ratio with LDL and HDL, and additionally, adjusted for HbA1c and a Z score of the inflammation biomarkers54 in model 3a. Fifth, we repeated the analyses in participants with two urine collections and after exclusion of 24-hour urine collections with a measured 24-hour urine creatinine excretion not within 30% of expected values56 to explore whether biologic variation and inaccurate collection, respectively, led to nondifferential misclassification with bias toward zero. Sixth, we repeated the analyses with quintiles and deciles of the respective capillaroscopy measures as independent variables. Seventh, we repeated the analyses with albuminuria defined as an albumin excretion ≥15 mg/24 h. Eighth, we performed multivariable linear regression analyses to examine whether the capillaroscopy measures were associated with urinary albumin excretion on a continuous scale. Urinary albumin excretion had to be transformed by taking the inverse square root of urinary albumin excretion to fulfill the normality assumption, because it was highly positively skewed and could not be transformed adequately using common57 transformations.
Disclosures
None.
Supplementary Material
Acknowledgments
The Maastricht Study is supported by the European Regional Development Fund through Operational Programme South Netherlands, the Province of Limburg, Dutch Ministry of Economic Affairs grant 31O.041, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the Cardiovascular Center, Cardiovascular Research Institute Maastricht, School for Public Health and Primary Care, School of Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag B.V., Novo Nordisk Farma B.V., and Sanofi-Aventis Netherlands B.V. In addition, this study is supported by an unrestricted grant from Fresenius Medical Care.
Abstracts on the basis of the results of this study have been published and presented previously at the Dutch Federation of Nephrology Fall Symposium 2014 (Utrecht, The Netherlands; October 10, 2014), the 3rd Joint Meeting of the Dutch Endothelial Biology Society and the Dutch Society for Microcirculation and Vascular Biology (Biezenmortel, The Netherlands; October 30–31, 2014), and the European Renal Association - European Dialysis and Transplant Association 52nd Congress (London, United Kingdom; May 28–31, 2015).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111219/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Stehouwer CD, Smulders YM: Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM: Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: Further evidence for a link between microalbuminuria and endothelial dysfunction--the Hoorn Study. Kidney Int Suppl 92: S42–S44, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Dogra G, Rich L, Stanton K, Watts GF: Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Diabetologia 44: 593–601, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Poredos P, Kek A: Relation of blunted dilation of the brachial artery in insulin-dependent diabetes mellitus to microalbuminuria. Am J Cardiol 86: 364–367, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B: Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 103: 1869–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Papaioannou GI, Seip RL, Grey NJ, Katten D, Taylor A, Inzucchi SE, Young LH, Chyun DA, Davey JA, Wackers FJ, Iskandrian AE, Ratner RE, Robinson EC, Carolan S, Engel S, Heller GV: Brachial artery reactivity in asymptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabetics-brachial artery reactivity study). Am J Cardiol 94: 294–299, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Zenere BM, Arcaro G, Saggiani F, Rossi L, Muggeo M, Lechi A: Noninvasive detection of functional alterations of the arterial wall in IDDM patients with and without microalbuminuria. Diabetes Care 18: 975–982, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Cosson E, Pham I, Valensi P, Pariès J, Attali JR, Nitenberg A: Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care 29: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ: Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet 340: 319–323, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH: Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: Progressive, interrelated, and independently associated with risk of death. Diabetes 51: 1157–1165, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Stehouwer CD, Fischer HR, van Kuijk AW, Polak BC, Donker AJ: Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes 44: 561–564, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD: C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol 22: 593–598, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Clausen P, Feldt-Rasmussen B, Jensen G, Jensen JS: Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: A 4-year prospective study. Clin Sci (Lond) 97: 37–43, 1999 [PubMed] [Google Scholar]
- 17.Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, Parving HH: Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest 68: 731–738, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Feldt-Rasmussen B: Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 29: 282–286, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Nannipieri M, Rizzo L, Rapuano A, Pilo A, Penno G, Navalesi R: Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care 18: 1–9, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Nannipieri M, Penno G, Rizzo L, Pucci L, Bandinelli S, Mattei P, Taddei S, Salvetti A, Navalesi R: Transcapillary escape rate of albumin in type II diabetic patients. The relationship with microalbuminuria and hypertension. Diabetes Care 20: 1019–1026, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Jensen EW, Espersen K, Knudsen JH, Nielsen SL: Increased transcapillary escape rate of albumin in elderly subjects due to long-term smoking habits. Clin Physiol 15: 159–167, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Guglielmi MD, Pierdomenico SD, Salvatore L, Romano F, Tascione E, Pupillo M, Porreca E, Imbastaro T, Cuccurullo F, Mezzetti A: Impaired left ventricular diastolic function and vascular postischemic vasodilation associated with microalbuminuria in IDDM patients. Diabetes Care 18: 353–360, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Schmiedel O, Schroeter ML, Harvey JN: Microalbuminuria in Type 2 diabetes indicates impaired microvascular vasomotion and perfusion. Am J Physiol Heart Circ Physiol 293: H3424–H3431, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Houben AJ, Schaper NC, Slaaf DW, Tangelder GJ, Nieuwenhuijzen Kruseman AC: Skin blood cell flux in insulin-dependent diabetic subjects in relation to retinopathy or incipient nephropathy. Eur J Clin Invest 22: 67–72, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Triantafyllou A, Anyfanti P, Zabulis X, Gavriilaki E, Karamaounas P, Gkaliagkousi E, Petidis K, Pyrpasopoulou A, Girasis C, Aslanidis S, Douma S: Accumulation of microvascular target organ damage in newly diagnosed hypertensive patients. J Am Soc Hypertens 8: 542–549, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Serné EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD: Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38: 238–242, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Imanishi M, Yoshioka K, Konishi Y, Okumura M, Okada N, Sato T, Tanaka S, Fujii S, Kimura G: Glomerular hypertension as one cause of albuminuria in type II diabetic patients. Diabetologia 42: 999–1005, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR: Long-term follow-up after partial removal of a solitary kidney. N Engl J Med 325: 1058–1062, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Holowatz LA, Thompson-Torgerson CS, Kenney WL: The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008 [DOI] [PubMed]
- 30.de Jongh RT, Serné EH, IJzerman RG, Stehouwer CD: Microvascular function: A potential link between salt sensitivity, insulin resistance and hypertension. J Hypertens 25: 1887–1893, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Serné EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Donker AJ, Gans RO: Birth weight relates to blood pressure and microvascular function in normal subjects. J Hypertens 18: 1421–1427, 2000 [DOI] [PubMed] [Google Scholar]
- 32.IJzerman RG, van Weissenbruch MM, Voordouw JJ, Yudkin JS, Serne EH, Delemarre-van de Waal HA, Stehouwer CD: The association between birth weight and capillary recruitment is independent of blood pressure and insulin sensitivity: A study in prepubertal children. J Hypertens 20: 1957–1963, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Mañalich R, Reyes L, Herrera M, Melendi C, Fundora I: Relationship between weight at birth and the number and size of renal glomeruli in humans: A histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, Stehouwer CD: Angiotensin II enhances insulin-stimulated whole-body glucose disposal but impairs insulin-induced capillary recruitment in healthy volunteers. J Clin Endocrinol Metab 95: 3901–3908, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Antonios TF, Rattray FE, Singer DR, Markandu ND, Mortimer PS, MacGregor GA: Maximization of skin capillaries during intravital video-microscopy in essential hypertension: Comparison between venous congestion, reactive hyperaemia and core heat load tests. Clin Sci (Lond) 97: 523–528, 1999 [PubMed] [Google Scholar]
- 37.Anderson S, Rennke HG, Garcia DL, Brenner BM: Short and long term effects of antihypertensive therapy in the diabetic rat. Kidney Int 36: 526–536, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Pelayo JC, Quan AH, Shanley PF: Angiotensin II control of the renal microcirculation in rats with reduced renal mass. Am J Physiol 258: F414–F422, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Morales E, Caro J, Gutierrez E, Sevillano A, Auñón P, Fernandez C, Praga M: Diverse diuretics regimens differentially enhance the antialbuminuric effect of renin-angiotensin blockers in patients with chronic kidney disease. Kidney Int 88: 1434–1441, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group : KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2: 337–414, 2012 [Google Scholar]
- 41.Birn H, Christensen EI: Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Schisterman EF, Cole SR, Platt RW: Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20: 488–495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA: Microcirculation in hypertension: A new target for treatment? Circulation 104: 735–740, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Remuzzi A, Sangalli F, Macconi D, Tomasoni S, Cattaneo I, Rizzo P, Bonandrini B, Bresciani E, Longaretti L, Gagliardini E, Conti S, Benigni A, Remuzzi G: Regression of renal disease by angiotensin II antagonism is caused by regeneration of kidney vasculature. J Am Soc Nephrol 27: 699–705, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, Stehouwer CD: Meal-related increases in microvascular vasomotion are impaired in obese individuals: A potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care 34[Suppl 2]: S342–S348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ: Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32: 1672–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ: Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RM, Stehouwer CD: The Maastricht Study: An extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol 29: 439–451, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Gronenschild EH, Muris DM, Schram MT, Karaca U, Stehouwer CD, Houben AJ: Semi-automatic assessment of skin capillary density: Proof of principle and validation. Microvasc Res 90: 192–198, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 52.Ward KM: Renal function (microalbuminuria). Anal Chem 67: 383R–391R, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Spauwen PJ, van Boxtel MP, Verhey FR, Köhler S, Sep SJ, Koster A, Dagnelie PC, Henry RM, Schaper NC, van der Kallen CJ, Schram MT, Kroon AA, Stehouwer CD: Both low and high 24-hour diastolic blood pressure are associated with worse cognitive performance in type 2 diabetes: The Maastricht Study. Diabetes Care 38: 1473–1480, 2015 [DOI] [PubMed] [Google Scholar]
- 54.van Dooren FE, Verhey FR, Pouwer F, Schalkwijk CG, Sep SJ, Stehouwer CD, Henry RM, Dagnelie PC, Schaper NC, van der Kallen CJ, Koster A, Schram MT, Denollet J: Association of Type D personality with increased vulnerability to depression: Is there a role for inflammation or endothelial dysfunction? - The Maastricht Study. J Affect Disord 189: 118–125, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Muller CJ, MacLehose RF: Estimating predicted probabilities from logistic regression: Different methods correspond to different target populations. Int J Epidemiol 43: 962–970, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, Rodby R, Torres VE, Zhang YL, Greene T, Levey AS: Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin J Am Soc Nephrol 6: 184–191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altman DG: Practical Statistics for Medical Research, London, Chapman and Hall/CRC, 1990, pp 143–145 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.