Abstract
Background: Previous studies have shown an inconsistent relation between habitual beverage consumption and insulin resistance and prediabetes.
Objective: The objective of the present study was to test the hypothesis that the consumption of sugar-sweetened beverages (SSBs), rather than diet soda, is associated with long-term progression of insulin resistance and the development of prediabetes.
Methods: We analyzed the prospective association between cumulative mean consumption of SSBs or diet soda and incident prediabetes (n = 1685) identified across a median of 14 y of follow-up in participants [mean ± SD age: 51.9 ± 9.2 y; 59.6% women; mean ± SD body mass index (BMI; kg/m2): 26.3 ± 4.4] of the Framingham Offspring cohort. The prospective association between beverage consumption and change in homeostasis model assessment of insulin resistance (HOMA-IR; n = 2076) over ∼7 y was also analyzed. The cumulative mean consumption of SSBs and diet soda was estimated by using food-frequency questionnaires. Multivariable Cox proportional hazards models and linear regression models were implemented to estimate the HRs of incident prediabetes and change in HOMA-IR, respectively.
Results: After adjustment for multiple potential confounders, including baseline BMI, we observed that SSB intake was positively associated with incident prediabetes (P-trend < 0.001); the highest SSB consumers (>3 servings/wk; median: 6 servings/wk) had a 46% higher risk of developing prediabetes than did the SSB nonconsumers (HR: 1.46; 95% CI: 1.16, 1.83). Higher SSB intake was also associated with a greater increase in HOMA-IR (P-trend = 0.006). No prospective associations were observed between diet soda intake and risk of prediabetes (P-trend = 0.24) or changes in HOMA-IR (P-trend = 0.25). These associations were similar after additional adjustment for change in BMI.
Conclusion: Regular SSB intake, but not diet soda intake, is associated with a greater increase in insulin resistance and a higher risk of developing prediabetes in a group of middle-aged adults.
Keywords: sugar-sweetened beverages, diet soda, insulin resistance, HOMA-IR, prediabetes
Introduction
Insulin resistance is a condition whereby the body’s sensitivity or responsiveness to the hormone insulin is decreased, leading to metabolic dysregulation (1). Insulin resistance is a major cause of type 2 diabetes (T2D)9 and is a key feature of many other cardiometabolic diseases (2). Sugar-sweetened beverages (SSBs), sweetened with either sucrose or high-fructose corn syrup, are the leading source of added sugars in the diets of American adults (3). Current evidence has linked excess SSB consumption to increased risk of T2D (4) and cardiovascular diseases (5). However, inconsistent findings have been observed with respect to SSB consumption and insulin resistance. Some short-term intervention studies found that high intakes of sucrose (6) or fructose (7, 8) appear to reduce insulin sensitivity, whereas others failed to show such effects (9, 10). Conflicting findings have also emerged from observational studies that examined habitual intakes of different types or sources of sugars and HOMA-IR in adults (11–14) and children (15–17). Furthermore, to our knowledge, few studies have examined the long-term association between SSB intake and the incidence of prediabetes, separately from T2D, in healthy adults.
Diet sodas, sweetened by low-calorie or artificial sweeteners in lieu of sugars, are often an alternative beverage to SSBs. Although a few prospective studies have found a link between diet soda intake and increased risk of T2D (18, 19), others observed a nonsignificant association (20, 21). To our knowledge, there is no prospective evidence that either supports or rejects a relation between habitual diet soda consumption and insulin resistance and prediabetes in adults.
Consequently, we hypothesized that the long-term intake of SSBs is associated with greater increases in insulin resistance and thus the development of prediabetes, whereas no such associations exist with diet soda consumption. In the present study, we tested this hypothesis by examining the longitudinal association between the intake of SSBs or diet soda and change in insulin resistance, as assessed by HOMA-IR, and incidence of prediabetes.
Methods
Study sample.
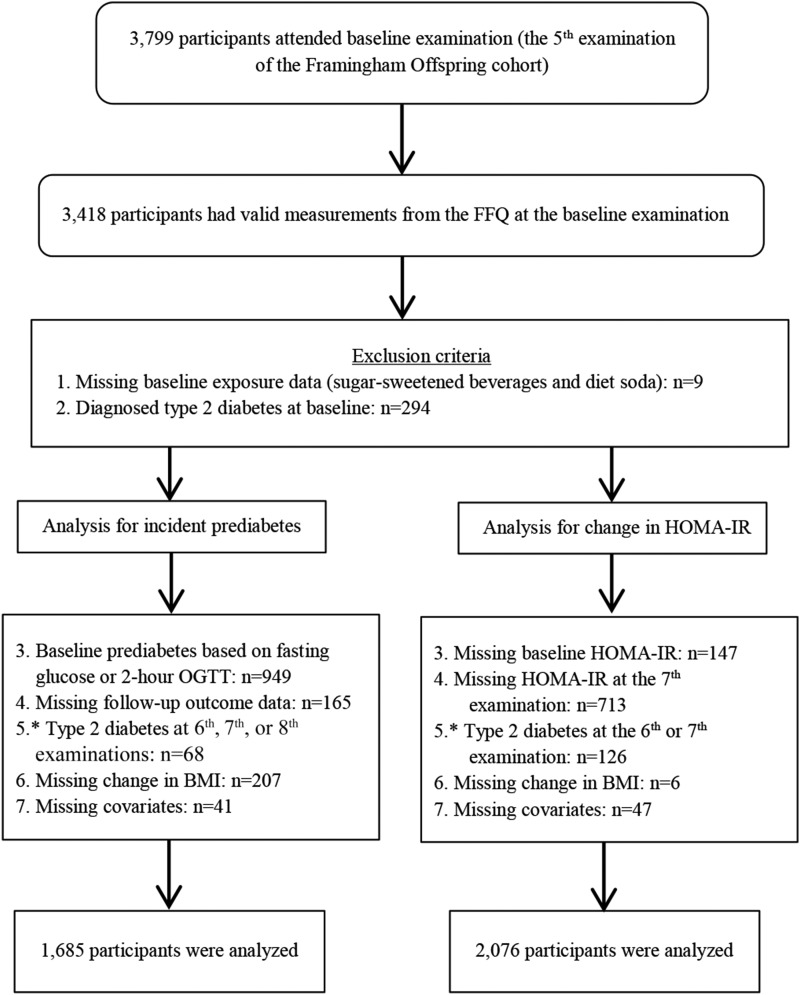
Study participants were from the Framingham Heart Study’s Offspring cohort and have been previously described (22). Participants in the Offspring cohort were evaluated every ∼3–4 y. A total of 3799 participants attended the fifth examination (baseline), of whom 3418 individuals had valid dietary data (Figure 1). In the present incident prediabetes analyses, we used data from the fifth (1991–1995) to the eighth (2005–2008) examinations. We excluded 303 participants who did not report beverage exposures (n = 9) or who had T2D at baseline (n = 294). For prediabetes, we additionally excluded 1430 participants who had prediabetes at baseline (n = 949), who were missing prediabetes status at baseline or follow-up (n = 165), who developed T2D during follow-up (n = 68), or who were missing change in BMI (n = 207) or other covariate data (n = 41) for a final sample of 1685 participants. In analyses for changes in HOMA-IR, because insulin was measured only at the fifth and seventh examinations, we excluded 860 participants with missing data on HOMA-IR at the fifth and seventh examinations. Participants who developed T2D after the baseline examination were also excluded (n = 126). After excluding participants with missing covariate or change in BMI data (n = 53), 2076 participants were available for the analysis of changes in HOMA-IR. In secondary analyses, we included those who developed T2D after baseline and who had complete covariate data in the analysis for a composite endpoint of prediabetes and T2D (n = 1751) and for changes in HOMA-IR (n = 2195). All participants provided written informed consent. The Framingham Heart Study protocols and procedures were approved by the Institutional Review Board for Human Research at Boston University Medical Center, and the current analyses were approved by the Tufts Medical Center and Tufts University Health Sciences Institutional Review Board.
FIGURE 1.
Study sample and exclusion criteria. *Participants who developed type 2 diabetes after baseline and had complete covariate data were included in the secondary analysis for a composite endpoint of prediabetes and type 2 diabetes (n = 1751) and for changes in HOMA-IR (n = 2195). OGTT, oral-glucose-tolerance test.
Dietary assessment.
We used a semiquantitative 126-item FFQ to assess the habitual dietary intakes of participants during the year preceding each examination cycle (23). The FFQ consisted of a list of foods with standard serving sizes and a selection of 9 frequency categories ranging from none or <1 serving/mo to ≥6 servings/d. SSB intake was captured by 4 FFQ items: 1) caffeinated colas with sugar, 2) caffeine-free colas with sugar, 3) other carbonated beverages with sugar, and 4) fruit punch, lemonade, or other noncarbonated fruit drinks. Diet soda intake was captured by 3 FFQ items: 1) low-calorie colas; 2) low-calorie, caffeine-free colas; and 3) other low-calorie carbonated beverages. One serving of SSBs or diet soda is equivalent to 360 mL (12 fluid ounces). The relative validity of the FFQ for beverage consumption has been examined in other cohorts (23). The correlation coefficients between intakes estimated from the FFQ and 7-d diet records for SSBs and diet soda were 0.51 and 0.66, respectively.
Outcome measures and definitions.
Fasting plasma glucose (FPG) was measured by using a hexokinase reagent. Fasting plasma insulin (FPI) was measured by standard RIAs at the fifth and seventh examinations. The Coat-A-Count immunoassay (Diagnostic Products) was used at the fifth examination, and the human-specific immunoassay (Linco Research, Inc.) was used at the seventh examination. HOMA-IR was calculated on the basis of FPI and FPG by using the formula by Matthews et al. (24): FPG (mmol/L) × FPI (μU/mL)/22.5. Because insulin was measured by using different approaches in the fifth and seventh examinations, we did not calculate the change in HOMA-IR. Instead, we used HOMA-IR measured at the seventh examination as the primary outcome, adjusted for HOMA-IR measured at the fifth examination in statistical models (see Statistical analysis).
At the baseline (fifth) examination, T2D was defined as an FPG concentration ≥7 mmol/L, a 2-h oral-glucose-tolerance test (OGTT) glucose concentration ≥11.1 mmol/L, or the reported use of hypoglycemic medications. At the baseline (fifth) examination, prediabetes was defined as an FPG ≥5.6 and <7 mmol/L or a 2-h OGTT glucose concentration ≥7.8 and <11.1 mmol/L without the use of hypoglycemic medications. Because a 2-h OGTT was not conducted in follow-up visits (sixth, seventh, and eighth examinations), we defined incident T2D as the first occurrence of an FPG concentration ≥7 mmol/L or the use of hypoglycemic medications, and incident prediabetes as the first occurrence of an FPG concentration ≥5.6 and <7 mmol/L in the absence of the use of hypoglycemic medications at follow-up. This definition of prediabetes excluded individuals who presented at a study examination with T2D without presenting with prediabetes at an earlier examination. Therefore, we also considered the incidence of a composite outcome of prediabetes or T2D defined as the first occurrence of either prediabetes or T2D at follow-up after exclusion of baseline prediabetes and T2D.
Anthropometric measurements and covariate assessment.
At each visit, participants underwent a physical examination with the use of standard protocols and completed a medical history questionnaire. Waist circumference was measured at the level of the umbilicus in the standing position. BMI was calculated as weight divided by height (kg/m2). Participants who reported that they smoked regularly in the past year were classified as current smokers. Physical activity level was calculated on the basis of questionnaire-derived time spent performing the activity in a typical day and the intensity of the activity (25). The 2010 Dietary Guidelines Adherence Index (DGAI) was used to capture overall diet quality (26). Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive drugs (27).
Statistical analysis.
To better estimate long-term consumption, cumulative mean intakes of foods and beverages were calculated. For analyses of HOMA-IR, the cumulative mean was calculated as the mean intake reported at the fifth, sixth, and seventh examinations. For analyses of incident prediabetes, the cumulative mean was calculated as the mean intake reported at examinations up to and including the examination of prediabetes diagnosis (e.g., fifth and sixth examinations for those who developed prediabetes by the sixth examination; the fifth, sixth, and seventh examinations for those who developed prediabetes by the seventh examination). For those who did not develop prediabetes during follow-up, the cumulative mean was calculated across all available examination data. The cumulative mean intake of SSBs or diet soda was then categorized into quartile categories.
HRs of incident prediabetes were estimated from multivariable proportional hazards models. Person-time was calculated from baseline until the first occurrence of prediabetes or the end of follow-up. The proportional hazards assumption was evaluated with a likelihood-ratio test comparing the model with and without an interaction term between time period and beverage category. In this analysis, covariates included in the models were FPG at baseline, age, sex, current smoking status, physical activity level, hypertension, BMI, DGAI, and intakes of energy, alcohol, and fruit juice [servings/d (1 serving = 240 mL or 8 fluid ounces)].
To estimate the change in insulin resistance, HOMA-IR assessed at the seventh examination was treated as the response variable, whereas HOMA-IR assessed at the fifth examination was adjusted for as a covariate. The least-squares means of HOMA-IR across SSB or diet soda quartile categories were calculated by using multiple linear regression models, with additional adjustment for age, sex, current smoking status, physical activity level, hypertension, BMI, DGAI, and intakes of energy, alcohol, and fruit juice. In addition, in analyses for both HOMA-IR and prediabetes, SSBs and diet soda were mutually adjusted (i.e., diet soda was adjusted for in analyses for SSBs and vice versa). The change in BMI during the follow-up period was additionally adjusted for in a separate model. The P values for linear trend were computed by modeling the median intake of beverage quartile categories as a continuous independent variable.
In secondary analyses, DGAI was replaced by individual foods, including coffee [servings/d (1 serving = 240 mL or 8 fluid ounces)], whole grains (grams per day), vegetables (grams per day), red meat (grams per day), fish (grams per day), and nuts (grams per day), to explore the potential effects of specific food groups on the associations of beverages with the outcomes. We repeated the above-mentioned analyses for HOMA-IR in a study sample without excluding participants who developed T2D after baseline (n = 2195). A composite endpoint of prediabetes or T2D was also analyzed (n = 1751). In separate models, we adjusted for baseline waist circumference and the change in waist circumference instead of baseline BMI and change in BMI.
In all of the analyses, cumulative mean intakes for dietary covariates were calculated by using the same method for beverage consumption as mentioned above. Baseline data, except for change in BMI or change in waist circumference, were used for nondietary covariates. All statistical analyses were conducted using SAS statistical software (version 9.3; SAS Institute). A 2-tailed P < 0.05 was considered significant unless otherwise specified.
Results
Baseline sample characteristics.
As shown in Table 1, higher SSB consumers were more likely to be younger, men, current smokers, and engaged in more physical activity, and to have higher energy and fruit juice intakes and a less healthy diet as assessed by the DGAI. In addition, SSB consumers were likely to have a higher HOMA-IR at baseline. There was an inverse correlation between SSBs and diet soda intake (r = –0.18, P < 0.001). Diet soda consumers were slightly younger and less likely to smoke, but there were no sex differences across diet soda consumption categories (Supplemental Table 1). Individuals with higher diet soda consumption had a higher BMI, waist circumference, fasting glucose, and HOMA-IR.
TABLE 1.
Baseline characteristics of participants according to quartile categories of sugar-sweetened beverage intake1
| Quartile of sugar-sweetened beverage intake |
|||||
| 1 (n = 394) | 2 (n = 447) | 3 (n = 423) | 4 (n = 421) | P-trend2 | |
| Median intake, servings/wk | 0 | 0.5 | 2 | 6 | |
| Age, y | 53.6 ± 8.5 | 53.0 ± 8.9 | 51.0 ± 9.2 | 50.2 ± 9.5 | <0.001 |
| Women, n (%) | 298 (75.6) | 303 (67.8) | 241 (57) | 163 (38.7) | <0.001 |
| Current smokers, n (%) | 59 (15.0) | 60 (13.4) | 75 (17.7) | 102 (24.2) | <0.001 |
| Alcohol intake, g/d | 9.6 ± 14.5 | 9.6 ± 14.1 | 9.4 ± 13.4 | 9.9 ± 15.1 | 0.009 |
| Physical activity score | 33.8 ± 4.7 | 34.4 ± 5.5 | 34.9 ± 6.3 | 35.8 ± 6.9 | 0.02 |
| BMI, kg/m2 | 26.3 ± 4.6 | 26.2 ± 4.3 | 26.2 ± 4.1 | 26.5 ± 4.5 | 0.17 |
| FPG, mmol/L | 5.01 ± 0.34 | 5.01 ± 0.33 | 5.01 ± 0.38 | 5.05 ± 0.33 | 0.79 |
| HOMA-IR | 5.49 (1.79) | 5.41 (1.76) | 5.57 (2.09) | 5.87 (2.2) | 0.002 |
| Hypertension, n (%) | 85 (21.6) | 95 (21.3) | 82 (19.4) | 87 (20.7) | 0.93 |
| Energy intake, kcal/d | 1614 ± 509 | 1724 ± 538 | 1891 ± 587 | 2195 ± 686 | <0.001 |
| DGAI score | 61.9 ± 10.7 | 60.6 ± 10.4 | 57.9 ± 10.7 | 54.2 ± 10.2 | <0.001 |
| Fruit juice, servings/wk | 4.1 ± 4.5 | 5.2 ± 5.9 | 6.6 ± 6.8 | 7.6 ± 9.8 | <0.001 |
| Diet soda, servings/wk | 5.7 ± 8.7 | 4.2 ± 7.8 | 3.8 ± 7.0 | 2.8 ± 6.0 | <0.001 |
Values are means ± SDs, medians (IQRs), or n (%); n = 1685. One serving of sugar-sweetened beverages or diet soda is equivalent to 360 mL (12 fluid ounces). DGAI, Dietary Guidelines Adherence Index; FPG, fasting plasma glucose.
P-trend was calculated after adjustment for sex and age.
HRs of incident prediabetes.
Among 1685 participants without prediabetes at baseline, 823 went on to develop prediabetes across 18,660 person-years of follow-up. As shown in Table 2, after adjustment for age, sex, and dietary and other potential confounders, those in the highest SSB consumption category, who had a median intake of 6 servings/wk, had an ∼46% higher risk (HR: 1.46; 95% CI: 1.16, 1.83) of incident prediabetes than did those in the lowest quartile category (essentially nonconsumers of SSBs). The linear trend across increasing quartile categories of SSBs was significant (P-trend < 0.001). Adjustment for change in BMI did not substantively change the results. In contrast, after adjusting for BMI and other covariates, no significant association was observed between diet soda intake and incident prediabetes (P-trend = 0.24). Compared with the lowest quartile category, HRs (95% CIs) across the second through the highest quartile categories were 0.87 (0.71, 1.07), 0.76 (0.63, 0.92), and 1.02 (0.85, 1.23), respectively. Further adjustment for change in BMI over the follow-up did not substantially change the observed associations.
TABLE 2.
HRs (95% CIs) of prediabetes according to cumulative mean beverage consumption in 1685 adults1
| Quartile of beverage intake |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Sugar-sweetened beverages | |||||
| Median intake,2 servings/wk | 0 | 0.5 | 2 | 6 | |
| n | 394 | 447 | 423 | 421 | |
| Number of incident cases | 182 | 196 | 197 | 248 | |
| Sex- and age-adjusted | 1 (Ref) | 0.90 (0.74, 1.10) | 0.89 (0.72, 1.09) | 1.24 (1.01, 1.52) | 0.001 |
| Model 1 | 1 (Ref) | 0.94 (0.76, 1.15) | 0.96 (0.77, 1.18) | 1.46 (1.16, 1.83) | <0.001 |
| Model 1 + BMI change | 1 (Ref) | 0.94 (0.76, 1.15) | 0.95 (0.77, 1.17) | 1.47 (1.17, 1.84) | <0.001 |
| Model 2 | 1 (Ref) | 0.91 (0.74, 1.12) | 0.88 (0.71, 1.09) | 1.26 (0.99, 1.59) | 0.003 |
| Model 2 + BMI change | 1 (Ref) | 0.91 (0.74, 1.11) | 0.87 (0.70, 1.08) | 1.27 (1.00, 1.61) | 0.002 |
| Diet soda | |||||
| Median intake,2 servings/wk | 0 | 0.4 | 2 | 9 | |
| n | 520 | 325 | 418 | 422 | |
| Number of incident cases | 268 | 143 | 189 | 223 | |
| Sex- and age-adjusted | 1 (Ref) | 0.83 (0.68, 1.02) | 0.80 (0.67, 0.97) | 1.09 (0.91, 1.31) | 0.05 |
| Model 1 | 1 (Ref) | 0.87 (0.71, 1.07) | 0.76 (0.63, 0.92) | 1.02 (0.85, 1.23) | 0.24 |
| Model 1 + BMI change | 1 (Ref) | 0.85 (0.69, 1.05) | 0.75 (0.62, 0.91) | 0.99 (0.82, 1.20) | 0.36 |
| Model 2 | 1 (Ref) | 0.89 (0.72, 1.10) | 0.77 (0.63, 0.93) | 1.02 (0.84, 1.23) | 0.30 |
| Model 2 + BMI change | 1 (Ref) | 0.87 (0.71, 1.07) | 0.76 (0.62, 0.92) | 0.99 (0.82, 1.20) | 0.40 |
Model 1 adjusted for baseline fasting glucose, age, sex, current smoking status, hypertension, physical activity level, BMI, DGAI score, and intakes of energy, alcohol, and fruit juice. Models were also mutually adjusted for sugar-sweetened beverage and diet soda intakes. Model 2 adjusted for the same covariates as for model 1, except that DGAI score was replaced with intakes of individual foods, including coffee, whole grains, fruit, vegetables, red meat, nuts, and fish. DGAI, Dietary Guidelines Adherence Index; Ref, reference.
One serving of sugar-sweetened beverages or diet soda is equivalent to 360 mL (12 fluid ounces).
Change in insulin resistance.
As shown in Table 3, higher SSB intake was associated with a higher HOMA-IR at follow-up after adjusting for baseline HOMA-IR and other potential confounders (P-trend = 0.006). The adjusted geometric means (95% CIs) were 2.90 (2.79, 3.01), 2.94 (2.84, 3.05), 3.07 (2.96, 3.18), and 3.15 (3.02, 3.27) from the lowest to the highest categories of SSB intake, respectively. Additional adjustment for change in BMI did not materially alter the association. Diet soda intake was not associated with changes in HOMA-IR after adjusting for BMI and other potential confounders. Adjusted geometric mean (95% CI) HOMA-IR values at follow-up across increasing diet soda quartile categories were 2.96 (2.86, 3.05), 2.93 (2.81, 3.06), 3.12 (3.01, 3.23), and 3.04 (2.93, 3.15), respectively (P-trend = 0.25).
TABLE 3.
Geometric means (95% CIs) of HOMA-IR according to cumulative mean beverage consumption in 2076 adults1
| Quartile of beverage intake |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Sugar-sweetened beverages | |||||
| Median intake,2 servings/wk | 0 | 1 | 2 | 6 | |
| n | 522 | 518 | 518 | 518 | |
| Sex- and age-adjusted | 2.94 (2.81, 3.07) | 2.88 (2.75, 3.01) | 3.00 (2.87, 3.14) | 3.24 (3.10, 3.39) | <0.001 |
| Model 1 | 2.90 (2.79, 3.01) | 2.94 (2.84, 3.05) | 3.07 (2.96, 3.18) | 3.15 (3.02, 3.27) | 0.006 |
| Model 1 + BMI change | 2.93 (2.82, 3.04) | 2.90 (2.81, 3.00) | 3.06 (2.96, 3.17) | 3.16 (3.05, 3.28) | 0.001 |
| Model 2 | 2.93 (2.82, 3.04) | 2.94 (2.84, 3.05) | 3.06 (2.96, 3.18) | 3.12 (3.00, 3.25) | 0.02 |
| Model 2 + BMI change | 2.95 (2.84, 3.06) | 2.90 (2.81, 3.00) | 3.06 (2.96, 3.16) | 3.14 (3.03, 3.26) | 0.006 |
| Diet soda | |||||
| Median intake,2 servings/wk | 0 | 0.3 | 3 | 9 | |
| n | 665 | 373 | 520 | 518 | |
| Sex- and age-adjusted | 2.83 (2.72, 2.94) | 2.82 (2.68, 2.97) | 3.15 (3.01, 3.29) | 3.27 (3.13, 3.42) | <0.001 |
| Model 1 | 2.96 (2.86, 3.05) | 2.93 (2.81, 3.06) | 3.12 (3.01, 3.23) | 3.04 (2.93, 3.15) | 0.25 |
| Model 1 + BMI change | 2.98 (2.89, 3.07) | 2.94 (2.83, 3.06) | 3.12 (3.01, 3.22) | 3.01 (2.91, 3.11) | 0.59 |
| Model 2 | 2.96 (2.87, 3.06) | 2.94 (2.81, 3.06) | 3.12 (3.01, 3.24) | 3.02 (2.92, 3.14) | 0.39 |
| Model 2 + BMI change | 2.98 (2.89, 3.07) | 2.94 (2.83, 3.06) | 3.12 (3.02, 3.23) | 3.00 (2.90, 3.10) | 0.74 |
Model 1 adjusted for baseline HOMA-IR, age, sex, current smoking status, hypertension, physical activity level, BMI, DGAI score, and intakes of energy, alcohol, and fruit juice. The model was also mutually adjusted for sugar-sweetened beverage and diet soda intakes. Model 2 adjusted for the same covariates as for model 1, except that the DGAI score was replaced with intakes of individual foods, including coffee, whole grains, fruit, vegetables, red meat, nuts, and fish. DGAI, Dietary Guidelines Adherence Index.
One serving of sugar-sweetened beverages or diet soda is equivalent to 360 mL (12 fluid ounces).
Secondary analyses.
In secondary analyses, in which we adjusted for individual foods rather than DGAI score, the observed associations remained similar (Tables 2 and 3). In addition, higher SSB intake was associated with a greater incidence of the composite outcome of prediabetes or T2D (Supplemental Table 2). Similarly, higher SSB intake was associated with a stepwise increased HOMA-IR in 2195 participants, including those who developed T2D after baseline (Supplemental Table 3). In analyses that substituted adjustment for baseline waist circumference and change in waist circumference for BMI and change in BMI, similar associations were observed (data not shown).
Discussion
In this prospective cohort study in middle-aged adults, higher SSB consumption was modestly associated with a higher incidence of prediabetes and a greater increase in insulin resistance as assessed by using HOMA-IR, after adjustment for multiple confounders, including change in general adiposity. In contrast, we observed no significant association between diet soda intake and incident prediabetes or changes in HOMA-IR. Therefore, our data suggest that regular SSB intake, but not diet soda intake, is associated with increased insulin resistance and a greater risk of developing prediabetes.
Several small randomized controlled trials examined the potential effects of added sugar on insulin resistance. Under conditions of weight maintenance, Black et al. (10) observed no difference in insulin resistance after 6 wk (n = 13) on intervention arms that provided 25% or 10% of total energy from sucrose. However, a parallel randomized controlled trial in 37 overweight adults showed that, after adjustment for baseline HOMA-IR and change in body weight, 10 wk of consuming a high-sucrose (∼25% energy from sucrose) ad libitum diet significantly increased HOMA-IR compared with a diet in which participants consumed artificial sweeteners (28).
To date, several observational studies in adults have examined the cross-sectional associations between intakes of SSBs (11, 29), total sugar (12), sucrose (14), and fructose (13) and HOMA-IR; the findings have been inconsistent, with some observing a positive association (11, 13, 29), whereas others found no association (12, 14). One recent meta-analysis, which summarized studies from 17 prospective cohorts, suggested that an additional serving per day of SSBs is associated with an ∼18% increased risk of developing T2D (30). Prospective cohort studies of SSBs and diet soda in relation to HOMA-IR and/or prediabetes in adults are scarce. A 2-y follow-up study in children (n = 564, aged 8–10 y) observed that a higher intake of added sugar from liquid sources was prospectively associated with an increased HOMA-IR (15). The present longitudinal study provides epidemiologic evidence that regular SSB consumption may be associated with increased insulin resistance in adults, although the observed difference in HOMA-IR between regular SSB consumers and nonconsumers was relatively low, with an 8% higher HOMA-IR in the highest SSB consumers. Nevertheless, our data also showed that regular SSB intake was associated with a greater risk of developing prediabetes.
In the Framingham Heart Study Offspring cohort, higher SSB or soda intake was cross-sectionally associated with increased insulin resistance (11) and a greater prevalence of a metabolically unhealthy phenotype (31); furthermore, combined SSB and diet soda consumption was associated with a higher incidence of impaired fasting glucose over 4 y (32). The current analysis extends these previous studies by separately examining SSBs and diet soda by using cumulative mean intake, thereby reducing within-participant variability in dietary intake, as well as extending the follow-up period to 14 y.
In the present study, we observed no significant association between diet soda intake and HOMA-IR, an observation that is consistent, for example, with findings from a small trial conducted in 37 adults in whom no change in HOMA-IR was observed after 10 wk of artificial sweetener consumption (28). However, the role of artificial sweeteners in stimulating insulin resistance is not conclusive. Although diet soda provides no extra calories, it is thought that other mechanisms, such as effects on enhancing appetite (33), may play a role in the development of insulin resistance and/or diabetes; in fact, several prospective cohort studies observed a direct association between diet soda intake and the risk of T2D after adjustment for BMI (18, 19). Thus, future studies are still needed to examine the metabolic effects of diet soda.
The underlying mechanisms that link excess sugar intake and insulin resistance are not completely understood, particularly whether insulin resistance occurs due to sugar intake itself or by weight gain caused by a positive energy balance. One potential pathway by which excess sugar intake may mediate the impairment of insulin sensitivity is through an increase in fat synthesis in liver and adipose tissues. In the process of lipogenesis, sugar may be converted to products such as diacylglycerols that are harmful to insulin signaling (34). SSB intake may also exert its influence on insulin resistance through its potential role in visceral adipose tissue (VAT) deposition (35). Excess FFAs released from enlarged VAT may directly flow through the portal vein to induce hepatic insulin resistance (36). Finally, adipokines secreted by adipose tissue, particularly VAT, may trigger insulin resistance in liver and peripheral tissue (37).
The strengths of our study include its relatively novel explorations of the long-term associations of SSB or diet soda intake with insulin resistance and prediabetes, as opposed to the more typically evaluated incident T2D. The study used a prospective design and comprehensive dietary, lifestyle, and clinical data collected in a well-characterized subgroup of the Framingham Heart Study. With respect to limitations, insulin was measured by using different assays at baseline and follow-up; we, therefore, could not calculate the change in HOMA-IR directly. In addition, using different assays may lead to misclassification of insulin resistance. However, our finding on HOMA-IR was supported by that of incident prediabetes. Although we adjusted for a variety of dietary and lifestyle factors, residual confounding cannot be ruled out due to the observational nature of this study. We evaluated only diet soda, not the consumption of other low- or noncalorie or artificially sweetened beverages, which were not uniformly assessed in the FFQ. Compared with those excluded from the analysis (i.e., those without prediabetes and/or with missing data), participants who were analyzed in the present study were younger and more likely to be women, had lower BMIs and waist circumferences, and had a better cardiometabolic profile (Supplemental Table 4). Therefore, this may limit the generalizability of our observations to other populations. In addition, the majority of our study population was middle-aged and white, which may minimize confounding from race/ethnicity and socioeconomic factors but may also limit the generalizability.
Our data suggest that the long-term consumption of SSBs predicts increased insulin resistance and a higher risk of developing prediabetes, independent of body weight. In contrast, long-term diet soda intake was not associated with elevated insulin resistance or prediabetes risk. Although these observational data provide further evidence to support the association between daily SSB consumption and increased cardiometabolic risk, well-designed metabolically controlled intervention trials with sufficient power and duration are required to further examine how SSB intake may influence insulin resistance and its underlying mechanisms.
Acknowledgments
JM and NMM designed the analysis; JM analyzed the data and wrote the manuscript; JM and GTR conducted the statistical analysis; PFJ, JBM, CSF, CES, AH, ES, and NMM provided critical editorial comments; and NMM had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DGAI, Dietary Guidelines Adherence Index; FPG, fasting plasma glucose; FPI, fasting plasma insulin; OGTT, oral-glucose-tolerance test; SSB, sugar-sweetened beverage; T2D, type 2 diabetes; VAT, visceral adipose tissue.
References
- 1.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–26. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynes AE, Mark Edwards C, Ghatei MA, Dornhorst A, Morgan LM, Bloom SR, Frost GS. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br J Nutr 2003;89:207–18. [DOI] [PubMed] [Google Scholar]
- 7.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–13. Erratum in: Diabetes 2006;55(2):563. [DOI] [PubMed] [Google Scholar]
- 9.Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–5. [DOI] [PubMed] [Google Scholar]
- 10.Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CN, McCance DR, Young IS, Bell PM, Hunter SJ. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006;55:3566–72. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida M, McKeown NM, Rogers G, Meigs JB, Saltzman E, D’Agostino R, Jacques PF. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr 2007;137:2121–7. [DOI] [PubMed] [Google Scholar]
- 12.Austin GL, Krueger PM. Increasing the percentage of energy from dietary sugar, fats, and alcohol in adults is associated with increased energy intake but has minimal association with biomarkers of cardiovascular risk. J Nutr 2013;143:1651–8. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez Coello S, Cabrera de Leon A, Rodriguez Perez MC, Borges Alamo C, Carrillo Fernandez L, Almeida Gonzalez D, Garcia Yanes J, Gonzalez Hernandez A, Brito Diaz B, Aguirre-Jaime A. Association between glycemic index, glycemic load, and fructose with insulin resistance: the CDC of the Canary Islands Study. Eur J Nutr 2010;49:505–12. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Faerch K, Glumer C, Tetens I, Pedersen O, Carstensen B, Jorgensen T, Borch-Johnsen K; Inter99 study. Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: the Inter99 study. Diabetes Care 2005;28:1397–403. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Light K, Henderson M, O’Loughlin J, Mathieu ME, Paradis G, Gray-Donald K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr 2014;144:81–6. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosini GL, Oddy WH, Huang RC, Mori TA, Beilin LJ, Jebb SA. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr 2013;98:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kynde I, Johnsen NF, Wedderkopp N, Bygbjerg IB, Helge JW, Heitmann BL. Intake of total dietary sugar and fibre is associated with insulin resistance among Danish 8–10- and 14–16-year-old girls but not boys. European Youth Heart Studies I and II. Public Health Nutr 2010;13:1669–74. [DOI] [PubMed] [Google Scholar]
- 18.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2013;97:517–23. [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 21.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J 1986;112:820–5. [DOI] [PubMed] [Google Scholar]
- 26.Sauder KA, Proctor DN, Chow M, Troy LM, Wang N, Vita JA, Vasan RS, Mitchell GF, Jacques PF, Hamburg NM, et al. Endothelial function, arterial stiffness and adherence to the 2010 Dietary Guidelines for Americans: a cross-sectional analysis. Br J Nutr 2015;113:1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr 2005;82:421–7. [DOI] [PubMed] [Google Scholar]
- 29.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green AK, Jacques PF, Rogers G, Fox CS, Meigs JB, McKeown NM. Sugar-sweetened beverages and prevalence of the metabolically abnormal phenotype in the Framingham Heart Study. Obesity (Silver Spring) 2014;22:E157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 33.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 2009;89:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC, Smith SR, Langin D, Moro C. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011;60:1734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma J, Sloan M, Fox CS, Hoffmann U, Smith CE, Saltzman E, Rogers GT, Jacques PF, McKeown NM. Sugar-sweetened beverage consumption is associated with abdominal fat partitioning in healthy adults. J Nutr 2014;144:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 2012;13(Suppl 2):30–9. [DOI] [PubMed] [Google Scholar]
- 37.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes 2012;19:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]