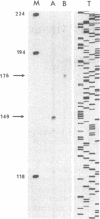
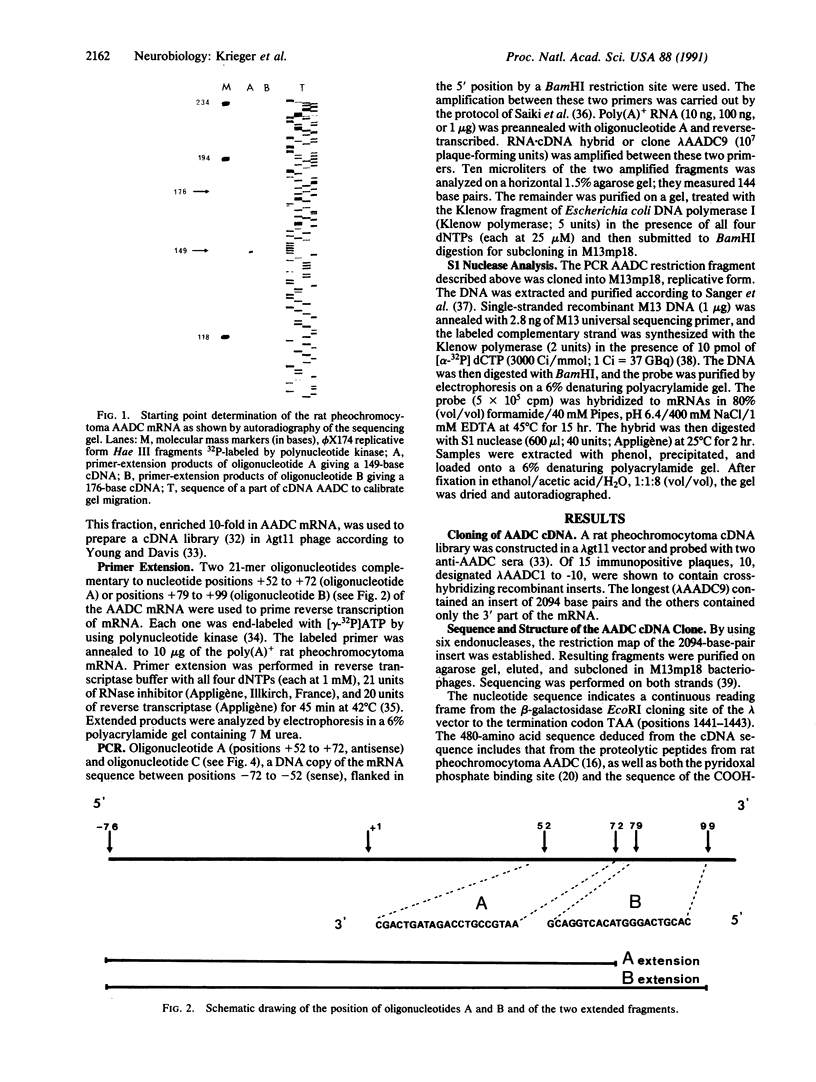
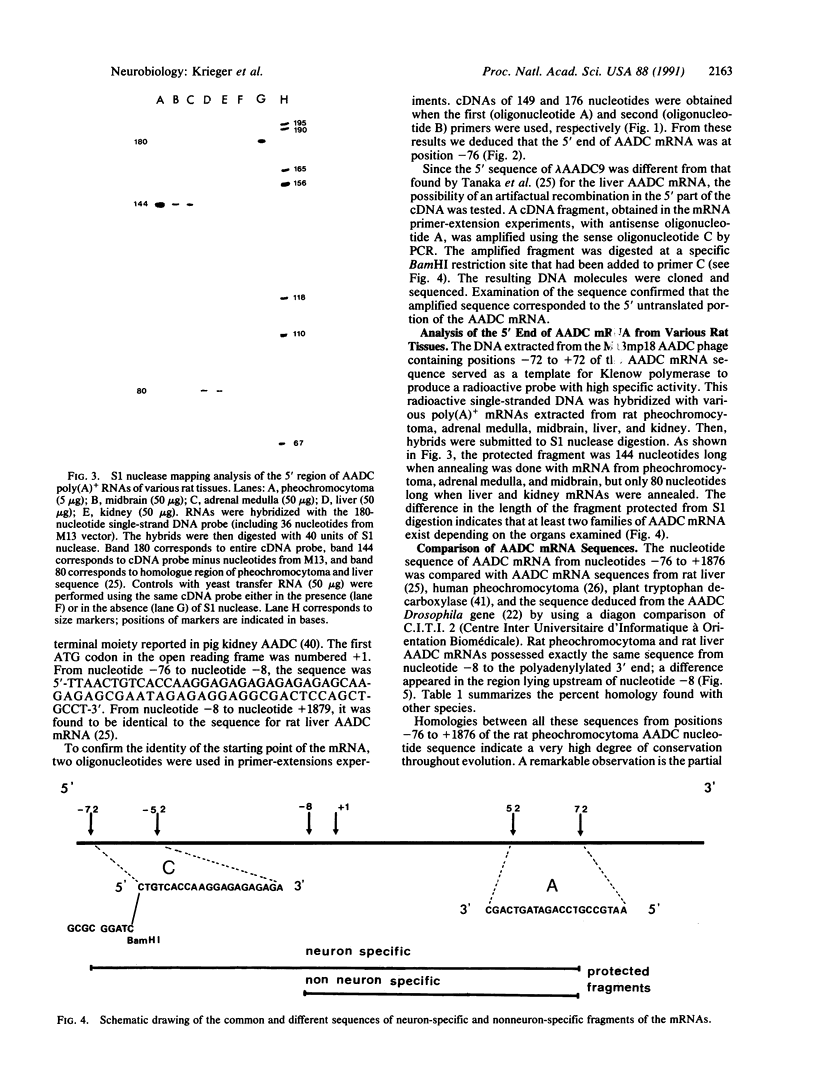
Abstract
A cDNA clone for dopa decarboxylase (EC 4.1.1.28) has been isolated from a rat pheochromocytoma cDNA library and the cDNA sequence has been determined. It corresponds to an mRNA of 2094 nucleotides. The length of the mRNA was measured by primer-extension of rat pheochromocytoma RNA and the 5' end of the sequence of the mRNA was confirmed by the PCR. A probe spanning the translation initiation site of the mRNA was used to hybridize with mRNAs from various organs of the rat. S1 nuclease digestion of the mRNAs annealed with this probe revealed two classes of mRNAs. The comparison of the cDNA sequence and published sequences for rat liver, human pheochromocytoma, and Drosophila dopa decarboxylase supported the conclusion that two mRNAs are produced: one is specific for tissue of neuronal origin and the other is specific for tissues of nonneuronal (mesodermal or endodermal) origin. The neuronal mRNA contains a 5' untranslated sequence that is highly conserved between human and rat pheochromocytoma including a GA stretch. The coding sequence and the 3' untranslated sequence of mRNAs from rat liver and pheochromocytoma are identical. The rat mRNA differs only in the 5' untranslated region. Thus a unique gene codes for dopa decarboxylase and this gene gives rise to at least two transcripts presumably in response to different signals during development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. R., Culvenor A. J., Hall J., Jarrott B., Wellard R. M. Aromatic L-amino acid decarboxylase: histochemical localization in rat kidney and lack of effect of dietary potassium or sodium loading on enzyme distribution. Clin Exp Pharmacol Physiol. 1986 Jan;13(1):47–53. doi: 10.1111/j.1440-1681.1986.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Albert V. R., Allen J. M., Joh T. H. A single gene codes for aromatic L-amino acid decarboxylase in both neuronal and non-neuronal tissues. J Biol Chem. 1987 Jul 5;262(19):9404–9411. [PubMed] [Google Scholar]
- Ando-Yamamoto M., Hayashi H., Sugiyama T., Fukui H., Watanabe T., Wada H. Purification of L-dopa decarboxylase from rat liver and production of polyclonal and monoclonal antibodies against it. J Biochem. 1987 Feb;101(2):405–414. doi: 10.1093/oxfordjournals.jbchem.a121925. [DOI] [PubMed] [Google Scholar]
- Bossa F., Martini F., Barra D., Voltattorni C. B., Minelli A., Turano C. The chymotryptic phosphopyridoxyl peptide of DOPA decarboxylase from pig kidney. Biochem Biophys Res Commun. 1977 Sep 9;78(1):177–184. doi: 10.1016/0006-291x(77)91237-2. [DOI] [PubMed] [Google Scholar]
- Bruneau G., Krieger-Poullet M., Coge F., Borri-Voltattorni C., Gros F., Thibault J. Characterization of DOPA decarboxylase mRNA in rat pheochromocytoma. Biochimie. 1990 Jan;72(1):73–76. doi: 10.1016/0300-9084(90)90175-g. [DOI] [PubMed] [Google Scholar]
- Chobert M. N., Lahuna O., Lebargy F., Kurauchi O., Darbouy M., Bernaudin J. F., Guellaen G., Barouki R., Laperche Y. Tissue-specific expression of two gamma-glutamyl transpeptidase mRNAs with alternative 5' ends encoded by a single copy gene in the rat. J Biol Chem. 1990 Feb 5;265(4):2352–2357. [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase (immunological titration-aromatic L-amino acid decarboxylase-serotonin-dopamine-norepinephrine). Proc Natl Acad Sci U S A. 1972 Feb;69(2):343–347. doi: 10.1073/pnas.69.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. Preparation and properties of a homogeneous aromatic L-amino acid decarboxylase from hog kidney. Arch Biochem Biophys. 1970 Nov;141(1):356–367. doi: 10.1016/0003-9861(70)90144-x. [DOI] [PubMed] [Google Scholar]
- Cogé F., Krieger-Poullet M., Guillemot J. C., Ferrara P., Gros F., Thibault J. Purification et séquençage partiel de la L-dopa décarboxylase de phéochromocytome de rat. C R Acad Sci III. 1989;309(14):587–592. [PubMed] [Google Scholar]
- De Luca V., Marineau C., Brisson N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2582–2586. doi: 10.1073/pnas.86.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Eveleth D. D., Gietz R. D., Spencer C. A., Nargang F. E., Hodgetts R. B., Marsh J. L. Sequence and structure of the dopa decarboxylase gene of Drosophila: evidence for novel RNA splicing variants. EMBO J. 1986 Oct;5(10):2663–2672. doi: 10.1002/j.1460-2075.1986.tb04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveleth D. D., Marsh J. L. Evidence for evolutionary duplication of genes in the dopa decarboxylase region of Drosophila. Genetics. 1986 Oct;114(2):469–483. doi: 10.1093/genetics/114.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Ichinose H., Kojima K., Togari A., Kato Y., Parvez S., Parvez H., Nagatsu T. Simple purification of aromatic L-amino acid decarboxylase from human pheochromocytoma using high-performance liquid chromatography. Anal Biochem. 1985 Nov 1;150(2):408–414. doi: 10.1016/0003-2697(85)90529-9. [DOI] [PubMed] [Google Scholar]
- Ichinose H., Kurosawa Y., Titani K., Fujita K., Nagatsu T. Isolation and characterization of a cDNA clone encoding human aromatic L-amino acid decarboxylase. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1024–1030. doi: 10.1016/0006-291x(89)91772-5. [DOI] [PubMed] [Google Scholar]
- Kaplan B. B., Bernstein S. L., Gioio A. E. An improved method for the rapid isolation of brain ribonucleic acid. Biochem J. 1979 Oct 1;183(1):181–184. doi: 10.1042/bj1830181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- Lindström P., Sehlin J. Mechanisms underlying the effects of 5-hydroxytryptamine and 5-hydroxytryptophan in pancreatic islets. A proposed role for L-aromatic amino acid decarboxylase. Endocrinology. 1983 Apr;112(4):1524–1529. doi: 10.1210/endo-112-4-1524. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Tempel B. L. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983 May 5;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Lunan K. D., Mitchell H. K. The metabolism of tyrosine-O-phosphate in Drosophila. Arch Biochem Biophys. 1969 Jul;132(2):450–456. doi: 10.1016/0003-9861(69)90388-9. [DOI] [PubMed] [Google Scholar]
- Luo X. C., Park K., Lopez-Casillas F., Kim K. H. Structural features of the acetyl-CoA carboxylase gene: mechanisms for the generation of mRNAs with 5' end heterogeneity. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4042–4046. doi: 10.1073/pnas.86.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A., Johnson W. A., Hirsh J. Regulated splicing produces different forms of dopa decarboxylase in the central nervous system and hypoderm of Drosophila melanogaster. EMBO J. 1986 Dec 1;5(12):3335–3342. doi: 10.1002/j.1460-2075.1986.tb04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2452–2457. [PubMed] [Google Scholar]
- Otten U., Schwab M., Gagnon C., Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977 Sep 16;133(2):291–303. doi: 10.1016/0006-8993(77)90765-x. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci. 1968 May 14;170(1018):71–80. doi: 10.1098/rspb.1968.0025. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Rost F. W., Pearse A. G. Fluorogenic amine tracing of neural crest derivatives forming the adrenal medulla. Gen Comp Endocrinol. 1971 Feb;16(1):132–136. doi: 10.1016/0016-6480(71)90215-2. [DOI] [PubMed] [Google Scholar]
- Rahman M. K., Nagatsu T., Kato T. Aromatic L-amino acid decarboxylase activity in central and peripheral tissues and serum of rats with L-DOPA and L-5-hydroxytryptophan as substrates. Biochem Pharmacol. 1981 Mar 15;30(6):645–649. doi: 10.1016/0006-2952(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Rudd E. A., Thanassi J. W. Inhibition of aromatic L-amino acid decarboxylase by coenzyme-amino acid adducts. Biochemistry. 1981 Dec 22;20(26):7469–7475. doi: 10.1021/bi00529a022. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Schweinfest C. W., Kwiatkowski R. W., Dottin R. P. Molecular cloning of a DNA sequence complementary to creatine kinase M mRNA from chickens. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4997–5000. doi: 10.1073/pnas.79.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Konarksa M. M., Grabowski P. J., Lamond A. I., Marciniak R., Seiler S. R. Splicing of messenger RNA precursors. Cold Spring Harb Symp Quant Biol. 1987;52:277–285. doi: 10.1101/sqb.1987.052.01.033. [DOI] [PubMed] [Google Scholar]
- Shirota K., Fujisawa H. Purification and characterization of aromatic L-amino acid decarboxylase from rat kidney and monoclonal antibody to the enzyme. J Neurochem. 1988 Aug;51(2):426–434. doi: 10.1111/j.1471-4159.1988.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Horio Y., Taketoshi M., Imamura I., Ando-Yamamoto M., Kangawa K., Matsuo H., Kuroda M., Wada H. Molecular cloning and sequencing of a cDNA of rat dopa decarboxylase: partial amino acid homologies with other enzymes synthesizing catecholamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8142–8146. doi: 10.1073/pnas.86.20.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancini B., Dominici P., Simmaco M., Schininà M. E., Barra D., Voltattorni C. B. Limited tryptic proteolysis of pig kidney 3,4-dihydroxyphenylalanine decarboxylase. Arch Biochem Biophys. 1988 Feb 1;260(2):569–576. doi: 10.1016/0003-9861(88)90483-3. [DOI] [PubMed] [Google Scholar]
- Thibault J., Vidal D., Gros F. In vitro translation of mRNA from rat pheochromocytoma tumors, characterization of tyrosine hydroxylase. Biochem Biophys Res Commun. 1981 Apr 15;99(3):960–968. doi: 10.1016/0006-291x(81)91256-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltattorni C. B., Giartosio A., Turano C. Aromatic-L-amino acid decarboxylase from pig kidney. Methods Enzymol. 1987;142:179–187. doi: 10.1016/s0076-6879(87)42027-2. [DOI] [PubMed] [Google Scholar]
- Voltattorni C. B., Minelli A., Vecchini P., Fiori A., Turano C. Purification and characterization of 3,4-dihydroxyphenylalanine decarboxyase from pig kidney. Eur J Biochem. 1979 Jan 2;93(1):181–188. doi: 10.1111/j.1432-1033.1979.tb12809.x. [DOI] [PubMed] [Google Scholar]
- Warren S., Chute R. N. Pheochromocytoma. Cancer. 1972 Feb;29(2):327–331. doi: 10.1002/1097-0142(197202)29:2<327::aid-cncr2820290210>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]