Abstract
Despite consistent observations of sex differences in depression and related emotional disorders, we do not yet know how these sex differences modulate the effects of genetic polymorphisms implicated in risk for these disorders. In this Mini-Review, we focus on genetic polymorphisms of the serotonergic system to illustrate how sex differences might modulate the neurobiological pathways involved in the development of depression. We consider the interacting role of environmental factors such as early life stress. Given limited current knowledge about this topic we highlight methodological considerations, challenges, and guidelines for future research.
Keywords: early life stress, amygdala, anxiety, suicide, conduct disorder, personality
Graphical Abstract
The effects of polymorphisms in serotonin transporter, monoamine oxidase a, and tyrosine hydroxylase genes on depression, clinical expressions, and latent risk factors are modulated by sex differences.
Introduction
Major depressive disorder is an issue of global concern. With an estimated lifetime prevalence of 10%, depression is a leading cause of disability in the US and worldwide (Demyttenaere et al., 2004, Kessler et al., 2003, Ustün et al., 2004). Depressed individuals have a 3.4% suicide risk, compared to 0.017% in the general population (Blair-West et al., 1999). Depression frequently co-occurs with other medical and psychiatric conditions, including a 51.2% comorbidity with anxiety, which is associated with slower recovery, higher rates of recurrence, and increased disability (Hirschfeld et al., 2001).
Despite the impact of depression, the development of preventative and remedial interventions is limited by our understanding of the multiple interacting biobehavioral pathways that contribute to the pathogenesis and expression of depression. To address these substantial gaps in knowledge, we might draw on findings regarding sex and genetic polymorphisms as risk factors for depression and related conditions. These findings are relatively consistent, especially compared to the work on biobehavioral factors, and also provide an important foundation for ultimately understanding individual differences in biobehavior.
Heritability data highlight the role of genetic factors in risk for depression and related conditions. Twin studies show that heritability of depression, anxiety, and suicide is estimated at, respectively, 37% (Sullivan et al., 2000), 45% (Stein et al., 2001) and 43% (McGuffin et al., 2010). Single gene variations involved in serotonergic metabolism in particular have been extensively studied for their role in depression. However, these single gene variations may confer depression risk through interactions with environmental factors rather than via their own discrete effects, and these gene-environment interactions may themselves be mediated by sexually dimorphic pathways.
Epidemiological studies have long highlighted sex differences in depression and its related phenomenology. Women have a 21.3% lifetime rate of depression, as compared to 12.7% for men (Kessler et al., 1993). Similarly, women have a higher lifetime rate of generalized anxiety disorder—7.7% as compared to 12.7% for men—as well as higher comorbidity with depression—38.3% as compared to 30% for men (McLean et al., 2011). However, the suicide rate for depressed men is 7% as compared to 1% for depressed women (Blair-West et al., 1999). The heritability of major depression is higher in women (42%) than in men (29%), which suggests modulation of genetic risk by sex (Kendler et al., 2006). Sex differences have also been observed in the environmental risk factors, such as early life stress (Bale and Epperson, 2015) and in bi-obehavioral factors implicated in the mechanisms by which risk for depression is conferred, including personality traits such as neuroticism (Goodwin and Gotlib, 2004) and brain measures such as amygdala reactivity (Williams et al., 2005). Here we focus on the current state of knowledge regarding sex differences in genetic variation implicated in risk for depression and related phenomena.
The scope of this review
Our intention is to provide a focused review and personal view highlighting the potential mechanisms for how sex differences might modulate the effect of genetic polymorphisms in depression and its related conditions (i.e., “gene-sex interactions”), rather than to provide a systematic review of gene-sex interactions. We hope to show the importance of considering sex differences, even when studying the brain at the genetic level.
We focus on major depressive disorder, including also evidence regarding anxiety and externalizing disorders highly comorbid with depression (Hirschfeld et al., 2001) and regarding suicidality given its association with depression (Blair-West et al., 1999). Though we focus our review on the serotonergic system, we do not intend to imply that it is the only system for which gene-sex interactions are relevant to depression and related phenomenology. Rather, we hope this focused review will serve as a template for considering sex differences in other biological genetic pathways implicated in depression.
We propose three potential pathways through which polymorphisms in genes involved in serotonin synthesis, transport, and breakdown may differentially impact behavior as a function of sex. First, we consider sex differences in response to early life stress and the effects of stress on gene-sex interactions involving the serotonin-transporter-linked polymorphic region (5-HTTLPR) in the serotonin transporter gene (SLC6A4) which codes for the protein that transports serotonin from the synaptic cleft to the pre-synaptic neuron. Next, we consider the effects of x-linkage, using as an example the monoamine oxidase-A linked polymorphic region (MAOA-LPR) which alters the degradation serotonin and other amine neurotransmitters. Third, we consider how sex hormones interact with gene products, focusing on polymorphisms in two tryptophan hydroxylase isozymes (TPH1 and TPH2) the rate-limiting enzymes in serotonin biosynthesis in the periphery and central nervous system (Zhang et al., 2004). We do not intend to suggest that these are the only pathways through which gene-sex interactions exert their effect within the serotonergic system, nor that genetic polymorphisms are only modulated by these pathways. Rather, our intention is to illustrate the central role of sex in the biobehavioral effects of polymorphisms involved in the serotonergic system.
For each polymorphism, we have summarized findings of association with depression, its related disorders (anxiety disorders, conduct disorder, and suicide), trait risk factors (neuroticism, harm avoidance, aggression, and impulsiveness), and neurobiological endophenotypes, including amygdala hyperactivation, limbic structure and function, and affective processing (Tables I–III). For all polymorphisms, we included studies with significant gene main effects, or significant gene-environment interactions, in one or both sexes. We classified a finding as showing a sex difference (1) if an allele conferred opposite risk in men and women, (2) if significant main effects emerged only for one sex, or (3) if differing interaction effects emerged. We classified a finding as having no sex difference only if the investigators explicitly tested and ruled out a sex interaction. Studies were classified as having unclear gene-sex interactions if (1) only one sex was studied, (2) sex interactions were not tested in a mixed group, (3) marginally significant sex differences were found, or (4) sex differences were found in measures not included in the table. Due to space constraints, we have limited the listing of the studies performed on the association between 5-HTTLPR and depression to those solely focusing on sex differences. A more complete listing may be found in Karg et al., 2011 or Sharpley et al., 2014. We chose to include studies performed on only one sex, because the risk directionality of some of the alleles may be sex-dependent. For example, while the preponderance of studies point toward MAOA-L conferring risk for conduct disorder in boys, the female-only study performed by Sjoberg et al. in 2007 supports the hypothesis that the other allele, MAOA-H, confers risk in girls. However, future studies, performed in both sexes, are necessary to confirm these associations. Studies that show gene-sex interactions are listed in Table IV, along with sample and effect sizes.
Table I.
Sex Modulation of 5-HTT-LPR in Depression, Related Disorders, Risk Factors, and Endophenotypes1
This tabular summary only reflects studies with positive findings in one or both sexes.
Due to space constraints, a listing of all studies on the main effect of 5-HTTLPR have been omitted. Refer to Karg et al., 2011 and Sharpley et al., 2014 for more complete meta-analyses.
No effects in gene-sex interactions: Denotes studies in which gene-sex interactions were tested and not found
Sex difference: Denotes studies in which (†) significant effects emerged for only one sex, (‡) significant effects emerged in opposite directions by sex, or interaction effects were different. Bold Underlining denotes studies that observed sex differences.
Did not examine/Unclear: Denotes studies in which (1) only one sex was studied, (2) sex interactions were not ruled out, (3) marginally significant sex differences were found, or (4) sex differences were found in measures not included in the table.
Conduct disorder: Includes measures of delinquency and other externalizing symptoms when applicable
Limbic structure: Includes volumetric and other anatomical measures of limbic components. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Limbic function: Includes functional connectivity, steady-state effects, and measures of limbic activity excluding amygdala hyperactivation. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Denotes significant effects arising in the context of stress or early life adversity
Denotes specifically the SL genotype
Denotes meta-analyses
Table III.
Sex Modulation of TPH1 and TPH2 in Depression, Related Disorders, Risk Factors, and Endophenotypes1
| Parameter | Depression | Anxiety | Conduct Disorder | Suicide | ||||
|---|---|---|---|---|---|---|---|---|
| Independent effects within sexes by gene2 | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 |
| Significant effects in males | Serretti 2001, Eley 2004, Gizatullin 2006, Jokela 2007*, Viikki 2010 | Zill 2004b, Zhang 2005, Zhou 2005, Van Den Bogaert 2006, Anttila 2009, Tsai 2009, Gao 2012, Nobile 2009*, Ma 2015* | Zhou 2005, Campos 2011 | Nielsen 1994, Nielsen 1998, Rujescu 2003, Bellivier 2004||, Galfalvy 2009, Gizatullin 2006, Li 2006, Gonzalez-Castro 2014 | Mann 1997, Zill 2004a, Zhou 2005, Ke 2006, Lopez de Lara 2007, Yoon 2009, Grohmann 2010, Perez-Rodiguez 2010, Zhang 2010 | |||
| Significant effects in females | Eley 2004, Sun 2004, Gizatullin 2006, Jokela 2007*, Viikki 2010 | Zill 2004b, Zhang 2005, Zhou 2005, Van Den Bogaert 2006, Anttila 2009, Lin 2009, Tsai 2009, Utge 2010, Shen 2011, Fasching 2012, Gao 2012, Nobile 2009*, Mandelli 2012*, Ma 2015* | Sun 2004 | Zhou 2005, Maron 2007, Kim 2009, Lin 2009, Campos 2010 | Rujescu 2003, Bellivier 2004||, Galfalvy 2009, Gizatullin 2006, Li 2006, Zaboli 2006, Gonzalez-Castro 2014 | Mann 1997, Zill 2004a, Zhou 2005, Ke 2006, Lopez de Lara 2007, Yoon 2009, Grohmann 2010, Perez-Rodiguez 2010, Zhang 2010 | ||
|
| ||||||||
| No effects in gene-sex interactions | Eley, Viikki | Lopez de Lara | ||||||
| Sex difference | Serretti† | Shen†, Utge | Kim†, Maron† | |||||
| Did not examine/unclear | Gizatullin, Jokela, Sun | Anttila, Fasching, Gao, Lin, Ma, Mandelli, Nobile, Tsai, Van Den Bogaert, Zhang, Zhou, Zill | Sun | Campos, Lin, Zhou | Bellivier||, Galfalvy, Gizatullin, González-Castro, Li, Nielsen 1994, Nielsen 1998, Rujescu, Zaboli | Grohmann, Ke, Mann, Perez-Rodriguez, Zhang, Zhou, Zill, Yoon | ||
|
| ||||||||
| Parameter | Neuroticism | Harm Avoidance | Aggression | Impulsiveness | ||||
|
| ||||||||
| Independent effects within sexes by gene | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 |
| Significant effects in males | Lehto 2015 | Andre 2013 | Reuter 2007, Gutknecht 2007 | New 1998, Manuck 1999, Rujescu 2002, Staner 2002, Koh 2012 | Perez-Rodiguez 2010 | New 1998, Staner 2002, Galfalvy 2009 | Stoltenberg 2012 | |
| Significant effects in females | Lehto 2015 | Keltikangas- Järvinen 2007, Andre 2013 | Reuter 2007, Gutknecht 2007 | Manuck 1999, Rujescu 2002, Staner 2002, Koh 2012 | Perez-Rodiguez 2010, Yang 2010 | Staner 2002, Galfalvy 2009 | ||
|
| ||||||||
| No effects in gene-sex interactions | Koh, Rujescu, Staner | Staner | ||||||
| Sex difference | Keltikangas-Järvinen† | Stoltenberg† | ||||||
| Did not examine/unclear | Lehto | Andre | Gutknecht, Reuter | Manuck, New | Perez-Rodiguez, Yang | Galfalvy, New | ||
|
| ||||||||
| Parameter | Amygdala Hyperactivation | Limbic Structure | Limbic Function | Affective Processing | ||||
|
| ||||||||
| Independent effects within sexes by gene | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 | TPH1 | TPH2 |
| Significant effects in males | Brown 2005, Canli 2005c, Canli 2008, Furmark 2009 | Inoue 2010, Yoon 2012 | Canli 2008, Hermann 2012* | Herrmann 2007, Armbruster 2010, Perez-Rodiguez 2010, Yoon 2012 | ||||
| Significant effects in females | Lee 2009 | Brown 2005, Canli 2005b, Canli 2008, Lee 2008b, Furmark 2009 | Inoue 2010, Yoon 2012 | Canli 2008, Hermann 2012* | Herrmann 2007, Armbruster 2010, Perez-Rodiguez 2010, Yoon 2012 | |||
|
| ||||||||
| No effects in gene-sex interactions | Inoue | |||||||
| Sex difference | Armbruster‡ | |||||||
| Did not examine/unclear | Lee | Brown, Canli 2005c, Canli 2008, Furmark, Lee | Yoon | Canli, Hermann | Herrmann, Perez-Rodriguez, Yoon | |||
This tabular summary only reflects studies with positive findings in one or both sexes.
As both TPH1 and TPH2 have multiple polymorphisms implicated in depression and related disorders, we segregated our analysis by gene, rather than by risk allele.
No effects in gene-sex interactions: Denotes studies in which gene-sex interactions were tested and not found
Sex difference: Denotes studies in which (†) significant effects emerged for only one sex, (‡) significant effects emerged in opposite directions by sex, or interaction effects were different. Bold Underlining denotes studies that observed sex differences.
Did not examine/Unclear: Denotes studies in which (1) only one sex was studied, (2) sex interactions were not ruled out, (3) marginally significant sex differences were found, or (4) sex differences were found in measures not included in the table.
Conduct disorder: Includes measures of delinquency and other externalizing symptoms when applicable
Limbic structure: Includes volumetric and other anatomical measures of limbic components. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Limbic function: Includes functional connectivity, steady-state effects, and measures of limbic activity excluding amygdala hyperactivation. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Denotes significant effects arising in the context of stress or early life adversity
Table IV.
Studies Reporting Gene-Sex Interactions in Depression, Related Disorders, Risk Factors, and Endophenotypes
| Authors | F/M | Parameter | Finding | Effect |
|---|---|---|---|---|
| 5-HTTLPR | ||||
|
| ||||
| Ancelin et al., 2010 | 1040/752 | Depression | S interacts with lipid levels in males | p = .02 |
| Antypa et al., 2011 | 186/59 | Facial emotion recognition | SS females recognize negative emotions at lower intensity | p < 0.05 |
| Aslund et al., 2009 | 717/765 | Depression | SS interacts with maltreatment in females | p = 0.034 |
| Aslund et al., 2013 | 714/753 | Delinquency | Interacts with high SES in L males, S females; with low SES in LL males, SS females | p = .022 |
| Baca-Garcia et al., 2002 | 214/178 | Suicide attempts | S associated in females | p = 0.02 |
| Baune et al., 2008 | 194/146 | Depression | L associated with melancholic depression in females | p = 0.05 |
| Beaver et al., 2012 | 924/778 | Depressive symptoms | SS interacts with stress in females | p < .05 |
| Brummett et al., 2003 | 129/73 | Neuroticism | S protective in males | p < 0.01 |
| Brummett et al., 2008a | 160/55 | Depression | S interacts with stress in females; L interacts with stress in males | p < 0.003 |
| Brummett et al., 2008b | 31/41 | Negative affect (tryptophan infusion) | LL associated in males; SS associated in females | p = .013 |
| Cadoret et al., 2003 | 59/39 | Conduct disorder; aggression | L associated in males; S associated in females | p < .05 |
| Cerasa et al., 2014 | 76/62 | Anxiety; Amygdala volume | SS associated with anxiety and increased volume in females, protective in males | p = 0.01; 0.002 |
| Douglas et al., 2011 | 567/814 | Antisocial personality disorder | S interacts with life events in females | p < 0.001 |
| Du et al., 2000 | 109/77 | Neuroticism | S associated in males | p = 0.018 |
| El-Hage et al., 2013 | 42/39 | Resting cerebral blood flow in amygdala | Higher in S males | p < 0.03 |
| Eley et al., 2004 | 220/157 | Depression | S associated in females | p = 0.03 |
| Everaerd et al., 2012 | 221/136 | Hippocampal volume | S associated with decrease in women; S interacts with childhood adversity in males | p = 0.023; 0.007 |
| Flory et al., 1999 | 135/135 | Anxiety | L associated in males | p = 0.03 |
| Gaysina et al., 2006 | 219/175 | Suicide attempts | L associated in females | p = 0.002 |
| Gelernter et al., 1998 | 132/190 | Harm Avoidance | S associated in males; S protective in females | p = 0.04 |
| Grabe et al., 2005 | 676/300 | Mental/physical distress | S interacts with unemployment and chronic disease in females | p < 0.001 |
| Hammen et al., 2010 | 214/132 | Depression | S interacts with stress in females | p = .01 |
| Hung et al., 2011 | 63/105 | Suicide attempts | L associated in males | p = 0.012 |
| Jabbi et al., 2007 | 31/33 | Cortisol stress response | SS associated with larger response in females | p < 0.006 |
| Lee et al., 2014 | 960/258 | Depression | S associated in females | p = 0.015 |
| Li et al., 2010 | 1144/1054 | Antisocial behavior | SS interacts with maltreatment in females | p < 0.01 |
| Li et al., 2013 | 453/577 | Suicide attempts; depression | S associated in males with low family support; S protective in males with high family support | p < .05 |
| Limosin et al., 2005 | 52/48 | Suicide attempts | S associated in males | p = 0.05 |
| Maron et al., 2004 | 18/11 | CCK-4-induced panic attacks | Lower rate in S females | p = 0.03 |
| McCaffery et al., 2003 | 191/191 | Cardiovascular response | Greater response in SS women | p < .05 |
| Ming et al., 2013 | 131/121 | Depressive symptoms | S interacts with stress in females | p < 0.0001 |
| Mizuno et al., 2006 | 59/45 | Anxiety | SL associated in females; SS associated in males | p < .05 |
| Nikolova et al., 2011 | 27/33 | Reward response | S associated with stress-related reduction in males | p = 0.002 |
| Paaver et al., 2008 | 261/222 | Impulsivity | S interacts with family relations in females | p = 0.036 |
| Price et al., 2013 | 25/26 | Hippocampal volume | S increases volume in females, decreases volume in males | p < .03 |
| Priess-Groben et al., 2013 | 129/180 | Depressive symptoms | Life stress and MAOA-L interacts with S in females and L in males | p = 0.009 |
| Rucci et al., 2009 | 147/75 | Depression | L decreases manic/hypomanic component in females | p=0.012 |
| Sakai et al., 2010 | 254/213 | Conduct problems | S associated in females | p < 0.05 |
| Sjoberg et al., 2006 | 119/81 | Depression | S confers risk in females and protection in males | p = 0.018; 0.032 |
| Starr et al., 2013 | 217/137 | Depression | S interacts with security in males | p=0.05 |
| Steffens et al., 2002 | 194/95 | Depression | SS associated in males | p = 0.02 |
| Uddin et al., 2010 | 560/524 | Depressive symptoms | SL protective in females; SL protective in males only with deprivation | p = .03; .04 |
| Van Strien et al., 2010 | 153/133 | Emotional eating | S moderates depression and emotional eating in females | p < .01 |
| Verona et al., 2006 | 56/55 | Aggression | SS interacts with acute stress in males | p < .05 |
| Volf et al., 2015 | 109/101 | Resting EEG | SL associated with more power in women | p = 0.041 |
| Vormfelde et al., 2006 | 98/97 | Neuroticism | L protective in males | p = 0.049 |
| Walderhaug et al., 2007 | 44/39 | Mood (tryptophan depletion) | SL protective in females | p = 0.019 |
| Walderhaug et al., 2010 | 14/38 | Impulsivity | S associated in males | p = 0.033 |
| Zhang et al., 2015 | 158/104 | Anxiety; Functional connectivity | SS associated in males | p = 0.006; < 0.005 |
|
| ||||
| MAOA-LPR | ||||
|
| ||||
| Adkins et al., 2012 | 987/922 | Depression | H males experience increased distress in late adolescence | p < .05 |
| Aslund et al., 2011 | 882/943 | Delinquency | L interacts with maltreatment in males; H interacts with maltreatment in females | p < 0.001 |
| Buckholtz et al., 2008 | 63/60 | Amygdala activity; Functional connectivity | S associated with dysregulated amygdala activity, increased vmPFC connectivity in males | p < 0.01; < 0.008 |
| Chen et al., 2013 | 193/152 | Happiness | L associated in females | p= 0.002 |
| Deckert et al., 1999 | 254/145 | Panic disorder | H associated in females | p = 0.001 |
| Du et al., 2002 | 39/97 | Depressed suicide | H associated in males | p = 0.012 |
| Eley et al., 2003 | 76/41 | Neuroticism | H associated in males | p < 0.01 |
| Frazetto et al., 2007 | 153/82 | Physical aggression | L interacts with life events in males | p = 0.009 |
| Guo et al., 2008 | 1324/1200 | Delinquency | L associated in males | p= 0.008 |
| Holz et al., 2016 | 53/72 | Activity in amygdala and hippocampus | Increased with life events in male L, decreasing in male H; reversed in females | p= 0.008; 0.005 |
| Huang et al., 2004 | 424/342 | Impulsivity | L interacts with abuse in males | p= 0.038 |
| Huang et al., 2009 | 281/309 | Depression | L associated with severe depression in females | p= 0.041 |
| Jabbi et al., 2007 | 31/33 | Baseline cortisol | H associated with higher cortisol in females | p < 0.009 |
| Lung et al., 2011 | 567/410 | Depression | H associated in males | p= 0.041 |
| Maron et al., 2004 | 18/11 | CCK-4-induced panic attacks | Higher rate in L females | p= 0.007 |
| Maron et al., 2005 | 286/87 | Panic disorder with agoraphobia | H associated in females | p= 0.016 |
| Melas et al., 2013 | 993/675 | Depression | L interacts with childhood adversity in females | p= 0.006 |
| Meyer-Lindenberg et al., 2006 | 72/70 | Limbic volume and activity | L associated with amygdalar, hippocampal, cingulate activity, orbitofrontal volume in males | p < 0.05 |
| Nikulina et al., 2012 | 280/295 | Dysthymia | H interacts with life stress in females | p < .05 |
| Nilsson et al., 2011 | 735/851 | Adolescent alcohol consumption | H associated in females; L associated in males | p = 0.006; < 0.001 |
| Prom-Wormley et al., 2009 | 721/578 | Conduct disorder | H associated in females | p= 0.05 |
| Reif et al., 2012 | 1636/739 | Panic disorder | H associated in females | p=0.006 |
| Rivera et al., 2009 | 884/344 | Depression | H associated in females | P < 0.05 |
| Samochowiec et al., 2004 | 225/78 | Anxiety disorders | H associated with panic attacks and generalized anxiety disorder in females | p < 0.05 |
| Schulze et al., 2000 | 170/77 | Depression | H associated in females | p= 0.029 |
| Verhoeven et al., 2012 | 332/100 | Aggression | H associated in females | p= 0.03 |
| Voltas et al., 2015 | 143/85 | Anxiety | H associated in females; L in males | p= .026; .031 |
| Wakschlag et al., 2010 | 99/77 | Conduct disorder; hostile attribution bias | L associated with conduct disorder in males; H associated with CD and bias in females | p= 0.03; 0.002; 0.04 |
| Williams et al., 2009 | 69/141 | Emotion-processing event related potentials | Differing response localization between L males and females | p < 0..05 |
| Yu et al., 2005a | 236/205 | Depression | H associated in females; smaller effect in males | p= 0.008 |
|
| ||||
| TPH1 and TPH2 | ||||
|
| ||||
| Keltikangas-Järvinen et al., 2007 | 186/155 | Harm avoidance | TPH1 haplotype (A218C A and A779C A) interacts with childhood environment in females | p = 0.002 |
| Serretti et al., 2001 | 851/573 | Depression | TPH1 A218C A protective in males | p = 0.016 |
| Armbruster et al., 2010 | 228/219 | Startle response | Higher in TPH2 −703 G/G females; higher in T males | p = 0.043; 0.039 |
| Kim et al., 2009 | 272/183 | Panic disorder | TPH2 rs4570625 T protective in females | p = 0.041 |
| Maron et al., 2007 | 375/141 | Panic disorder | TPH2 rs1386494 G protective in females | p = 0.01 |
| Shen et al., 2011 | 278/90 | Depression | TPH2 haplotype (rs4290270 A and rs7305115 A) associated in females | p = 0.001 |
| Stoltenberg et al., 2012 | 309/168 | Impulsivity | TPH2 rs1386483 A associated in males | p = 0.018 |
| Utge et al., 2010 | 967/687 | Clinical manifestations of depression | TPH2 rs12229394 associated with depression accompanied by fatigue in females | p = 0.005 |
F/M: Numbers of females and male participants p: Reflects main effects in studies with positive findings only in one sex, or gene-sex interaction effects when examined
We conclude by highlighting the conceptual and methodological difficulties of studying gene-sex interactions and suggesting directions for future research to advance our understanding of gene-sex interactions, particularly in regard to mechanistic pathways and their translational relevance for developing a neurobehavioral taxonomy for depression and related phenotypes.
Depression and related conditions
Existing diagnostic categories of clinical depression and related disorders, currently defined by symptom criteria, may in fact comprise an ensemble of multiple underlying dysfunctions that are more cohesive when defined by neurobiobehavioral measures (Williams 2016). These underlying neurobiobehavioral dysfunctions may not map on to symptom-based boundaries but define consistent subtypes present across different diagnoses. Sex differences may be an important consideration for anchoring a neurobiobehavioral understanding of depression. For example, women have been reported to have a tendency to internalize distress and men, to externalize distress (Eaton et al., 2012). Internalizing might reflect the action of particular biobehavioral mechanisms for depressed or anxious outcomes in women, and externalizing might reflect the action of different mechanisms for antisocial or aggressive outcomes in men, such that investigation of sex differences could help disentangle the pathways of genetic risk for “clinical expressions” of depression and emotional dysregulation. In this mini-review we consider these clinical expressions as including depression, anxiety, conduct disorders, and suicidality.
Another collection of depression-related phenomena may be considered “latent expressions” of risk for overt clinical states; these include personality traits, as well as alterations in brain anatomy and functional activity. The personality trait of neuroticism in the Revised NEO Personality inventory, reflecting a dispositional bias toward negative information, is higher in women than men across 26 cultures, with US women scoring 0.51 SD higher than their male counterparts (Costa et al., 2001). These higher levels of neuroticism are thought to be a latent trait that moderates the expression of a greater prevalence of depression in females (Goodwin and Gotlib, 2004). Similarly, trait harm avoidance is associated with panic disorder and general anxiety (Starcevic et al., 1996) and trait impulsiveness, thought to be reflective of low serotonin turnover, is associated with suicide (Fawcett et al., 1997). Likewise, structural and functional alterations of the brain are subject to sex differences that exist on the spectrum of subclinical to healthy brains. Dysfunctional activity in the amygdala and other limbic structures correlate with depression severity, probability of relapse, as well as dysregulated processing of emotionally valenced stimuli, which may reflect a trait risk for depression (for review; Drevets 2000). Healthy women exhibit more persistent amygdala activity in response to fear signals than men (Williams et al., 2005). In regard to neuroanatomy, decreased hippocampal volume, thought to reflect the effects of chronic stress, has been observed both in both male and female depressed patients (Videbech and Ravnkilde 2004), though hippocampal size and microstructure is altered in men, but not women, with subclinical depression (Spalletta et al., 2014).
The serotonin system in men and women
We focus our review on the serotonergic system because it (1) plays an important role in mood and mood disorders, (2) is widely accepted to be sexually dimorphic, (3) encompasses polymorphisms that have been extensively studied with respect to mood disorders, and (4) is directly relevant to the efficacy of SSRIs, the most commonly used treatments for depression and anxiety, making it an important aspect of individualized treatment and precision medicine. Apart from its role as a neurotransmitter, serotonin also plays a role in brain development by regulating neurite outgrowth, synaptogenesis, and cell survival (Gaspar et al, 2003), all of which have important consequences for neurobiological function.
Sexual dimorphisms within the serotonin system have been known for the past four decades. Males and females exhibit different rates of serotonin synthesis (Nishizawa et al, 1997), different levels of serotonin metabolites (Gottfries et al, 1974), different receptor and transporter binding potentials (Jovanovic et al., 2008), and different SSRI response and tolerance (Kornstein et al., 2000). Furthermore, acute tryptophan depletion, which induces lower mood in recovered depressed patients by temporarily decreasing serotonin levels, leads to larger mood-lowering effects in women than in men (Booji et al., 2002).
Sex differences within the serotonergic system might account on their own for some of the sex differences in genetic risk for depression and related clinically expressed phenomena. In addition, sex differences multiply when serotonergic gene products interact with, regulate, and are modulated by other sexually dimorphic biological pathways. In this review, we will discuss the interface of serotonergic genetic polymorphisms with three such pathways: the effects of early life stress, the effects of sex chromosome differences, and the effects of sex hormones.
Potential Pathways of Sex Modulation of Genetic Polymorphisms
Mechanism: Early Life Stress
Exposure to early life stress is a risk factor for developing mood and anxiety disorders, due in part to long-term stress response dysregulation, cognitive coping strategies, and neurobiological anatomy (Heim and Nemeroff, 2001). Many genes that have been implicated in depression, including BDNF, COMT, and CRHR1, have more pronounced effects in the context of early life stress (Heim and Binder, 2012).
Psychological and biological responses to stress, particularly early life stress, are sexually dimorphic (reviewed in Bale and Epperson, 2015). Men and women differ in the types of stressors that most impact depression risk (Chu et al., 2013). Though women are more likely to develop a depressive disorder, men may be more susceptible to the immediate neurobiological effects of stress, including stress-related c-fos expression, enhanced fear conditioning, and increased HPA axis response (reviewed in Altemus 2006). Animal work suggest neurobiological mechanisms for the observed sex difference in stress response. For example, male rats exposed to perinatal stress show a period in adolescence of increased neurogenesis, BDNF expression, and spatial learning, which is reduced by adulthood, while female rats exhibit the opposite pattern of decreased neurogenesis in adolescence followed by an increase in adulthood (Loi et al., 2014). In examining three-way interactions between stress, sex, and genotype, it is important to remember that observed differences in subclinical traits, neuroimaging, and neuroanatomy may represent either risk mechanisms, or protective compensatory mechanisms.
5-HTTLPR
The 5-HTTLPR polymorphism is associated with the largest body of research regarding sex differences—a recent review of sex differences in 5-HTTLPR included 78 studies (Gressier et al, 2016). The 5-HTTLPR polymorphism consists of 16-repeat long variant and a 14-repeat short variant, which causes decreased SLC6A4 transcription (Lesch et al., 1996). The long variant is further modified by a single nucleotide polymorphism, A/G SNP rs25531, with L(A) variants expressing normally and L(G) variants expressing similar to the S allele (Wendland et al., 2006). In this review, we designate the S and L(G) alleles as low-expressing alleles.
While 5-HTTLPR and gender may modulate depression risk, severity, and suicide risk independently of environmental stress (see Table I) the depressogenic effect of the low-expressing alleles may be potentiated by stressful life events, particularly in early childhood (Caspi et al., 2003). This finding was supported by subsequent meta-analyses ((Karg et al., 2011, Sharpley et al., 2014) though others have yielded negative results (Risch et al., 2009; Munafo et al., 2009). The gene-environment interaction becomes stronger when taking sex into account, with a majority of studies finding that the low-expressing alleles interact with stress to confer risk more specifically in females (see Table I).
Neuroimaging evidence supports the hypothesis that the 5-HTTLPR polymorphism differently influences hippocampal, amygdalar, and cortical structure in men and women in the context of early life stress (see Figure 1). However, though a number of studies have established a relationship between low-expressing alleles and amygdala hyperreactivity, no sex differences have been found, as few studies explicitly examined gene-sex interactions (see Table I). Connections between genotype and personality traits provide a mechanism by which 5-HTTLPR may differentially interact with sex to alter preclinical risk factors, independent of early life stress exposure (Table I).
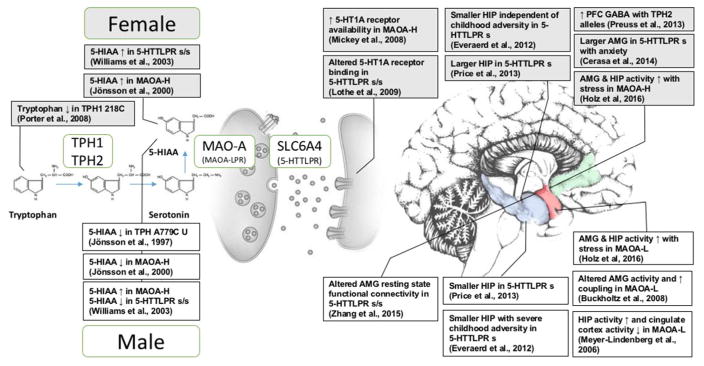
Figure 1.
Different effects of serotonergic genotype in the female (top) and male (bottom) brain; AMG: amygdala, HIP: hippocampus
The mechanistic basis of how 5-HTTLPR variation leads to biobehavioral sex differences are still unclear, but multiple lines of evidence illustrate sex-specific effects on serotonin metabolism. 5-HTTLPR genotype interacts with sex to modulate resting state cerebral blood flow in the amygdala (El-Hage et al., 2013) as well as resting state electroencephalography activity (Volf et al., 2015). Depressed women, but not men, exhibit lower levels of serotonin transporter availability relative to their healthy counterparts (Staley et al., 2006). The low-expressing allele is associated with lower 5-HIAA in males and higher 5-HIAA in females, indicative of differing rates of CNS serotonin turnover (Williams et al., 2003). Women homozygous for low-expressing alleles exhibit altered 5-HT1A receptor binding, which may indicate either a higher 5-HT1A receptor density or a lower level of serotonin with respect to high-expressing allele carriers, a difference not observed in men (Lothe et al., 2009). Tryptophan depletion leads to increased impulsivity in men, and increased caution and mood reduction in women, particularly marked in women homozygous for either allele (Walderhaug et al., 2007). The effect of 5-HTTPLR genotype on women is further underscored by another study showing tryptophan depletion induced mood reductions in women homozygous for the low-expressing alleles, no change in women homozygous for the high-expressing allele, and intermediate effects in heterozygote women, depending on the presence of a family history of depression (Neumeister et al., 2002).
Given findings of gene-sex-environment interactions, another important mechanism may be the interaction of 5-HTTLPR genotype and stress. The low-expressing alleles correlate with greater cortisol reactivity to stress in both a mixed group (Way et al., 2010) and a group of girls (Gotlib et al., 2008). Sex also interacts with the 5-HTTLPR polymorphism to predict cortisol awakening response, ACTH levels after dexamethasone administration, (Wust et al., 2009) diurnal cortisol (Wankerl et al., 2010) and cortisol response to stress (Jabbi et al., 2007). A study of macaques showed a higher ACTH stress response only in females with a history of adversity (Barr et al., 2004). Together, these data provide evidence that 5-HTTLPR confers depression risk through differential susceptibility to stress, with the low-expressing alleles associated with sensitivity to the environment and the high-expressing allele associated with immunity to environmental effects (Paaver et al., 2008; Nilsson et al., 2015). The low-expressing allele predicts increased stress generation in both males and females with low relational security, and decreased stress generation with high relational security (Starr et al., 2013). A similar differential susceptibility model has been shown to underlie the influence of other serotonin system genes in an additive multilocus score (Vrshek-Schallhorn et al., 2015). These findings underscore the need to further research the biological mechanisms of interactions between 5-HTTLPR genotype, sex, and environmental stress.
Mechanism: Sex Chromosomes
A systematic coverage of epigenetic sex differences is beyond the scope of this review. In the current content we highlight a special case of epigenetic modification: X-inactivation of the sex chromosome, the consequences of which are to date not well specified. The choice of which X-chromosome will be inactivated in a given cell is random, creating a mosaic of different cell populations (Migeon et al., 2007). Furthermore, approximately 15% of genes escape inactivation, while an additional 10% show heterogeneous inactivation, creating differences in gene expression levels between males and females, as well as variability among females (Carrel et al., 2005). In addition, the sex-determining region Y (SRY), found only on the male Y-chromosome, plays a role in modulating autosomal gene expression (Wijchers et al., 2010). Given the complexity of gene-sex interactions, it is difficult to conclude with certainty when X-inactivation is a primary factor. However, inheritance patterns in family studies have raised the possibility of sex-linked genes playing a role in depression: maternal grandfather longevity was associated with mental health in a male group, though maternal mental health was not associated, suggestive of an x-linked recessive genetic basis (Vaillant et al, 2005). There are several X-linked genes that have been associated with depression endophenotypes, including HTR2C, though its interactions with sex remain unclear (Avery and Vrshek-Schallhorn, 2016).
MAOA-LPR
MAOA is an x-linked gene that regulates monoamine neurotransmission by degrading serotonin, noradrenaline, and dopamine; MAOA knockout mice are characterized by higher levels of serotonin and noradrenaline and increased aggressive behavior (Cases et al, 1995). In humans, the polymorphic region located upstream of the coding sequence has been widely studied with regard to conduct disorder, which co-occurs with depression and other affective disorders in both children and adults (Puig-Antich et al., 1982; Marriage et al., 1986; Zoccolillo et al., 1992). The polymorphism consists of 2, 3, 3.5, 4, or 5 copies of a repeat sequence, with the rarer 2, 3 and 5 repeats exhibiting lower promoter activity (Sabol et al., 1998; Guo et al., 2008).
Males carrying low activity alleles (MAOA-L) are more likely to develop a conduct disorder, and exhibit increased aggression and impulsivity, particularly in the presence of childhood maltreatment (Table II; reviewed also in Byrd et al., 2014), whereas the high activity allele (MAOA-H) has been associated with increased ventro-lateral prefrontal activity (Cerasa et al., 2008a) and gray matter loss (Cerasa et al., 2008b). In females, however, conduct disorder and aggression has been linked to the high activity alleles (MAOA-H) (Wakschlag et al., 2010; Aslund et al., 2011). MAOA-H also confers risk for depression and anxiety disorders in women specifically (see Table II). Other polymorphisms in MAOA, including 1460T, MAOA-CA, and 941T, have also shown sex differences in conferring risk for depression and anxiety (Slopien et al., 2012; Tadic et al., 2003).
Table II.
Sex Modulation of MAOA-LPR in Depression, Related Disorders, Risk Factors, and Endophenotypes1
| Parameter | Depression | Anxiety | Conduct Disorder | Suicide | ||||
|---|---|---|---|---|---|---|---|---|
| Independent effects within sexes by allele | L Risk | H Risk | L Risk | H Risk | L Risk | H Risk | L Risk | H Risk |
| Significant effects in males | Cicchetti 2007* | Yu 2005a, Lung 2011, Adkins 2012 | Voltas 2015 | Liu 2013, Reif 2014 | Kim-Cohen 2006, Reif 2007, Guo 2008, Stetler 2014, Tiihonen 2015, Caspi 2002*, Foley 2004*, Nilsson 2006*, Widom 2006*, Frazzetto 2007*, Nilsson 2007*, Edwards 2010*, Wakschlag 2010*, Aslund 2011*, Derringer 2010*, Nilsson 2011* | Du 2002 | ||
| Significant effects in females | Huang 2009, Cicchetti 2007*, Melas 2013* | Schulze 2000, Yu 2005a, Rivera 2009, Nikulina 2012* | Maron 2004 | Deckert 1999, Samochowiec 2004, Maron 2005, Reif 2012, Liu 2013, Reif 2014, Voltas 2015 | Tiihonen 2015, Caspi 2002*, Widom 2006*, Ducci 2008*, Prom-Wormley 2009, Derringer 2010* | Sjoberg 2007*, Nilsson 2008*, Wakschlag 2010*, Aslund 2011*, Nilsson 2011* | ||
|
| ||||||||
| No effects in gene-sex interactions | Tiihonen, Widom | |||||||
| Sex difference | Adkins, Huang, Lung†, Melas†, Nikulina†, Rivera†, Schulze†, Yu | Deckert†, Maron 2004†, Maron 2005†, Reif 2012†, Samochowiec†, Voltas‡ | Aslund‡, Frazzetto, Guo 2008†, Nilsson 2011‡, Prom-Wormley†, Wakschlag‡ | Du† | ||||
| Did not examine/unclear | Cicchetti | Liu, Reif 2014 | Caspi, Derringer, Ducci, Edwards, Foley, Kim-Cohen, Nilsson 2006, Nilsson 2007, Nilsson 2008, Reif, Sjoberg, Stetler | |||||
|
| ||||||||
| Parameter | Neuroticism | Harm Avoidance | Aggression | Impulsiveness | ||||
|
| ||||||||
| Independent effects within sexes by allele | L Risk | H Risk | L Risk | H Risk | L Risk | H Risk | L Risk | H Risk |
| Significant effects in males | Eley 2003 | Eisenberger 2007, Kuepper 2013, Weder 2009*, Beaver 2013, Gorodetsky 2014* | Manuck 2000, Beitchman 2004, Gorodetsky 2014 | Stetler 2014, Huang 2004*, Enoch 2010* | Manuck 2000 | |||
| Significant effects in females | Yu 2005b | Eisenberger 2007, Kuepper 2013, Weder 2009* | Verhoeven 2012 |
Kinnally 2009 Kinnally 2009*, Enoch 2010* |
||||
|
| ||||||||
| No effects in gene-sex interactions | Eisenberger, Kuepper, Weder | |||||||
| Sex difference | Eley† | Verhoeven† | Huang | |||||
| Did not examine/unclear | Yu | Beaver, Beitchman, Gorodetsky, Manuck | Enoch, Kinnally, Manuck, Stetler | |||||
|
| ||||||||
| Parameter | Amygdala Hyperactivation | Limbic Structure | Limbic Function | Affective Processing | ||||
|
| ||||||||
| Independent effects within sexes by allele | L (More Activity) | H (More Activity) | L Risk | H Risk | ||||
| Significant effects in males | Meyer-Lindenberg 2006, Buckholtz 2008, Denson 2014, Holz 2016* | Holz 2016 | Meyer-Lindenberg 2006 | Fan 2003, Meyer-Lindenberg 2006, Passamonti 2006, Eisenberger 2007, Buckholtz 2008, Passamonti 2008, Alia-Klein 2009, Dannlowski 2009, Williams 2009, Denson 2014, Lei 2014, Reif 2014, Clemens 2015, Holz 2016* | Brummett 2008c, Alia-Klein 2009, Bouma 2012 | Buckholtz 2008, Reif 2014 | ||
| Significant effects in females | Lee 2008a, Holz 2016 | Holz 2016* | Meyer-Lindenberg 2006 | Fan 2003, Eisenberger 2007, Lee 2008a, Dannlowski 2009, Williams 2009, Reif 2014, Clemens 2015, Holz 2016* | Bouma 2012 | Jabbi 2007, Chen 2013, Wakschlag 2010*, Reif 2014 | ||
|
| ||||||||
| No effects in gene-sex interactions | Eisenberger, Fan | |||||||
| Sex difference | Holz‡, Meyer-Lindenberg† | Meyer-Lindenberg† | Holz‡, Meyer-Lindenberg†, Williams | Chen†, Jabbi, Wakschlag† | ||||
| Did not examine/unclear | Buckholtz, Denson, Lee | Alia-Klein, Buckholtz, Clemens, Dannlowski, Denson, Lee, Lei, Passamonti 2006, Passamonti 2008, Reif 2014 | Alia-Klein, Bouma, Brummett, Buckholtz, Reif | |||||
This tabular summary only reflects studies with positive findings in one or both sexes.
No effects in gene-sex interactions: Denotes studies in which gene-sex interactions were tested and not found
Sex difference: Denotes studies in which (†) significant effects emerged for only one sex, (‡) significant effects emerged in opposite directions by sex, or interaction effects were different. Bold Underlining denotes studies that observed sex differences.
Did not examine/Unclear: Denotes studies in which (1) only one sex was studied, (2) sex interactions were not ruled out, (3) marginally significant sex differences were found, or (4) sex differences were found in measures not included in the table.
Conduct disorder: Includes measures of delinquency and other externalizing symptoms when applicable
Limbic structure: Includes volumetric and other anatomical measures of limbic components. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Limbic function: Includes functional connectivity, steady-state effects, and measures of limbic activity excluding amygdala hyperactivation. Because the directionality of risk assignment in these measures is not always clear, the alleles have been merged.
Denotes significant effects arising in the context of stress or early life adversity
Studies on brain structure and activity indicate subclinical sex differences that may explain the opposing findings in the clinical literature (Figure 1). Amygdala activity during emotional face-matching increases with childhood stress in male MAOA-L carriers and decreases with childhood stress in male MAOA-H carriers, with the reverse holding in females (Holz et al., 2016). Amygdala volume, however, was not affected by MAO-A genotype or an interaction effect between genotype and sex (Cerasa et al., 2011). Increased hippocampal activity (Meyer Lin-denberg et al., 2006) and dysregulated vmPFC (Buckholtz et al., 2008) were observed in MAOA-L males but not females (see Figure 1). Studies on personality provide minimal evidence for sex differences (see Table II) with no sex difference seen in trait aggression, reactive aggression, or dACC reactivity to social exclusion (Eisenberger et al., 2007; Kuepper et al., 2013).
Like 5-HTTLPR, MAOA-LPR may exert its effects through sex-dependent differential susceptibility. Sex differences may be due to differential responses to stress, or to sex-dependent methylation patterns, which are themselves also affected by stress. Sex interacts with MAOA genotype to influence both baseline cortisol and subjective stress (Jabbi et al., 2007). Depressed females, particularly those who have experienced early life stress, exhibit lower methylation at the MAOA locus (Domschke et al., 2012; Melas et al., 2013). Another possible mechanism of sex difference is the SRY element found on the Y chromosome, which activates and regulates MAOA transcription (Wu et al., 2009). A more thorough understanding of how sex-dependent methylation and sex-based gene dose effects contribute to the impact of risk polymorphisms may yield further insight into biological mechanisms.
Mechanism: Hormones
There is a strong case to be made for the role of estrogen and other sex steroids as a factor in mood and depression. The highest rates of depression onset in women correspond with major hormonal changes, peaking in puberty, in the post-partum period, and at the age of menopause onset (Joffe et al., 1998). Testosterone has been shown to have antidepressant and anxiolytic effects in both men and women (McHenry et al., 2014) and changes in salivary testosterone over the course of a day correlate with depression and anxiety measures (Granger et al., 2003). Hormones may also affect mood indirectly by altering gene expression, changing the rate of gene transcription, or regulating mRNA stability (Ing 2005). The expression of both serotonin transporter (McQueen et al., 1997) and MAO-A (Gundlah et al., 2002) is regulated by estrogen. Exogenous hormone administration has also been shown to alter the functioning of the serotonergic system: female-to-male transsexuals undergoing androgen treatment have increased serotonin transporter binding, while male-to-female transsexuals undergoing antiandrogen and estrogen treatments have decreased serotonin transporter binding (Kranz et al., 2015).
Tryptophan Hydroxylase
Tryptophan hydroxylase is the rate-limiting enzyme in serotonin synthesis. The TPH2 isozyme is the predominant form in the brain, expressed highly in the serotonergic raphe nuclei (Bach-Mizrachi et al., 2005); however, the TPH1 isozyme is also highly expressed in the amygdala (Zill et al., 2007). Both forms are regulated by both estrogen (Gutknecht et al., 2015; Hiroi et al., 2006; Hiroi et al., 2013) and testosterone (Goldstein et al., 1992), and both have been linked to depression and related conditions in both men and women (see Table III). The TPH A218C polymorphism is associated with depression in males only (Serretti et al., 2001). Other alleles play a stronger role in conferring risk for depression and anxiety to women in the peripartum phase (Lin et al., 2009; Sun et al., 2004) suggestive of an intermediary role for hormones.
Variation in TPH2 has also been associated with depression and suicide in both men and women, as well as amygdala activity (see Table III). TPH2 variations also exhibit gene-sex interactions, predicting depression (Shen et al., 2011) and panic disorder (Maron et al., 2007) in women, but not men. Adult male -703 T-carriers have a stronger overall startle response, while the effect is reversed in adult females; notably, this finding in females achieves significance only after accounting for menstrual cycle phase, while no sex interaction effects were seen in children or older adults, suggestive of a modulatory role for hormones (Armbruster et al., 2010).
The majority of studies do not address sex differences, but there is evidence from metabolic and animal studies to further support those that have been found in both TPH1 and TPH2. The TPH1 218C allele is associated with decreased plasma TRP in women, but not in men (Porter et al., 2008). TPH2 knockout mice exhibit anxiety- and depression- like behaviors, with males showing increased impulsivity and aggression and females showing increased reactivity to aversive conditions (Gutknecht et al., 2015).
Conclusions and considerations for future research
In giving three examples of how sex modulates genotype to yield differing risk and expression of depression and related conditions, we hope to have made clear the importance of considering sex differences in the context of genetic variation. We expect that there are many yet undiscovered gene-sex interactions, as many of the genetic polymorphisms that have previously been associated with depression have not been explicitly studied with regard to sex differences. More research is needed to clarify the mechanisms through which genes contribute differentially to depression in men and women, and we conclude by raising methodological considerations and guidelines for future research.
Our review of sex differences raises a number of methodological considerations for future research into sex differences. To address them, we must re-evaluate how we conceptualize genetic risk, diagnostic categories, and sex itself. As we have shown, the same genotype can lead to different conditions in men and women, and alleles that confer risk to one sex may confer protection to the other; risk therefore cannot be directly attributed to a particular allele outside of the context of its effects on biological pathways and its interactions with the environment. We have also seen how similar underlying biological dysfunctions may give rise to different behavioral outputs in men and women. This raises the issue of how to group participants for further experimental studies of gene-sex interactions, when they may be heterogeneous with respect both to diagnosis and genetic risk profile. Another consideration is how to conceptualize sex. Some sex differences, such as those stemming from x-inactivation, arise from the most simplistic, chromosome-based formulation of sex, while others, such as hormones, arise from factors that vary with sex. While this review focuses on the interplay of biological sex and genes, genetic effects might also be modulated by gender, self-identity, and cultural expectations.
It is essential that future research in the genetics of depression explicitly check for interactions with sex and sex-related factors known to be relevant. For example, as some sex differences are dependent on levels of cycling hormones, it is vital to incorporate the hormonal status of female participants. Otherwise, the effect of sexually asymmetric alleles may be diluted to the point of statistical insignificance, or even bidirectionally cancel out. In addition, it is important to check for three-way-interactions between environment, sex, and genotype. The sex differences relevant to depression and related conditions may be obscured by compensatory mechanisms in healthy individuals, as sexual dimorphisms in the brain may in fact exist to prevent, rather than cause, sexually dimorphic behavior in the healthy brain (De Vries 2004). It is possible that some sex differences only emerge in the context of pathology, as in the case of early life stress modulation of gene-sex interactions. Therefore, researchers must be careful in extrapolating sex differences, or lack thereof, in healthy individuals to patients.
Future studies of gene-sex differences may move beyond understanding the basis of genetic risk, toward understand the pathogenic mechanism of disease. Studies that consider multiple time points and longitudinal trajectories, rather than cross-sectional grouping of participants, are needed in order to elucidate the role of sex differences and genetic risk in causal pathways for depression and emotional disorder. Such studies might incorporate intermediate measures such as subclinical manifestations of depression, stress response dysregulation, and personality traits to elucidate the mechanisms by which risk converts to overt psychopathology. Studies on groups of individuals that are biologically homogenous, rather than symptomatically homogenous, may help in isolating the distinct mechanisms of depression pathogenesis that create epidemiological differences between the sexes. Studies that cross traditional diagnostic boundaries may better capture the full range of behavioral output in men and women and better elucidate how sex differences in behavior and psychopathology emerge from similar underlying biology.
Finally, as the ultimate goal of research into depression is to treat individuals, it is vital to extend these considerations of gene-sex interactions into the domain of treatment. Given that men and women exhibit different pathways to pathology, it is also reasonable to expect different pathways to recovery. Research to date has supported the existence of gender differences in the response to pharmacological treatment (Gorman 2006). Understanding the different mechanisms that contribute to psychopathology in men and women will be essential in developing and targeting personalized interventions.
While it is premature to define generalizable rules about these interactions, or even to draw strong conclusions about the examples discussed, we may in the interim outline some central considerations that emerged from reviewing our current state of knowledge. We note that 1) that there is a paucity of information on the impact of sex and gene interactions, 2) that evidence from limited studies do support that there are sex and gene interactions with clinically relevant outcomes, 3) that these differences might be potentiated by environmental stressors, 4) that these gene-sex and gene-sex-environment interactions need to be tested explicitly, 5) that adopting more nuanced conceptions of genetic risk, diagnostic categories, and sex itself will help to clarify these interactions, and 5) that further research utilizing these methodological recommendations will be essential to understand underlying mechanisms. Although by necessity we have focused our review on depression and sex differences related to the serotonergic system, we hope that this review will facilitate a broader consideration of the topic, as there are likely many more differences between sexes relevant to understanding the trajectory of mental disorders.
Significance Statement.
Depression and related disorders, such as anxiety, conduct disorder, and suicidality, exhibit sex differences. These differences stem from underlying biological differences in men and women, including response to early life stress, sex chromosome expression, and hormonal control. In this Mini-Review, we consider how these known differences might alter the effects of genetic polymorphisms of the serotonergic system. In doing so, we highlight the importance of considering gene-sex and gene-sex-environment interactions in the study of depression and related conditions.
Acknowledgments
Support/Grant Information: ANG-P and LMW were supported by the NIMH grant R01MH101496 during the preparation of this review.
Footnotes
Role of Authors
LMP conceived the scope of this Mini-Review. ANG and LMW developed the intellectual content with LMP and all authors wrote the manuscript.
Declaration of Conflicts of Interests
LMW has received consultant fees from Humana for projects not related to this work.
References
- Adkins DE, Daw JK, McClay JL, van den Oord EJ. The influence of five monoamine genes on trajectories of depressive symptoms across adolescence and young adulthood. Dev Psychopathol. 2012;24:267–285. doi: 10.1017/S0954579411000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N, Klucken T, Koppe G, Osinsky R, Walter B, Vaitl D, Sammer G, Stark R, Hennig J. Interaction of the serotonin transporter-linked polymorphic region and environmental adversity: increased amygdala-hypothalamus connectivity as a potential mechanism linking neural and endocrine hyperreactivity. Biol Psychiatry. 2012;72:49–56. doi: 10.1016/j.biopsych.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tomasi D, Woicik PA, Moeller SJ, Williams B, Craig IW, Telang F, Biegon A, Wang G, Fowler JS, Volkow ND. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion (Washington, DC) 2009;9:385–396. doi: 10.1037/a0015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–538. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Ancelin M, Carrière I, Boulenger J, Malafosse A, Stewart R, Cristol J, Ritchie K, Chaudieu I, Dupuy A. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study) Biol Psychiatry. 2010;68:125–132. doi: 10.1016/j.biopsych.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Andre K, Kampman O, Viikki M, Illi A, Setälä-Soikkeli E, Poutanen O, Mononen N, Leinonen E, Lehtimäki T. TPH1 A218C polymorphism and temperament in major depression. BMC Psychiatry. 2013;13:118. doi: 10.1186/1471-244X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila S, Viikki M, Huuhka K, Huuhka M, Huhtala H, Rontu R, Lehtimäki T, Leinonen E. TPH2 polymorphisms may modify clinical picture in treatment-resistant depression. Neurosci Lett. 2009;464:43–46. doi: 10.1016/j.neulet.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Antypa N, Cerit H, Kruijt AW, Verhoeven FEA, Van dD. Relationships among 5-HTT genotype, life events and gender in the recognition of facial emotions. Neuroscience. 2011;172:303–313. doi: 10.1016/j.neuroscience.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Kirschbaum C, Lesch K, Brocke B. Influence of functional tryptophan hydroxylase 2 gene variation and sex on the startle response in children, young adults, and older adults. Biol Psychol. 2010;83:214–221. doi: 10.1016/j.biopsycho.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav Genet. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Aslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav Genet. 2011;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- Aslund C, Comasco E, Nordquist N, Leppert J, Oreland L, Nilsson KW. Self-reported family socioeconomic status, the 5-HTTLPR genotype, and delinquent behavior in a community-based adolescent population. Aggressive Behav. 2013;39:52–63. doi: 10.1002/ab.21451. [DOI] [PubMed] [Google Scholar]
- Avery BM, Vrshek-Schallhorn S. Nonsynonymous HTR2C polymorphism predicts cortisol response to psychosocial stress I: Effects in males and females. Psychoneuroendocrinology. 2016;70:134–141. doi: 10.1016/j.psyneuen.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca-García E, Vaquero C, Diaz-Sastre C, Saiz-Ruiz J, Fernández-Piqueras J, de Leon J. A gender-specific association between the serotonin transporter gene and suicide attempts. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2002;26:692–695. doi: 10.1016/S0893-133X(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal Tryptophan Hydroxylase mRNA Expression in the Human Dorsal and Median Raphe Nuclei: Major Depression and Suicide. Neuropsychopharmacology. 2005;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA. 2004;101:12358–63. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Hohoff C, Mortensen LS, Deckert J, Arolt V, Domschke K. Serotonin transporter polymorphism (5-HTTLPR) association with melancholic depression: a female specific effect? Depress Anxiety. 2008;25:920–925. doi: 10.1002/da.20433. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Wright JP, Delisi M. An interaction between perceived stress and 5HTTLPR genotype in the prediction of stable depressive symptomatology. Am J Orthopsychiatry. 2012;82:260–266. doi: 10.1111/j.1939-0025.2012.01148.x. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Barnes JC, Boutwell BB. The 2-Repeat Allele of the MAOA Gene Confers an Increased Risk for Shooting and Stabbing Behaviors. Psychiatr Q. 2013;85:257–265. doi: 10.1007/s11126-013-9287-x. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Pacheco J, Clasen P, McGeary JE, Schnyer D. Prefrontal morphology, 5-HTTLPR polymorphism and biased attention for emotional stimuli. Genes, Brain, and Behavior. 2010a;9:224–233. doi: 10.1111/j.1601-183X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Ellis AJ, Wells TT, McGeary JE. Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biol Psychol. 2010b;83:260–265. doi: 10.1016/j.biopsycho.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Mik HM, Ehtesham S, Douglas L, Kennedy JL. MAOA and persistent, pervasive childhood aggression. Mol Psychiatry. 2004;9:546–547. doi: 10.1038/sj.mp.4001492. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, Ehtesham S, Kennedy JL. Serotonin Transporter Polymorphisms and Persistent, Pervasive Childhood Aggression. Am J Psychiatry. 2006;163:1103–1105. doi: 10.1176/ajp.2006.163.6.1103. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Szöke A, Henry C, Lacoste J, Bottos C, Nosten-Bertrand M, Hardy P, Rouillon F, Launay J, Laplanche J, Leboyer M. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biol Psychiatry. 2000;48:319–322. doi: 10.1016/s0006-3223(00)00891-x. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Chaste P, Malafosse A. Association between the TPH gene A218C polymorphism and suicidal behavior: a meta-analysis. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2004;124B:87–91. doi: 10.1002/ajmg.b.20015. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Blair-West G, Cantor CH, Mellsop GW, Eyeson-Annan M. Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord. 1999;55:171–178. doi: 10.1016/s0165-0327(99)00004-x. [DOI] [PubMed] [Google Scholar]
- Bondy B, Erfurth A, de Jonge S, Krüger M, Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Mol Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- Booij L, Van dD, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van dK. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Doornbos B, Ormel J, Oldehinkel AJ. Genetically based reduced MAOA and COMT functioning is associated with the cortisol stress response: a replication study. Mol Psychiatry. 2012;17:119–121. doi: 10.1038/mp.2011.115. [DOI] [PubMed] [Google Scholar]
- Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch K, Kirschbaum C, Strobel A. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Mol Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Brown GW, Ban M, Craig TKJ, Harris TO, Herbert J, Uher R. Serotonin transporter length polymorphism, childhood maltreatment, and chronic depression: a specific gene-environment interaction. Depress Anxiety. 2013;30:5–13. doi: 10.1002/da.21982. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–888. 805. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Siegler IC, McQuoid DR, Svenson IK, Marchuk DA, Steffens DC. Associations among the NEO Personality Inventory, Revised and the serotonin transporter gene-linked polymorphic region in elders: effects of depression and gender. Psychiatr Genet. 2003;13:13–18. doi: 10.1097/00041444-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Züchner S, Collins A, Williams RB. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008a;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Muller CL, Collins AL, Boyle SH, Kuhn CM, Siegler IC, Williams RB, Ashley-Koch A. 5-HTTLPR and gender moderate changes in negative affect responses to tryptophan infusion. Behav Genet. 2008b;38:476–483. doi: 10.1007/s10519-008-9219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Surwit RS, Garrett ME, Collins A, Ashley-Koch A, Williams RB. HPA axis function in male caregivers: Effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR) Biol Psychol. 2008c;79:250–255. doi: 10.1016/j.biopsycho.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret RJ, Langbehn D, Caspers K, Troughton EP, Yucuis R, Sandhu HK, Philibert R. Associations of the serotonin transporter promoter polymorphism with aggressivity, attention deficit, and conduct disorder in an adoptee population. Compr Psychiatry. 2003;44:88–101. doi: 10.1053/comp.2003.50018. [DOI] [PubMed] [Google Scholar]
- Campos SB, Miranda D, Souza BR, Pereira PA, Neves FS, Tramontina J, Kapczinski F, Romano-Silva M, Correa H. Association study of tryptophan hydroxylase 2 gene polymorphisms in bipolar disorder patients with panic disorder comorbidity. Psychiatr Genet. 2011;21:106–111. doi: 10.1097/YPG.0b013e328341a3a8. [DOI] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JDE, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005a;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005b;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. Journal of Neural Transmission (Vienna, Austria: 1996) 2005c;112:1479–1485. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Todd Constable R, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biol Psychol. 2008;79:118–125. doi: 10.1016/j.biopsycho.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Carli V, Mandelli L, Zaninotto L, Roy A, Recchia L, Stoppia L, Gatta V, Sarchiapone M, Serretti A. A protective genetic variant for adverse environments? The role of childhood traumas and serotonin transporter gene on resilience and depressive severity in a high-risk population. European Psychiatry: The Journal of the Association of European Psychiatrists. 2011;26:471–478. doi: 10.1016/j.eurpsy.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science (New York, NY) 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY) 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science (New York, NY) 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Fera F, Passamonti L, Liguori M, Lanza P, Muglia M, Magariello A, Quattrone A. Ventro-lateral prefrontal activity during working memory is modulated by MAO A genetic variation. Brain Res. 2008a;1201:114–121. doi: 10.1016/j.brainres.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Labate A, Lanza P, Magariello A, Muglia M, Quattrone A. MAO A VNTR polymorphism and variation in human morphology: a VBM study. Neuroreport. 2008b;19:1107–1110. doi: 10.1097/WNR.0b013e3283060ab6. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A, Gioia MC, Magariello A, Muglia M, Assogna F, Bernardini S, Caltagirone C, Bossù P, Spalletta G. MAO A VNTR polymorphism and amygdala volume in healthy subjects. Psychiatry Res. 2011;191:87–91. doi: 10.1016/j.pscychresns.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A, Piras F, Mangone G, Magariello A, Fagioli S, Girardi P, Muglia M, Caltagirone C, Spalletta G. 5-HTTLPR, anxiety and gender interaction moderates right amygdala volume in healthy subjects. Social Cognitive and Affective Neuroscience. 2014;9:1537–1545. doi: 10.1093/scan/nst144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Pine DS, Ernst M, Gorodetsky E, Kasen S, Gordon K, Goldman D, Cohen D. The MAOA gene predicts happiness in women. Prog Neuro-Psychoph. 2013;40:122–125. doi: 10.1016/j.pnpbp.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DA, Williams LM, Harris AWF, Bryant RA, Gatt JM. Early life trauma predicts self-reported levels of depressive and anxiety symptoms in nonclinical community adults: relative contributions of early life stressor types and adult trauma exposure. J Psychiatr Res. 2013;47:23–32. doi: 10.1016/j.jpsychires.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple M. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Clemens B, Voß B, Pawliczek C, Mingoia G, Weyer D, Repple J, Eggermann T, Zerres K, Reetz K, Habel U. Effect of MAOA Genotype on Resting-State Networks in Healthy Participants. Cerebral Cortex (New York, NY: 1991) 2015;25:1771–1781. doi: 10.1093/cercor/bht366. [DOI] [PubMed] [Google Scholar]
- Costa P, Jr, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: Robust and surprising findings. J Pers Soc Psychol. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Courtet P, Baud P, Abbar M, Boulenger JP, Castelnau D, Mouthon D, Malafosse A, Buresi C. Association between violent suicidal behavior and the low activity allele of the serotonin transporter gene. Mol Psychiatry. 2001;6:338–341. doi: 10.1038/sj.mp.4000856. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T. Serotonergic genes modulate amygdala activity in major depression. Genes, Brain, and Behavior. 2007;6:672–676. doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33:418–424. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schöning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nöthen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora M, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell B, Zaslavsky AM, Ustun TB, Chatterji S WHO World Mental Health, Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Jama. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Denson TF, Dobson-Stone C, Ronay R, von Hippel W, Schira MM. A functional polymorphism of the MAOA gene is associated with neural responses to induced anger control. J Cogn Neurosci. 2014;26:1418–1427. doi: 10.1162/jocn_a_00592. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Irons DE, Iacono WG. Harsh discipline, childhood sexual assault, and MAOA genotype: an investigation of main and interactive effects on diverse clinical externalizing outcomes. Behav Genet. 2010;40:639–648. doi: 10.1007/s10519-010-9358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Kuithan H, Schwarte K, Klauke B, Ambrée O, Reif A, Schmidt H, Arolt V, Kersting A, Zwanzger P, Deckert J. Monoamine oxidase A gene DNA hypomethylation - a risk factor for panic disorder? Int J Neuropsychopharmacol. 2012;15:1217–1228. doi: 10.1017/S146114571200020X. [DOI] [PubMed] [Google Scholar]
- Douglas K, Chan G, Gelernter J, Arias AJ, Anton RF, Poling J, Farrer L, Kranzler HR. 5-HTTLPR as a potential moderator of the effects of adverse childhood experiences on risk of antisocial personality disorder. Psychiatr Genet. 2011;21:240–248. doi: 10.1097/YPG.0b013e3283457c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Ramel W, Edge MD, Hyde LW, Kuo JR, Goldin PR, Hariri AR, Gross JJ. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. Am J Psychiatry. 2012;169:397–405. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, Demeter E, Bakish D, Lapierre YD, Sótonyi P, Hrdina PD. Frequency of long allele in serotonin transporter gene is increased in depressed suicide victims. Biol Psychiatry. 1999;46:196–201. doi: 10.1016/s0006-3223(98)00376-x. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Hrdina PD. Gender differences in association between serotonin transporter gene polymorphism and personality traits. Psychiatr Genet. 2000;10:159–164. doi: 10.1097/00041444-200010040-00002. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, Sotonyi P, Bakish D, Hrdina PD. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch M, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE, Grant BF, Hasin DS. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J Abnorm Psychol. 2012;121:282–288. doi: 10.1037/a0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Dodge KA, Latendresse SJ, Lansford JE, Bates JE, Pettit GS, Budde JP, Goate AM, Dick DM. MAOA-uVNTR and early physical discipline interact to influence delinquent behavior. Journal of Child Psychology and Psychiatry. 2010;51:679–687. doi: 10.1111/j.1469-7610.2009.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biol Psychiatry. 2007;61:1100–1108. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Eker MC, Kitis O, Okur H, Eker OD, Ozan E, Isikli S, Akarsu N, Gonul AS. Smaller hippocampus volume is associated with short variant of 5-HTTLPR polymorphism in medication-free major depressive disorder patients. Neuropsychobiology. 2011;63:22–28. doi: 10.1159/000321834. [DOI] [PubMed] [Google Scholar]
- El-Hage W, Zelaya F, Radua J, Gohier B, Alsop DC, Phillips ML, Surguladze SA. Resting-state cerebral blood flow in amygdala is modulated by sex and serotonin transporter genotype. Neuroimage. 2013;76:90–97. doi: 10.1016/j.neuroimage.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, Riemann R, Spinath F, Craig I. Association analysis of MAOA and COMT with neuroticism assessed by peers. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2003;120B:90–96. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- Enoch M, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes, Brain, and Behavior. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D, Gerritsen L, Rijpkema M, Frodl T, van Oostrom I, Franke B, Fernández G, Tendolkar I. Sex modulates the interactive effect of the serotonin transporter gene polymorphism and childhood adversity on hippocampal volume. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37:1848–1855. doi: 10.1038/npp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching PA, Faschingbauer F, Goecke TW, Engel A, Häberle L, Seifert A, Voigt F, Amann M, Rebhan D, Burger P, Kornhuber J, Ekici AB, Beckmann MW, Binder EB. Genetic variants in the tryptophan hydroxylase 2 gene (TPH2) and depression during and after pregnancy. J Psychiatr Res. 2012;46:1109–1117. doi: 10.1016/j.jpsychires.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Busch KA, Jacobs D, Kravitz HM, Fogg L. Suicide: A four-pathway clinical-biochemical model. Ann N Y Acad Sci. 1997;836:288–301. doi: 10.1111/j.1749-6632.1997.tb52366.x. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Dent KM, Peters DG, Muldoon MF. Neuroticism is not associated with the serotonin transporter (5-HTTLPR) polymorphism. Mol Psychiatry. 1999;4:93–96. doi: 10.1038/sj.mp.4000466. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, et al. CHildhood adversity, monoamine oxidase a genotype, and risk for conductdisorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fortier E, Noreau A, Lepore F, Boivin M, Pérusse D, Rouleau GA, Beauregard M. Early impact of 5-HTTLPR polymorphism on the neural correlates of sadness. Neurosci Lett. 2010;485:261–265. doi: 10.1016/j.neulet.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Frazzetto G, Lorenzo GD, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype. Plos One. 2007;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, Möller H, Meisenzahl EM. Childhood Stress, Serotonin Transporter Gene and Brain Structures in Major Depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Zill P, Baghai T, Schüle C, Rupprecht R, Zetzsche T, Bondy B, Reiser M, Möller H, Meisenzahl EM. Reduced hippocampal volumes associated with the long variant of the tri- and diallelic serotonin transporter polymorphism in major depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2008;147B:1003–1007. doi: 10.1002/ajmg.b.30680. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Joormann J, Gotlib IH. Altered timing of amygdala activation during sad mood elaboration as a function of 5-HTTLPR. Social Cognitive and Affective Neuroscience. 2011;6:270–276. doi: 10.1093/scan/nsq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Långström B, Oreland L, Fredrikson M. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett. 2004;362:189–192. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- Furmark T, Henningsson S, Appel L, Ahs F, Linnman C, Pissiota A, Faria V, Oreland L, Bani M, Pich EM, Eriksson E, Fredrikson M. Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. Journal of Psychiatry & Neuroscience: JPN. 2009;34:30–40. [PMC free article] [PubMed] [Google Scholar]
- Galfalvy H, Huang Y, Oquendo MA, Currier D, Mann JJ. Increased risk of suicide attempt in mood disorders and TPH1 genotype. J Affect Disord. 2009;115:331–338. doi: 10.1016/j.jad.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Pan Z, Jiao Z, Li F, Zhao G, Wei Q, Pan F, Evangelou E. TPH2 gene polymorphisms and major depression--a meta-analysis. PloS One. 2012;7:e36721. doi: 10.1371/journal.pone.0036721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews. Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gaysina D, Zainullina A, Gabdulhakov R, Khusnutdinova E. The serotonin transporter gene: polymorphism and haplotype analysis in Russian suicide attempters. Neuropsychobiology. 2006;54:70–74. doi: 10.1159/000096041. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro EF, Siever LJ, New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–1338. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Rao H, Brennan L, Wang DJJ, Detre JA, Sankoorikal GMV, Brodkin ES, Farah MJ. Serotonin transporter genotype modulates the association between depressive symptoms and amygdala activity among psychiatrically healthy adults. Psychiatry Res. 2011;193:161–167. doi: 10.1016/j.pscychresns.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizatullin R, Zaboli G, Jönsson EG, Asberg M, Leopardi R. Haplotype analysis reveals tryptophan hydroxylase (TPH) 1 gene variants associated with major depression. Biol Psychiatry. 2006;59:295–300. doi: 10.1016/j.biopsych.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Goldstein ME, Tank AW, Fossom LH, Hamill RW. Molecular aspects of the regulation of tyrosine hydroxylase by testosterone. Brain Research. Molecular Brain Research. 1992;14:79–86. doi: 10.1016/0169-328x(92)90013-2. [DOI] [PubMed] [Google Scholar]
- Gonda X, Juhasz G, Laszik A, Rihmer Z, Bagdy G. Subthreshold depression is linked to the functional polymorphism of the 5HT transporter gene. J Affect Disord. 2005;87:291–297. doi: 10.1016/j.jad.2005.05.007. [DOI] [PubMed] [Google Scholar]
- González-Castro TB, Juárez-Rojop I, López-Narváez ML, Tovilla-Zárate CA. Association of TPH-1 and TPH-2 gene polymorphisms with suicidal behavior: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:196. doi: 10.1186/1471-244X-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Gotlib IH. Gender differences in depression: the role of personality factors. Psychiatry Res. 2004;126:135–142. doi: 10.1016/j.psychres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gender Medicine. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Gorodetsky E, Bevilacqua L, Carli V, Sarchiapone M, Roy A, Goldman D, Enoch M. The interactive effect of MAOA-LPR genotype and childhood physical neglect on aggressive behaviors in Italian male prisoners. Genes, Brain, and Behavior. 2014;13:543–549. doi: 10.1111/gbb.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, Batel P, Adès J, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biol Psychiatry. 2000;48:259–264. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfries CG, Roos BE, Winblad B. Determination of 5-hydroxytryptamine, 5-hydroxyindoleacetic acid and homovanillic acid in brain tissue from an autopsy material. Acta Psychiatr Scand. 1974;50:496–507. doi: 10.1111/j.1600-0447.1974.tb09711.x. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Völzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003;15:431–449. [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet. 2000;96:202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gressier F, Calati R, Serretti A. 5-HTTLPR and gender differences in affective disorders: A systematic review. J Affect Disord. 2016;190:193–207. doi: 10.1016/j.jad.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Grochans E, Jurczak A, Szkup M, Samochowiec A, Wloszczak-Szubzda A, Karakiewicz B, Grzywacz A, Brodowska A, Samochowiec J. Evaluation of the Relationship between 5-HTT and MAO Gene Polymorphisms, Mood and Level of Anxiety among Postmenopausal Women. International Journal of Environmental Research and Public Health. 2015;12:268–281. doi: 10.3390/ijerph120100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann M, Hammer P, Walther M, Paulmann N, Büttner A, Eisenmenger W, Baghai TC, Schüle C, Rupprecht R, Bader M, Bondy B, Zill P, Priller J, Walther DJ. Alternative Splicing and Extensive RNA Editing of Human TPH2 Transcripts. Plos One. 2010:5. doi: 10.1371/journal.pone.0008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl ) 2002;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Guo G, Ou X, Roettger M, Shih JC. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. European Journal of Human Genetics. 2008;16:626–634. doi: 10.1038/sj.ejhg.5201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, Markert C, Escher A, Wendland J, Reif A, Mössner R, Gross C, Brocke B, Lesch K. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int J Neuropsychopharmacol. 2007;10:309–320. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Popp S, Waider J, Sommerlandt FMJ, Göppner C, Post A, Reif A, van dH, Strekalova T, Schmitt A, Colaςo MBN, Sommer C, Palme R, Lesch K. Interaction of brain 5-HT synthesis deficiency, chronic stress and sex differentially impact emotional behavior in Tph2 knockout mice. Psychopharmacology (Berl ) 2015;232:2429–2441. doi: 10.1007/s00213-015-3879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Smolen A, Hewitt JK. Family-Based Association Test of the 5HTTLPR and Aggressive Behavior in a General Population Sample of Children. Biol Psychiatry. 2006;59:836–843. doi: 10.1016/j.biopsych.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynänen OP, Kauhanen J, Syvälahti E, Hietala J, Tiihonen J. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4:385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions predicting depression symptoms in youth. J Child Psychol Psychiatry. 2010;51:180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science (New York, NY) 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grüsser SM, Flor H, Schumann G, Mann K, Büchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, Büchel C, Hariri AR, Weinberger DR. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61:1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Hermann A, Küpper Y, Schmitz A, Walter B, Vaitl D, Hennig J, Stark R, Tabbert K. Functional gene polymorphisms in the serotonin system and traumatic life events modulate the neural basis of fear acquisition and extinction. PloS One. 2012;7:e44352. doi: 10.1371/journal.pone.0044352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Huter T, Müller F, Mühlberger A, Pauli P, Reif A, Renner T, Canli T, Fallgatter AJ, Lesch K. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cerebral Cortex (New York, NY: 1991) 2007;17:1160–1163. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Handa RJ. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J Neurochem. 2013;127:487–495. doi: 10.1111/jnc.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RMA. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Primary Care Companion to the Journal of Clinical Psychiatry. 2001;3:244–254. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Bogdan R, Pizzagalli DA. Serotonin Transporter Genotype and Action Monitoring Dysfunction: A Possible Substrate Underlying Increased Vulnerability to Depression. Neuropsychopharmacology. 2010;35:1186–1197. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz N, Boecker R, Buchmann AF, Blomeyer D, Baumeister S, Hohmann S, Jennen-Steinmetz C, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer-Lindenberg A, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Laucht M. Evidence for a Sex-Dependent MAOA× Childhood Stress Interaction in the Neural Circuitry of Aggression. Cerebral Cortex (New York, NY: 1991) 2016;26:904–914. doi: 10.1093/cercor/bhu249. [DOI] [PubMed] [Google Scholar]
- Huang S, Lin M, Lin W, Huang C, Shy M, Lu R. Association of monoamine oxidase A (MAOA) polymorphisms and clinical subgroups of major depressive disorders in the Han Chinese population. The World Journal of Biological Psychiatry. 2009;10:544–551. doi: 10.1080/15622970701816506. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Hung C, Lung F, Chen C, O’Nions E, Hung T, Chong M, Wu C, Wen J, Lin P. Association between suicide attempt and a tri-allelic functional polymorphism in serotonin transporter gene promoter in Chinese patients with schizophrenia. Neurosci Lett. 2011;504:242–246. doi: 10.1016/j.neulet.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, Takei K, Suga M, Abe O, Yamada H, Rogers MA, Aoki S, Sasaki T, Kasai K. Effect of tryptophan hydroxylase-2 gene variants on amygdalar and hippocampal volumes. Brain Res. 2010;1331:51–57. doi: 10.1016/j.brainres.2010.03.057. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van dP, Minderaa RB, Ormel J, den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- Jaworska N, MacMaster FP, Foster J, Ramasubbu R. The influence of 5-HTTLPR and Val66Met polymorphisms on cortical thickness and volume in limbic and paralimbic regions in depression: a preliminary study. BMC Psychiatry. 2016:16. doi: 10.1186/s12888-016-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- Jokela M, Räikkönen K, Lehtimäki T, Rontu R, Keltikangas-Järvinen L. Tryptophan hydroxylase 1 gene (TPH1) moderates the influence of social support on depressive symptoms in adults. J Affect Disord. 2007;100:191–197. doi: 10.1016/j.jad.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Goldman D, Spurlock G, Gustavsson JP, Nielsen DA, Linnoila M, Owen MJ, Sedvall GC. Tryptophan hydroxylase and catechol-O-methyltransferase gene polymorphisms: relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Eur Arch Psychiatry Clin Neurosci. 1997;247:297–302. doi: 10.1007/BF02922258. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Norton N, Gustavsson JP, Oreland L, Owen MJ, Sedvall GC. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatr Res. 2000;34:239–244. doi: 10.1016/s0022-3956(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin Å, Saijo T, Varrone A, Halldin C, Nordström A. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi S, Kunugi H, Sano A, Tsutsumi T, Isogawa K, Nanko S, Akiyoshi J. Association between serotonin transporter gene polymorphism and anxiety-related traits. Biol Psychiatry. 1999;45:368–370. doi: 10.1016/s0006-3223(98)00090-0. [DOI] [PubMed] [Google Scholar]
- Ke L, Qi ZY, Ping Y, Ren CY. Effect of SNP at position 40237 in exon 7 of the TPH2 gene on susceptibility to suicide. Brain Res. 2006;1122:24–26. doi: 10.1016/j.brainres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Keltikangas-Järvinen L, Puttonen S, Kivimäki M, Elovainio M, Rontu R, Lehtimäki T. Tryptophan hydroxylase 1 gene haplotypes modify the effect of a hostile childhood environment on adulthood harm avoidance. Genes, Brain and Behavior. 2007;6:305–313. doi: 10.1111/j.1601-183X.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish National Twin Study of Lifetime Major Depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity SR. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee H, Yang J, Hwang J, Yoon H. A tryptophan hydroxylase 2 gene polymorphism is associated with panic disorder. Behav Genet. 2009;39:170–175. doi: 10.1007/s10519-008-9254-8. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Huang Y, Haverly R, Burke AK, Galfalvy H, Brent DP, Oquendo MA, Mann JJ. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatr Genet. 2009;19:126–133. doi: 10.1097/YPG.0b013e32832a50a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, Merz CJ, Kagerer S, Osinsky R, Walter B, Vaitl D, Hennig J, Stark R. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Social Cognitive and Affective Neuroscience. 2013a;8:318–325. doi: 10.1093/scan/nss005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, Merz CJ, Hennig J, Vaitl D, Stark R. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum Brain Mapp. 2013b;34:2549–2560. doi: 10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Blecker C, Walter B, Kuepper Y, Hennig J, Stark R. The association between the 5-HTTLPR and neural correlates of fear conditioning and connectivity. Social Cognitive and Affective Neuroscience. 2015;10:700–707. doi: 10.1093/scan/nsu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshöfer DE, Ikonomidou VN, Vollmert C, Vollstädt-Klein S, Rietschel M, Reischl G, Heinz A, Smolka MN. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Translational Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KB, Kim CH, Choi EH, Lee Y, Seo WY. Effect of tryptophan hydroxylase gene polymorphism on aggression in major depressive disorder and undifferentiated somatoform disorder. J Clin Psychiatry. 2012;73:e574–579. doi: 10.4088/JCP.11m07342. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, Lanzenberger R. High-Dose Testosterone Treatment Increases Serotonin Transporter Binding in Transgender People. Biol Psychiatry. 2015;78:525–533. doi: 10.1016/j.biopsych.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper Y, Grant P, Wielpuetz C, Hennig J. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behav Brain Res. 2013;247:73–78. doi: 10.1016/j.bbr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Lee B, Ham B. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 2008a;19:515–519. doi: 10.1097/WNR.0b013e3282f94294. [DOI] [PubMed] [Google Scholar]
- Lee B, Ham B. Serotonergic genes and amygdala activity in response to negative affective facial stimuli in Korean women. Genes, Brain and Behavior. 2008b;7:899–905. doi: 10.1111/j.1601-183X.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Lee H, Lee B, Pae C, Yoon B, Ryu S, Choi I, Lee M, Ham B. Impact of the tryptophan hydroxylase 1 gene A218C polymorphism on amygdala activity in response to affective facial stimuli in patients with major depressive disorder. Genes, Brain, and Behavior. 2009;8:512–518. doi: 10.1111/j.1601-183X.2009.00500.x. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong SH, Kim SH, Ahn YM, Kim YS, Jung HY, Bang YW, Joo E. Genetic Role of BDNF Val66Met and 5-HTTLPR Polymorphisms on Depressive Disorder. Psychiatry Investigation. 2014;11:192–199. doi: 10.4306/pi.2014.11.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto K, Vaht M, Mäestu J, Veidebaum T, Harro J. Effect of tryptophan hydroxylase-2 gene polymorphism G-703 T on personality in a population representative sample. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57:31–35. doi: 10.1016/j.pnpbp.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Lei H, Zhang X, Di X, Rao H, Ming Q, Zhang J, Guo X, Jiang Y, Gao Y, Yi J, Zhu X, Yao S. A functional polymorphism of the MAOA gene modulates spontaneous brain activity in pons. BioMed Research International. 2014;2014:243280. doi: 10.1155/2014/243280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Boni C, Pessiglione M, Lehéricy S, Fossati P. Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Hum Brain Mapp. 2011;32:1856–1867. doi: 10.1002/hbm.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY) 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li D, He L. Further clarification of the contribution of the tryptophan hydroxylase (TPH) gene to suicidal behavior using systematic allelic and genotypic meta-analyses. Hum Genet. 2006;119:233–240. doi: 10.1007/s00439-005-0113-x. [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent Class Analysis of Antisocial Behavior: Interaction of Serotonin Transporter Genotype and Maltreatment. J Abnorm Child Psychol. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Berk MS, Lee SS. Differential susceptibility in longitudinal models of gene-environment interaction for adolescent depression. Dev Psychopathol. 2013;25:991–1003. doi: 10.1017/S0954579413000321. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze J, Boni C, Hamon M, Adès J, Rouillon F, Gorwood P. Male-specific association between the 5-HTTLPR S allele and suicide attempts in alcohol-dependent subjects. J Psychiatr Res. 2005;39:179–182. doi: 10.1016/j.jpsychires.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Ko H, Chang F, Yeh T, Sun HS. Population-specific functional variant of the TPH2 gene 2755C>A polymorphism contributes risk association to major depression and anxiety in Chinese peripartum women. Archives of Women’s Mental Health. 2009;12:401–408. doi: 10.1007/s00737-009-0088-z. [DOI] [PubMed] [Google Scholar]
- Little K, Olsson CA, Whittle S, Youssef GJ, Byrne ML, Simmons JG, Yücel M, Foley DL, Allen NB. Association between serotonin transporter genotype, brain structure and adolescent-onset major depressive disorder: a longitudinal prospective study. Translational Psychiatry. 2014;4:e445. doi: 10.1038/tp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Mo Y, Ge T, Wang Y, Luo X, Feng J, Li M, Su B. Allelic variation at 5-HTTLPR is associated with brain morphology in a Chinese population. Psychiatry Res. 2015;226:399–402. doi: 10.1016/j.psychres.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu Z. The Relationship Between MAOA Gene Polymorphism and Test Anxiety. Twin Research and Human Genetics. 2013;16:1103–1106. doi: 10.1017/thg.2013.71. [DOI] [PubMed] [Google Scholar]
- Loi M, Koricka S, Lucassen PJ, Joëls M. Age- and Sex-Dependent Effects of Early Life Stress on Hippocampal Neurogenesis. Frontiers in Endocrinology. 2014:5. doi: 10.3389/fendo.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez dL, Brezo J, Rouleau G, Lesage A, Dumont M, Alda M, Benkelfat C, Turecki G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol Psychiatry. 2007;62:72–80. doi: 10.1016/j.biopsych.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lothe A, Boni C, Costes N, Gorwood P, Bouvard S, Le Bars D, Lavenne F, Ryvlin P. Association between triallelic polymorphism of the serotonin transporter and 18F]MPPF binding potential at 5-HT1A receptors in healthy subjects. Neuroimage. 2009;47:482–492. doi: 10.1016/j.neuroimage.2009.04.067. [DOI] [PubMed] [Google Scholar]
- Lung F, Tzeng D, Huang M, Lee M. Association of the MAOA promoter uVNTR polymorphism with suicide attempts in patients with major depressive disorder. BMC Medical Genetics. 2011;12:74. doi: 10.1186/1471-2350-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Xiao H, Yang Y, Cao D, Wang L, Yang X, Qiu X, Qiao Z, Song J, Liu Y, Wang P, Zhou J, Zhu X. Interaction of tryptophan hydroxylase 2 gene and life events in susceptibility to major depression in a Chinese Han population. J Affect Disord. 2015;188:304–309. doi: 10.1016/j.jad.2015.07.041. [DOI] [PubMed] [Google Scholar]
- Mandelli L, Antypa N, Nearchou FA, Vaiopoulos C, Stefanis CN, Serretti A, Stefanis NC. The role of serotonergic genes and environmental stress on the development of depressive symptoms and neuroticism. J Affect Disord. 2012;142:82–89. doi: 10.1016/j.jad.2012.03.047. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Nielsen DA, Goldman D, Erdos J, Gelernter J. Possible association of a polymorphism of the tryptophan hydroxylase gene with suicidal behavior in depressed patients. Am J Psychiatry. 1997;10:1451–3. doi: 10.1176/ajp.154.10.1451. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Markus CR, De Raedt R. Differential effects of 5-HTTLPR genotypes on inhibition of negative emotional information following acute stress exposure and tryptophan challenge. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36:819–826. doi: 10.1038/npp.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Tasa G, Tõru I, Lang A, Vasar V, Shlik J. Association between Serotonin-related Genetic Polymorphisms and CCK-4-induced Panic Attacks with or without 5-hydroxytryptophan Pretreatment in Healthy Volunteers. The World Journal of Biological Psychiatry. 2004;5:149–154. doi: 10.1080/15622970410029927. [DOI] [PubMed] [Google Scholar]
- Maron E, Lang A, Tasa G, Liivlaid L, Tõru I, Must A, Vasar V, Shlik J. Associations between serotonin-related gene polymorphisms and panic disorder. Int J Neuropsychopharmacol. 2005;8:261–266. doi: 10.1017/S1461145704004985. [DOI] [PubMed] [Google Scholar]
- Maron E, Tõru I, Must A, Tasa G, Toover E, Vasar V, Lang A, Shlik J. Association study of tryptophan hydroxylase 2 gene polymorphisms in panic disorder. Neurosci Lett. 2007;411:180–184. doi: 10.1016/j.neulet.2006.09.060. [DOI] [PubMed] [Google Scholar]
- Marriage K, Fine S, Moretti M, Haley G. Relationship between depression and conduct disorder in children and adolescents. J Am Acad Child Psychiatry. 1986;25:687–691. doi: 10.1016/s0002-7138(09)60295-8. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Bleil M, Pogue-Geile M, Ferrell RE, Manuck SB. Allelic variation in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cardiovascular reactivity in young adult male and female twins of European-American descent. Psychosom Med. 2003;65:721–728. doi: 10.1097/01.psy.0000088585.67365.1d. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Perroud N, Uher R, Butler A, Aitchison KJ, Craig I, Lewis C, Farmer A. The genetics of affective disorder and suicide. European Psychiatry: The Journal of the Association of European Psychiatrists. 2010;25:275–277. doi: 10.1016/j.eurpsy.2009.12.012. [DOI] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35:42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender Differences in Anxiety Disorders: Prevalence, Course of Illness, Comorbidity and Burden of Illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17β increase serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Mol Brain Res. 1997;45:13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Melas PA, Wei Y, Wong CCY, Sjöholm LK, Åberg E, Mill J, Schalling M, Forsell Y, Lavebratt C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int J Neuropsychopharmacol. 2013;16:1513–1528. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- Melke J, Landén M, Baghei F, Rosmond R, Holm G, Björntorp P, Westberg L, Hellstrand M, Eriksson E. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. Am J Med Genet. 2001;105:458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey BJ, Ducci F, Hodgkinson CA, Langenecker SA, Goldman D, Zubieta J. Monoamine Oxidase A Genotype Predicts Human Serotonin 1A Receptor Availability In Vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:11354–11359. doi: 10.1523/JNEUROSCI.2391-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gender Medicine. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- Ming Q, Zhang Y, Chai Q, Chen H, Hou C, Wang M, Wang Y, Cai L, Zhu X, Yi J, Yao S. Interaction between a serotonin transporter gene promoter region polymorphism and stress predicts depressive symptoms in Chinese adolescents: a multi-wave longitudinal study. BMC Psychiatry. 2013;13:142. doi: 10.1186/1471-244X-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Q, Zhang Y, Yi J, Wang X, Zhu X, Yao S. Serotonin transporter gene polymorphism (5-HTTLPR) L allele interacts with stress to increase anxiety symptoms in Chinese adolescents: a multiwave longitudinal study. BMC Psychiatry. 2015;15:248. doi: 10.1186/s12888-015-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, Itoyama Y, Kanazawa M, Utsumi A, Endo Y, Nomura T, Hiratsuka M, Mizugaki M, Goto J, Hongo M, Fukudo S. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res. 2006;60:91–97. doi: 10.1016/j.jpsychores.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry. 2004;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- New AS, Gelernter J, Yovell Y, Trestman RL, Nielsen DA, Silverman J, Mitropoulou V, Siever LJ. Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: a preliminary study. Am J Med Genet. 1998;81:13–17. doi: 10.1002/(sici)1096-8628(19980207)81:1<13::aid-ajmg3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Virkkunen M, Lappalainen J, Eggert M, Brown GL, Long JC, Goldman D, Linnoila M. A tryptophan hydroxylase gene marker for suicidality and alcoholism. Arch Gen Psychiatry. 1998;55:593–602. doi: 10.1001/archpsyc.55.7.593. [DOI] [PubMed] [Google Scholar]
- Nikolova Y, Bogdan R, Pizzagalli DA. Perception of a Naturalistic Stressor Interacts with 5-HTTLPR/rs25531 Genotype and Gender to Impact Reward Responsiveness. Neuropsychobiology. 2011;65:45–54. doi: 10.1159/000329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina V, Widom CS, Brzustowicz LM. Child abuse and neglect, MAOA, and mental health outcomes: a prospective examination. Biol Psychiatry. 2012;71:350–357. doi: 10.1016/j.biopsych.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, Lindström L, Oreland L. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Wargelius H, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius H, Sjöberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Åslund C, Nordquist N, Leppert J, Oreland L. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 2011;16:347–355. doi: 10.1111/j.1369-1600.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Hodgins S, Oreland L, Åslund C. Genotypes do not confer risk for delinquency but rather alter susceptibility to positive and negative environmental factors: gene-environmentinteractions of BDNF Val66Met, 5-HTTLPR, and MAOA-uVNTR corrected. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Vanzin L, Molteni M, Battaglia M. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50:317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Paaver M, Kurrikoff T, Nordquist N, Oreland L, Harro J. The effect of 5-HTT gene promoter polymorphism on impulsivity depends on family relations in girls. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1263–1268. doi: 10.1016/j.pnpbp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40:1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, Nicoletti G, Gallo O, Provinciali L, Quattrone A. Monoamine oxidase-a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez M, Weinstein S, New AS, Bevilacqua L, Yuan Q, Zhou Z, Hodgkinson C, Goodman M, Koenigsberg HW, Goldman D, Siever LJ. Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatr Res. 2010;44:1075–1081. doi: 10.1016/j.jpsychires.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Mulder RT, Joyce PR, Miller AL, Kennedy M. Tryptophan hydroxylase gene (TPH1) and peripheral tryptophan levels in depression. J Affect Disord. 2008;109:209–212. doi: 10.1016/j.jad.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Preuss N, Salehi B, Veen JWvd, Shen J, Drevets WC, Hodgkinson C, Goldman D, Hasler G. Associations between prefrontal Œ≥-aminobutyric acid concentration and the tryptophan hydroxylase isoform 2 gene, a panic disorder risk allele in women. International Journal of Neuropsychopharmacology. 2013;16:1707–1717. doi: 10.1017/S1461145713000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, Strong J, Eliassen J, McQueeny T, Miller M, Padula CB, Shear P, Lisdahl K. Serotonin transporter gene moderates associations between mood, memory and hippocampal volume. Behav Brain Res. 2013;242:158–165. doi: 10.1016/j.bbr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess-Groben H, Hyde JS. 5-HTTLPR X stress in adolescent depression: moderation by MAOA and gender. J Abnorm Child Psychol. 2013;41:281–294. doi: 10.1007/s10802-012-9672-1. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley E, Eaves LJ, Foley DL, Gardner CO, Archer KJ, Wormley BK, Maes HH, Riley BP, Silberg JL. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol Med. 2009;39:579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J. Major depression and conduct disorder in prepuberty. J Am Acad Child Psychiatry. 1982;21:118–128. doi: 10.1016/s0002-7138(09)60910-9. [DOI] [PubMed] [Google Scholar]
- Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, Popovic A, Scharinger C, Huemer J, Kalcher K, Pail G, Haslacher H, Perkmann T, Windischberger C, Brocke B, Sitte HH, Pollak DD, Dreher J, Kasper S, Praschak-Rieder N, Moser E, Esterbauer H, Pezawas L. Additive gene-environment effects on hippocampal structure in healthy humans. Journal of Neuroscience. 2014;34:9917–9926. doi: 10.1523/JNEUROSCI.3113-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Rösler M, Freitag CM, Schneider M, Eujen A, Kissling C, Wenzler D, Jacob CP, Retz-Junginger P, Thome J, Lesch K, Retz W. Nature and Nurture Predispose to Violent Behavior: Serotonergic Genes and Adverse Childhood Environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Reif A, Weber H, Domschke K, Klauke B, Baumann C, Jacob CP, Ströhle A, Gerlach AL, Alpers GW, Pauli P, Hamm A, Kircher T, Arolt V, Wittchen H, Binder EB, Erhardt A, Deckert J. Meta-analysis argues for a female-specific role of MAOA-uVNTR in panic disorder in four European populations. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2012;159B:786–793. doi: 10.1002/ajmg.b.32085. [DOI] [PubMed] [Google Scholar]
- Reif A, Richter J, Straube B, Höfler M, Lueken U, Gloster AT, Weber H, Domschke K, Fehm L, Ströhle A, Jansen A, Gerlach A, Pyka M, Reinhardt I, Konrad C, Wittmann A, Pfleiderer B, Alpers GW, Pauli P, Lang T, Arolt V, Wittchen H, Hamm A, Kircher T, Deckert J. MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry. 2014;19:122–128. doi: 10.1038/mp.2012.172. [DOI] [PubMed] [Google Scholar]
- Reuter M, Kuepper Y, Hennig J. Association between a polymorphism in the promoter region of the TPH2 gene and the personality trait of harm avoidance. Int J Neuropsychopharmacol. 2007;10:401–404. doi: 10.1017/S1461145706007073. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M, Gutierrez B, Molina E, Torres-Gonzalez F, Bellon JA, Moreno-Kuestner B, King M, Nazareth I, Martinez-Gonzalez L, Martinez-Espin E, Munoz-Garcia M, Motrico E, Martinez-Canavate T, Lorente JA, Luna JD, Cervilla JA. High-Activity Variants of the uMAOA Polymorphism Increase the Risk for Depression in a Large Primary Care Sample. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2009;150B:395–402. doi: 10.1002/ajmg.b.30829. [DOI] [PubMed] [Google Scholar]
- Rucci P, Nimgaonkar VL, Mansour H, Miniati M, Masala I, Fagiolini A, Cassano GB, Frank E. Gender moderates the relationship between mania spectrum and serotonin transporter polymorphisms in depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2009;150B:907–913. doi: 10.1002/ajmg.b.30917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Möller H. Association of anger-related traits with SNPs in the TPH gene. Mol Psychiatry. 2002;7:1023–1029. doi: 10.1038/sj.mp.4001128. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Sato T, Hartmann AM, Möller HJ. Genetic variations in tryptophan hydroxylase in suicidal behavior: analysis and meta-analysis. Biol Psychiatry. 2003;54:465–473. doi: 10.1016/s0006-3223(02)01748-1. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Sakado K, Sakado M, Muratake T, Mundt C, Someya T. A psychometrically derived impulsive trait related to a polymorphism in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) in a Japanese nonclinical population: assessment by the Barratt impulsiveness scale (BIS) American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2003;121B:71–75. doi: 10.1002/ajmg.b.20063. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Boardman JD, Gelhorn HL, Smolen A, Corley RP, Huizinga D, Menard S, Hewitt JK, Stallings MC. Using trajectory analyses to refine phenotype for genetic association: conduct problems and the serotonin transporter (5HTTLPR) Psychiatr Genet. 2010;20:199–206. doi: 10.1097/YPG.0b013e32833a20f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Słopień G, Grzywacz A, Kucharska-Mazur J. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Res. 2004;128:21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Scherk H, Gruber O, Menzel P, Schneider-Axmann T, Kemmer C, Usher J, Reith W, Meyer J, Falkai P. 5-HTTLPR genotype influences amygdala volume. Eur Arch Psychiatry Clin Neurosci. 2009;259:212–217. doi: 10.1007/s00406-008-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Müller DJ, Krauss H, Scherk H, Ohlraun S, Syagailo YV, Windemuth C, Neidt H, Grässle M, Papassotiropoulos A, Heun R, Nöthen MM, Maier W, Lesch KP, Rietschel M. Association between a functional polymorphism in the monoamine oxidase A gene promoter and major depressive disorder. Am J Med Genet. 2000;96:801–803. [PubMed] [Google Scholar]
- Selvaraj S, Godlewska BR, Norbury R, Bose S, Turkheimer F, Stokes P, Rhodes R, Howes O, Cowen PJ. Decreased regional gray matter volume in S’ allele carriers of the 5-HTTLPR triallelic polymorphism. Mol Psychiatry. 2011;16:472–473. doi: 10.1038/mp.2010.112. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Serretti A, Lilli R, Lorenzi C, Lattuada E, Cusin C, Smeraldi E. Tryptophan hydroxylase gene and major psychoses. Psychiatry Res. 2001;103:79–86. doi: 10.1016/s0165-1781(01)00269-4. [DOI] [PubMed] [Google Scholar]
- Sharpley CF, Palanisamy SKA, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. 2014;273:89–105. doi: 10.1016/j.bbr.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Shen X, Wu Y, Qian M, Wang X, Hou Z, Liu Y, Sun J, Zhong H, Yang J, Lin M, Li L, Guan T, Shen Z, Yuan Y. Tryptophan hydroxylase 2 gene is associated with major depressive disorder in a female Chinese population. J Affect Disord. 2011;133:619–624. doi: 10.1016/j.jad.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindström L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Wargelius H, Leppert J, Lindström L, Oreland L. Adolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factors. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Słopień R, Słopień A, Różycka A, Warenik-Szymankiewicz A, Lianeri M, Jagodziński PP. The c.1460C>T polymorphism of MAO-A is associated with the risk of depression in postmenopausal women. Thescientificworldjournal. 2012;2012:194845. doi: 10.1100/2012/194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. 2014;152:105–112. doi: 10.1016/j.jad.2013.05.068. [DOI] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, Charney D, Innis RB. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Staner L, Uyanik G, Correa H, Tremeau F, Monreal J, Crocq M, Stefos G, Morris-Rosendahl D, Macher JP. A dimensional impulsive-aggressive phenotype is associated with the A218C polymorphism of the tryptophan hydroxylase gene: a pilot study in well-characterized impulsive inpatients. Am J Med Genet. 2002;114:553–557. doi: 10.1002/ajmg.10405. [DOI] [PubMed] [Google Scholar]
- Starcevic V, Uhlenhuth EH, Fallon S, Pathak D. Personality dimensions in panic disorder and generalized anxiety disorder. J Affect Disord. 1996;37:75–79. doi: 10.1016/0165-0327(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Starr LR, Hammen C, Brennan PA, Najman JM. Relational security moderates the effect of serotonin transporter gene polymorphism (5-HTTLPR) on stress generation and depression among adolescents. J Abnorm Child Psychol. 2013;41:379–388. doi: 10.1007/s10802-012-9682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Svenson I, Marchuk DA, Levy RM, Hays JC, Flint EP, Krishnan KR, Siegler IC. Allelic Differences in the Serotonin Transporter-Linked Polymorphic Region in Geriatric Depression. The American Journal of Geriatric Psychiatry. 2002;10:185–191. [PubMed] [Google Scholar]
- Stein MB, Gorman JM. Unmasking social anxiety disorder. J Psychiatry Neurosci. 2001;26:185–189. [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Campbell-Sills L, Gelernter J. Genetic variation in 5HTTLPR is associated with emotional resilience. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2009;150B:900–906. doi: 10.1002/ajmg.b.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler DA, Davis C, Leavitt K, Schriger I, Benson K, Bhakta S, Wang LC, Oben C, Watters M, Haghnegahdar T, Bortolato M. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J Psychiatr Res. 2014;58:69–75. doi: 10.1016/j.jpsychires.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Christ CC, Highland KB. Serotonin system gene polymorphisms are associated with impulsivity in a context dependent manner. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:182–191. doi: 10.1016/j.pnpbp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Sun HS, Tsai H, Ko H, Chang F, Yeh T. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes, Brain, and Behavior. 2004;3:328–336. doi: 10.1111/j.1601-183X.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Elkin A, Ecker C, Kalidindi S, Corsico A, Giampietro V, Lawrence N, Deeley Q, Murphy DGM, Kucharska-Pietura K, Russell TA, McGuffin P, Murray R, Phillips ML. Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes, Brain and Behavior. 2008;7:543–551. doi: 10.1111/j.1601-183X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- Tadic A, Rujescu D, Szegedi A, Giegling I, Singer P, Möller H, Dahmen N. Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2003;117B:1–6. doi: 10.1002/ajmg.b.10013. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rautiainen M, Ollila HM, Repo-Tiihonen E, Virkkunen M, Palotie A, Pietiläinen O, Kristiansson K, Joukamaa M, Lauerma H, Saarela J, Tyni S, Vartiainen H, Paananen J, Goldman D, Paunio T. Genetic background of extreme violent behavior. Mol Psychiatry. 2015;20:786–792. doi: 10.1038/mp.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Hong C, Liou Y, Yu YW, Chen T, Hou S, Yen F. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsycho-pharmacol Biol Psychiatry. 2009;33:637–641. doi: 10.1016/j.pnpbp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Uddin M, Koenen KC, de LS, Bakshis E, Aiello AE, Galea S. Gender differences in the genetic and environmental determinants of adolescent depression. Depress Anxiety. 2010;27:658–666. doi: 10.1002/da.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustün TB, Ayuso-Mateos J, Chatterji S, Mathers C, Murray CJL. Global burden of depressive disorders in the year 2000. The British Journal of Psychiatry: The Journal of Mental Science. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Utge S, Soronen P, Partonen T, Loukola A, Kronholm E, Pirkola S, Nyman E, Porkka-Heiskanen T, Paunio T. A population-based association study of candidate genes for depression and sleep disturbance. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2010;153B:468–476. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Batalden M, Orav J, Roston D, Barrett JE. Evidence for a possibly X-linked trait related to affective illness. Aust N Z J Psychiatry. 2005;39:730–735. doi: 10.1080/j.1440-1614.2005.01658.x. [DOI] [PubMed] [Google Scholar]
- Van DB, Sleegers K, De Zutter S, Heyrman L, Norrback K, Adolfsson R, Van Broeckhoven C, Del-Favero J. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- van Strien T, van dZ, Engels RCME. Emotional eating in adolescents: a gene (SLC6A4/5- HTT) - depressive feelings interaction analysis. J Psychiatr Res. 2010;44:1035–1042. doi: 10.1016/j.jpsychires.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Verhoeven FEA, Booij L, Kruijt A, Cerit H, Antypa N, Does W. The effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggression. Brain and Behavior. 2012;2:806–813. doi: 10.1002/brb3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Joiner TE, Johnson F, Bender TW. Gender specific gene-environment interactions on laboratory-assessed aggression. Biol Psychol. 2006;71:33–41. doi: 10.1016/j.biopsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Viikki M, Kampman O, Illi A, Setälä-Soikkeli E, Anttila S, Huuhka M, Nuolivirta T, Poutanen O, Mononen N, Lehtimäki T, Leinonen E. TPH1 218A/C polymorphism is associated with major depressive disorder and its treatment response. Neurosci Lett. 2010;468:80–84. doi: 10.1016/j.neulet.2009.10.069. [DOI] [PubMed] [Google Scholar]
- Volf NV, Belousova LV, Knyazev GG, Kulikov AV. Gender differences in association between serotonin transporter gene polymorphism and resting-state EEG activity. Neuroscience. 2015;284:513–521. doi: 10.1016/j.neuroscience.2014.10.030. [DOI] [PubMed] [Google Scholar]
- Voltas N, Aparicio E, Arija V, Canals J. Association study of monoamine oxidase-A gene promoter polymorphism (MAOA-uVNTR) with self-reported anxiety and other psychopathological symptoms in a community sample of early adolescents. J Anxiety Disord. 2015;31:65–72. doi: 10.1016/j.janxdis.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Hoell I, Tzvetkov M, Jamrozinski K, Sehrt D, Brockmöller J, Leibing E. Anxiety- and novelty seeking-related personality traits and serotonin transporter gene polymorphisms. J Psychiatr Res. 2006;40:568–576. doi: 10.1016/j.jpsychires.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Stroud CB, Mineka S, Zinbarg RE, Adam EK, Redei EE, Hammen C, Craske MG. Additive genetic risk from five serotonin system polymorphisms interacts with interpersonal stress to predict depression. J Abnorm Psychol. 2015;124:776–790. doi: 10.1037/abn0000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Dukic V, Blair RJR, Leventhal BL, Cox NJ, Burns JL, Kasza KE, Wright RJ, Cook EH. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychiatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderhaug E, Herman AI, Magnusson A, Morgan MJ, Landrø NI. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci Lett. 2010;473:208–211. doi: 10.1016/j.neulet.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderhaug E, Magnusson A, Neumeister A, Lappalainen J, Lunde H, Refsum H, Landrø NI. Interactive Effects of Sex and 5-HTTLPR on Mood and Impulsivity During Tryptophan Depletion in Healthy People. Biol Psychiatry. 2007;62:593–599. doi: 10.1016/j.biopsych.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Wankerl M, Zyriax B, Bondy B, Hinkelmann K, Windler E, Otte C. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) and diurnal cortisol: A sex by genotype interaction. Biol Psychol. 2010;85:344–346. doi: 10.1016/j.biopsycho.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch K, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Yandim C, Panousopoulou E, Ahmad M, Harker N, Saveliev A, Burgoyne PS, Festenstein R. Sexual dimorphism in mammalian autosomal gene regulation is determined not only by Sry but by sex chromosome complement as well. Developmental Cell. 2010;19:477–484. doi: 10.1016/j.devcel.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage. 2005;28:618–626. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, Costa PT, Schofield PR, Gordon E. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- Williams LM. Lancet Psychiatry. 2016. Precision Psychiatry: A neural circuit taxonomy for depression and anxiety. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsy-chopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Wu JB, Chen K, Li Y, Lau YC, Shih JC. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. The FASEB Journal. 2009;23:4029–4038. doi: 10.1096/fj.09-139097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee M, Lee S, Lee B, Kim S, Joe S, Jung I, Choi I, Ham B. Association between Tryptophan Hydroxylase 2 Polymorphism and Anger-Related Personality Traits among Young Korean Women. Neuropsychobiology. 2010;62:158–163. doi: 10.1159/000318572. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kim Y. TPH2 -703G/T SNP may have important effect on susceptibility to suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:403–409. doi: 10.1016/j.pnpbp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Yoon H, Lee H, Kim L, Lee M, Ham B. Impact of tryptophan hydroxylase 2 G-703T polymorphism on anger-related personality traits and orbitofrontal cortex. Behav Brain Res. 2012;231:105–110. doi: 10.1016/j.bbr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai S, Hong C, Chen T, Chen M, Yang C. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005a;30:1719–1723. doi: 10.1038/sj.npp.1300785. [DOI] [PubMed] [Google Scholar]
- Yu YW, Yang C, Wu H, Tsai S, Hong C, Chen M, Chen T. Association study of a functional MAOA-uVNTR gene polymorphism and personality traits in Chinese young females. Neuropsychobiology. 2005b;52:118–121. doi: 10.1159/000087556. [DOI] [PubMed] [Google Scholar]
- Zaboli G, Gizatullin R, Nilsonne A, Wilczek A, Jönsson EG, Ahnemark E, Asberg M, Leopardi R. Tryptophan hydroxylase-1 gene variants associate with a group of suicidal borderline women. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31:1982–1990. doi: 10.1038/sj.npp.1301046. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu X, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu L, Li X, Song Y, Liu J. Serotonin transporter gene polymorphism (5-HTTLPR) influences trait anxiety by modulating the functional connectivity between the amygdala and insula in Han Chinese males. Hum Brain Mapp. 2015;36:2732–2742. doi: 10.1002/hbm.22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan Hydroxylase-2 Controls Brain Serotonin Synthesis. Science. 2004;305:217–217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu J, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang C, Yuan G, Yao J, Cheng Z, Liu C, Liu Q, Wan G, Shi G, Cheng Y, Ling Y, Li K. Effect of tryptophan hydroxylase-2 rs7305115 SNP on suicide attempts risk in major depression. Behavioral and Brain Functions: BBF. 2010;6:49. doi: 10.1186/1744-9081-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, Enoch M, Virkkunen M, Goldman D. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005;62:1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]
- Zill P, Büttner A, Eisenmenger W, Möller H, Bondy B, Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry. 2004a;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schüle C, Eser D, Rupprecht R, Möller H, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004b;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- Zill P, Büttner A, Eisenmenger W, Möller H, Ackenheil M, Bondy B. Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: A post-mortem study. J Psychiatr Res. 2007;41:168–173. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M. Co-occurrence of conduct disorder and its adult outcomes with depressive and anxiety disorders: a review. J Am Acad Child Adolesc Psychiatry. 1992;31:547–556. doi: 10.1097/00004583-199205000-00024. [DOI] [PubMed] [Google Scholar]