Abstract
Background
Non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen are common medications with multiple useful effects including pain relief and reduction of inflammation. However, surgeons commonly hold all NSAIDs peri-operatively because of bleeding concerns. However, not all NSAIDs irreversibly block platelet function. We hypothesized that the use of ibuprofen would have no effect on postoperative bleeding in plastic surgery patients.
Methods
A literature review was performed using Medline (PubMed), EMBASE, and the Cochrane Collaboration Library for primary research articles on ibuprofen and bleeding. Inclusion criteria were primary journal articles examining treatment of acute postoperative based on any modality. Data related to pain assessment, postoperative recovery, and complications were extracted. Bias assessment and meta-analysis were performed.
Results
A total of 881 publications were reviewed. Four primary randomized controlled trials were selected for full analysis. Articles were of high quality by bias assessment. No significant difference was noted regarding bleeding events (p = 0.32) and pain control was noted to be equivalent.
Conclusion
Ibuprofen is a useful medication in the setting of surgery with multiple beneficial effects. This meta-analysis represents a small set of high quality studies that suggests ibuprofen provides equivalent pain control to narcotics. Importantly, ibuprofen was not associated with an increased risk of bleeding. Further large studies will be necessary to elucidate this issue further, but ibuprofen is a safe postoperative analgesic in patients undergoing common plastic surgery soft tissue procedures.
Keywords: ibuprofen, NSAID, bleeding, plastic surgery
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are cyclooxygenase (COX) inhibitors with effects including reduction of inflammation and pain control. NSAIDs are widely taken and easily available medications. Further, they have a variety of uses pertinent to surgical patients. Worldwide, varieties of formulations are available for over-the-counter (OTC) consumption by patients without a prescription. In the United States, OTC oral formulations are limited to ibuprofen and naproxen.1 Little is known about the perioperative risk of these common OTC analgesics. Despite the temporary effect of ibuprofen on platelet function, there is no evidence that risk of operative bleeding or postoperative hematoma increases. In fact, there may be minimal clinically significant risk even when the bleeding time is elevated by a NSAID.2 However, concern for these complications prompts many plastic surgeons to hold ibuprofen for up to a week before and after surgery.
There are many reasons to consider NSAID therapy postoperatively. For instance, there is growing federal, medical, and public concern regarding the potential over-prescribing and abuse of opiate pain medications.3 In the US alone, over 244 million prescriptions were issued for narcotics in 2010, with an estimated cost at over $8.4 billion.4 The current guidelines of the American Society of Anesthesiologists (ASA) suggest that multimodal pain management should be initiated immediately following surgery and a NSAID should be considered in that regimen.5 Further, the ASA agreed that the regimen should include around-the-clock scheduling of either acetaminophen or a NSAID. Ibuprofen is frequently given to surgical patients postoperatively, but its use is more limited in flap or soft tissue plastic surgery procedures where postoperative bleeding may sacrifice viability or aesthetic outcomes. It is therefore important to understand the quality of this treatment in patients compared to other options.
In order to understand the perioperative effects and to summarize the known literature of ibuprofen, a systematic review with meta-analysis was performed. We hypothesize that ibuprofen will offer equivalent pain control in comparison with other analgesics and will not cause an increased risk of postoperative bleeding. We specifically targeted plastic surgery type procedures with limitation to soft tissues, including aesthetic, breast, muscle, or skin-limited surgeries, to evaluate risk of bleeding in these very sensitive procedures.
MATERIALS AND METHODS
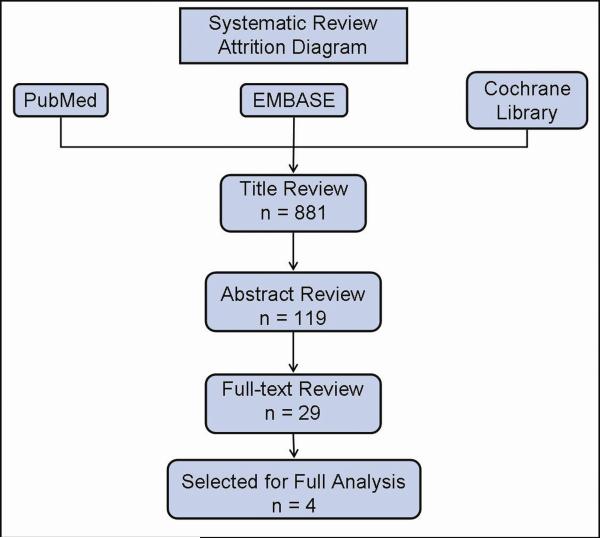
A systematic review of the literature was performed per PRISMA guidelines.6, 7 A thorough review of randomized control trials was performed using Medline (PubMed), EMBASE, and the Cochrane Collaboration Library by two independent authors (B.P.K. and K.G.B.) using the keywords “ibuprofen,” “Motrin,” “Advil,” “surgery,” “post-operative bleeding,” “post-operative hemorrhage,” and “post-operative hematoma.” Articles were then vetted against predefined inclusion and exclusion criteria at stages by title, abstract review, and finally full-text review (Fig. 1). Any disagreements were resolved by consensus.
Fig. 1.
Study attrition diagram, outline of search process and excluded studies.
Inclusion and exclusion criteria were developed a priori and are displayed in Table 1. We used a restrictive set of inclusion criteria, in order to include only the highest level of evidence. Inclusion criteria were limited to randomized, controlled trials (RCTs) that assessed ibuprofen use in the peri-operative setting. Eligible studies were double-blinded and limited to human subjects. Qualified studies were considered only if there were at least 20 patients included. Studies in open access journals or those without peer review were excluded.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria: |
Exclusion Criteria: |
|---|---|
| Human | Bleeding not included in outcomes |
| RCT | Non-RCT study design |
| Double-blind | Non-soft tissue surgery: |
| English Language | Dental procedures |
| Perioperative Ibuprofen Use | Ophthalmologic surgery |
| Comparison on pain control | Orthopedic procedures |
| Comparison on bleeding | Intrathoracic surgery |
| Intraabdominal surgery | |
| Obstetric surgery | |
| Neurologic procedures | |
| Vascular/Endovascular surgery | |
| Anesthesia / block procedures | |
| Known coagulopathy / anticoagulation | |
| Pediatric |
RCT, randomized controlled trial
Following full-text screening, four studies met criteria for inclusion into the analysis (Table 2). Two investigators (BPK and KGB) reviewed the studies independently. Data were extracted from text and tables related to pain assessment, adverse events, and incidence of bleeding. Pain assessments included were satisfaction with the selected pain regimen (“yes” or “no”), need for “rescue” medications, and averaged daily pain score based on standard 100mm visual analog scale (VAS). Jadad scores were tabulated for bias assessment. Additionally, studies were assessed for bias by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.8 Guideline analysis was performed using the GRADEPro software package by the GRADE Working Group (Fig. 2).9 Statistical meta-analysis was performed by random effects model using RevMan review software package.10 Analysis was performed by Mantel-Haenzel risk ratio analysis for dichotomous data and inverse variance risk difference for continuous data. Subgroup analysis was performed on ibuprofen versus acetaminophen + codeine (T3).
Table 2.
General Study Characteristics, organized by alphabetical order
| Study | JADAD score | Type | Study Design* | Participants (n) | Surgical Procedures |
|---|---|---|---|---|---|
| Chen et al. 2000 | 4 | Prospective | RCT | 35 | Variable cosmetic facial procedures** |
| Mitchell et al. 2001 | 5 | Prospective | RCT | 141 | Lumpectomy, mastectomy with/or without ALND/SLNB*** |
| Mixter et al. 2001 | 5 | Prospective | RCT | 59 | Laparoscopic inguinal hernia repair |
| Sniezek et al. 2003 | 4 | Prospective | RCT | 208 | Complex linear repair, skin grafting, local/regional flap |
RCT – Randomized, controlled trial
Rhinoplasty, submentoplasty, trichophytic forehead lift, otoplasty, facelift (the author's do not elaborate on type of rhytidectomy), upper and lower blepharoplasty
ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy
Fig. 2.
Summary of study risk bias analysis.
RESULTS
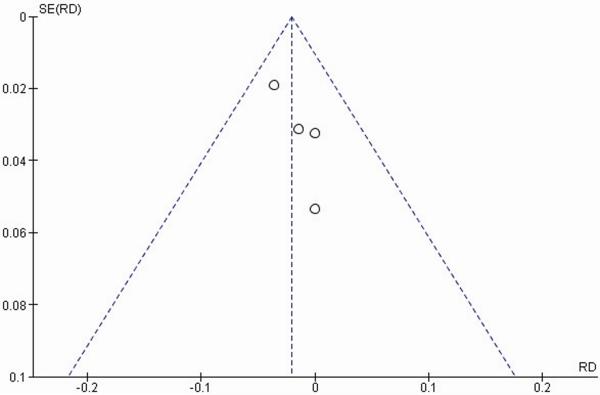
Four primary RTCs were selected for analysis following screening of 881 total studies by inclusion and exclusion criteria. Demographic data are summarized in Table 2. All studies were within the 95 percent confidence interval (95 percent CI) on a funnel plot, suggesting no significant publication bias (Fig. 3).
Fig. 3.
Funnel plot of major bleeding events graphically assessing for publication bias. The plot represents the standard error for each study plotted against the measured effect size. The vertical line represents the combined effect for all studies. The diagonal lines represent the 95 percent confidence interval. All studies were confined within the 95 percent confidence interval, suggesting a lack of significant publication bias.
Two studies included patients undergoing operations with general anesthesia11, 12 while 2 studies did not mention the type of anesthesia provided.13, 14 A range of plastic surgery related operations were performed including facial aesthetic surgery, breast surgery, inguinal herniorraphy, and cutaneous reconstructive procedures following Mohs.11–14 A single study began ibuprofen dosing just prior to incision12 while the other studies began ibuprofen after surgery.11, 13, 14 Ibuprofen was given orally in all studies with a dose of 400mg every four hours.11–14 The control groups included acetaminophen alone,14 T3,11, 13, 14 or ketorolac.12 In studies utilizing T3 as a control, 2 studies used a dosing of 600mg / 60mg11, 13 and 1 study used a lower dose of 300 mg / 30mg.14 One study gave ibuprofen combined with acetaminophen in the ibuprofen group.14 All 4 studies discussed pain management utilizing a standardized 100mm VAS. Two studies looked at pain control using the 100mm VAS for mean daily pain on the day of surgery (post-operative day #0, POD #0).11, 14 The remainder of time points evaluated did not overlap enough for analysis and were excluded from this analysis. The study findings are summarized in Tables 3.
Table 3.
Reported Outcomes in Randomized Controlled Trials of Ibuprofen
| Medications, dose | Bleeding Incidence | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Authors, Year | IB | KT | IB | KT | Result |
| Chen et al., 2009 | 400mg | T3 | 0 | 0 | No significant difference in bleeding. Noted less pain on POD3 and any side effects with IB. More patients requesting to change medications with T3 vs IB (p = 0.045) |
| Mitchell et al., 2012 | 400mg | T3 | 2 | 3 | No significant difference in bleeding, postop pain or incidence of adverse events. More patients requesting to change medications with T3 vs IB (p = 0.018). |
| Mixter et al., 1998 | 400mg | ketorolac | 0 | 0 | No significant difference in bleeding or pain. |
| Sniezek et al., 2011 | Ac/IB, 1000/400mg | T3 or Ac | 0 | 5 | No bleeding complications noted in IB group. Noted significant difference between T3 vs IB for overall complication rate (p = 0.001) |
IB, ibuprofen; KT, control; Ac, acetaminophen; T3, acetaminophen + codeine; POD3, postoperative day 3
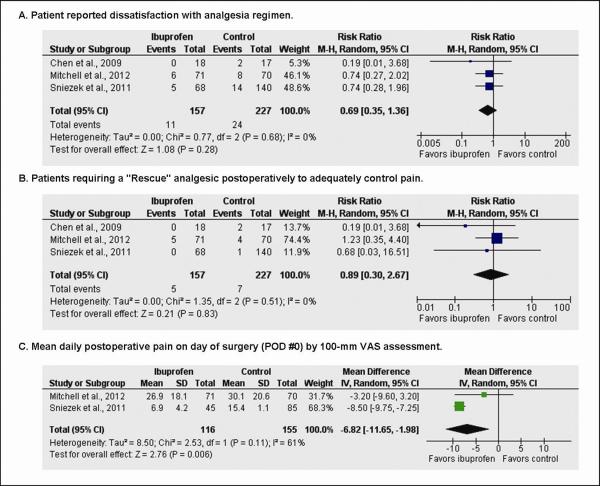
Pain treatment was evaluated by three variables: overall satisfaction, the need for “rescue” pain medications, and pain scores on the day of surgery (POD #0). Three studies showed overall patient satisfaction with pain treatment using a “yes or no” response.11, 13, 14 A total of 384 patients answered the satisfaction question, with 157 in the ibuprofen group and 227 in the controls (Fig. 4A). Events were tallied as a patient response of “No,” signifying dissatisfaction with their pain regimen. Patients reported dissatisfaction with their pain regimen in 11 of 157 (7.0%) patients in the ibuprofen group and 24 of 227 (10.6%) patients in the control group (RR 0.67, 95 percent CI, 0.34 to 1.31, p = 0.24). Three studies commented on the need for a “rescue medication” to achieve appropriate pain control.11, 13, 14 A total of 384 patients answered the rescue medication question, 157 in the ibuprofen group and 227 in the control group (Fig. 4B). Patients reported the need for an additional rescue medication with their pain regimen in 5 of 127 (3.2%) patients in the ibuprofen group and 7 of 227 (3.1%) patients in the control group (RR 0.89, 95 percent CI, 0.30 to 2.67, p = 0.83). Two studies evaluated mean pain using the 100mm VAS on POD#0.11, 14 A total of 271 patient responses were reported on POD#0, 116 in the ibuprofen group and 155 in the control group including both acetaminophen alone and T3 with a mean difference favoring ibuprofen over control (MD −6.82, 95 percent CI, − 11.65 to −1.98, p < 0.01; Fig. 4C).
Fig. 4.
Forest plot representing pooled effect of ibuprofen versus control on (A) pain satisfaction, (B) need for rescue medication, (C) mean daily pain on POD#0. Each square represents the effect size for a particular study, with the size of the square proportional to the study size. Horizontal lines represent 95 percent confidence intervals. A random effects model was used for all analyses. Diamonds represent pooled data for each subgroup, and the overall effect size. M-H, Mantel-Haenszel; SD, standard deviation.
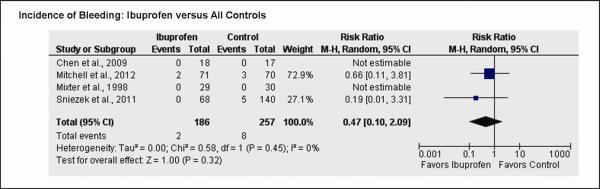
Four studies commented on post-operative bleeding.11–14 A total of 443 patients were assessed after surgery for bleeding by the participating surgeon, 186 in the ibuprofen group and 257 in the control group (Fig. 5). Surgically significant post-operative bleeding was noted in 2 of 186 (3.5%) patients in the ibuprofen group and 8 of 257 (4.1%) patients in the control groups (RR 0.47, 95 percent CI, 0.10 to 2.09, p = 0.32).
Fig. 5.
Forest plot representing pooled effect of ibuprofen versus control on incidence of bleeding events. Each square represents the effect size for a particular study, with the size of the square proportional to the study size. Horizontal lines represent 95 percent confidence intervals. A random effects model was used for all analyses. Diamonds represent pooled data for each subgroup, and the overall effect size. M-H, Mantel-Haenszel; SD, standard deviation.
A subset of studies compared ibuprofen to T3 and these were further analyzed.11, 13, 14 Three studies commented on patient satisfaction with their pain control with 314 patients responding to this survey question (Fig. 5a). Patients reported dissatisfaction in 11 of 157 (7.0%) in the ibuprofen group and 19 of 157 (12.1%) in the T3 group (RR 0.61, 95 percent CI, 0.30 to 1.23, p = 0.17). Two studies evaluated pain on POD #0, with a significant difference noted favoring ibuprofen over T3 (MD −6.34, 95 percent CI, −8.43 to −4.25, p < 0.01; Fig. 5b).11, 14 Three studies compared incidence of significant post-operative bleeding between ibuprofen and T3 groups (Fig. 5c).11, 13, 14 Significant bleeding was noted in 2 of 157 (1.3%) in the ibuprofen group and 6 of 157 (3.8%) in the control group (RR 0.44, 95 percent CI, 0.10 to 2.01, p = 0.29).
DISCUSSION
The results of this meta-analysis demonstrated no significant difference in clinical bleeding with ibuprofen. All studies continued ibuprofen use for at least 1 week after surgery and either began immediately prior to surgery or in post-operative recovery. Furthermore, ibuprofen appears to be equivalent for treatment of postoperative pain compared to tramadol. Of the included studies, none noted a statistically significant difference in pain control in their series. One study noted significantly improved pain control in the early post-operative period in patients after Mohs reconstruction.14 The remainder of the studies noted no significant different in patient satisfaction of pain control. Three studies evaluated the need for a “rescue” medication and no significant difference was noted.11, 13, 14 Two studies evaluated the mean pain on the day of surgery and ibuprofen appeared to be especially effective on the day of surgery.11,14
Bleeding is a significant concern for plastic surgeons and NSAIDs are routinely held with this in mind. Ketorolac was recently shown in meta-analysis to be safe in the perioperative period in a combined surgical meta-analysis, but this medication is only available in IV formulation.2 Furthermore, this study was limited by inclusion of orthopedic, intra-abdominal, gynecologic, and dental procedures, which could confound bleeding incidence based on tissue type or distribution in a body cavity. Ibuprofen is often avoided for weeks peri-operatively despite having a short, temporary effect on platelet function. Previous studies have demonstrated complete platelet functional recovery within 8–24 hrs of the last dose of ibuprofen in healthy subjects.15 Studies have demonstrated that post-operative hematomas in surgery can occur frequently in the first 48 hours after surgery, but factors influencing these events are still not fully elucidated.16, 17 One proposed theory is that inadequate post-operative pain control could lead to postoperative hypertension and possibly increase risk for bleeding. Better pain control, therefore, may reduce bleeding incidence, thus overruling the anti-platelet effects from NSAIDs.
Consideration of alternative postoperative analgesics, such as ibuprofen, is increasingly important given the global concerns for opiate misuse. Over 256 million prescriptions were written for opiate medications in 2009.18 Furthermore, results of the 2010 National Survey on Drug Use and Health showed that approximately 5.1 million Americans, age 12 or older, had used opiate pain medication for non-medical purposes within the past month.19 The cost of narcotics is equally alarming. For example, opiates have been shown to significantly increase postoperative hospital length of stay with an estimated cost increase of $6,500 per admission.20 However, OTC analgesics like ibuprofen are more cost effective given their effectiveness in pain control, well-established public tolerance, and low-risk qualities for abuse. Furthermore, this meta-analysis suggests that there is no increased risk of bleeding or hematoma from use of ibuprofen in the postoperative setting for this particular set of procedures.
Despite being available over-the-counter and inexpensive, ibuprofen has not been studied in major plastic surgery operations. This may be because surgeons are hesitant to randomize patients to get ibuprofen because they have no evidence to support its safety. Cases with increased exposed tissue surface area, such as breast surgery, body contouring, or facial rhytidectomy, are often associated with higher bleeding risk. This meta-analysis does not specifically address questions of safety in these plastic surgery operations. However, this study suggests that in procedures where bleeding and hematoma are easily detectable, ibuprofen does not increase the risk for this complication. Non-plastic surgery operations, such as extraperitoneal laparoscopic inguinal hernia, were included because a dead space, though small, is created during the procedure. Furthermore, laparoscopic inguinal hernia repair has detectable hematoma rates of 1.5 to 3.6 percent.21, 22 Similarly, the terms bleeding and hematoma are often used interchangeably. This may be especially useful when considering small surgical procedures such as Mohs defect reconstruction. For instance, hematoma and bleeding have been noted in large-scale Mohs databases as frequently as 8.4 percent.23 However, this bleeding includes, for example, bleeding on a raw flap surface that later required intervention by the surgeon. Though this is certainly not the same as a breast hematoma and the threat to the patient may be considered significantly less, the ability to detect this minor amount of bleeding is not insignificant when evaluating for subtle changes in coagulability.
Limitations of this meta-analysis include the sample size and the low number of studies. Additionally, despite selecting for studies specific to soft-tissue and plastic surgery, a wide variety of procedures were included. Furthermore, by restricting this study to plastic surgery and procedures with soft-tissue dead space, these results may not be applicable to other anatomic tissues or areas. However, the quality of the included studies is high based on bias analysis. Additionally, these results should have general application to plastic surgery and dermatologic procedures with less concern for confounders. This study is also likely to be more sensitive to bleeding events as the surgical areas are limited to small spaces where hematoma or bleeding would be easily apparent. On the other hand, there were no studies including patients with large surface area surgeries, such as body contouring, and the meta-analysis conclusions may be more limited when discussing these operations.
Ibuprofen is a useful medication in surgical patients with multiple beneficial effects. This meta-analysis represents a small set of high quality studies. The findings suggest that aversion to ibuprofen after surgery may be unfounded as it provides a satisfactory adjunct to postoperative pain treatment with no significant difference in bleeding risk and equivalent pain control in soft tissue and selected plastic surgery procedures. Furthermore, the avoidance of ibuprofen and other NSAIDs may lead to increased narcotic consumption or more difficult postoperative pain control. Ibuprofen may be of the most benefit in the immediate post-operative period starting on the day of surgery to achieve adequate, early pain control. Given previous findings on platelet function in conjunction with the findings of this study, ibuprofen is likely throughout the peri-operative period in plastic surgery patients. However, further studies will be necessary to fully elucidate this question in larger aesthetic, body contouring, and reconstructive procedures.
Acknowledgement
This manuscript and the research described complied with the Declaration of Helsinki.
Financial Disclosures: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 2 K24-AR053120-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Ibuprofen: Overview. Food and Drug Administration; Washington, D.C.: 2014. [Google Scholar]
- 2.Gobble RM, Hoang HL, Kachniarz B, et al. Ketorolac Does Not Increase Perioperative Bleeding: A Meta-Analysis of Randomized Controlled Trials. Plast Reconstr Surg. 2014;133:741–755. doi: 10.1097/01.prs.0000438459.60474.b5. [DOI] [PubMed] [Google Scholar]
- 3.Meier B. New York Times. New York: 2012. Doctors Petition for Limits on Painkillers; p. B2. [Google Scholar]
- 4.Informatics., I. I. f. H. The Use of Medicines in the United States: Review of 2010. 2011. [Google Scholar]
- 5.Practice Guidelines for Acute Pain Management in the Perioperative Setting: An Updated Report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 6.Haase SC. Systematic Reviews and Meta-Analysis. Plast Reconstr Surg. 2011;127:955–966. doi: 10.1097/PRS.0b013e318200afa9. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Oxman AD, Vist GE, et al. Grade: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brozek J, Oxman A, Schünemann H, Gradepro . Version 3.6 for Windows Ed 2008. [Google Scholar]
- 10.Version 5.2 Ed The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2012. [Google Scholar]
- 11.Mitchell A, McCrea P, Inglis K, et al. A Randomized, Controlled Trial Comparing Acetaminophen Plus Ibuprofen Versus Acetaminophen Plus Codeine Plus Caffeine (Tylenol 3) after Outpatient Breast Surgery. Ann Surg Oncol. 2012;19:3792–3800. doi: 10.1245/s10434-012-2447-7. [DOI] [PubMed] [Google Scholar]
- 12.Mixter CG, 3rd, Meeker LD, Gavin TJ. Preemptive Pain Control in Patients Having Laparoscopic Hernia Repair: A Comparison of Ketorolac and Ibuprofen. Arch Surg. 1998;133:432–437. doi: 10.1001/archsurg.133.4.432. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Adamson PA. Comparison of Ibuprofen and Acetaminophen with Codeine Following Cosmetic Facial Surgery. J Otolaryngol Head Neck Surg. 2009;38:580–586. [PubMed] [Google Scholar]
- 14.Sniezek PJ, Brodland DG, Zitelli JA. A Randomized Controlled Trial Comparing Acetaminophen, Acetaminophen and Ibuprofen, and Acetaminophen and Codeine for Postoperative Pain Relief after Mohs Surgery and Cutaneous Reconstruction. Dermatol Surg. 2011;37:1007–1013. doi: 10.1111/j.1524-4725.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief Communication: Duration of Platelet Dysfunction after a 7-Day Course of Ibuprofen. Ann Intern Med. 2005;142:506–509. doi: 10.7326/0003-4819-142-7-200504050-00009. [DOI] [PubMed] [Google Scholar]
- 16.Seth AK, Hirsch EM, Kim JY, et al. Hematoma after Mastectomy with Immediate Reconstruction: An Analysis of Risk Factors in 883 Patients. Ann Plast Surg. 2013;71:20–23. doi: 10.1097/SAP.0b013e318243355f. [DOI] [PubMed] [Google Scholar]
- 17.Maricevich MA, Adair MJ, Maricevich RL, et al. Aesthetic Plast Surg. 2014. Facelift Complications Related to Median and Peak Blood Pressure Evaluation. [DOI] [PubMed] [Google Scholar]
- 18.Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid Epidemic in the United States. Pain physician. 2012;15:ES9–38. [PubMed] [Google Scholar]
- 19.Substance Abuse and Mental Health Services Administration. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. (NSDUH Series H-41). HHS Publication No. (SMA) 11-4658. [Google Scholar]
- 20.Oderda G. Challenges in the Management of Acute Postsurgical Pain. Pharmacotherapy. 2012;32:6S–11S. doi: 10.1002/j.1875-9114.2012.01177.x. [DOI] [PubMed] [Google Scholar]
- 21.Dehal A, Woodward B, Johna S, et al. Bilateral Laparoscopic Totally Extraperitoneal Repair without Mesh Fixation. JSLS : Journal of the Society of Laparoendoscopic Surgeons / Society of Laparoendoscopic Surgeons. 2014;18 doi: 10.4293/JSLS.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah NS, Fullwood C, Siriwardena AK, et al. Mesh Fixation at Laparoscopic Inguinal Hernia Repair: A Meta-Analysis Comparing Tissue Glue and Tack Fixation. World journal of surgery. 2014;38:2558–2570. doi: 10.1007/s00268-014-2547-6. [DOI] [PubMed] [Google Scholar]
- 23.Newlove T, Cook J. Safety of Staged Interpolation Flaps after Mohs Micrographic Surgery in an Outpatient Setting: A Single-Center Experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.] 2013;39:1671–1682. doi: 10.1111/dsu.12338. [DOI] [PubMed] [Google Scholar]