Abstract
Regulatory T cells (Tregs) are critical regulators of peripheral immune tolerance. Treg insufficiency can lead to autoimmune disorders, including Type 1 Diabetes (T1D). Increasing evidence in mouse models of T1D, as well as other autoimmune disorders, suggest that there are defects in Treg-mediated suppression. Indeed, while Treg frequency in the peripheral blood of T1D patients is unaltered, their suppressive abilities are diminished compared to Tregs in healthy controls. Although expression of the transcription factor Foxp3 is a prerequisite for Treg development and function, there are many additional factors that can alter their stability, survival and function. Much has been learned in other model systems, such as tumors, about the mechanism and pathways that control Treg stability and function. This review poses the question: can we use these findings to develop new therapeutic approaches that might boost Treg stability, survival and/or function in T1D, and possibly other autoimmune disorders?
Introduction
T1D, also known as Juvenile Diabetes, is a chronic autoimmune disorder where a targeted immune response by both T and B cells leads to destruction of insulin producing β-cells in the islets of the pancreas (1). T1D is one of the most common chronic diseases of children. Around 70,000 children are diagnosed with T1D each year, a number that is rising by 3-5% each year in developing countries (2). Defects in the control of effector populations is a common culprit in many autoimmune disorders including T1D (3), and this may be due to dysfunctions in Treg-mediated suppression.
Tregs are either generated within the thymus, known as thymic-derived Tregs (tTregs), or in the periphery, known as peripherally-derived Tregs (pTregs), where pTreg generation requires TGFβ for their differentiation (4, 5). While pTregs have been shown to play an important role at mucosal sites and at the fetal-maternal interface (6, 7), we will be focusing on tTregs as they are the dominant regulatory population that are impacted in T1D. tTregs arise in the thymus upon high-affinity T cell receptor (TCR) signals to self-antigens and have a diverse repertoire (8, 9), suggesting that they have broad antigen specificity. tTregs are typically found in lymphoid tissues and can traffic to peripheral tissues during times of inflammation.
Tregs express the transcription factor forkhead box 3 (Foxp3), which is required for their development and function. In the absence of functional Foxp3, humans succumb to a lymphoproliferative disorder known as immunodysregulation polyendocrinopathyenteropathy X-linked syndrome (IPEX). Scurfy mice, which have a point mutation in Foxp3, develop a similar phenotype and succumb to disease early in life (10, 11). Bone marrow transplantation in IPEX patients and adoptive transfer of Foxp3+ Tregs or T cell-enriched splenocytes into Foxp3−/− or Scurfy mice restores normal immune homeostasis, supporting the necessity for Tregs in preventing autoimmune responses (12, 13). pTregs arising from CD4+Foxp3− splenocytes have also been suggested to play a role in immune homeostasis as their TCR repertoire is non-overlapping with tTregs (14, 15). Of note, splenocyte transfer may also limit the expansion of recipient diabetogenic T cells independently of any impact of tTregs and pTregs (16). Tregs can suppress immune responses by both cell-cell (CTLA-4, Granzyme B) and soluble factor- (TGFβ, IL-10, IL-35, adenosine) mediated mechanisms (17, 18). These effector functions may become deficient upon Treg instability, which may lead to the development of autoimmunity, in this case T1D.
A two-checkpoint hypothesis has been suggested in the progression of T1D from insulitis to overt diabetes where Tregs play a central role at these checkpoints based on studies performed in mice (19). During the first checkpoint, autoreactive T cells begin entering the islet but are still under Treg-mediated control and therefore insulitic. The transition from insulitis to overt diabetes occurs when Tregs lose their ability to suppress effector cell responses. Is the loss of stability in Tregs a factor in T1D progression from insulitis to overt diabetes? While many factors, including genetics and environment, contribute to the development of T1D, this review will focus on the failure of Tregs to control autoreactive T cells and how this may relate to Treg instability. This review will summarize the contributions from other models in understanding what factors are important for Treg stability (Fig. 1). Can we use what has been learned toward stabilizing Tregs in T1D?
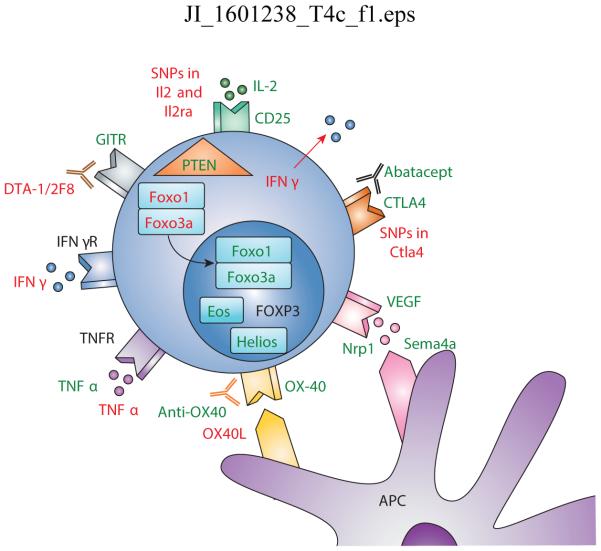
Figure 1. Mechanisms of Treg stability and instability.
IL-2 is critical for Treg stability and maintenance where polymorphisms in both Il2 and Il2ra have been seen in diabetes. Pro-inflammatory cytokines including IFNγ and TNFα may alter the Treg phenotype. Many Treg-associated molecules are important for optimal suppressive function including CTLA4, GITR, and OX-40. Interestingly, agonistic antibodies to GITR are detrimental to Treg mediated stability and suppression. Intracellular molecules including Helios, Eos, and PTEN are also key molecules in optimal Treg function. Foxo1/3a localization into the nucleus is necessary to stabilize Foxp3 in Tregs. Green: stabilizing signal; Red: destabilizing signal
Loss of Treg phenotype and function in T1D and autoimmune diabetes
While the majority of studies have reported no differences in the frequency of Tregs in peripheral blood isolated from T1D patients, defects in Treg phenotype and suppressive capacity have been reported (20-24). Unfortunately, the majority of data obtained from T1D patients is from peripheral blood, due to the feasibility of obtaining pancreas samples from T1D patients. Therefore, whether Tregs are actively playing a role in limiting β cell destruction or have an altered phenotype of function in the islets during the disease course is unknown. Thus, mouse models of TID have been employed to investigate disease progression in the islet microenvironment.
The most commonly used model for T1D is the non-obese diabetic (NOD) mouse. NOD mice spontaneously develop autoimmune diabetes starting at ~10 weeks of age in females and with increasing incidence over time until ~25 weeks (25). Both diabetes onset and progression is delayed in male NOD mice. Diabetes incidence in females and males is usually ~80% and ~30%, respectively. This may be due to differences in the gut microbiome between females and males, due to hormonal differences (26). Other environmental factors, including housing conditions and diet, can also affect the development of autoimmune diabetes (25). Genetic analyses have uncovered susceptibility loci in NOD mice that are known as the insulin-dependent diabetes (Idd) loci. Over 40 Idd loci have been identified with the major histocompatibility complex (MHC) exhibiting the highest linkage with T1D incidence, (25, 27). The NOD mouse shares many similarities to T1D in humans, but with some notable differences (25). Nevertheless, the NOD mouse has proven to be a useful model to study the role of Tregs in autoimmune diabetes.
Treg modulation studies have highlighted their importance in limiting autoimmune diabetes and controlling immune responses in the islet, despite some contradictory observations. Whereas Treg depletion using anti-CD25 (PC61) has been shown to accelerate autoimmune diabetes development in several studies (28-30), one group observed complete protection from the development of autoimmune diabetes (31), perhaps due to the depletion of activated diabetogenic CD25+ effector T cells in addition to CD25+ Tregs as a consequence of late initiation of PC61 treatment (>9weeks). However, mice that lack Tregs, due to Foxp3 deficiency rapidly develop autoimmune diabetes (32). Indeed, temporal depletion of Tregs, due to diphtheria toxin (DT) treatment of Foxp3-DTR (diphtheria toxin receptor) mice showed strong immune infiltrates in the pancreas two weeks after DT treatment (33). Of note, NOD.Foxp3-DTR mice (Foxp3 bacterial artificial chromosome (BAC) Tg, DEREG mouse model) do not develop diabetes at an accelerated rate (34). These conflicting observations with two independently generated BAC Tg NOD.Foxp3-DTR strains may be due to differences in expression and deletional efficiency and warrant further investigation. Interestingly, mice expressing the BDC2.5 TCR transgene (expressed on CD4+ T cells specific for the islet antigen chromogranin A), which are immunocompetent only develop insulitis (35). However, when the BDC2.5 TCR transgene is expressed on a Rag−/− background, in which CD4+ effector T cells develop but Tregs do not, mice succumb to diabetes rapidly. Indeed, diphtheria toxin treatment of NOD.Foxp3-DTR mice crossed to BDC2.5 Tg also rapidly develops diabetes (33). Collectively, these studies suggest that diabetes onset may be associated with decreased Treg numbers or function.
If humans and mice are not Treg deficient, why do they succumb to T1D and autoimmune diabetes, respectively? What is affecting their functionality? Interestingly, islet infiltrating Tregs in mouse models still express high levels of Foxp3, but have decreased expression of the high affinity IL-2 receptor CD25 and survival factor Bcl2 (36). Likewise in T1D patients, Tregs found in PBMCs have low expression of another Treg-associated marker GITR (37), which will be further discussed later in the review. In children with T1D, a higher proportion of Tregs produce the pro-inflammatory cytokines IL-12 and IL-18, which are also found at increase levels in serum, compared to healthy controls (23). Consequently, this altered Treg phenotype has been implicated in T1D pathogenesis. Thus, if altered Treg numbers and/or function are primary contributors to the development of T1D, boosting either parameter in vivo may provide a therapeutic opportunity.
Boosting Tregs in mice and humans
Pharmacological-based therapy
Inducing Treg proliferation via multiple pharmacological methods has been proposed and attempted in both the NOD mouse model and in clinical trials. IL-2, which is important for maintenance of Tregs, has been a potential target for Treg therapy (38) (Fig. 1). While high dose IL-2 has been used as a therapeutic approach in the treatment of melanoma and renal cancers, low dose IL-2 in NOD mice can reverse established disease by increasing Treg numbers and function (36, 39). IL-2/anti-IL-2 Ab complexes have also been used to preferentially promote Treg expansion (40). Modulation of mTOR activity with rapamycin has been shown to promote Treg expansion, survival and function (41). Although no difference in Treg number, proliferation or cytokine production was seen with rapamycin therapy prior to islet transplantation, Tregs do have increased suppressive capabilities (42). A combinational therapy has also been assessed with the use of IL-2/anti-IL-2 Ab complexes in combination with rapamycin and islet antigen peptide treatment. Treg expansion was observed and mice protected from diabetes development in both spontaneous and induced models of diabetes (43).
Non-activating, non-FcR binding CD3 antibodies may currently be the most promising treatment for T1D. More than 8 clinical trials have targeted this approach, with five of which are using teplizumab, a humanized non-FcR-binding anti-CD3 monoclonal antibody (44). C-peptide is a byproduct of insulin production and is produced at equimolar concentrations and thus can be used to determine the amount of insulin produced by β-cells. Short-term treatment of younger individuals and recent onset patients with teplizumab has shown promising results in four-year follow-up studies, based on C-peptide levels, with limited toxicity (45-48). While its mechanism of action is currently unclear, a two-fold tolerance induction has been suggested through depletion of pathogenic T cells and preservation of Tregs and their function (49, 50). Although the mechanisms of action of all these therapeutic approaches is different, in all cases the common denominator is increased Treg number and function.
Cell-based therapy
As Treg insufficiency may be a key driver of T1D and autoimmune diabetes, increasing the number of Tregs in circulation may overcome this deficiency. Repeated Treg adoptive transfer into neonatal NOD mice can delay the onset of autoimmune diabetes (51), suggesting that Treg number or functionality may be deficient in NOD mice over time thereby requiring supplementation. Adoptive transfer of pre-diabetic NOD splenocytes or BDC2.5 TCR Tg Teff cells into immunodeficient NOD mice develop autoimmune diabetes ~14 days post-transfer. Interestingly, disease can be prevented following co-transfer with >106 polyclonal Tregs or as few as 5×104 BDC2.5 TCR Tg Tregs (34). Adoptive transfer of a low number of DC-expanded BDC2.5 TCR Tg Tregs into pre-diabetic NOD mice also blocks diabetes development and can rescue mice with overt diabetes (52). While low numbers of antigen-specific Tregs are able to reverse autoimmune diabetes, adoptive transfer of ten-fold more polyclonal Tregs is not as effective in treating NOD mice therapeutically (53), suggesting that specificity for β cell antigens is critically important for optimal Treg functionality.
In vitro-expanded polyclonal Tregs are currently in clinical trials as a promising alternative to pharmacological-based therapies. Phase 1 clinical trials have been performed in both children and adults with no safety concerns thus far (54-56). Interestingly, some potential efficacy has been observed in children at 4-5 week follow-up based on C-peptide levels. However, while C-peptide levels were increased initially at one- and two-year follow-ups, they declined over time. Approximately 25% of the transferred Tregs with a naïve/memory-like phenotype were still present in patients at one-year follow-up based on deuterium incorporation. A similar trial has also been conducted in Poland with promising results. At a one-year follow-up of 12 children with T1D, increased C-peptide levels and diminished use of insulin was observed in 8 of 12 patients and, remarkably, complete insulin independence was achieved in 2 of 12 patients (55). Whether these observations are durable and can be replicated in Phase 2 clinical trials remains to be determined.
While these initial observations are encouraging, the key challenge is likely to focus on understanding what the primary limitations are for successful, durable responses and can these be overcome with [i] increased Treg numbers, [ii] islet antigen specificity, and/or [iii] approaches that increased stability, survival, functionality and longevity. There is a growing consensus that future clinical trails need to focus on the development of Tregs with β cell antigen-specificity to maximize [i] islet homing and therapeutic index, and [ii] retention of Tregs over time to endure a durable response. Also, is the adoptive transfer of more Tregs the only viable therapeutic approach or could the Tregs that are already present in the patient be ‘reinvigorated’? Clearly, gaining a greater understanding of the mechanisms and factors that control Treg stability and function will greatly inform future clinical development.
Loss of Treg stability and function
What is Treg stability, how does this differ from plasticity, and what drives instability? Treg plasticity and stability have been used interchangeably in the past, but represent two distinct Treg fates. A stable Treg expresses the transcription factor Foxp3, is suppressive, produces anti-inflammatory cytokines (such as IL-10 and IL-35), and a minimal amount of effector cytokines (eg. IFNγ, TNFα, IL-2) (57). When Tregs exhibit plasticity, they still express Foxp3 and remain functionally suppressive but gain distinct migratory and functional programs that can enhance their capacity to suppress certain Th subsets (58). In contrast, destabilized Tregs lose their suppressive abilities and gained effector functions, while either retaining Foxp3 expression (59) and eventually losing Foxp3 expression and becoming pathogenic “ex-Tregs” in inflammatory environments (60). In both the NOD mice and humans with T1D, Tregs are identified based on Foxp3 expression, yet exhibit defective suppressive activity suggesting that they may be destabilized. Further analysis of Treg stability has recently been extended to include epigenetic modifications by assessing the methylation pattern of the conserved noncoding sequence 2 (CNS2 or TSDR) in the 5’ UTR of the Foxp3 gene, where tTregs are demethylated at this locus and loss of stability has been associated with re-methylation at this locus (61). Foxp3 CNS2 hypomethylation appears to be important for the binding of key transcription factors including NF-kB, CREB/ATF, Ets1 and STAT5 (62-64). Methylation studies have expanded to other Treg associated genes Il2ra (CD25), Ikzf4 (Eos), Ctla4 (cytotoxic T-lymphocyte-associated protein 4), and Tnfrsf18 (GITR), which are also hypomethylated (65).
In addition to epigenetic modifications of target genes, other mechanisms including microRNAs (miRNAs) may also modulate disease development. These short non-coding RNAs are transcribed and processed via the RNAses Drosha and Dicer to generate mature miRNAs that silence genes either through repressing translation or accelerating transcript degradation (66). Mice with a Treg-restricted deletion of Dicer or Drosha possess unstable Tregs with poor suppressive ability, diminished expression of Treg-associated molecules, increased effector cytokines, and succumb to a Scurfy-like disease (67-69). Similar results have also been seen upon gene silencing of miR-126 in a breast cancer tumor model leading to increased anti-tumor immunity by altering activation of the PI3K/AKT pathway (70). While miR-155 is not necessary for Treg homeostasis or its suppressive function, its role in downregulating SOCS1, which increases responsiveness of STAT5, can make Tregs better responders to IL-2, even under suboptimal conditions (71). Treg-specific ablation of the miR-17-92 cluster results in exacerbated EAE with decreased IL-10 producing Tregs (72), but is not required for thymic generation of Tregs (73). miR-10a is selectively expressed in Tregs and expression has been correlated to autoimmune disease susceptibility as the autoimmune-resistant C57BL/6 strain expresses high levels of miR-10a while the autoimmune-susceptible NOD strain expresses lower levels (74). These studies suggest that certain miRNAs may be important in maintaining Treg stability and function. Indeed, miR-342, miR-191, and miR-510 are differentially expressed in Tregs of patients with T1D, but whether these are biomarkers or contribute to disease still needs to be further elucidated (75).
Understanding the factors and pathways that control Treg stability would clearly facilitate their therapeutic utilization in T1D, as well as other autoimmune and inflammatory diseases, and potentially in transplantation. While Foxp3 is the master transcription factor that is required for Treg development and functionality, a variety of external signals from cytokines and surface receptors, via intracellular signaling molecules impinge on Tregs and impact their stability.
Factors that impact Treg stability
Cytokines
Several cytokines have a substantive impact on Treg development and function (Fig. 1). IL-2, produced by effector T cells, is necessary for the maintenance and function of Tregs, as they do not make their own autocrine IL-2 (76-78). The majority of Tregs express the high affinity IL-2 receptor (Il2ra, CD25) that signals via STAT5 (79). Genetically manipulated mice deficient in Il2 or Il2ra phenocopy Foxp3-deficient or Treg-ablated mice, yet still harbor T cells that express diminished levels of Foxp3 (80, 81). Humans with CD25 deficiency also have many of the same symptoms as seen in patients with IPEX (82). IL-2 reverses anergic, non-proliferative phenotype of Tregs in vitro and promotes their capacity to suppress immune responses (83). IL-2 withdrawal has been shown in vitro to limit Treg suppressive ability (84). Under sub-optimal IL-2 conditions, the CNS2 element sustains Foxp3 expression, while in its absence, actively proliferating Tregs lose Foxp3 expression at an accelerated rate (85). Genome wide association studies have identified IL-2 pathway polymorphisms in both T1D (Il2ra) and autoimmune diabetes (Il2) (86-88). Indeed, reduced IL-2 signaling, via pSTAT5 analysis, has been documented in T1D patients with diminished Treg suppressive capabilities (89, 90). In NOD mice, Tregs have decreased Bcl2 and CD25 expression only in inflamed islets. This may be due to decreased levels of IL-2 in the islet as low-dose IL-2 treatment increases Treg survival and protection (36). These studies highlight the importance of IL-2 in Treg function and possible defects that might lead to the development of T1D.
Inflammatory environments have been shown to destabilize Tregs in many models due to their interaction with or production of pro-inflammatory cytokines. While several cytokines may destabilize Tregs, we will focus here on those that may be relevant to T1D. IFNγ is highly expressed in many inflammatory conditions and may limit Treg function. Upon stimulation with IFNγ in vitro, Tregs downregulate CD25, lose Foxp3 expression, and exhibit limited expansion (91). Under high salt conditions, Tregs can begin to produce IFNγ and lose suppressive activity, which can be restored upon antibody blockade of IFNγ (92). Whether this Treg-derived IFNγ acts on Tregs or Teffs still needs to be further elucidated (92). In T1D patients, increases in IFNγ+Foxp3+ Tregs has been observed in peripheral blood. These cells are predominantly hypermethylated at the CNS2 locus but still exhibit suppressive function (93).
Tregs constitutively express TNFRII, which upon signaling leads to diminished Foxp3 mRNA and protein levels, and reduced suppressive actvity. Not surprisingly, patients with active rheumatoid arthritis (RA) possess Tregs that express lower Foxp3 expression and suppressive ability, and this could be reversed with anti-TNF (infliximab) treatment (94). In contrast, others have shown the requirement for TNF signaling in the generation of functional Tregs within the thymus and their function in inflammatory settings. In colitis models, expression of TNFRII expression is critical for Treg function (95, 96). Likewise, in NOD mice, TNF receptor deficiency protects mice from autoimmune diabetes and increases Treg mediated suppression (97).
The role of IL-27 in Treg stability has been quite conflicting. IL-27 has been shown to antagonize pTreg generation (98), but has been shown to enhance tTreg function in a T-cell mediated colitis model via a Lag3-mediated mechanism (99). In a tumor model, IL-27Rα-deficient mice have decreased Tregs in the tumor microenvironment suggesting IL-27 may act indirectly on Tregs via suppressing IL-2 generation by effector T cells (100). Nevertheless, the role of IL-27 specifically on Tregs has yet to be clarified. Increased IL-27 has been documented in autoimmune diabetes and blockade of IL-27 in NOD mice delays the onset of autoimmune diabetes (101).
Extensive studies still need to be performed to assess whether these cytokines directly impact Tregs before conclusions can be drawn regarding their role in modulating Treg function in T1D and autoimmune diabetes.
Surface molecules
Several cell surface molecules have been shown to impact Treg stability and function (Fig. 1). OX40 (Tnfrsf4, CD134) is part of the tumor necrosis factor superfamily receptors (TNFRs) and is expressed on Tregs (102), yet its role in Treg-mediated suppression has led to conflicting results both in vitro (103-105) and in vivo. OX40 expression on Tregs may play a role in suppressing inflammatory responses in vivo as mice with a Treg-restricted deficiency in OX40 retain Foxp3 expression yet develop gut inflammation in a T cell-mediated gut inflammation model (106). Indeed, use of an agonist anti-OX40 (OX86) protects NOD mice from the development of autoimmune diabetes (107). However, disease is inhibited in Ox40l−/− mice and neutralizing anti-OX40L-treated NOD mice (108). Whether Tregs are the primary subset responding to OX40L has not been fully addressed as CD4+ and CD8+ T cells also express OX40 during autoimmune diabetes progression (109).
GITR (Tnfrsf18, CD357) is another TNFR family member that is found at high levels on the surface of Tregs (102). Paradoxically, use of an agonist anti-GITR (DTA-1) undermines Treg-mediated suppression and tolerance in tumor models. Both a decrease in Treg frequency and expression of Foxp3 in intratumoral Tregs has been seen (110). This loss of Foxp3, and Helios, expression is mediated by the c-Jun N-terminal kinase (JNK) pathway. Treatment of lung allergy mice with a JNK inhibitor led to reversal of GITR-induced changes in phenotype and function; and therefore was rescued from disease (111). Indeed, accelerated development of autoimmune diabetes has also been seen using a different agonistic anti-GITR antibody (2F8) (112), suggesting that activation of this pathway may be detrimental to Treg stability.
Cytotoxic T lymphocyte antigen 4 (Ctla4, CD152) is highly expressed on Tregs and has extensively been studied as an inhibitory molecule important for T cell homeostasis and tolerance (113). Ctla4−/− mice succumb to fatal lymphoproliferative disease (114), while Treg numbers are increased (115, 116). Results from in vivo models of autoimmunity have been quite conflicting, where Ctla4−/− Tregs are suppressive in some instances but not in others (115, 117). Ctla4 is a susceptibility gene in autoimmune diseases, including T1D, where many polymorphisms have been identified (118-120). Costimulation blockade using anti-CTLA4 (Abatacept) has recently been shown in phase II clinical trials to delay the progression of T1D (121), but whether Tregs are playing a direct role still needs to be assessed further.
Neuropilin-1 (Nrp1) is an important factor in axonal guidance during embryonic development, but its role in the immune system has only recently been appreciated. Nrp1 is highly expressed on tTregs but is expressed at lower levels in pTregs (122-124). A role for Nrp1 in promoting the stability, survival and function of Tregs has been suggested (125). Nrp1 on Tregs has been shown to interact with both Semaphorin-4a (Sema4a) and VEGF. Mice with a Treg-specific Nrp1 deletion had substantially reduced tumor growth in multiple models suggesting that Treg-mediated suppression of anti-tumor immunity has been lost (125, 126). Interestingly, these mice did not succumb to autoimmunity and inflammatory disease and the frequency of Foxp3+ Tregs was not altered (125). Stabilization via the Nrp1:Sema4a pathway enhances expression of the survival factor Bcl2, effector molecules IL-10 and CD73, limits expression of lineage-associated transcription factors, including T-bet, IRF4 and RORγt, and the pro-inflammatory cytokine IFNγ (125). Boosting Treg function by engaging the Nrp1:Sema4a pathway may be a possible therapeutic approach to stabilize Tregs in vivo or prior to adoptive transfer.
Intracellular signaling molecules
There are also several intracellular proteins that appear to modulate Treg stability and function by dependently or indirectly modulating Foxp3 function or stability (Fig. 1). Eos (Ikzf4), a zinc-finger transcription factor, is a member of the Ikaros family of transcription factors and is highly expressed in Tregs. Eos interacts directly with Foxp3 and is necessary for gene silencing (Il2, Ifng, etc.) while maintaining expression of key Treg-associated genes including Ctla4 and GITR (127). Silencing of Eos using siRNA does not result in loss of Foxp3 expression but does result in the loss of Treg suppression in a T cell-mediated colitis model and induction of effector cytokines, such as IFNγ and IL-2 (127). Downregulation of Eos expression is required for the reprograming of Tregs into helper-like cells that retain Foxp3 expression (128). These Eos−Foxp3+ Tregs (Eos-liable) exhibit reduced regulatory function and enhanced expression of CD40L, IL-2 and IL-17 (128). Of note, global deletion of Eos in mice does not affect the function or phenotype of Tregs in vivo and in vitro, but does result in the development of more severe EAE. This observation was attributed to the function of Eos in Teff populations (129).
Helios (Ikzf2), another member of the Ikaros transcription factor family, was once thought to distinguish tTregs from pTregs, however, it now appears that Helios expression is highly dependent on antigen stimulation via the TCR (130, 131). While Helios does not form a complex with Foxp3 or bind to the Foxp3 locus, Helios plays an indirect role in supporting Treg stability (132, 133). In mice with a Treg-specific Helios deficiency develop autoimmunity development and appear to possess unstable Tregs with diminished Foxp3 expression, increased effector cytokine expression and reduced suppressive activity (133). ChIP-Seq and pathway analysis of Helios targeting genes in Tregs highlighted deficiencies in the IL-2Ra/STAT5b pathway suggesting that Helios may be important in regulating IL-2 signaling and Treg survival (133).
Foxo1 and Foxo3, which are also forkhead box transcription factors, pay a key role in maintaining Foxp3 expression in Tregs (134). Mice deficient in Foxo1 in Tregs succumb to a Scurfy-like phenotype by 5 weeks. This lymphoproliferative disease is not due to the loss of Treg number but rather their loss of function (135, 136). This phenotype can be rescued by expression of Foxo1AAA, where Foxo1 is insensitive to 14-3-3-mediated cytosolic restriction and is this confined to the nucleus where it can facilitate Foxp3 function. Autoimmunity is further exacerbated by dual deletion of Foxo1 and Foxo3 (136). Foxo1/3 binds directly to the Foxp3 locus and controls promoter activity (136, 137).
The phosphatase, PTEN, has recently been shown to paly a pivotal role in mediating Treg stability. PTEN is an upstream inhibitor of the PI(3)K-Akt pathway and therefore inhibits mechanistic target of rapamycin complex (mTORC)1 and mTORC2 activity (138). Upon genetic deletion of PTEN in Tregs, mice have increased levels of auto-antibodies, renal pathology and ongoing age-related autoimmunity. Nevertheless, Tregs are found in high numbers and readily proliferate compared to PTEN-sufficient Tregs. These Tregs are highly activated and express higher levels of ICOS, PD-1 and IFNγ, decreased levels of CD25, and have a higher proportion of “ex-Tregs” based on the use of lineage-tracing experiments (61, 139). The mechanism of Treg-mediated loss of suppression is via upregulation of mTORC2 activity upon PTEN loss (139). Indeed, inhibition of mTOR in Tregs leads to heightened stability of Foxp3 expression (140) and Treg specific loss of mTOR inhibitor tuberous sclerosis 1 (TSC1) results in loss of Foxp3 expression, suppressive functionality, and increased expression of IL-17 (141). Interestingly, Nrp1, which as discussed above promotes Treg stability and function, has been shown to signal via PTEN that in turn limits Akt activity, reduces Foxo phosphorylation and thus nuclear exclusion, thereby promoting Foxp3 activity (125). Taken together, these observations provide a potential causal link between Nrp1, PTEN and Foxo in mediating Treg stability and function.
Conclusions
In summary, many factors impinge on Tregs to either promote or undermine their stability, survival and function (Fig. 1). Some of these pathways are inherent, while others are induced or selectively utilized in inflammatory environments (59). We postulate that a primary driver of autoimmunity may be Treg insufficiency caused by a failure to promote pathways that enforce their stability survival and function. In tumors, where Treg activity is arguably at its most robust, Treg stability is enforced by an Nrp1:PTEN:Foxo axis, and potentially other mechanisms, to prevent effective anti-tumor immunity. This also appears to protect Tregs from destabilizing forces that may be quite severe given the hostile intratumoral microenvironment, which is hypoxic, acidic, and nutrient and glucose starved. Thus, under normal circumstances Tregs seem to be well adapted to respond to cues from diverse microenvironments to maintain Treg stability and function. However, we posit that genetic, environmental or contextual factors conspire to undermine these programs that ultimately leads to Treg insufficiency and autoimmunity.
This hypothesis and the information outlined above raise several key questions. (1) Can we boost Tregs that are already present but appear to exhibit insufficiency? This could be achieved by developing therapeutics that promote utilization of the Nrp1:PTEN:Foxo axis. For example, Sema4a-Ig fusion proteins may act as Nrp1 agonists thereby promoting Treg stability and function. Alternatively, intracellular delivery of therapies that promote Foxo stability and nuclear translocation may produce a similar Treg stabilizing effect. (2) Can we inhibit pathways that lead to instability? While we need to gain a greater understanding of the factors that promote Treg instability, approaches that limit the factors that are known to drive these processes may be beneficial. The use of blocking antibodies against cytokines that can destabilize Tregs may be useful in a manner analogous to TNFα blockade in RA. We could also develop antibodies to block OX40L from interacting with OX-40 on Tregs. (3) Is a combinatorial therapy possible and necessary? Given that there may be a two-fold defect in Treg number and function in T1D, combinatorial therapy may be most useful. One could combine Treg adoptive transfer with approaches that promote Treg stability, prior to and/or following transfer. Of course, these approaches may also be combined with current therapies that are in clinical trials for T1D, such as teplizumab (non-FcR binding anti-CD3). Indeed, one might argue that as combinatorial approaches are the mainstay of effective cancer therapy it is likely that combinatorial approaches will be required for the treatment of T1D, with perhaps the inclusion of therapies that promote Tregs stability and function.
Acknowledgments
This work was supported by the National Institutes of Health (R01 DK089125 and P01 AI108545 to D.A.A.V; T32 AI089443 to A.V.).
References
- 1.Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nature reviews. Immunology. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burn P. Type 1 diabetes. Nat Rev Drug Discov. 2010;9:187–188. doi: 10.1038/nrd3097. [DOI] [PubMed] [Google Scholar]
- 3.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 6.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 11.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyk-Pearson SK, Bakke AC, Held PK, Wildin RS. Rescue of the autoimmune scurfy mouse by partial bone marrow transplantation or by injection with T-enriched splenocytes. Clin Exp Immunol. 2003;133:193–199. doi: 10.1046/j.1365-2249.2003.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang X, Zheng P, Liu Y. Homeostatic proliferation in the mice with germline FoxP3 mutation and its contribution to fatal autoimmunity. J Immunol. 2008;181:2399–2406. doi: 10.4049/jimmunol.181.4.2399. [DOI] [PubMed] [Google Scholar]
- 17.Collison LW, Vignali DA. In vitro Treg suppression assays. Methods Mol Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 21.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 22.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 23.Ryba-Stanislawowska M, Rybarczyk-Kapturska K, Mysliwiec M, Mysliwska J. Elevated levels of serum IL-12 and IL-18 are associated with lower frequencies of CD4(+)CD25 (high)FOXP3 (+) regulatory t cells in young patients with type 1 diabetes. Inflammation. 2014;37:1513–1520. doi: 10.1007/s10753-014-9878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haseda F, Imagawa A, Murase-Mishiba Y, Terasaki J, Hanafusa T. CD4(+) CD45RA(−) FoxP3high activated regulatory T cells are functionally impaired and related to residual insulin-secreting capacity in patients with type 1 diabetes. Clin Exp Immunol. 2013;173:207–216. doi: 10.1111/cei.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 26.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 27.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 28.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billiard F, Lobry C, Darrasse-Jeze G, Waite J, Liu X, Mouquet H, DaNave A, Tait M, Idoyaga J, Leboeuf M, Kyratsous CA, Burton J, Kalter J, Klinakis A, Zhang W, Thurston G, Merad M, Steinman RM, Murphy AJ, Yancopoulos GD, Aifantis I, Skokos D. Dll4-Notch signaling in Flt3-independent dendritic cell development and autoimmunity in mice. J Exp Med. 2012;209:1011–1028. doi: 10.1084/jem.20111615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, Gondorf F, Hoerauf A, Killoran KE, Stocker JT, Davies SJ, Tarbell KV, Mitre E. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-beta. J Immunol. 2012;188:559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. The Journal of experimental medicine. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petzold C, Riewaldt J, Watts D, Sparwasser T, Schallenberg S, Kretschmer K. Foxp3(+) regulatory T cells in mouse models of type 1 diabetes. J Diabetes Res. 2013;2013:940710. doi: 10.1155/2013/940710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol. 2001;2:1117–1125. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xufre C, Costa M, Roura-Mir C, Codina-Busqueta E, Usero L, Pizarro E, Obiols G, Jaraquemada D, Marti M. Low frequency of GITR+ T cells in ex vivo and in vitro expanded Treg cells from type 1 diabetic patients. Int Immunol. 2013;25:563–574. doi: 10.1093/intimm/dxt020. [DOI] [PubMed] [Google Scholar]
- 38.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 41.Chapman NM, Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy. 2014;6:1295–1311. doi: 10.2217/imt.14.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manirarora JN, Wei CH. Combination Therapy Using IL-2/IL-2 Monoclonal Antibody Complexes, Rapamycin, and Islet Autoantigen Peptides Increases Regulatory T Cell Frequency and Protects against Spontaneous and Induced Type 1 Diabetes in Nonobese Diabetic Mice. J Immunol. 2015;195:5203–5214. doi: 10.4049/jimmunol.1402540. [DOI] [PubMed] [Google Scholar]
- 44.Skyler JS. The compelling case for anti-CD3 in type 1 diabetes. Diabetes. 2013;62:3656–3657. doi: 10.2337/db13-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C, Candon S, Waldmann H, Ziegler AG, Chatenoud L, Pipeleers D. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 46.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 47.Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 49.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–2022. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol. 2013;149:268–278. doi: 10.1016/j.clim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, Owczuk R, Szadkowska A, Witkowski P, Mlynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153:23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 59.Overacre AE, Vignali DA. Treg stability: to be or not to be. Curr Opin Immunol. 2016;39:39–43. doi: 10.1016/j.coi.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature immunology. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mouly E, Chemin K, Nguyen HV, Chopin M, Mesnard L, Leite-de-Moraes M, Burlen-defranoux O, Bandeira A, Bories JC. The Ets-1 transcription factor controls the development and function of natural regulatory T cells. J Exp Med. 2010;207:2113–2125. doi: 10.1084/jem.20092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeker LT, Zhou X, Blelloch R, Bluestone JA. DGCR8-mediated production of canonical microRNAs is critical for regulatory T cell function and stability. PLoS One. 2013;8:e66282. doi: 10.1371/journal.pone.0066282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin A, Wen Z, Zhou Y, Li Y, Li Y, Luo J, Ren T, Xu L. MicroRNA-126 regulates the induction and function of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J Cell Mol Med. 2013;17:252–264. doi: 10.1111/jcmm.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Kouchkovsky D, Esensten JH, Rosenthal WL, Morar MM, Bluestone JA, Jeker LT. microRNA-17-92 regulates IL-10 production by regulatory T cells and control of experimental autoimmune encephalomyelitis. J Immunol. 2013;191:1594–1605. doi: 10.4049/jimmunol.1203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, Wan Y, He L, Li QJ. Molecular dissection of the miR-17-92 cluster's critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118:5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, Lund AH, Bluestone JA. MicroRNA 10a marks regulatory T cells. PLoS One. 2012;7:e36684. doi: 10.1371/journal.pone.0036684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hezova R, Slaby O, Faltejskova P, Mikulkova Z, Buresova I, Raja KR, Hodek J, Ovesna J, Michalek J. microRNA-342, microRNA-191 and microRNA-510 are differentially expressed in T regulatory cells of type 1 diabetic patients. Cell Immunol. 2010;260:70–74. doi: 10.1016/j.cellimm.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 76.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 78.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 80.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 81.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 84.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 85.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Wicker LS, Santamaria P. IL-2 and its high-affinity receptor: genetic control of immunoregulation and autoimmunity. Semin Immunol. 2009;21:363–371. doi: 10.1016/j.smim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, Jones R, Ring SM, McArdle W, Pembrey ME, Strachan DP, Dunger DB, Twells RC, Clayton DG, Todd JA. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang ZY, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang JH, Cutler AJ, Ferreira RC, Reading JL, Cooper NJ, Wallace C, Clarke P, Smyth DJ, Boyce CS, Gao GJ, Todd JA, Wicker LS, Tree TI. Natural Variation in Interleukin-2 Sensitivity Influences Regulatory T-Cell Frequency and Function in Individuals With Long-standing Type 1 Diabetes. Diabetes. 2015;64:3891–3902. doi: 10.2337/db15-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.St Rose MC, Taylor RA, Bandyopadhyay S, Qui HZ, Hagymasi AT, Vella AT, Adler AJ. CD134/CD137 dual costimulation-elicited IFN-gamma maximizes effector T-cell function but limits Treg expansion. Immunol Cell Biol. 2013;91:173–183. doi: 10.1038/icb.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, Hafler DA. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186:6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 96.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190:1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chee J, Angstetra E, Mariana L, Graham KL, Carrington EM, Bluethmann H, Santamaria P, Allison J, Kay TW, Krishnamurthy B, Thomas HE. TNF receptor 1 deficiency increases regulatory T cell function in nonobese diabetic mice. J Immunol. 2011;187:1702–1712. doi: 10.4049/jimmunol.1100511. [DOI] [PubMed] [Google Scholar]
- 98.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 99.Do JS, Visperas A, Sanogo YO, Bechtel JJ, Dvorina N, Kim S, Jang E, Stohlman SA, Shen B, Fairchild RL, Baldwin Iii WM, Vignali DA, Min B. An IL-27/Lag3 axis enhances Foxp3(+) regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol. 2016;9:137–145. doi: 10.1038/mi.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li MS, Liu Z, Liu JQ, Zhu X, Liu Z, Bai XF. The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy. 2015;7:191–200. doi: 10.2217/imt.14.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang R, Han G, Wang J, Chen G, Xu R, Wang L, Li X, Shen B, Li Y. The pathogenic role of interleukin-27 in autoimmune diabetes. Cell Mol Life Sci. 2008;65:3851–3860. doi: 10.1007/s00018-008-8540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 103.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 104.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 105.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bresson D, Fousteri G, Manenkova Y, Croft M, von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. Journal of autoimmunity. 2011;37:342–351. doi: 10.1016/j.jaut.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nature reviews. Immunology. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. European journal of immunology. 2004;34:3039–3046. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 110.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, Merghoub T, Houghton AN, Wolchok JD. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joetham A, Ohnishi H, Okamoto M, Takeda K, Schedel M, Domenico J, Dakhama A, Gelfand EW. Loss of T regulatory cell suppression following signaling through glucocorticoid-induced tumor necrosis receptor (GITR) is dependent on c-Jun N-terminal kinase activation. J Biol Chem. 2012;287:17100–17108. doi: 10.1074/jbc.M111.316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.You S, Poulton L, Cobbold S, Liu CP, Rosenzweig M, Ringler D, Lee WH, Segovia B, Bach JF, Waldmann H, Chatenoud L. Key role of the GITR/GITRLigand pathway in the development of murine autoimmune diabetes: a potential therapeutic target. PloS one. 2009;4:e7848. doi: 10.1371/journal.pone.0007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 114.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 115.Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, Abbas AK, Sharpe AH, Sansom DM, Walker LS. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 116.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 119.Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143–155. doi: 10.1111/j.1600-065X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 120.Qu HQ, Bradfield JP, Grant SF, Hakonarson H, Polychronakos C, I. D. G. C. Type Remapping the type I diabetes association of the CTLA4 locus. Genes Immun. 2009;10(Suppl 1):S27–32. doi: 10.1038/gene.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS, G. Type 1 Diabetes TrialNet Abatacept Study Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 123.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. S1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. S1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, Helfrich I. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, Drake CG, Liu JO, Ostrowski MC, Pardoll DM. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi H, Munn DH. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rieder SA, Metidji A, Glass DD, Thornton AM, Ikeda T, Morgan BA, Shevach EM. Eos Is Redundant for Regulatory T Cell Function but Plays an Important Role in IL-2 and Th17 Production by CD4+ Conventional T Cells. J Immunol. 2015;195:553–563. doi: 10.4049/jimmunol.1500627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 132.Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR, Rudensky AY. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13:1010–1019. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207:1347–1350. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 138.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Raju J, Leveque L, Markey KA, Varelias A, Clouston AD, Lane SW, MacDonald KP, Hill GR. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J Immunol. 2013;191:5291–5303. doi: 10.4049/jimmunol.1301181. [DOI] [PubMed] [Google Scholar]
- 141.Park Y, Jin HS, Lopez J, Elly C, Kim G, Murai M, Kronenberg M, Liu YC. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]