Abstract
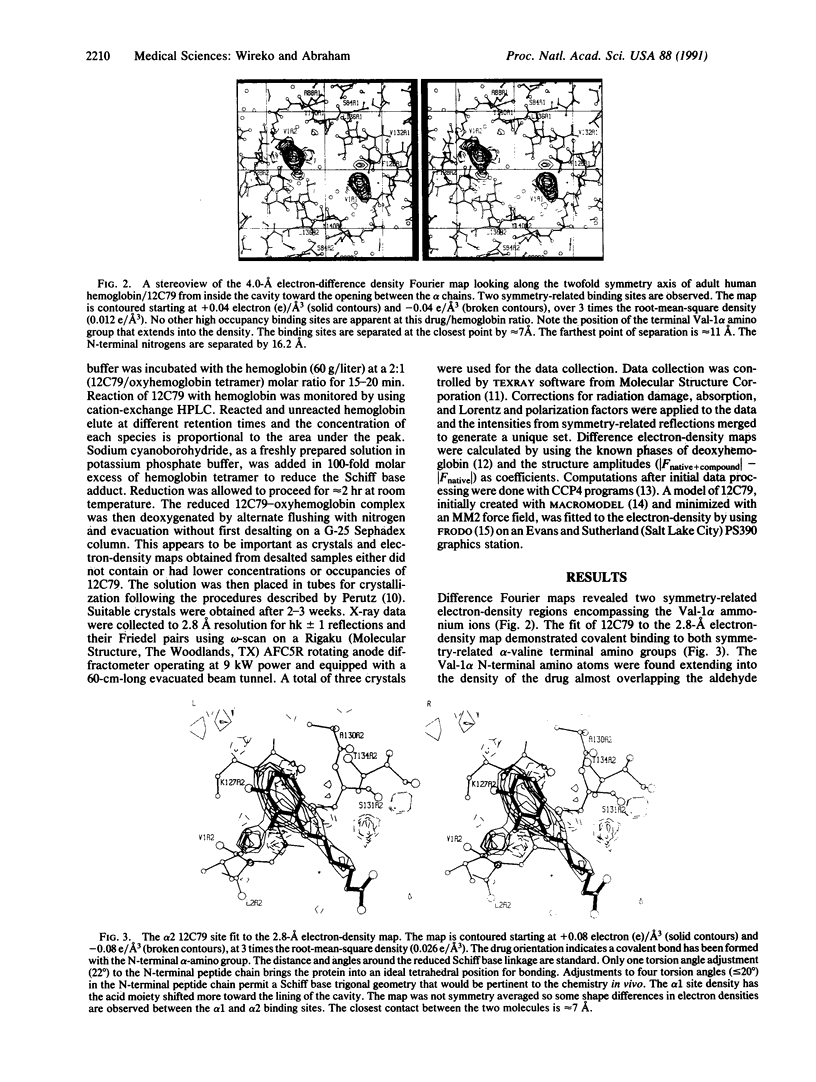
The hemoglobin binding site of the antisickling agent 12C79 has been determined by x-ray crystallography. 12C79 is recognized as one of the first molecules to reach clinical trials that was designed, de novo, from x-ray-determined atomic coordinates of a protein. Several previous attempts to verify the proposed Hb binding sites via crystallographic studies have failed. Using revised experimental procedures, we obtained 12C79-deoxyhemoglobin crystals grown after reaction with oxyhemoglobin and cyanoborohydride reduction to stabilize the Schiff base linkage. The difference electron-density Fourier maps show that two 12C79 molecules bind covalently to both symmetry-related N-terminal amino groups of the hemoglobin alpha chains. This is in contrast to the original design that proposed the binding of one drug molecule that spans the molecular dyad to interact with both N-terminal alpha-amino groups.
Full text
PDF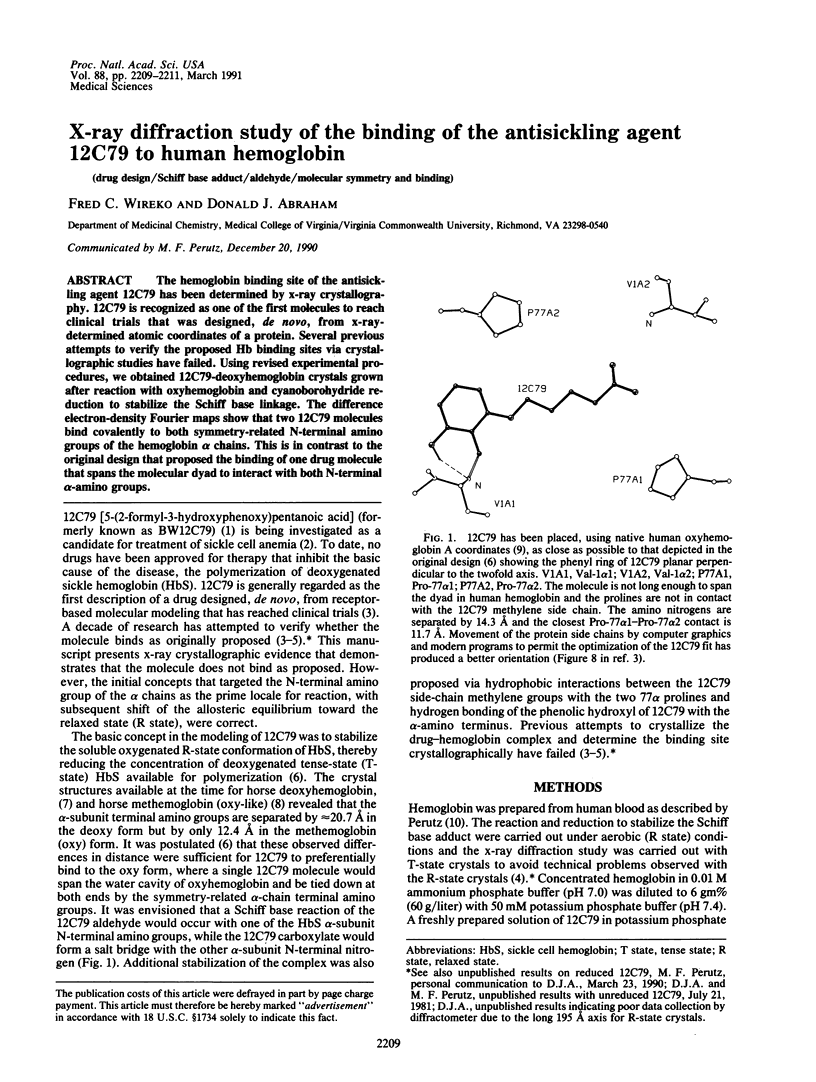

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A., Perutz M. F. Structure of inositol hexaphosphate--human deoxyhaemoglobin complex. Nature. 1974 May 3;249(452):34–36. doi: 10.1038/249034a0. [DOI] [PubMed] [Google Scholar]
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Kneen G., White R. D., Wilkinson S., Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984 Jun;82(2):397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Walder R. Y., Arnone A., Walder J. A. Mechanism for the increase in solubility of deoxyhemoglobin S due to cross-linking the beta chains between lysine-82 beta 1 and lysine-82 beta 2. Biochemistry. 1982 Nov 9;21(23):5901–5909. doi: 10.1021/bi00266a027. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Welty E. V., Walder R. Y., Pruitt S. L., Rogers P. H., Arnone A., Walder J. A. Isolation and characterization of a new hemoglobin derivative cross-linked between the alpha chains (lysine 99 alpha 1----lysine 99 alpha 2). J Biol Chem. 1986 Jul 25;261(21):9929–9937. [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Lalezari I., Lalezari P., Poyart C., Marden M., Kister J., Bohn B., Fermi G., Perutz M. F. New effectors of human hemoglobin: structure and function. Biochemistry. 1990 Feb 13;29(6):1515–1523. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- Lalezari I., Rahbar S., Lalezari P., Fermi G., Perutz M. F. LR16, a compound with potent effects on the oxygen affinity of hemoglobin, on blood cholesterol, and on low density lipoprotein. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6117–6121. doi: 10.1073/pnas.85.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna A. S., Abraham D. J. Comparison of crystal and solution hemoglobin binding of selected antigelling agents and allosteric modifiers. Biochemistry. 1990 Apr 24;29(16):3944–3952. doi: 10.1021/bi00468a022. [DOI] [PubMed] [Google Scholar]
- Merrett M., Stammers D. K., White R. D., Wootton R., Kneen G. Characterization of the binding of the anti-sickling compound, BW12C, to haemoglobin. Biochem J. 1986 Oct 15;239(2):387–392. doi: 10.1042/bj2390387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Walder R. Y., Arnone A. Development of antisickling compounds that chemically modify hemoglobin S specifically within the 2,3-diphosphoglycerate binding site. J Mol Biol. 1980 Aug 5;141(2):195–216. doi: 10.1016/0022-2836(80)90385-x. [DOI] [PubMed] [Google Scholar]
- Wireko F. C., Kellogg G. E., Abraham D. J. Allosteric modifiers of hemoglobin. 2. Crystallographically determined binding sites and hydrophobic binding/interaction analysis of novel hemoglobin oxygen effectors. J Med Chem. 1991 Feb;34(2):758–767. doi: 10.1021/jm00106a042. [DOI] [PubMed] [Google Scholar]




