Abstract
The beneficial microbial-plant interaction plays important role in the soil health, crop growth and productivity. Plant growth promoting rhizobacteria (PGPR) are such beneficial microorganisms, which in association with plant roots not only promote their growth but also help in counteracting the detrimental effects of soil stresses. Salt stress is one such stress, frequently confronted by the plants. The present study aimed at isolation and identification of PGPR inhabiting the mungbean rhizosphere, testing them for salt (NaCl) tolerance and subsequently in salt-supplemented mungbean crop. For this purpose, two salt-tolerant bacterial strains belonging to genus Pantoea and Enterococcus, characterized for their P-solubilization ability, indole acetic acid and siderophore production were selected. These two PGPR were further evaluated for their effect on the salt-stressed mungbean plants, grown at two salt concentrations (5 and 10 dS/m). The plants treated with the combination of PGPR showed better performance in growth (16–37 %) and yield (22–32 %), under salt stress, as compared with control. The increasing salt concentration was found to increase the membrane damage, Na+ concentration in the plants. PGPR treatments effectively reduced the Na+ concentration (17–41 %), membrane damage (1.1–1.5 folds) and enhanced the antioxidants i.e. ascorbic acid (8–26 %) and glutathione (10–30 %) in salt-stressed plants, in comparison to uninoculated stressed plants. Overall, the results indicated that both PGPR were effective as stress mitigators however, in combination they showed relatively better improvement in growth, yield as well as oxidative parameters of the salt-affected plants. These findings about the effects of native salt-tolerant PGPR Pantoea and Enterococcus sp. in mungbean crop are novel.
Keywords: Salt stress, PGPR, Mungbean, Stress injury, Growth, Sodium, Chlorophyll
Introduction
Saline stress is one of the major abiotic stresses limiting crop growth and productivity (Shrivastava and Kumar 2015). Worldwide, about 20 % of the total cultivable land and 33 % of irrigated agricultural land is affected by high salinity. This salinized area is increasing by 10 % every year and estimated to reach above 50 % (of arable land) by the year 2050 (Shrivastava and Kumar 2015). With the burgeoning population and shrinking agricultural land, it has become imperative to manage saline stress and increase the crop productivity under stressed conditions in order to fulfill food demands.
Salinity induces osmotic and ionic stress in plants, which leads to nutritional imbalance, morphological damages and reduced photosynthesis resulting in diminished growth/productivity and leads to plant death (Ahmad et al. 2013). However, to improve the plant’s growth in stress conditions, it is important to increase the ability of the crop to survive and grow under salt stress, and one such option is application of PGPR. These microbes are associated with plant roots and are known to be beneficial for the overall growth of the plants (Shrivastava and Kumar 2015). Some of the PGPR effects involve increased germination rate, root-shoot growth, yield including grains, leaf area, chlorophyll content, protein content, nutrient availability, delayed leaf senescence and tolerance to stresses (Habib et al. 2016).
Previous studies have indicated that PGPR effectively mitigate the impact of various stresses on plants via different mechanisms and act as plant-helpers in combating stress by enhancing the plant’s tolerance (Palaniyandi et al. 2014). PGPR have been reported for their beneficial effect on the growth enhancement and stress amelioration effects in different crops such as wheat; chickpea; onion and groundnut etc. (Dasgupta et al. 2015). Some of these PGPR belong to genera Azospirillum, Azotobacter, Bacillus, Burkholderia, Erwinia, Enterobacter, Flavobacterium, Micrococcus, Pseudomonas and Serratia etc. (Singh 2015). Another aspect involves the combination of these microorganisms with beneficial attributes to get an outcome with numerous or complementing beneficial effects (Damam et al. 2014). Reports are available on the synergistic effects of PGPR combinations on different crops (Kalita et al. 2015).
Research on beneficial bacteria for development of inoculants has gained special interest of researchers. The purpose of developing such inoculants is, using a biological option to stimulate plants’ responses against stressed conditions. At the same time, it is prudent to isolate and test the indigenous bacterial strains, which would be more effective in combating the salt stress than the exotic strains. In the present study, we have focused on a salt-sensitive leguminous crop mungbean (Vigna radiata L.), an important protein-rich legume crop of India, and widely cultivated in Asia. It is quite versatile and short duration crop, considered as golden bean because of its high nutritional values, seeds, forage and suitability for increasing fertility of soil (Reetha et al. 2014). In this study, we have evaluated some selective native PGPR, isolated from the rhizosphere of mungbean, applied individually as well as in consortium to test their effects on this crop grown under simulated salt conditions (5 dS/m and 10 dS/m NaCl i.e. ≈ 50 mM and 100 mM NaCl concentration). The objective of this study was to ascertain the effects of salt conditions on mungbean growth and the potential of these bacterial strains as stress mitigators in relation to their effects on growth, productivity and antioxidant system of stress affected mungbean plants. To the best of our knowledge, this is the first report about mitigation effects of Pantoea dispersa and Enterococcus faecium on salt- stressed mungbean plants.
Materials and methods
Collection of phosphate solubilizing microorganisms
The phosphate (P) solubilizing microorganisms were isolated from the rhizospheric soil of mungbean (Vigna radiata L. cv. SML 668) plants grown in pots (8 kg soil capacity, 12 cm diameter) maintained at green house, Department of Botany, Panjab University, Chandigarh (latitude and longitude 30°44′13″N, 76°47′13″E, respectively), India. The rhizospheric soil was collected aseptically into sterile petriplates and processed for the isolation of P-solubilizing bacteria on Pikovskaya’s agar plates by serial dilution (1:10) method. The bacterial colonies showing clearance zone around them were selected and streaked on fresh plates. The selected colonies were further screened for their growth, P-solubilization ability and siderophore production on salt-supplemented agar plates (final concentration up to 150 mM).
Identification of isolates
The selected isolates were further examined microscopically as well by physiological and biochemical characteristics. Further characterization was done by basic biochemical tests for Gram positive and Gram negative bacteria, including indole, methyl red, oxidase, catalase, sugar fermentation and lysine-ornithine-arginine carboxylase test according to Bergey’s Manual of Systemic Bacteriology (Breed et al. 1957). For final identification, the isolated bacteria were subjected to 16S rRNA gene sequence analysis. The 16S rRNA gene sequences were submitted to GenBank.
Assessment of salt tolerance of isolates
The selected isolates were screened for their growth and salt tolerance at various NaCl concentrations. The actively growing cultures were inoculated in nutrient broth supplemented with increasing concentration of salt (0, 200, 400, 800 mM and 1200 mM) and incubated at 30 °C at 150 rpm. The bacterial growth was monitored using viable count technique. The highest concentration of salt with no significant effect on the growth pattern of the isolates was taken as their tolerance level.
Quantitative estimation of P-solubilizing activity and siderophore production
The bacterial isolates were assessed for their P-solubilizing ability in salt amended Pikovskaya’s broth (50, 100, 150 mM). The actively growing bacterial cultures were inoculated in 100 ml liquid medium (with and without supplemented salt) and incubated at 30 °C, 150 rpm. Medium without salt was taken as control and P-solubilization in salt-amended media was compared with the control values. Five milliliters of the culture was withdrawn at regular intervals and used for quantitative estimation of P-solubilization using soluble P chlorostannous reduced molybdophosphoric acid method (Jackson 1973). Final concentrations for solubilized P by isolates were calculated from standard curve prepared using 0–10 µg/ml phosphate (KH2PO4) solution following linear regression analysis.
P-solubilizing isolates were further analyzed for their siderophore production ability using universal chrome azurol method (Schwyn and Neilands 1987). The isolates were spot inoculated on CAS agar plates followed by incubation at 30 °C for 24–72 h and observed for the orange halo zones around the colonies. Quantitative estimation was carried out in salt supplemented (up to 150 mM) iron (Fe) free succinate liquid medium (Barbhaiya and Rao 1985) inoculated with 1 % primary inoculum. Medium without salt was taken as control. Samples in replicates were taken regularly after 24 h interval from each flask and siderophore estimation was performed according to universal chrome azurol liquid assay (Schwyn and Neilands 1987). Siderophore units were calculated as follows
where Ar is absorbance of uninoculated media and CAS solution; As is absorbance of test sample i.e. culture supernatant and CAS solution.
Analysis for IAA production and ACC (1-aminocyclopropane-1-carboxylate) deaminase activity
The bacterial isolates were screened for IAA production with and without supplemented salt, by colorimetric method (Gorden and Paleg 1957). One ml of culture was inoculated in 100 ml minimal medium containing tryptophan (Frankenberg and Poth 1988) and incubated at 30 °C (150 rpm). Two ml of Salper’s reagent (1.0 ml 0.5 M FeCl3, in 50.0 ml of 35 % HClO4) was added to 1.0 ml cell free culture supernatant (taken at 24 h intervals) and incubated for 30 min in dark at room temperature. Un-inoculated media was used as blank and the intensity of pink color developed was measured at 530 nm using UV/VIS spectrophotometer. The final concentration of IAA was calculated from IAA (0–350 µg/ml) standard curve.
The isolates were further screened for their ACC deaminase activity with and without salt supplementation, by measuring the amount of α-ketobutyrate produced by ACC using 2,4-dinitrophenylhydrazine reagent (Honma and Shimomura 1978; Penrose and Glick 2003). The bacterial cells were grown in a tryptone soya broth up to late log phase and later grown and induced in minimal media (with and without supplemented salt) with additional 3.0 mM ACC as sole nitrogen source. After reaction with 2,4-dinitrophenylhydrazine reagent, absorbance was taken at 540 nm wavelength. The amount of α-ketobutyrate produced was determined using the standard curve of α-ketobutyrate ranging between 0.1 and 1.0 mmol. ACC deaminase activity was expressed as nmol α-ketobutyrate produced/mg protein/h and the protein concentrations were determined using Bradford’s protein determination method (Bradford 1976). All the assays were performed in triplicates.
Biocompatibility test
The isolates were analyzed for the biocompatibility with Rhizobium by streaking one isolate in the centre and others streaked perpendicularly across the initial culture. After incubation at 30 °C for 48 h, the absence of any growth inhibition at the intersections indicated biocompatibility of the isolates.
Pot experiment
The selected halo-tolerant bacterial strains were used as inoculants to determine their effects on mungbean SML-668 cultivar grown in salt (5 and 10 dS/m) supplemented soil The isolates were grown in nutrient broth and the final concentration of bacterial cells were adjusted up to 1 × 109 cells, followed by confirmation through viable counting method. The microbial consortium was prepared by mixing equal volumes of each bacterial suspension. The pot experiment was carried out under natural out-door conditions in a wired enclosure at Department of Botany, Panjab University, Chandigarh. Disinfected pots were filled with soil i.e. soil:manure in 3:1 ratio (0.77 % organic matter, 4 mg/kg available P, 30 mg/kg available N, 108 mg/kg K, 0.417 mEq/100 g soil Ca, 0.084 mEq/100 g soil Mg) also supplemented with tri-calcium phosphate (200 mg/kg). The soil-filled pots were saturated with NaCl solutions i.e. 50 mM and 100 mM concentrations, a week before the sowing.
Mungbean seeds, procured from Punjab Agriculture University, Ludhiana, India, were surface sterilized by soaking in 5 % NaOCl for 10 min followed by thorough washing with distilled water and soaking in the Rhizobium inoculum. Six seeds per pot were kept in soil at a depth of 2 cm and were then inoculated with 1.0 ml of respective inoculum according to treatment plan. The treatment plan included different sets of individual and combination treatments.
Three sets of treatments were used in each experiment where set 1, set 2 and set 3 contained pots with 0, 5 and 10 dS/m NaCl respectively. The treatment plan for each set included (i) PSB3 (ii) PSB5 (iii) PSB3 + PSB5 (iv) no bacterial strain(s) or control. Five replications of pots were kept for each treatment. Plants were watered regularly according to the requirement and no fertilizers were added. The plants height and pods were recorded at regular intervals and harvested after maturity.
Determination of membrane damage, Relative leaf water content and Chlorophyll content
The leaves were collected from five plants of each treatment at the time of peak flowering and subjected to various tests. Membrane damage was determined on the basis of electrolyte leakage of the leaf tissue. The washed leaf samples (1 g) were placed in closed vials containing 10 ml of deionized water and incubated (25 °C) for 24 h, subsequently the electrical conductivity of the solution (L1) was determined. Samples were then autoclaved at 120 °C for 20 min and the final electrical conductivity (L2) was obtained after equilibration at 25 °C. The electrolyte leakage (%) was calculated as (L1/L2) × 100 (Lutts et al. 1996).
For relative leaf water content (RLWC) determination, fresh leaves were excised into smaller segments, weighed and kept in distilled water under low light for 3 h to obtain the turgid weight. Leaf samples were oven-dried at 80 °C for 24 h and weighed (Barrs and Weatherley 1962). RLWC was calculated as shown below and expressed as %:
The chlorophyll concentration was measured from leaves by extracting in 80 % acetone, followed by centrifugation at 13,000 rpm for 10 min. The absorbance of the supernatant was read at 645 and 663 nm, and the total chlorophyll was calculated (Arnon 1949), against 80 % acetone as a blank. The amount of chlorophyll present (mg g−1 dw) in the extract was calculated according to the following equations stated below:
Determination of Na+ and K+ concentrations
The dried leaf samples (6 per treatment) were homogenized into powder and transferred to Erlenmeyer flasks, to which 6 ml nitric acid + perchloric acid solutions were added. The samples were kept for digestion (30 min, 40 °C) in a water bath and the solution removed by heating at 150–180 °C until the volume reduced to 1 ml. This residue was dissolved in distilled water and final volume was made up to 100 ml. The Na+ and K+ concentrations in the samples were determined by flame photometry following the modified method of Holiday and Preedy (1953) and expressed as mg g−1 dw.
Effect on oxidative stress parameters of mungbean
The plants were analyzed for the oxidative stress parameters i.e. antioxidant enzymes and metabolites. The leaves were collected from five plants of each treatment at the time of peak flowering and subjected to various tests.
Malondialdehyde (MDA)
Lipid peroxidation (LPO) was determined by estimating the MDA content, a product of LPO (Heath and Packer 1968). In 1.0 ml of extract (prepared in 0.1 % trichloroacetic acid (TCA)) supernatant, 4.0 ml of 0.5 % thiobarbituric acid (in 20 % TCA) was added and boiled for 30 min. The mixture was centrifuged at 10,000 rpm for 10 min, after cooling and absorbance taken at 532 nm. MDA content was calculated by extinction coefficient of 155 mM−1 cm−1.
Hydrogen peroxide (H2O2)
Hydrogen peroxide was estimated based on formation of titanium hydro-peroxide complex. Five milliliters of ammonia and 4.0 ml of titanium reagent were added, to 5.0 ml of extract (prepared in 90 % acetone), to precipitate the titanium-hydro peroxide complex followed by overnight incubation at 4 °C. The precipitate was dissolved by adding 5.0 ml of 1 M H2SO4 and absorbance was recorded at 410 nm against reagent blank. The extinction coefficient of H2O2 is 0.28 μmol−1 cm−1. Calculations were made with standard curve plotted with pure H2O2 (Mukherji and Chaudhari 1983).
Catalase (CAT)
For CAT activity, the reaction mixture (3.0 ml) consisted of 200 µl enzyme extract (prepared in 50 mM phosphate buffer), 50 mM phosphate buffer (2.6 ml) and 200 mM hydrogen peroxide (200 µl). The absorbance was taken at 410 nm and decrease in absorbance was observed up to 3 min. CAT activity was measured using extinction coefficient 40 mM−1 cm−1 and expressed in µmol H2O2 reduced s−1 g−1 dw (Teranishi et al. 1974).
Glutathione reductase (GR)
For GR activity, reaction mixture constituted water, buffer, 30 mM GSSG, 0.8 mM NADPH and 1 % BSA. In 0.2 ml enzyme extract, 2.8 ml reaction mix was added and absorbance recorded at 340 nm for 3 min, taking reaction mixture (without enzyme) as blank (Mavis and Stellwagen 1968). The enzyme activity was expressed in µmol ascorbate oxidised min−1 g−1 dw.
Superoxide dismutase (SOD)
SOD activity was determined based on the ability to inhibit photochemical reduction of nitro-blue tetrazolium (NBT) (Dhindsa et al. 1981). The reaction mixture (3.0 ml) contained 13 mM methionine (0.1 ml), 50 mM NaHCO3 (0.1 ml), 25 mM NBT (0.1 ml), 0.1 mM EDTA (0.1 ml), 50 mM buffer (2.3 ml) and 0.2 ml of enzyme extract. Riboflavin (2 mM) was added at the end and reaction mixture was exposed to 15 W fluorescent tubes (10 min) followed by incubation in dark for 20 min. The non-radiated reaction mixture without enzyme was taken as blank whereas the radiated reaction mixture without enzyme was taken as control and absorbance was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme, which causes 50 % inhibition of photochemical reduction of NBT.
Ascorbate peroxidase (APO)
The activity of APO was determined using method given by Nakano and Asada (1981). The reaction mixture (3 ml) contained 50 mM phosphate buffer (2.6 ml), 0.1 mM EDTA (0.1 ml), 0.5 mM ascorbic acid (0.1 ml), enzyme extract (0.1 ml) and 0.1 mM H2O2 (0.1 ml). The absorbance was recorded at 290 nm up to 3 min, to determine the change in activity. The rate constant was calculated using the extinction coefficient of 2.8 mM−1 cm−1 and enzyme activity was expressed in µmol H2O2 reduced s−1 g−1 dw.
Reduced Glutathione (GSH)
For GSH estimation, the extract was prepared in 2 % metaphosphoric acid and centrifuged at 17,000 rpm for 10 min. The supernatant enzyme extract (0.9 ml) was then neutralized by 0.6 ml of 10 % sodium citrate. The reaction mix included 0.3 mM NADPH (0.7 ml), 6 mM DTNB (6 mM), distilled water (0.1 ml), buffer (1.9 ml) and enzyme extract (0.1 ml), stabilized at 25 °C for 3–4 min. At the end, 10.0 µl of GR was added and absorbance was taken at 412 nm. Glutathione was calculated from a standard graph (Griffith 1980).
Ascorbic acid
Ascorbic acid estimation was done by method given by Mukherji and Chaudhari (1983). The reaction mixture contained extract (2.0 ml) prepared in 6 % TCA, 2 % DNPH (1.0 ml) followed by 1 drop of 10 % thiourea, boiled for 15 min in water bath. 80 % chilled H2SO4 (5 ml) was added after cooling and absorbance was read at 530 nm against blank. Ascorbic acid content (mg/g dw) was calculated from a standard curve plotted with known concentration of ascorbic acid.
Analysis of root colonization by inoculated bacteria
At the end of the pot experiment, the rhizospheric soil samples were taken from the harvested plants and processed by dilution (1:10) method for isolation of P-solubilizers on Pikovskaya’s agar plate. The positive isolates with similar colony morphology to bacterial inoculants were analyzed for DNA banding pattern using random amplified polymorphic DNA- polymerase chain reaction (RAPD PCR).
RAPD PCR was carried out using the decamer arbitrary primer OPA-04 (5′-AATCCGGCTG-3′) (Betancor et al. 2010). The reaction mixture (25 µl) containing 2.5 µl of 1X Taq buffer, 3.5 µl of 25 mM MgCl2, 0.5 µl of 200 µM dNTP mix, 5.0 µl of 10 µM primer, 1.0 µl of 1U Taq polymerase and 1 µl of genomic DNA (~100 ng). The thermocycling conditions included the initial denaturation at 94 °C for 5 min followed by 40 cycles including denaturation at 94 °C for 40 s, annealing at 33 °C for 40 s, extension at 72 °C for 2 min followed by final extension at 72 °C for 10 min. PCR products were observed on 1.5 % agarose gel run at 100 V and the bands size were estimated by comparing with 100 bp and 1 Kb DNA marker. The gel was photographed and band pattern analysis was carried out on the basis of banding pattern of inoculants.
Statistical analysis
Three replicated values were pooled, and mean values were worked out, which have been presented along with standard errors in tables. ANOVA analysis of recorded data was conducted using statistical package (Agristat, IASRI, New Delhi) at P < 0.05 level of significance. The correlation coefficients were calculated in MS excel.
Results
Isolation of P-solubilizing bacteria
Eleven colonies showing zone of solubilization on Pikovskaya’s agar medium were isolated and labeled as PSB (P-solubilizing bacteria). These isolates were further screened for their growth on salt-amended Pikovskaya’s agar plates (with 150 mM). Based on the growth and P-solubilization in salt’s presence, two promising isolates PSB3 and PSB5 were selected. PSB3 was found to have higher P-solubilization zone diameter (12–16 mm) and siderophore production (halo diameter 6–9 mm) on Pikovskaya’s agar medium and CAS agar medium, respectively after 48 h of incubation whereas PSB5 showed clearance zone of 6–10 mm on Pikovskaya’s agar and 4–6 mm on CAS agar plate.
Identification of bacterial isolates by colony morphology and biochemical tests
PSB3 was found to be gram-negative unicellular rods and PSB5 was observed as gram-positive coccobacilli, mainly in pairs under microscope. On nutrient agar plates, PSB3 produced yellow colored round colonies and PSB5 produced white, opaque, circular colonies with 1.0 and 0.5 mm diameter respectively. Presumptive characterization was done using basic biochemical tests based on Bergey’s Manual of Determinative Bacteriology. Based on biochemical characterization, both isolates were found to be indole, oxidase negative and positive for glucose fermentation as represented in Table 1.
Table 1.
Preliminary characterization of the bacterial isolates
| PSB3 | PSB5 | |
|---|---|---|
| Gram character | Gram negative unicellular rods | Gram positive coccobacilli, mostly in pairs |
| Colony characteristics | Yellow colored, round colony (1.0 mm) | White, opaque, circular colonies (0.5 mm) |
| Indole | − | − |
| Oxidase | − | − |
| Glucose fermentation | + (g) | + (g) |
| Catalase | + | + |
| Methyl red | − | − |
| Lys-Orn-Arg | − − − | − − + |
Lys-Orn-Arg represents lysine ornithine arginine decarboxylase test, (g) represents gas production
Molecular characterization of bacterial isolates
The final confirmation of the bacterial identity was done using 16S rRNA gene sequencing. According to the sequencing results, PSB3 exhibited sequence homology with Pantoea dispersa and PSB5 showed homology with Enterococcus faecium. The 16S gene sequences for strains P. dispersa and E. faecium were deposited in GenBank with accession numbers KM095646 and KM095647, respectively.
Growth of bacterial isolates with different salt concentrations
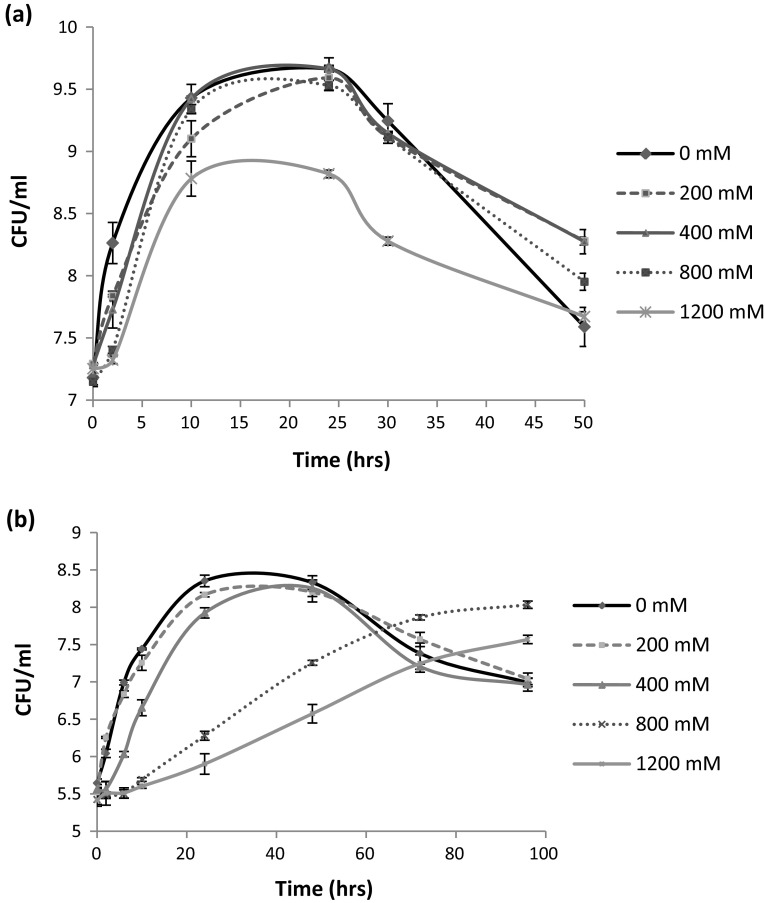
The growth of P. dispersa and E. faecium was observed at different salt concentrations i.e. 200–1200 mM, in salt supplemented nutrient broth using plate count method. Initially, the bacterial growth was comparatively slow in presence of salt, as compared to their without salt counterparts. With time, the bacterial growth rate was increased and the maximum growth remained unaffected (no significant difference from the bacterial viability without salt) up to the salt concentration of 400 and 800 mM in case of E. faecium and P. dispersa, respectively. However, the bacterial viable cell count decreased with further increase in salt concentration (Fig. 1). The results indicated that E. faecium was comparatively less tolerant to salt as significant decrease in CFU/ml was observed at 800 and 1200 mM, in comparison to the control value (Fig. 1b). In contrast, less reduction was observed in the growth of P. dispersa at 1200 mM NaCl, in comparison to control (Fig. 1a).
Fig. 1.
Effect of different salt concentrations on the growth of P. dispersa and E. faecium
Plant growth promoting attributes of isolates
Both the isolates were found positive for different PGP attributes and showed different levels of P-solubilization, siderophore production, IAA production and ACC deaminase activity. The isolates were examined both with and without added salt, in the growth medium (as mentioned in materials and methods section) to determine the effect of salt on their PGP attributes. P. dispersa showed higher P-solubilization (3.64 µg/ml), siderophore production (60.3 %) and ACC deaminase activity (207.4 nmol α-ketobutyrate/mg protein/hr), as compared to E. faecium in medium without any salt supplementation (Table 2). However, E. faecium possessed higher IAA producing ability i.e. 652.1 µg/ml in medium without any salt.
Table 2.
Effect of different NaCl concentrations on the plant growth promoting attributes of P. dispersa and E. faecium
| NaCl (mM) | P-solubilization (µg/ml) | Siderophore unit (%) | IAA concentration (µg/ml) | ACC deaminase activity (nmol/mg/h) | ||||
|---|---|---|---|---|---|---|---|---|
| P. dispersa | E. faecium | P. dispersa | E. faecium | P. dispersa | E. faecium | P. dispersa | E. faecium | |
| 0 (control) | 3.64 ± 0.05a | 2.02 ± 0.15b | 60.33 ± 3.77 | 32.11 ± 2.23d | 218.3 ± 7.8a | 652.09 ± 7.0b | 207.45 ± 8.6a | 123.34 ± 9.62c |
| 50 | 4.64 ± 0.13b | 2.07 ± 0.16b | 60.68 ± 3.67 | 33.79 ± 1.34c | 222.6 ± 3.3ab | 702.43 ± 20.6a | 263.45 ± 19.6ab | 288.96 ± 32.3b |
| 100 | 4.75 ± 0.11b | 2.3 ± 0.06a | 61.47 ± 6.8 | 36.43 ± 0.97b | 237.6 ± 6.5bc | 718.88 ± 35.98a | 328.01 ± 7.9b | 318.49 ± 41.6b |
| 150 | 5.18 ± 0.49bc | 2.35 ± 0.19a | 54.67 ± 3.01 | 39.23 ± 2.67a | 253.86 ± 2.7c | 730.42 ± 28.7a | 355.42 ± 11.8c | 476.47 ± 38.1a |
| LSD (P < 0.05) | 0.461 | 0.210 | – | 1.598 | 17.52 | 35.49 | 75.31 | 94.59 |
Values represent mean of triplicates. Means sharing same alphabets represents statistically non-significant according to ANOVA analysis
In the salt-supplemented medium (50, 100 and 150 mM NaCl), the PGP attributes showed a gradual increase in the values with increase in NaCl concentration (Table 2). At 150 mM NaCl concentration, 42.3, 16.3 % and 1.7 folds increment was found for P-solubilization, IAA production and ACC deaminase, respectively in P. dispersa with respect to the controls (0 mM). Likewise with E. faecium, significant increment by 16.3, 12.0 %, 1.2 fold and 3.8 folds for P-solubilization, IAA production, siderophores and ACC deaminase activity was recorded at 150 mM NaCl concentration in comparison to control values (Table 2).
Pot experiment
Salt stress conditions were created by watering the soil with saline solutions (i.e. 5 and 10 dS/m) to maximum water holding capacity. Different parameters including height, biomass, pods/seeds numbers and pods/seeds weight were measured in controls, salt-stressed plants and PGPR-inoculated salt-stressed plants.
Effects on vegetative traits of mungbean
At 5 and 10 dS/m salt stress, a reduction of 9.7 and 15.9 % was observed in the height of mungbean plants, compared with control. A significant increase in height was noticed with individual PGPR treatments under salt stress conditions. Maximum improvement was observed in case of combination treatment (P. dispersa and E. faecium), which exhibited 22.6, 30.5 and 15.7 % increase at no salt, 5 and 10 dS/m salt treatments, respectively (Table 3).
Table 3.
Effect of P. dispersa and E. faecium on the growth and biomass of mungbean cultivar grown under salt stress
| Treatments | Height (cm) | Biomass (g) |
|---|---|---|
| Control (no salt) | 41.17 ± 1.04cde | 4.14 ± 0.20b |
| Control (5 dS/m) | 37.17 ± 0.76fg | 3.43 ± 0.18de |
| Control (10 dS/m) | 35.0 ± 1.80g | 3.03 ± 0.19e |
| P. dispersa (5 dS/m) | 39.0 ± 3.0ef | 3.78 ± 0.18bcd |
| E. faecium (5 dS/m) | 40.33 ± 2.8de | 4.05 ± 0.33bc |
| P. dispersa + E. faecium (5 dS/m) | 43.0 ± 0.5bcd | 4.21 ± 0.39b |
| P. dispersa (10 dS/m) | 42.5 ± 1.73cd | 3.49 ± 0.52cde |
| E. faecium (10 dS/m) | 43.67 ± 1.75bc | 3.72 ± 0.25bcd |
| P. dispersa + E. faecium (10 dS/m) | 45.67 ± 1.55b | 4.16 ± 0.32b |
| P. dispersa (no salt) | 44.17 ± 3.01bc | 4.92 ± 0.50a |
| E. faecium (no salt) | 46.0 ± 1.32b | 5.28 ± 0.11a |
| P. dispersa + E. faecium (no salt) | 50.5 ± 0.87a | 5.45 ± 0.61a |
| LSD (P < 0.05) | 3.07 | 0.588 |
Values represent mean of triplicate treatments. Means sharing same alphabets represents statistically non-significant according to ANOVA analysis
At 5 and 10 dS/m salt treatments, biomass (above ground dry weight) decreased by 17.0 and 26.8 %, respectively, as compared to controls. With PGPR application to salt-stressed plants, a significant improvement was noticed (Table 3). Maximum enhancement in biomass was observed with combination of P. dispersa and E. faecium (37.0 %), applied to the plants growing in 10 dS/m salt stress. PGPR were also effective in plants growing without salt and the combination of both the strains resulted in around 32.0 % increase in biomass over the control plants.
Effects on the yield parameters of mungbean
Number of pods and seeds
At 5 and 10 dS/m salt conditions, the number of pods per plant decreased by 15.4 and 23.1 % whereas seeds decreased by 16.5 and 20.3 %, over control. However, with PGPR treatment, improvement in pods’ and seeds number was observed at both the salt concentrations, in comparison to the un-inoculated stressed plants. At 5 dS/m NaCl, an improvement in pods’ number and seeds’ number by 18–27 and 22.5–16.0 %, respectively was noticed with PGPR treatments, maximum effect was found with the combination treatment. Whereas, at 10 dS/m salt concentration an improvement ranging 30–25 and 32–27 % was noticed in number of pods and seeds, respectively. The plants growing only with single PGPR showed little increase compared to control (Table 4).
Table 4.
Effect of P. dispersa and E. faecium on the yield components/plant of mungbean cultivar grown under salt stress
| Treatments | Number of pods | Number of seeds | Weight of pods (g) | Weight of seeds (g) |
|---|---|---|---|---|
| Control (no salt) | 8.67 ± 0.57ab | 44.33 ± 2.51bc | 1.23 ± 0.09abcd | 0.98 ± 0.19bcd |
| Control (5 dS/m) | 7.33 ± 1.52b | 37.0 ± 1.73de | 0.96 ± 0.06ef | 0.79 ± 0.05ef |
| Control (10 dS/m) | 6.67 ± 0.58c | 35.33 ± 3.21e | 0.90 ± 0.14f | 0.7 ± 0.04f |
| P. dispersa (5 dS/m) | 8.67 ± 0.71ab | 43.0 ± 2.64cd | 1.15 ± 0.11cd | 0.90 ± 0.18de |
| E. faecium (5 dS/m) | 9.33 ± 1.41ab | 44.0 ± 5.29bc | 1.23 ± 0.09abcd | 0.97 ± 0.14cd |
| P. dispersa + E. faecium (5 dS/m) | 9.0 ± 0.71ab | 45.33 ± 3.78bc | 1.19 ± 0.07bcd | 1.01 ± 0.87bcd |
| P. dispersa (10 dS/m) | 8.0 ± 1.0b | 43.67 ± 5.13cd | 1.1 ± 0.08de | 0.90 ± 0.12de |
| E. faecium (10 dS/m) | 8.33 ± 0.58b | 45.0 ± 3.60bc | 1.14 ± 0.07cde | 0.91 ± 0.16de |
| P. dispersa + E. faecium (10 dS/m) | 8.67 ± 1.41ab | 46.67 ± 4.16abc | 1.19 ± 0.11bcd | 0.95 ± 0.21cd |
| P. dispersa (no salt) | 9.33 ± 0.58ab | 48.6 ± 5.03abc | 1.30 ± 0.08abc | 1.06 ± 0.11abc |
| E. faecium (no salt) | 9.67 ± 1.41ab | 50.67 ± 3.05ab | 1.36 ± 0.14ab | 1.10 ± 0.19ab |
| P. dispersa + E. faecium (no salt) | 10.0 ± 0.71a | 53.33 ± 5.03a | 1.39 ± 0.19a | 1.13 ± 0.19a |
| LSD (P < 0.05) | 1.426 | 6.9 | 0.184 | 0.129 |
Values represent mean of triplicate treatments. Means sharing same alphabets represents statistically non-significant according to ANOVA analysis
Weight of pods and seeds
Salt stress of 5 and 10 dS/m resulted in 22.4 and 26.5 % reduction in pods’ weight and 18.6 and 25.9 % reduction in seeds’ weight per plant over control. At 5 dS/m salt stress, a significant improvement in weight of pods (28.6–20.5 %) and seeds (26.4–13.0 %) was found with PGPR treatments. At 10 dS/m salt stress too, a significant increase in weight of pods (31.4–21.5 %) and seeds (31.0–25.0 %) was observed, which was maximum with combination treatment (Table 4). Under non-stress situations also, the combination of both the bacterial strains resulted in about 13.2 and 16.3 % improvement in pods and seeds yield, respectively.
Effects on the stress-related parameters
Membrane damage, RLWC and chlorophyll content
Salt stress of 5 and 10 dS/m resulted in 19.3 and 24.7 % membrane damage, respectively in the leaves. The damage was reduced by approximately 1.5 folds at 5 dS/m salt treatment with all the PGPR treatments. Similarly 1.1–1.3 folds decrease in membrane damage was found with PGPR treatments at 10 dS/m salt stress treatment, maximum reduction was noticed with the combination treatment. However, no significant changes occurred under non stress conditions (Table 5).
Table 5.
Effect of PGPR treatments on membrane damage, RLWC, Chlorophyll concentration, Na+/K+ concentration of mungbean plants grown under salt stress conditions
| Treatments | Membrane damage (%) | RLWC (%) | Chl (mg/g dw) | Na+ (mg/g dw) | K+ (mg/g dw) | Na+/K+ ratio |
|---|---|---|---|---|---|---|
| Control (no salt) | 12.3 ± 1.6a | 85.2 ± 2.5c | 17.3 ± 1.7c | 2.11 ± 0.8a | 18.3 ± 1.4c | 0.11 ± 0.04a |
| Control (5 dS/m) | 19.3 ± 1.4b | 84.1 ± 1.4c | 13.2 ± 1.5b | 14.6 ± 2.6cd | 12.1 ± 1.6bc | 1.2 ± 0.05e |
| Control (10 dS/m) | 24.7 ± 1.5c | 71.3 ± 1.6a | 10.6 ± 1.6a | 23.4 ± 2.1e | 7.9 ± 1.2a | 2.9 ± 0.08g |
| P. dispersa (5 dS/m) | 12.6 ± 1.4a | 85.1 ± 1.7c | 16.3 ± 1.8c | 12.1 ± 1.4c | 12.8 ± 1.5bc | 0.94 ± 0.06de |
| E. faecium (5 dS/m) | 12.7 ± 1.3a | 82.5 ± 1.8c | 16.3 ± 1.5c | 11.4 ± 1.6bc | 16.6 ± 1.5c | 0.68 ± 0.05c |
| P. dispersa + E. faecium (5 dS/m) | 12.7 ± 1.5a | 83.5 ± 1.6c | 16.9 ± 1.8c | 8.65 ± 0.6b | 17.4 ± 1.7c | 0.49 ± 0.06b |
| P. dispersa (10 dS/m) | 22.7 ± 1.5c | 72.4 ± 1.6a | 12.7 ± 1.7ab | 18.2 ± 1.6d | 10.3 ± 1.4ab | 1.76 ± 0.07f |
| E. faecium (10 dS/m) | 20.5 ± 1.4bc | 77.2 ± 1.8b | 13.5 ± 1.4b | 16.3 ± 1.7d | 15.3 ± 1.4bc | 1.06 ± 0.06e |
| P. dispersa + E. faecium (10 dS/m) | 18.4 ± 1.6b | 78.9 ± 1.7bc | 15.5 ± 1.6b | 13.8 ± 1.6cd | 15.8 ± 1.6c | 0.87 ± 0.07d |
| P. dispersa (no salt) | 11.4 ± 1.6a | 84.2 ± 2.2c | 16.3 ± 1.5c | 2.45 ± 0.7a | 16.7 ± 1.3c | 0.14 ± 0.03a |
| E. faecium (no salt) | 13.2 ± 1.6a | 83.5 ± 1.7c | 16.4 ± 1.8c | 2.36 ± 0.6a | 19.4 ± 1.6c | 0.12 ± 0.03a |
| P. dispersa + E. faecium (no salt) | 13.2 ± 1.5a | 82.8 ± 1.6c | 18.9 ± 1.7d | 2.22 ± 0.5a | 19.3 ± 1.6c | 0.11 ± 0.1a |
| LSD (P < 0.05) | 2.2 | 3.9 | 2.5 | 3.2 | 3.6 | 0.13 |
Values represent mean of triplicate treatments. Means sharing same alphabets represents statistically non-significant according to ANOVA analysis
RLWC remained unaffected at 5 dS/m salt, however reduction of 1.2 folds was observed at 10 dS/m salt from control. With PGPR treatments, a significant increase by approximately 1.1 fold was observed with E. faecium and combination treatment, in comparison to control (10 dS/m) plants.
Salt stress of 5 and 10 dS/m resulted in 23.7 and 38.7 % reduction in chlorophyll concentration, respectively. However, the PGPR treatments were found to mitigate the adverse effects of salt stress on the chlorophyll of leaves to some extent. A significant increase (23.5–28.0 %) and (20.0–46.2 %) was noticed with PGPR treatments at 5 and 10 dS/m, respectively. No significant differences were noticed when PGPR were applied to un-stressed plants (Table 5).
Na+ and K+ concentration
Salt stress increased the Na+ accumulation by 7 and 11-folds at 5 dS/m and 10 dS/m, respectively, as compared to control plants. PGPR treatments were found to effectively decrease the sodium concentration in the leaves of stressed plants. At 5 dS/m salt stress, the application of PGPR resulted in 17.1–40.1 % reduction in the leaf Na+ concentration while at 10 dS/m salt, sodium accumulation was reduced by 22.2–41.0 % (Table 5). Salt stress significantly decreased the K+ uptake in leaves. At 5 and 10 dS/m, a reduction of 33.9 and 56.8 % was noticed, respectively. PGPR treatments effectively increased the K+ concentration in leaves of the stressed plants (Table 5). At 5 dS/m salt stress, an improvement by 37.2 and 43.8 % was recorded with application of E. faecium and P. dispersa + E. faecium strains, respectively. Similar trend was observed at 10 dS/m salt stress, where increase by 93.7 and 100 % was observed with E. faecium and P. dispersa + E. faecium. In case of un-stressed plants, a slight increase was noticed in K+ uptake with PGPR treatments. Likewise, PGPR treatments resulted in significant reductions in Na+/K+ ratio (Table 5).
Effects on the oxidative damage and antioxidants
MDA and H2O2 concentration
Malondialdehyde (MDA) serves as an index to determine the redox status of the plant. In salt-stressed plants, 1.9 and 4.3 fold increase in MDA concentration was observed at 5 and 10 dS/m NaCl, respectively. However with PGPR treatments, 22.3–38.5 and 14.5–39.0 % reduction in MDA concentration was noticed at 5 and 10 dS/m salt, respectively, compared to respective controls. No appreciable effect was observed with individual PGPR treatments in case of plants without stress, although a slight reduction was recorded with combination treatment in this case (Table 6). H2O2 concentration was increased by 2.0 and 4.6 fold with 5 and 10 dS/m salt stress respectively, in comparison to control. The treatment of plants with PGPR significantly decreased the H2O2 concentrations by 34.4–18.75 and 40.6–26.1 % in salt-stressed plants at 5 and 10 dS/m, respectively (Table 6). On the contrary, no reduction was found with individual PGPR inoculation in unstressed plants although the PGPR combination reduced the H2O2 concentration (13 %) in un- stressed plants too.
Table 6.
Effect of PGPR treatment on the antioxidant enzyme activities and metabolites in the mungbean plants grown under salt stress
| Treatments | MDA (nmol/gdw) | H2O2 (µmol/gdw) | SOD (µmol/gdw) | CAT (µmol/min/gdw) | APO (µmol/min/gdw) | GR (mmol/min/mg dw) | ASC (nmol/gdw) | GSH (µmol/gdw) |
|---|---|---|---|---|---|---|---|---|
| Control (no salt) | 12.8 ± 1.8ab | 1.5 ± 0.2ab | 12.7 ± 1.8a | 2.2 ± 0.4a | 3.1 ± 0.5a | 4.3 ± 0.4ab | 134.5 ± 6.3ab | 34.6 ± 2.7ab |
| Control (5 dS/m) | 24.7 ± 2.1d | 3.2 ± 0.2bc | 20.3 ± 1.6bc | 3.8 ± 0.6bc | 4.6 ± 0.5c | 5.8 ± 0.4c | 287.4 ± 5.3f | 58.2 ± 3.1e |
| Control (10 dS/m) | 55.3 ± 2.2h | 6.9 ± 0.7d | 25.3 ± 2.1d | 4.9 ± 0.5c | 5.9 ± 0.4d | 6.6 ± 0.4d | 210.5 ± 6.3c | 43.1 ± 3.3c |
| P. dispersa (5 dS/m) | 27.3 ± 2.1d | 2.6 ± 0.4b | 18.4 ± 1.8bc | 3.1 ± 0.4b | 4.1 ± 0.5bc | 5.1 ± 0.4b | 234.3 ± 6.9d | 63.5 ± 3.6ef |
| E. faecium (5 dS/m) | 19.2 ± 1.7c | 2.4 ± 0.8ab | 17.5 ± 1.8b | 2.9 ± 0.4ab | 4.4 ± 0.4c | 4.8 ± 0.4b | 294.3 ± 6.8f | 61.3 ± 4.6ef |
| P. dispersa + E. faecium (5 dS/m) | 15.2 ± 1.8b | 2.1 ± 0.7ab | 15.3 ± 1.6ab | 2.6 ± 0.4ab | 3.8 ± 0.4b | 4.3 ± 0.5ab | 310.2 ± 6.9g | 64.2 ± 3.2f |
| P. dispersa (10 dS/m) | 47.3 ± 2.4g | 5.1 ± 0.8c | 22.1 ± 1.7c | 4.1 ± 0.2c | 4.9 ± 0.4c | 5.9 ± 0.5c | 254.1 ± 7.3e | 51.2 ± 4.4d |
| E. faecium (10 dS/m) | 43.2 ± 2.6f | 4.2 ± 0.7c | 20.5 ± 2.2c | 3.8 ± 0.3bc | 4.3 ± 0.4bc | 5.1 ± 0.5b | 286.4 ± 8.1f | 54.3 ± 3.8de |
| P. dispersa + E. faecium (10 dS/m) | 33.7 ± 2.6e | 4.1 ± 0.6c | 19.5 ± 1.7bc | 3.4 ± 0.5bc | 3.1 ± 0.4a | 5.3 ± 0.4bc | 234.2 ± 5.8d | 56.2 ± 3.7de |
| P. dispersa (no salt) | 15.3 ± 1.8b | 1.8 ± 0.4ab | 13.3 ± 1.3a | 2.5 ± 0.3ab | 3.4 ± 0.5ab | 4.7 ± 0.4b | 141.6 ± 7.1b | 29.4 ± 3.8a |
| E. faecium (no salt) | 14.1 ± 1.6ab | 1.6 ± 0.7ab | 14.2 ± 1.6a | 2.6 ± 0.3ab | 3.8 ± 0.5b | 4.1 ± 0.4ab | 129.2 ± 7.7a | 31.4 ± 3.1ab |
| P. dispersa + E. faecium (no salt) | 11.9 ± 1.5a | 1.3 ± 0.1a | 14.3 ± 1.6a | 2.3 ± 0.4ab | 2.9 ± 0.3a | 4.0 ± 0.4a | 140.2 ± 7.2ab | 36.2 ± 3.4b |
| LSD (P < 0.05) | 3.2 | 1.1 | 2.8 | 0.86 | 0.57 | 0.62 | 11.3 | 5.8 |
Values represent mean of triplicate treatments. Means sharing same alphabets represents statistically non-significant according to ANOVA analysis. MDA malondialdehyde, H 2 O 2 hydrogen peroxide, SOD superoxide dismutase, CAT catalase, APO ascorbate peroxidase, GR glutathione reductase, ASC ascorbic acid, GSH reduced glutathione
Antioxidant enzymes activity
Superoxide dismutase (SOD) is one of the first enzymes to take part in the antioxidative response. Increasing salt concentration (5 and 10 dS/m) raised the SOD activity by 1.6 and 1.2 fold, respectively. However, PGPR treatments decreased the enzyme activity by 9.3–24.6 and 12.6–23.0 % at 5 and 10 dS/m salt stress, respectively, as compared to un-inoculated plants (Table 6).
In the plants exposed to salt stress, catalase (CAT) activity also increased by 1.73 and 2.22 folds at 5 and 10 dS/m salt respectively. However, activity was reduced in the PGPR treated plants by 18.4, 23.7 and 31.6 % with P. dispersa, E. faecium and P. dispersa + E. faecium treatments, respectively at 5 dS/m salt stress in comparison to untreated plants. Similar trend was noticed (16.3, 22.4 and 30.6 % reduction) at 10 dS/m salt stress. The saline stress of 5 and 10 dS/m NaCl, resulted in rise in APO activity by 1.5–1.9 fold with respect to unstressed control plants. APO activity decreased with PGPR treatments i.e. 10.8 % by P. dispersa, 4.3 % by E. faecium and 17.4 % with combination of both, in the salt-stressed plants (5 dS/m). In the same way, at 10 dS/m salt, application of P. dispersa, E. faecium and P. dispersa + E. faecium reduced the enzyme activity by 16.9, 27.1 and 47.4 % respectively, in comparison to control (10 dS/m; Table 6). GR activity increased by 1.3 and 1.5 fold in the plants exposed to 5 and 10 dS/m salt stress, compared to control plants. A drop in enzyme activity by 12.1–25.9 and 10.6–22.7 % was found with PGPR treatments at 5 and 10 dS/m salt stress respectively, as compared to their respective controls (Table 6).
Antioxidant metabolites: Ascorbic acid and GSH concentration
Under salt stress of 5 and 10 dS/m, the ascorbic acid concentration increased by 2.1 and 1.6 fold, respectively from control. Ascorbic acid concentration was found to increase slightly with E. faecium and its combination with P. dispersa at 5 dS/m salt stress (Table 6). At 10 dS/m salt, PGPR treatments resulted in significant enhancement in ascorbic acid (36.0 %) with E. faecium, followed by P. dispersa (20.7 %) and their combination treatment (11.2 %). GSH (reduced glutathione) concentration was found to increase (1.68 and 1.24 folds) at 5 and 10 dS/m salt concentrations, respectively. PGPR treatments resulted in 9.1, 5.3 and 10.3 % increase in GSH with P. dispersa, E. faecium and P. dispersa + E. faecium treatments, respectively in 5 dS/m salt-stressed plants, compared to untreated salt-stressed plants (5 dS/m). Likewise, 18.8, 25.9 and 30.4 % enhancement was observed in GSH concentration with P. dispersa, E. faecium and P. dispersa + E. faecium, respectively at 10 dS/m salt stress (Table 6).
Correlation studies demonstrated a negative correlation in growth (r = − 0.62) and yield parameters (−0.77 to −0.87) with Na+ concentration in the salt-stressed plants, whereas a significant positive correlation was observed between K+ concentration and growth, yield, chlorophyll, RLWC of the treated plants. A positive correlation (r = 0.87) between Na+ concentration and stress damage parameter (electrolyte leakage) was noticed i.e. increased Na+ uptake resulted in enhanced membrane damage in salt-stressed plants. The chlorophyll as well as leaf water content of the PGPR treated plants were found to be negatively correlated (r = − 0.89, −0.79) with the Na+ concentration. Antioxidants play important role in stress tolerance, and these molecules showed a positive correlation ranging from 0.55 to 0.98, with Na+ concentration in the PGPR treated plants.
RAPD PCR
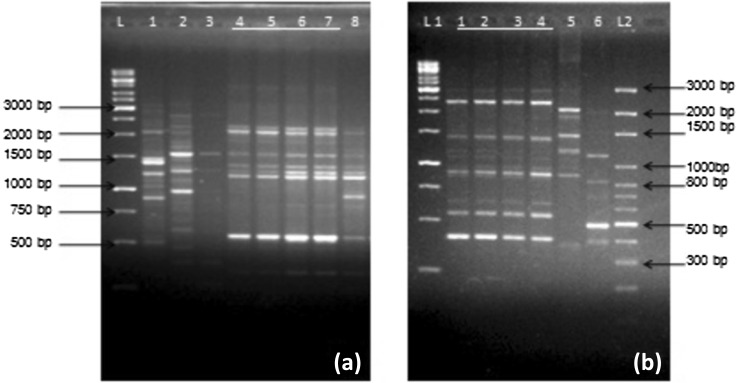
To confirm the survival of inoculated bacteria in the rhizosphere of mungbean, bacteria were re-isolated from the roots of the mungbean plants, after they reach maturity. RAPD analysis was performed with decamer arbitrary primer OPA-04 on the isolated bacteria as well as inoculated PGPR (P. dispersa and E. faecium). The presence of the inoculated bacteria in the rhizosphere was confirmed by simple matching the banding patterns obtained from RAPD gels. RAPD profile of isolates obtained from rhizosphere of grown mungbean plants treated with P. dispersa and E. faecium represented similar banding pattern as the initial inoculums used in the pot experiment. RAPD profile of 5, 6, 7 represented same banding pattern as P. dispersa in lane 4, with major amplicons of size 500, 1200, 1400, 1500, 2000 bp as depicted in Fig. 2. Similarly, RAPD profile of isolates 2, 3, 4 represented same banding pattern as E. faecium in lane 1, with major amplicons of approximate size 400, 550, 850, 1900 and 2500 bp as depicted in Fig. 2.
Fig. 2.
RAPD gel for P. dispersa and E. faecium. a Lane L represents 1 Kb DNA marker; lanes 1–8: RAPD profiles of isolates where 5, 6 and 7 represents same profile as P. dispersa (lane 4) showing amplified fragment size ranging from 300 to 2000 bp. b Lane L1 represents 1 Kb and L2 represents 100 bp DNA marker; lanes 1–6: RAPD profiles of the isolates, where 2, 3, 4 represents same profile as E. faecium (lane 1) showing amplified fragment size ranging from 400 to 2500 bp
Discussion
The present study was done to evaluate the effects of two native bacterial isolates, individually, as well as in combination, in salt-stressed mungbean crop. Both isolates were screened for their PGP traits, followed by their identification using 16S rRNA gene sequencing. The bacterial isolates were identified as Pantoea dispersa and Enterococcus faecium and possessed different levels of PGP traits namely P-solubilization, IAA and siderophore production and ACC deaminase activity, with salt (up to 150 mM) and without salt. Studies on PGP attributes in presence of salt has been done previously by different groups (Deshwal and Kumar 2013; Paul et al. 2014) supporting their tolerance without any reduction/inverse effects on PGP attributes up to 0.4 M and 0.3 M NaCl concentrations, respectively.
In our study, salt (NaCl) caused inhibitory effects on the growth and yield of mungbean. A reduction of 9.7–14.9 % (in height); 17.0–26.8 % (in biomass); 15.4–23.1 % (pods number); 16.5–20.3 % (seeds number); 22.4–26.5 % (pods weight) and 18.6–26.0 % (seeds weight) was observed in mungbean under saline stress of 5 and 10 dS/m NaCl. Salinity affects the growth of the plants by obstructing the cell division and enlargement in meristematic region; water deficit due to osmotic effect, inhibition of nutrient absorption or other metabolic disorders due to salt (Del Amor and Cuadra-Crespo 2012).
Our results illustrated that the combined PGPR inoculation has higher potential to improve mungbean’s growth and yield by mitigating the negative effects of salt. A significant improvement was recorded in vegetative and yield parameters of mungbean by PGPR inoculations under salt stress conditions with respect to controls (with salt). The better performance of combination treatments can be attributed to the synergistic interaction between these microorganisms. Also, the results were more promising with PGPR treatments at higher salinity level i.e. 10 dS/m, as compared to lower salt concentration (5 dS/m). The better performance at 10 dS/m can be accredited to the comparatively higher levels of PGP attributes at 10 dS/m (≈ 100 mM NaCl) than lower salt concentration, in our lab studies. The in vitro studies demonstrated that the PGP attributes showed gradual increase with increase in salt concentrations (50–150 mM). These results are supported by the findings of Chookietwattana and Maneewan (2012). The data also demonstrates that the performance of PGPR in the environment (in vivo) was in concordance with the in vitro study.
PGPR inoculations were effective in stress amelioration and have shown positive effects on the measured parameters in our study, which can be attributed to P-solubilization, siderophore production, IAA production or ACC deaminase activity. Auxins play important role in formation of lateral primordial in shoot and root apical meristem; promotes cytokinin synthesis in roots by feedback signaling thus helping in cell division and growth promotion (Dodd and Perez-Alfocea 2012). P-solubilization and siderophores can be responsible for making nutrients available for the plants thus contributing to growth promotion (Hayat et al. 2010). Another mechanism can be reduction of stress-induced ethylene by ACC deaminase enzyme produced by the bacteria, thereby improving the plant growth in stress conditions (Chaudhary and Sindhu 2015). In single PGPR treatments, E. faecium was found to be more effective as compared to P. dispersa. The better performance of E. faecium can be attributed to its higher IAA production and ACC deaminase activity in presence of salt. Therefore, improvement in vegetative growth and stress alleviation can also be attributed to the ACC deaminase activity and IAA production ability of PGPR (Glick 2014).
It was observed that RLWC and other parameters like chlorophyll concentration and electrolyte leakage were also improved with PGPR, maximum improvement was noticed with combination treatment. These effects are possibly due to reduced sodium uptake in PGPR-treated plants (discussed later). Photosynthesis and cell growth are among primary processes to be affected by salt stress (Munns and Tester 2008). Salinity affects the photosynthetic machinery, leading to decreased chlorophyll due to suppression of specific enzymes responsible for synthesis of photosynthetic pigments or also via membrane damage (Younesi et al. 2013). Our findings are in accordance with Barnawal et al. (2014) where microbial treatment was reported to improve chlorophyll concentration under salt stress of 10 and 20 dS/m, and maximum improvement shown by the combination (Arthrobacter protophormiae, Rhizobium and Glomus mosseae) treatment with respect to untreated stressed pea plants. An earlier study indicates that PGPR affects the stomatal conductance and PS II function and chlorophyll concentration, and thus photosynthetic efficiency (Del Amor and Cuadra-Crespo 2012).
Data in present illustration, showed a significant reduction in leaf Na+ concentration by approximately 1.2–1.6 folds and increment in K+ by 1.0–2.0 folds with PGPR (P. dispersa and E. faecium) application at 5 and 10 dS/m salt stress. During salt stress, the excessive Na+ ions compete with another major macronutrient like K+ leading to nutritional imbalance as well as metabolic disorders thus negatively affecting the plants. PGPR treatment can promote the plant growth by changing ion selectivity and maintaining the higher K+ concentration, as compared to Na+, as noticed in our study. High K+/Na+ ratio is one of the indicators of effective mechanisms to defend against salt stress (Jeschke and Wolf 1988). Comparatively, higher Na+ and less K+ concentration were reported in salt-stressed plants (10 dS/m), with respect to control, which was antagonized by PGPR application, in our study. The decreased Na+ accumulation by the PGPR treatment can also be attributed to the bacterial exopolysaccharides, which bind cations (especially Na+) in roots, thus prevent their transfer to leaves and help alleviate salt stress in plants (Ashraf et al. 2004).
Salt stress is linked to oxidative stress with respect to overproduction of ROS (OH−, O2 −, H2O2), detrimental for plant’s survival (Patel and Saraf 2013). ROS is major parameter to determine destruction caused by salt stress. ROS accumulation leads to membrane damage and oxidation of cellular macro molecules like nucleic acids, proteins etc. Lipid peroxidation is one of the biochemical markers for oxidative stress and is estimated by MDA concentration produced. MDA concentration was increased by 2.0–4.3 folds at both salt treatments i.e. 5 and 10 dS/m, implying the ROS accumulation and membrane damaging effects. Although PGPR application reduced the MDA concentration, maximum reduction (by 39 %) was recorded with combination treatment at both the salt stress conditions. Lower MDA concentration represents less membrane damage or increased salt tolerance of the plants (Jha and Subramanian 2014).
Accumulation of ROS like (O2 −) results in activation of SOD activity, which leads to synthesis of H2O2, a toxic signal molecule for oxidative stress (Miller 2010). Accumulation of H2O2 triggers the CAT (catalase), APO (ascrobate peroxidase) and POD (peroxidase) activities to reduce its concentration by converting it into oxygen and water (Tausz 2004). With salt stress, an increment in H2O2 content (2.1–4.6 folds) occurred however; it decreased with the PGPR treatment. Furthermore, decrease in antioxidant enzyme activities with PGPR in salt stressed plants in comparison to untreated stressed plants, were observed, which were antagonistic to the findings by Kang et al. (2014). In our study, the reduction in expression of enzymatic antioxidants in PGPR-treated plants growing under salt stress might be associated with decreased sodium uptake and consequently less oxidative damage. However, the non-enzymatic antioxidants i.e. glutathione and ascorbic acid concentrations were increased in response to the PGPR treatments. Glutathione can be responsible for the ROS scavenging activity and maintenance of reduced conditions in cells (Gill and Tuteja 2010). The increase in chlorophyll concentration and decrease in membrane damage can also be attributed to the increased concentration of ascorbic acid (Sayed et al. 2015).
To the best of our knowledge, this is first report on the PGPR attributes of E. faecium and its application on mungbean in combination with P. dispersa, on salt stressed mungbean plants. In this study, P. dispersa and E. faecium were able to perform efficiently under the unfavorable salt stress conditions to confer partial protection to mungbean plants. Our data also confirmed that PGPR mitigated the salt stress effects by reducing the sodium uptake. The lower oxidative stress in the inoculated mungbean plants under salt stress indicates the bacterial capacity to reduce ROS along with membrane damage and improve yield and vegetative parameters, primarily due to reduction in sodium uptake. We conclude that the native rhizobacteria existing in the rhizosphere of mungbean have the potential to adapt and resist the negative effects of salt stress, more efficiently. Further studies should be done in the field-raised plants growing under natural saline soils to validate the efficacy of these PGPR.
Acknowledgments
We sincerely thank Council of Scientific and Industrial Research, India for providing the financial assistance to the first author and Dr. Arvind Gulati from Institute of Himalayan Bioresource Technology (CSIR), Palampur, Himachal Pradesh, India for identification of strains using 16S rRNA gene sequencing.
Compliance with ethical standards
Conflict of interest
The author declares that they have no conflict of interest.
References
- Ahmad M, Zahir ZA, Khalid M, Nazli F, Arshad M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol Biochem. 2013;63:170–176. doi: 10.1016/j.plaphy.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Hasnain S, Berge O, Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils. 2004;40:157–162. [Google Scholar]
- Barbhaiya HB, Rao KK. Production of pyoverdine, the fluorescent pigment of Pseudomonas aeruginosa PA01. FEMS Microbiol Lett. 1985;27:233–235. doi: 10.1111/j.1574-6968.1985.tb00673.x. [DOI] [Google Scholar]
- Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A. ACC deaminase containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidasse activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol. 2014;171:884–894. doi: 10.1016/j.jplph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Betancor L, Pereira M, Martinez A, Giossa G, Fookes M, Flores K, Barrios P, Repiso V, Vignoli R, Cordeiro N, Algorta G, Thomson N, Maskell D, Schelotto F, Chabalgoity JA. Prevalence of Salmonella enterica in poultry and eggs in Uruguay during an epidemic due to Salmonella enterica serovar Enteritidis. J Clin Microbiol. 2010;48:2413–2423. doi: 10.1128/JCM.02137-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breed RS, Murray EGD, Smith NR. Bergey’s manual of determinative bacteriology. 7. Baltimore: Williams and Wilkins; 1957. p. 332. [Google Scholar]
- Chaudhary D, Sindhu SS. Inducing salinity tolerance in chickpea (Cicer arietinum L.) by inoculation of 1-aminocyclopropane-1-carboxylic acid deaminase containing Mesorhizobium strains. Afr J Microbiol Res. 2015;9:117–124. doi: 10.5897/AJMR2014.7087. [DOI] [Google Scholar]
- Chookietwattana K, Maneewan K. Selection of efficient salt-tolerant bacteria containing ACC deaminase for promotion of tomato growth under salinity stress. Soil Environ. 2012;31:30–36. [Google Scholar]
- Damam M, Gaddam B, Kausar R. Effect of plant growth promoting rhizobacteria (PGPR) on Coleus forskohlii. Int J Curr Microbiol App Sci. 2014;3:266–274. [Google Scholar]
- Dasgupta D, Ghati A, Sarkar A. Application of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Sesbania bispinosa on the growth of chickpea (Cicer arietinum L.) Int J Curr Microbiol App Sci. 2015;4:1033–1042. [Google Scholar]
- Del Amor FM, Cuadra-Crespo P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct Plant Biol. 2012;39:82–90. doi: 10.1071/FP11173. [DOI] [PubMed] [Google Scholar]
- Deshwal V, Kumar P. Effect of salinity on growth and PGPR activity of Pseudomonads. J Acad Ind Res. 2013;2:353–356. [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescense correlated with increased level of membrane permeability and lipid peroxidation and decreased level of superoxide dismutase and catalase. J Exp Bot. 1981;23:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Dodd IC, Perez-Alfocea F. Microbial amelioration of crop salinity stress. J Exp Bot. 2012;63:3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- Frankenberg WT, Jr, Poth M. L-tryptophan transaminase of a bacterium isolated from rhizosphere of Festuca octoflora. Soil Biol Biochem. 1988;20:299–304. doi: 10.1016/0038-0717(88)90007-7. [DOI] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Gorden SA, Paleg LG. Observations on the quantitative determination of indole acetic acid. Plant Physiol. 1957;10:39–47. doi: 10.1111/j.1399-3054.1957.tb07608.x. [DOI] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int. Article ID 6284547. doi:10.1155/2016/6284547 [DOI] [PMC free article] [PubMed]
- Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–598. doi: 10.1007/s13213-010-0117-1. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts-I: kinetics stoichiometry of fatty peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Holiday ER, Preedy JRK. Precision of a direct reading flame photometer for the determination of sodium and potassium in biological fluids. Biochem J. 1953;55:214–220. doi: 10.1042/bj0550214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem. 1978;42:1825–1831. [Google Scholar]
- Jackson ML. Methods of chemical analysis. New Delhi: Prentice Hall of India (Pvt) Ltd.; 1973. [Google Scholar]
- Jeschke WD, Wolf O. External potassium supply is not required for root growth in saline conditions: experiments with Ricinus communis L. grown in a reciprocal split-root system. J Exp Bot. 1988;39:1149–1167. doi: 10.1093/jxb/39.9.1149. [DOI] [Google Scholar]
- Jha Y, Subramanian RB. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants. 2014;20:201–207. doi: 10.1007/s12298-014-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita M, Bharadwaz M, Dey T, Gogoi K, Dowarah P, Unni BG, Ozah D, Saikia I. Developing novel bacterial based bioformulation having PGPR properties for enhanced production of agricultural crops. Indian J Exp Biol. 2015;53:56–60. [PubMed] [Google Scholar]
- Kang S, Khan AL, Waqas M, You Y, Kim JH, Kim JG, Humayun M, Lee I. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 2014;9:673–768. doi: 10.1080/17429145.2014.894587. [DOI] [Google Scholar]
- Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot. 1996;78:389–398. doi: 10.1006/anbo.1996.0134. [DOI] [Google Scholar]
- Mavis RD, Stellwagen E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J Biol Chem. 1968;243:809–814. [PubMed] [Google Scholar]
- Miller G, Suzuki N, Cifti-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mukherji SP, Chaudhari MA. Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Plant Physiol. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Palaniyandi SA, Damodharan K, Yang SH, Suh JW. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of “Micro Tom” tomato plants. J Appl Microbiol. 2014;117:766–773. doi: 10.1111/jam.12563. [DOI] [PubMed] [Google Scholar]
- Patel D, Saraf M. Influence of soil ameliorants and microflora on induction of antioxidant enzymes and growth promotion of Jatropha curcas L. under saline condition. Eur J Soil Biol. 2013;55:47–54. doi: 10.1016/j.ejsobi.2012.12.004. [DOI] [Google Scholar]
- Paul S, Aggarwal C, Thakur JK, Rathi MS. Effect of salt on growth and plant growth promoting activities of Azotobacter chroococcum isolated from saline soils. Environ Ecol. 2014;32:1255–1259. [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Reetha S, Bhuvaneswari G, Thamizhiniyan P, Mycin TR. Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allium cepa L.) Int J Curr Microbiol Appl Sci. 2014;3:568–574. [Google Scholar]
- Sayed E, Ahmed HES, Baziad SAM, Basaba RAAS. Application of exogenous ascorbic acid on tomato (Solanum lycopersicum L.) seeds under NaCl salinity stress. Int J Curr Res Biosci Plant Biol. 2015;2:33–46. [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GG. Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol. 2015;7:96–102. [Google Scholar]
- Tausz M. The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid. J Exp Bot. 2004;55:1955–1962. doi: 10.1093/jxb/erh194. [DOI] [PubMed] [Google Scholar]
- Teranishi Y, Tanaka A, Osumi M, Fukui S. Catalase activity of Hydrocarbon utilizing Candida yeast. Agric Biol Chem. 1974;38:1213–1216. doi: 10.1080/00021369.1974.10861301. [DOI] [Google Scholar]
- Younesi O, Chaichi MR, Postini K. Salt tolerance in Alfalfa following inoculation with Pseudomonas. Middle-East J Sci Res. 2013;16:101–107. [Google Scholar]