Abstract
Mosquito-borne viruses are known to cause disease in humans and livestock and are often difficult to control due to the lack of specific antivirals and vaccines. The Wolbachia endosymbiont has been widely studied for its ability to restrict positive-strand RNA virus infection in mosquitoes, although little is known about the precise antiviral mechanism. In recent years, a variety of insect-specific viruses have been discovered in mosquitoes and an interaction with mosquito-borne viruses has been reported for some of them; however, nothing is known about the effect of Wolbachia on insect-specific virus infection in mosquitoes. Here, we show that transinfection of the Drosophila-derived wMelPop Wolbachia strain into Aedes aegypti-derived cells resulted in inhibition and even clearance of the persistent cell-fusing agent flavivirus infection in these cells. This broadens the antiviral activity of Wolbachia from acute infections to persistent infections and from arboviruses to mosquito-specific viruses. In contrast, no effect on the Phasi Charoen-like bunyavirus persistent infection in these cells was observed, suggesting a difference in Wolbachia inhibition between positive- and negative-strand RNA viruses.
Keywords: Wolbachia, insect-specific virus, CFAV, PCLV, A. aegypti
Introduction
Arboviruses are comprised of human and animal pathogens that are transmitted via blood-feeding arthropod vectors, including mosquitoes. Due to the lack of efficient antivirals and vaccines against most of these viruses, vector control is an important intervention strategy to reduce the impact of these viruses on human and animal health (Kean et al., 2015; Weaver & Reisen, 2010). In recent years, the use of the endosymbiotic intracellular bacterium, Wolbachia, has been a well-studied approach to control arbovirus transmission by mosquitoes and in particular by Aedes aegypti (Iturbe-Ormaetxe et al., 2011; Rainey et al., 2014). Wolbachia was first shown to confer resistance to RNA viruses in Drosophila-virus systems (Hedges et al., 2008; Teixeira et al., 2008). Later, transinfection of Drosophila-derived Wolbachia into A. aegypti (which is not known to naturally harbour these endosymbionts) or its derived cell lines resulted in resistance to the important mosquito-borne dengue (DENV) and chikungunya viruses (Moreira et al., 2009; Walker et al., 2011). This has resulted in successful field trials of A. aegypti transinfected with Wolbachia, proving its ability to reduce DENV transmission in natural settings (Frentiu et al., 2014). Moreover, Wolbachia can be stably maintained in nature, as crosses between non-infected females and infected males do not result in any offspring (Hoffmann et al., 2011, 2014). This unique feature is called cytoplasmic incapability (McMeniman et al., 2009) and gives a reproductive advantage to infected female mosquitoes, resulting in the spread of Wolbachia through the mosquito population (Sinkins, 2004).
The mechanism(s) of virus inhibition through Wolbachia is not known. Inhibition has been linked to Wolbachia density, with the resistant phenotype observed only with Wolbachia strains producing high concentrations of bacteria in infected cells (Osborne et al., 2009, 2012). Recent findings show the ability of Wolbachia to interfere with early events in virus replication, suggesting an intrinsic mechanism for viral resistance (Rainey et al., 2016).
It should be noted that Wolbachia-mediated virus resistance has only been reported for positive-stranded RNA viruses and no resistance has yet been reported for negative-stranded RNA viruses (Rainey et al., 2014), which include a variety of important mosquito-borne viruses such as Rift Valley fever virus (Bunyaviridae).
Further to arboviruses, mosquitoes have also been shown to be infected with additional viruses, called insect-specific viruses (ISVs) as they replicate exclusively in insect cells. The list of ISVs is steadily increasing through novel identification methods, including next-generation sequencing. ISVs belong to different virus families/genera, including the Bunyaviridae and Flaviviridae families, which also include important arboviruses. ISVs belonging to the Flavivirus genus share sequence similarities with their arbovirus counterparts, but cluster as a single defined group suggesting independent evolution. In contrast, ISVs belonging to the Bunyaviridae cluster into several defined groups across the virus family (Bolling et al., 2015; Marklewitz et al., 2015). ISV infections, at least in cell culture, normally result in initial cytopathic effect, followed by progression into a persistent, non-cytopathic infection (Bolling et al., 2015; Marklewitz et al., 2015).
The increasing numbers of ISVs identified in mosquitoes and derived cells suggest that a large number of mosquitoes in the wild are naturally infected with ISVs and that vertical transmission is the main infection and maintenance route. Thereby one can expect that mosquitoes in the wild can be infected by several viruses, including ISVs and/or arboviruses. Moreover, the interaction between ISV and arbovirus infections (either co-infected or sequentially infected) results in either inhibition or increased replication/infection of one of the viruses (Kean et al., 2015). It is suggested that such interactions could partly define vector competence of a mosquito in the wild to a given arbovirus.
No information is available at the moment about the interaction of Wolbachia with these ISVs or what effect Wolbachia transinfection could have on mosquitoes already persistently infected with RNA viruses. The inhibitory effect of Wolbachia on RNA viruses has only been investigated in light of an acute virus infection following a persistent Wolbachia transinfection (Rainey et al., 2014).
In order to address these questions and to understand if Wolbachia interacts with acute or persistent infections of ISVs, we have used the A. aegypti-derived Aag2 cell line previously transinfected with the Drosophila-derived Wolbachia strain wMelPop (known to grow to high titres and mediate DENV resistance) (Hedges et al., 2008; Teixeira et al., 2008) to investigate the effect of Wolbachia on two ISVs, known to be present in Aag2 cells and belonging to different families: positive-strand RNA cell-fusing agent virus (CFAV, Flaviviridae) (Scott et al., 2010) and the negative-strand RNA Phasi Charoen-like bunyavirus (PCLV, Bunyaviridae) (Maringer et al., 2015). Our results show that Wolbachia can refer resistance to CFAV infection independently of the time of Wolbachia transinfection. In contrast, no viral inhibition by Wolbachia was observed for PCLV in these experiments.
Results
Effect of Wolbachia on small RNA production in Aag2 cells
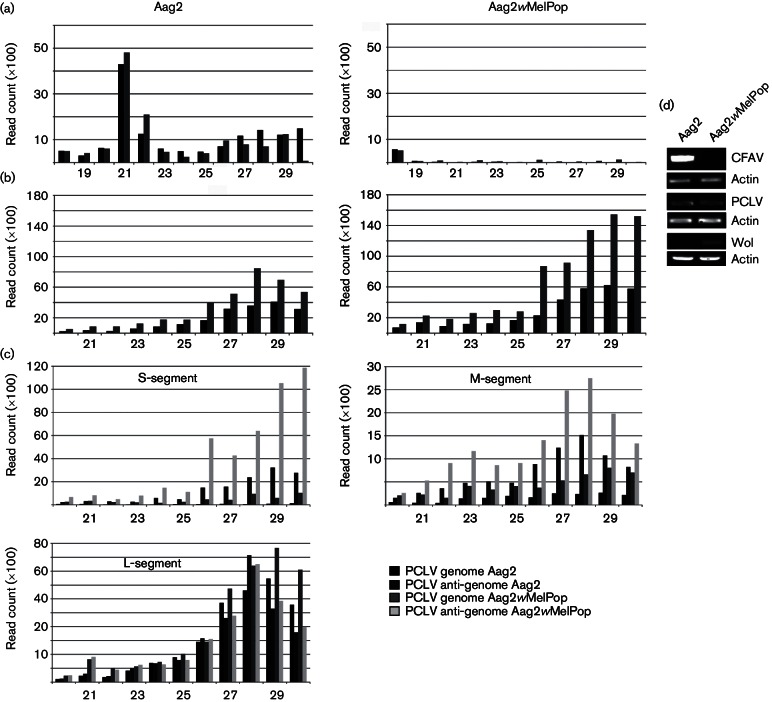
Aag2 cells can be stably transinduced with the wMelP strain of Drosophila, resulting in a reduction of small RNAs in the cytoplasm due to inhibition of small RNA transport from the nucleus to the cytoplasm (Mayoral et al., 2014). Aag2 cells are known to be persistently infected with the insect-specific flavivirus, CFAV and as result produce CFAV-specific small RNAs (Scott et al., 2010). Recently it has also been reported that Aag2 cells produce transcripts and proteins from another ISV, PCLV (suggesting a persistent infection) (Maringer et al., 2015). However, it is not yet known if this is due to an active virus infection. This virus has also been recently discovered in wild mosquitoes in Brazil (Aguiar et al., 2015). Therefore, we re-analysed the previously reported small RNA data of Aag2 and Aag2wMelPop cells (Mayoral et al., 2014) and mapped them to CFAV or PCLV. Almost no small RNA reads were detected in Aag2wMelPop cells mapping to CFAV, despite being observed in the parental Aag2 cells (Fig. 1a). The majority of CFAV small RNAs in the parental Aag2 cells were 21 nt in size, with similar amounts mapping to the genome and the antigenome. In contrast, small RNAs mapping against PCLV were identified in Aag2 cells and Aag2wMelPop cells, with a higher percentage in the Aag2wMelPop cells (Fig. 1b). The majority of PCLV small RNAs were 26–30 nt, mapped to the anti-genome and had sequence specificities seen for ping-pong-derived PIWI-interacting RNAs (piRNAs) (adenine at positionp 10, A10, and uridine at position 1, U1) (Fig. S1, available in the online Supplementary Material). The S-segment could be considered as the highest producer of PCLV-specific small RNAs, followed by the L- and M-segments. For the S- and M-segments, a bias could be observed for small RNAs of 26–30 nt mapping mainly to the anti-genome. For the L-segment, similar amounts of small RNAs mapping to the genome/anti-genome were detected with a slight bias for the genome (Fig. 1c). Small RNAs of 26–30 nt mapping to the genome and antigenome of CFAV were detected only in parental Aag2 cells and were absent from Aag2wMelPop cells (Fig. 1a). These 26–30 nt RNAs contained the U1 bias, but lacked the A10 bias (Fig. S2). The small number of CFAV-specific small RNAs 26–30 nt in length meant it was not possible to analyse the sequence logos for the CFAV-specific sequences in Aag2wMelPop cells. The presence or absence of wMelPop, as well as PCLV and CFAV in these cells, was determined by reverse transcription PCR (RT-PCR) (Fig. 1d). These data suggested that wMelPop reduces or even clears CFAV infection in persistently infected Aag2 cells, but has no or little effect on PCLV.
Fig. 1.
Presence or absence of CFAV, PCLV and wMelPop in Aag2 and Aag2wMelPop cells. Size distribution of small RNA molecules mapping to the CFAV (a) or PCLV (b) genome (black)/anti-genome (grey) in A. aegypti-derived Aag2 or wMelPop-transinfected Aag2 cells. (c) Size distribution of small RNA molecules mapping to the different segments of PCLV (S, M and L) genome/anti-genome in A. aegypti-derived Aag2 or wMelPop-transinfected Aag2 cells. (d) Detection of CFAV or PCLV in Aag2 and Aag2wMelPop cells by RT-PCR. Actin was used as loading control.
Effect of Wolbachia on persistent or acute ISV infection in Aag2 cells
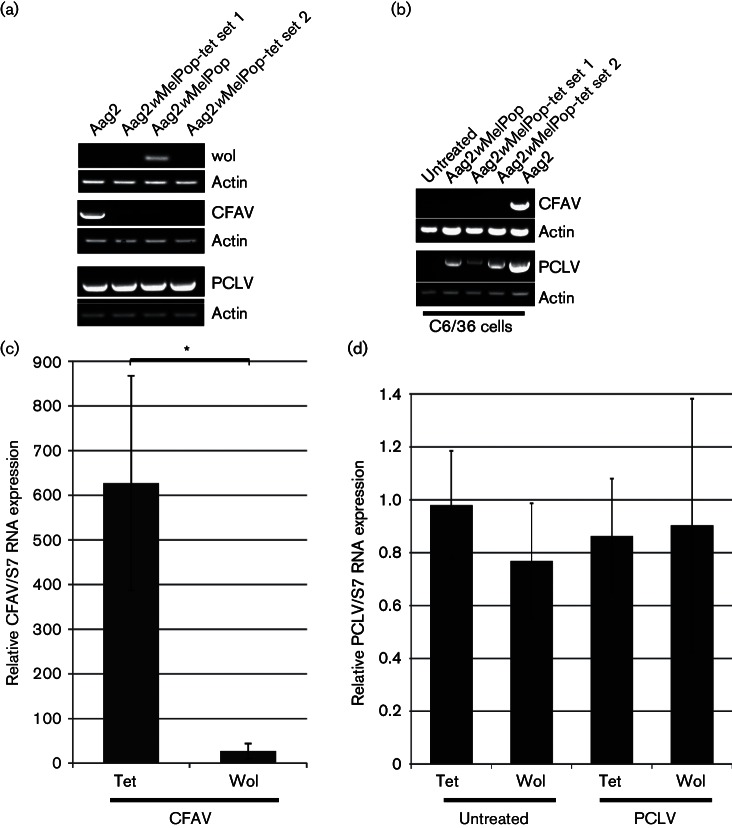
The presence of active PCLV production/infection in Aag2 and Aag2wMelPop cells was further confirmed by RT-PCR and was also detected following the transfer of supernatant from these cells to C6/36 cells, resulting in PCLV-positive C6/36 cells (Fig. 2a, b). CFAV was easily detected by RT-PCR in Aag2 cells, as well as in C6/36 cells incubated with Aag2 supernatant, in contrast to Aag2wMelPop or C6/36 cells incubated with Aag2wMelPop supernatant (Fig. 2a, b). To determine if the presence of wMelPop in Aag2 cells cured the cells from CFAV infection or just strongly inhibited CFAV replication/infection, Aag2wMelPop cells were treated with tetracycline over several passages, resulting in the loss of Wolbachia. The absence of Wolbachia in Aag2wMelPop-tetracyline-treated cells (called Aag2wMelPop-tet) was confirmed by RT-PCR (Fig. 2a). Similar to what is seen in the parental Aag2wMelPop cells, no CFAV could be detected in Aag2wMelPop-tet cells (Fig. 2a), even when a different region of the CFAV genome was used for detection (Fig. S3a) or in C6/36 cells incubated with Aag2wMelPop-tet supernatant (Fig. 2b). In contrast, PCLV was detected in each of these samples (Fig. 2a, b). This suggested that wMelPop transinfection cures Aag2 cells of persistent CFAV infection, but has no effect on PCLV. To exclude the possibility that tetracycline treatment per se inhibits CFAV, Aag2 cells were treated with tetracycline and CFAV levels were monitored over time. No effect on CFAV could be detected in tetracycline-treated Aag2 cells compared to untreated cells (Fig. S3b).
Fig. 2.
Effect of wMelPop on CFAV and PCLV infection in Aag2 cells. (a) Detection of CFAV, Wolbachia or PCLV in Aag2, Aag2wMelPop and two different cultures of Aag2wMelPop cells treated with tetracycline (Aag2wMelPop-tet sets 1 and 2) cells by RT-PCR. Actin was used as loading control. (b) Detection of CFAV or PCLV in C6/36 cells incubated with supernatant of Aag2, Aag2wMelPop or Aag2wMelPop treated with tetracycline (two different cultures, Aag2wMelPop-tet sets 1 and 2) by RT-PCR. Actin was used as a loading control. (c) Quantification of CFAV RNA in Aag2wMelPop (Wol) or Aag2wMelPop treated with tetracycline (Tet) cells after incubation with Aag2 supernatant containing CFAV by SYBR Green. S7 was used as internal control. Relative RNA expression is represented as (CFAV/S7). Error bars show sem from three independent experiments. (d) Quantification of PCLV RNA in Aag2wMelPop (Wol) or Aag2wMelPop treated with tetracycline (Tet) cells, either after incubation with Aag2 supernatant harbouring PCLV or untreated by SYBR Green. S7 was used as an internal control. Relative RNA expression is represented as (PCLV/S7) and mock-infected tetracycline cells were set to 1. Error bars show sem from three independent experiments. *P≤0.05.
To determine whether wMelPop has a similar effect on acute ISV infection, Aag2wMelPop and Aag2wMelPop-tet cells were incubated with Aag2 supernatant containing both CFAV and PCLV, and viral RNA detected by quantitative RT-PCR (Fig. 2c, d, respectively). Significantly less CFAV RNA was detected in Aag2wMelPop compared to Aag2wMelPop-tet cells. In contrast, no significant difference in PCLV RNA was observed under any of the test conditions.
In summary, these results show that wMelPop can inhibit CFAV infection in Aag2 cells, regardless of whether it is an acute or persistent infection, even resulting in total loss of CFAV in case of persistently infected cells. In contrast, no effect of PCLV was observed by wMelPop in Aag2 cells.
Discussion
Wolbachia endosymbionts have been studied for their ability to restrict RNA virus infection in Drosophila and A. aegypti mosquitoes, as well as their derived cell lines (Kean et al., 2015; Rainey et al., 2014). Little is known about the effects mediated by Wolbachia to induce antiviral activity, although density has been reported to be important (Osborne et al., 2009, 2012). Moreover, Wolbachia has recently been shown to inhibit early events during viral infection (Rainey et al., 2016). Over the last decade, a variety of ISVs have been discovered in mosquitoes and for some of them an interaction with mosquito-borne viruses has been reported that may be either beneficial or disadvantageous for these viruses (Bolling et al., 2015; Kean et al., 2015). However, nothing is known about the effect of Wolbachia transinfection on ISVs present in mosquitoes and whether there is a difference in the interaction depending on the virus (e.g. positive- versus negative-strand RNA virus). Transinfection of wMelPop into A. aegypti-derived Aag2 cells resulted in the inhibition and even clearance of persistent CFAV infection in these cells, broadening the antiviral activity of Wolbachia from acute infection to persistent infection. This could also be observed at the level of small RNA production, which was produced in Aag2 cells but not wMelPop Aag2 cells. Similar antiviral effects by Wolbachia were observed when these cured cells were freshly infected with acute CFAV infection. In contrast, no effect on PCLV persistent infection in these cells was observed after wMelPop transinfection; in addition, superinfection of PCLV in previously transinfected wMelPop cells resulted in no difference in PCLV replication. As expected from these results, small RNAs against PCLV were produced in both Aag2 and wMelPop Aag2 cells.
CFAV-specific small RNAs showed a bias for 21 nt, the typical size of Dicer-2-produced small interfering RNAs, as previously reported for CFAV (Scott et al., 2010) and other arthropod-borne flaviviruses (West Nile virus and DENV). In contrast, PCLV-specific small RNAs were mainly 26–30 nt in size, had a bias for the anti-genome and showed sequence-specific features for ping-pong-derived piRNAs (A10 and U1 bias) (Fig. S1). Similar results have been reported for other arthropod-borne bunyaviruses (Léger et al., 2013; Schnettler et al., 2013b), specifically for Rift Valley fever virus infection at later time points of infection (Léger et al., 2013). Interestingly, CFAV small RNAs of length 26–30 nt show the classic ping-pong signature of U1 bias in the positive (genome) orientation, but lack the A10 bias in the negative (anti-genome) orientation (Fig. S2). This raises the question whether these small RNAs are in fact piRNAs, whether just one type of piRNAs is produced in CFAV infection of Aag2 cells or whether some small RNAs are products of some other RNA decay pathway.
These results illustrated a difference in the ability of the endosymbiont to interfere with persistently infecting ISVs from different families. Until now Wolbachia has only been reported to have an antiviral effect against positive-strand RNA viruses during an acute infection (Frentiu et al., 2014; Martinez et al., 2014; Rainey et al., 2014, 2016), and the lack of effect of Wolbachia on PCLV is the first study to look at the interaction with a negative-strand RNA virus. Whether the observed lack of antiviral activity by Wolbachia is PCLV specific, or could be broadened to other negative-strand RNA viruses, still requires investigation. No antiviral effect was observed when persistently PCLV-infected and wMelPop-positive cells were superinfected with PCLV. It is not yet known whether this is due to the inability of wMelPop to inhibit PCLV infection, even at an acute stage of infection, or due to the inability of Aag2 cells to be superinfected with PCLV. Nonetheless, this raises some important questions for the field. For example, is Wolbachia-mediated inhibition limited to certain virus families and, if so, why is this the case? Could this be linked with the different small RNA profiles observed for flaviviruses versus bunyaviruses? How does this drive evolution of arboviruses or ISVs following the artificial introduction of Wolbachia into vector mosquitoes? What are the interactions between Wolbachia and ISVs and how do they influence vector competence in ISV-infected mosquitoes? Moreover, could it, for example during larger outbreaks involving many arboviruses, channel certain types of mosquito-borne pathogens and result in preferential amplification? Co-infection studies in mosquito systems with different families of arboviruses as well as ISVs are required to answer such questions.
In summary, wMelPop was able to efficiently inhibit persistent and acute infection of the positive-strand RNA insect-specific CFAV in Aag2 cells, but had no effect on persistent infection by the negative-strand RNA PCLV. Future research will have to investigate the effect of Wolbachia transinfection on other ISVs, as well as its effect on the complex interplay among ISVs, arboviruses and the mosquito vector, and how this influences/changes vector competence to different mosquito-borne viruses.
Methods
Cells and viruses.
A. aegypti-derived Aag2 wt, wMelPop-transinfected or wMelPop-transinfected and treated with tetracycline were maintained in Mitsuhashi and Maramorosch/Schneider’s (50 : 50) media supplemented with 10 % FCS and 10 % tryptose phosphate broth and PenStrep at 26 °C. Aag2- and wMelPop-transinfected cells were received from S. O'Neill and have been described previously (Mayoral et al., 2014). Aag2wMelPop-tet cells were produced by passaging Aag2wMelPop cells with 10 µg ml−1 tetracycline for four passages and maintained as described. C6/36 cells were maintained in L15 medium supplemented with 10 % FCS and 10 % tryptose phosphate broth and PenStrep at 28 °C. CFAV and PCLV were derived from Aag2 wt supernatant.
Reverse transcription, PCR and quantitative RT-PCR.
RT-PCR was performed with total RNA (1500 ng) isolated using TRIzol (Invitrogen), Superscript III and oligo-dT primer, according to the manufacturer’s protocol. CFAV, PCLV, Wolbachia and actin were detected and amplified by PCR (2 µl of the cDNA reaction) using corresponding primers [PCLV-N-FW: CAGTTAAAGCATTTAATCGTATGATAA, PCLV-N-RV: CACTAAGTGTTACAGCCCTTGGT, CFAV (3359 nt)-FW: GTTGACGACATATTGAAGAGATACG, CFAV (4060)-RV: GCCAAGGATACAGTCCAAAAC, CFAV-3UTR-FW: TAGACGTGATCGAATAGAGCCG, CFAV-3UTR-RV: GCGCATCTATGGTATAGAAAAGATAAT or as described previously (Rainey et al., 2016; Schnettler et al., 2013a)]. Quantitative detection of CFAV, PCLV and the housekeeping gene S7 was performed using specific primers [PCLV-N-qRT-FW: ATAGTGTGGGACGAGGAGGG, PCLV-N-qRT-RV: AGGTGCCAACAGGAAACACT, CFAV-qRT-FW: CTGATGTGCGTGCAGTTCTT, CFAV-qRT-RV: CACAACGGTAGCGAGAGACA or as described previously (McFarlane et al., 2014)], SYBR Green Master Mix (Applied Biosystems) and an ABI7500 Fast cycler according to manufacturer’s protocol.
Virus infection.
Aag2wMelPop or Aag2wMelPop-tet cells were incubated with 200 µl Aag2 supernatant for 24 h, followed by 3× PBS washes and addition of fresh culture medium. RNA was isolated at 48 h post-infection.
Small RNA analysis.
Small RNA reads from Aag2 (SRR1174240 and SRR1174241) and Aag2wMelPop cells (SRR1174242 and SRR1174243) published previously (Mayoral et al., 2014) were re-analysed. The datasets were downloaded from the SRA database and FastqQ reads were extracted using SRA toolkit. Using blastn, these reads were mapped to the CFAV (NCBI accession number NC_001564.1) and PCLV (NCBI accession numbers KR003786.1, KR003784.1 and KR003785.1 correspond to L, M and S segments, respectively) genome and anti-genome. Hits that matched and 20–30 nt with one maximum mismatch were taken for later analysis. These hits were further categorized into two groups, mapping to both the genome and the anti-genome.
Acknowledgements
T. M. was supported by Biotechnology and Biological Sciences Research Council (grant number BBSRC BB/J013854/1) and this work was supported by the MRC (MC_UU_12014). The authors wish to thank Scott O'Neill (Monash University) for providing the Aag2 and Aag2wMelPop cells, and members of the Kohl lab for critical reading of the manuscript.
Supplementary Data
References
- Aguiar E. R., Olmo R. P., Paro S., Ferreira F. V., de Faria I. J., Todjro Y. M., Lobo F. P., Kroon E. G., Meignin C., et al. (2015). Sequence-independent characterization of viruses based on the pattern of viral small RNAs produced by the host. Nucleic Acids Res 43 6191–6206. 10.1093/nar/gkv587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling B. G., Weaver S. C., Tesh R. B., Vasilakis N.(2015). Insect-specific virus discovery: significance for the arbovirus community. Viruses 7 4911–4928. 10.3390/v7092851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu F. D., Zakir T., Walker T., Popovici J., Pyke A. T., van den Hurk A., McGraw E. A., O'Neill S. L.(2014). Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia . PLoS Negl Trop Dis 8 e2688. 10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O'Neill S. L., Johnson K. N.(2008). Wolbachia and virus protection in insects. Science 322 702. 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Montgomery B. L., Popovici J., Iturbe-Ormaetxe I., Johnson P. H., Muzzi F., Greenfield M., Durkan M., Leong Y. S., et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Iturbe-Ormaetxe I., Callahan A. G., Phillips B. L., Billington K., Axford J. K., Montgomery B., Turley A. P., O'Neill S. L.(2014). Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8 e3115. 10.1371/journal.pntd.0003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I., Walker T., O' Neill S. L.(2011). Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 12 508–518. 10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean J., Rainey S. M., McFarlane M., Donald C. L., Schnettler E., Kohl A., Pondeville E.(2015). Fighting arbovirus transmission: natural and engineered control of vector competence in Aedes mosquitoes. Insects 6 236. 10.3390/insects6010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger P., Lara E., Jagla B., Sismeiro O., Mansuroglu Z., Coppée J. Y., Bonnefoy E., Bouloy M.(2013). Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J Virol 87 1631–1648. 10.1128/JVI.02795-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringer K., Yousuf A., Heesom K., Frnandez-sesma A., Matthews D. A., Davidson A. D.(2015). De novo ‘proteomics informed by transcriptomics’ analysis of aedes aegypti cells identifies novel ORFs, active transposable elements and persistent viruses. Conference Paper. International meeting on arboviruses and their vectors. 7-8. september 2015. Glasgow, UK. [Google Scholar]
- Marklewitz M., Zirkel F., Kurth A., Drosten C., Junglen S.(2015). Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc Natl Acad Sci U S A 112 7536–7541. 10.1073/pnas.1502036112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Longdon B., Bauer S., Chan Y. S., Miller W. J., Bourtzis K., Teixeira L., Jiggins F. M.(2014). Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10 e1004369. 10.1371/journal.ppat.1004369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral J. G., Etebari K., Hussain M., Khromykh A. A., Asgari S.(2014). Wolbachia infection modifies the profile, shuttling and structure of microRNAs in a mosquito cell line. PLoS One 9 e96107. 10.1371/journal.pone.0096107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane M., Arias-Goeta C., Martin E., O'Hara Z., Lulla A., Mousson L., Rainey S. M., Misbah S., Schnettler E., et al. (2014). Characterization of Aedes aegypti innate-immune pathways that limit chikungunya virus replication. PLoS Negl Trop Dis 8 e2994. 10.1371/journal.pntd.0002994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman C. J., Lane R. V., Cass B. N., Fong A. W., Sidhu M., Wang Y. F., O'Neill S. L.(2009). Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti . Science 323 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., Lu G., Pyke A. T., Hedges L. M., Rocha B. C., Hall-Mendelin S., Day A., et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 139 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Osborne S. E., Leong Y. S., O'Neill S. L., Johnson K. N.(2009). Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans . PLoS Pathog 5 e1000656. 10.1371/journal.ppat.1000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S. E., Iturbe-Ormaetxe I., Brownlie J. C., O'Neill S. L., Johnson K. N.(2012). Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans . Appl Environ Microbiol 78 6922–6929. 10.1128/AEM.01727-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey S. M., Shah P., Kohl A., Dietrich I.(2014). Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol 95 517–530. 10.1099/vir.0.057422-0 [DOI] [PubMed] [Google Scholar]
- Rainey S. M., Martinez J., McFarlane M., Juneja P., Sarkies P., Lulla A., Schnettler E., Varjak M., Merits A., et al. (2016). Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog 12 e1005536. 10.1371/journal.ppat.1005536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnettler E., Donald C. L., Human S., Watson M., Siu R. W. C., McFarlane M., Fazakerley J. K., Kohl A., Fragkoudis R.(2013a). Knockdown of piRNA pathway proteins results in enhanced Semliki forest virus production in mosquito cells. J Gen Virol 94 1680–1689. 10.1099/vir.0.053850-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnettler E., Ratinier M., Watson M., Shaw A. E., McFarlane M., Varela M., Elliott R. M., Palmarini M., Kohl A.(2013b). RNA interference targets arbovirus replication in culicoides cells. J Virol 87 2441–2454. 10.1128/JVI.02848-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Brackney D. E., Campbell C. L., Bondu-Hawkins V., Hjelle B., Ebel G. D., Olson K. E., Blair C. D.(2010). Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and -incompetent mosquito cells. PLoS Negl Trop Dis 4 e848. 10.1371/journal.pntd.0000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins S. P.(2004). Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem Mol Biol 34 723–729. 10.1016/j.ibmb.2004.03.025 [DOI] [PubMed] [Google Scholar]
- Teixeira L., Ferreira A., Ashburner M.(2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biol 6 e1000002. 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T., Johnson P. H., Moreira L. A., Iturbe-Ormaetxe I., Frentiu F. D., McMeniman C. J., Leong Y. S., Dong Y., Axford J., et al. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Reisen W. K.(2010). Present and future arboviral threats. Antiviral Res 85 328–345. 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.