Abstract
Background
Sex influences susceptibility to many infectious diseases, including some manifestations of leishmaniasis. The disease is caused by parasites that enter to the skin and can spread to the lymph nodes, spleen, liver, bone marrow, and sometimes lungs. Parasites induce host defenses including cell infiltration, leading to protective or ineffective inflammation. These responses are often influenced by host genotype and sex. We analyzed the role of sex in the impact of specific gene loci on eosinophil infiltration and its functional relevance.
Methods
We studied the genetic control of infiltration of eosinophils into the inguinal lymph nodes after 8 weeks of Leishmania major infection using mouse strains BALB/c, STS, and recombinant congenic strains CcS-1,-3,-4,-5,-7,-9,-11,-12,-15,-16,-18, and -20, each of which contains a different random set of 12.5% genes from the parental “donor” strain STS and 87.5% genes from the “background” strain BALB/c. Numbers of eosinophils were counted in hematoxylin-eosin-stained sections of the inguinal lymph nodes under a light microscope. Parasite load was determined using PCR-ELISA.
Results
The lymph nodes of resistant STS and susceptible BALB/c mice contained very low and intermediate numbers of eosinophils, respectively. Unexpectedly, eosinophil infiltration in strain CcS-9 exceeded that in BALB/c and STS and was higher in males than in females. We searched for genes controlling high eosinophil infiltration in CcS-9 mice by linkage analysis in F2 hybrids between BALB/c and CcS-9 and detected four loci controlling eosinophil numbers. Lmr14 (chromosome 2) and Lmr25 (chromosome 5) operate independently from other genes (main effects). Lmr14 functions only in males, the effect of Lmr25 is sex independent. Lmr15 (chromosome 11) and Lmr26 (chromosome 9) operate in cooperation (non-additive interaction) with each other. This interaction was significant in males only, but sex-marker interaction was not significant. Eosinophil infiltration was positively correlated with parasite load in lymph nodes of F2 hybrids in males, but not in females.
Conclusions
We demonstrated a strong influence of sex on numbers of eosinophils in the lymph nodes after L. major infection and present the first identification of sex-dependent autosomal loci controlling eosinophilic infiltration. The positive correlation between eosinophil infiltration and parasite load in males suggests that this sex-dependent eosinophilic infiltration reflects ineffective inflammation.
Keywords: Leishmania major, Mouse model, Eosinophil infiltration, Genetic control, QTL, Sex influence
Background
Sex influences susceptibility to many infectious diseases [1], including some manifestations of leishmaniasis [2], a disease that threatens several hundred million people in 98 countries [3]. Disability-adjusted life years (DALYs) due to leishmaniasis are globally increasing [4]. The disease is caused by intracellular protozoan parasites of the genus Leishmania and is transmitted to the vertebrates by the bite of female phlebotomine sand flies.
Leishmania parasites infect the so-called professional phagocytes (neutrophils, monocytes, and macrophages), as well as dendritic cells and fibroblasts. The major host cell is the macrophage, where parasites multiply, eventually rupturing the cell and spread to the uninfected cells (reviewed in [5]). Infected monocytes and macrophages circulating in the peripheral blood are believed to be carriers of the parasite to distal sites [6]. In the dermis, parasites cause the cutaneous form of the disease (which can be localized or diffuse), whereas infection of the mucosa gives rise to mucocutaneous leishmaniasis. The metastatic spread of the infection to the spleen and liver results in visceral leishmaniasis. Although these are the major sites of visceral disease, parasites can also enter other organs, such as the bone marrow, lymph nodes, and lungs (reviewed in [5]). Presence of parasites in organs usually induces inflammation through cascade of signals that leads to recruitment of inflammatory cells, such as neutrophils, macrophages, eosinophils, and dendritic cells. These innate immune cells might phagocytose parasites and/or produce cytokines and chemokines that activate both innate and adaptive immune responses. Resulting responses can be protective and eliminate parasites, or ineffective and lead to chronic inflammation [7].
The sex of the host influences the incidence of disease, parasite burden, pathology, mortality, and immunological response against various parasites, including Leishmania both in humans and in rodents (reviewed in [7–12]).
In general, sex bias is observed after infection with Leishmania parasites, and men are more frequently infected than women ([13–15]; reviewed in [11, 12]), although in certain areas no sex bias in prevalence of disease was observed [16]. The higher susceptibility of males also applies to hamster [17] and mouse [18, 19]; reviewed in [12] models of leishmaniasis. The effect of male orchidectomy and female testosterone replacement studies suggests that the hormone testosterone can modulate systemic L. major infection in BALB/cAnPt, DBA/2N, DBA/2J, and F1 hybrids (BALB/cAnPt x DBA/2N) mouse strains [18].
Importantly, the host genes, including those regulated differently in males and females, play a significant role in determining susceptibility and organ tropism for infectious diseases. Experimental data have shown different sex influence on susceptibility to relatively closely related pathogen species [20, 21], different sex biases in susceptibility to the same Leishmania species in different host genotypes [21, 22], and different sex and genetic influence on organ-specific pathology [21, 23, 24]. For example, high resistance to skin lesions induced by L. mexicana was observed in females but not in males of DBA/2 mice, but the sex effect was opposite in L. major infection [20].
Genotype influence on sex differences was defined in the studies of L. major infection [22, 24]. Giannini [22] found no sex effect on L. major-induced skin pathology and mortality in BALB/cJ mice, but a higher susceptibility of B10.129(10M)ScSn females than males. The comparison of L. major susceptibility in two strains, BALB/cHeA and CcS-11 [24], has shown that there is no significant sex influence on skin lesion development, splenomegaly, and hepatomegaly in these strains. However, parasite numbers in lymph nodes are higher in both BALB/c and CcS-11 males; moreover, CcS-11 males have higher parasite load in spleens, showing an organ-specific, sex-, and genotype-dependent pathology [24].
In the present study, we address influence of genotype and sex on infiltration of eosinophil leukocytes into the inguinal lymph nodes of L. major-infected mice. Eosinophils are granulocytes that develop in the bone marrow from pluripotent progenitors. They are released into the peripheral blood in phenotypically mature state and can be activated and recruited into tissues in response to appropriate stimuli, most notable IL-5, and the eotaxin chemokines [25].
Eosinophils contribute to the initiation of inflammatory and adaptive responses due to their bidirectional interactions with dendritic cells and T cells, as well as their large spectrum of secreted cytokines and soluble mediators. They have key immunoregulatory roles as professional antigen-presenting cells and modulators of functions of CD4+ T cells, dendritic cells, B cells, mast cells, neutrophils, and basophils [26].
Eosinophil-associated disorders can affect practically all tissues and organs in the body, either individually or in combination. They are involved in inflammatory conditions affecting the skin, cardiovascular, nervous and renal system, gastrointestinal tract, and upper and lower airways [27, 28], are key effector cells in eosinophilic asthma [29], and their interaction with peripheral nerves has impact on pathology of many diseases. In addition, they are also involved in regulatory mechanisms modulating local and systemic immune responses and remodeling and repair mechanisms [30].
Eosinophils may have an important role in maintaining host survival in life-threatening viral infections [31]. They combat worms such as Angiostrongylus cantonensis [32], Nippostrongylus brasiliensis [33], Litomosoides sigmodontis [34], and Brugia pahangi [35]; but their role in response to other nematoda is more complex. Eosinophils have no role in protection against Schistosoma mansoni [36]. They even promote larval growth in primary infection with Trichinella spiralis [37], but they mediate protective immunity against secondary infection with this nematode [38].
Activated eosinophils can kill [39] or support killing of L. major parasites [40]; however, in chronic disease, eosinophil infiltration might be a consequence of an ineffective elimination of these parasites and/or an excessive inflammatory response to the present pathogens [41].
Here, we analyzed genetic influence on eosinophil infiltration after L. major infection into the lymph nodes of strains BALB/cHeA (BALB/c), STS/A (STS), and selected 12 (out of 20) RC strains of CcS/Dem series [42]. Each of the 20 RC CcS/Dem strains contains a different unique set of approximately 12.5% genes of the donor strain STS on the genetic background of BALB/c. We found surprisingly high numbers of eosinophils in the inguinal lymph nodes of the strain CcS-9, males containing higher numbers of eosinophils than females. We analyzed genetics of this infiltration using microsatellite DNA markers and mapped four loci that control eosinophil numbers after L. major infection, one of them being strongly influenced by sex. We also found that the numbers of eosinophils in the lymph nodes correlate positively with the parasite load and that this correlation is partly genetically controlled and is higher in males than in females.
Methods
Mice
Tests of strain differences in eosinophil infiltration: Mice of the strains BALB/c (27 females, 27 males), STS (8 females, 9 males), CcS-1 (10 females, 13 males), CcS-3 (10 females, 10 males), CcS-4 (13 females, 12 males), CcS-5 (19 females, 27 males), CcS-7 (8 females, 12 males), CcS-9 (15 females, 10 males), CcS-11 (13 females, 13 males), CcS-12 (16 females, 12 males), CcS-15 (7 females, 12 males), CcS-16 (10 females, 13 males), CcS-18 (5 females, 3 males), and CcS-20 (13 females, 18 males) were infected with L. major as described previously [43, 44]. Mice were tested in eight successive experimental groups and were euthanized 8 weeks after infection. The age of mice at the time of infection was 7 to 47 weeks (mean 15 weeks, median 14 weeks).
A linkage study of eosinophil infiltration: F2 hybrids between CcS-9 and BALB/c (age 11 to 21 weeks at the time of infection, mean and median age 14.8 and 15 weeks, respectively) were produced at the Institute of Molecular Genetics. When used for these experiments, the CcS-9 was in the 40th generation of inbreeding and therefore highly homozygous. Two hundred fifty-four F2 hybrids between BALB/c and CcS-9 comprised 139 females and 115 males. Mice of the background parental strains BALB/c (18 females, 17 males) and STS (8 females, 6 males) and the RC strain CcS-9 (16 females, 14 males), 7 to 20 weeks old at the time of infection (mean 13 weeks, median 13 weeks), were used as controls. During the experiment, male and female mice were placed into separate rooms and males were caged individually. F2 mice were tested in three independent experimental groups.
Ethical statement
All experimental procedures in this study comply with the Czech Government Requirements under the Policy of Animal Protection Law (No.246/1992) and with the regulations of the Ministry of Agriculture of the Czech Republic (No.207/2004), which are in agreement with all relevant European Union guidelines for work with animals and were approved by the Institutional Animal Care Committee of the Institute of Molecular Genetics AS CR and by Departmental Expert Committee for the Approval of Projects of Experiments on Animals of the Academy of Sciences of the Czech Republic (permissions Nr. 274/2011; 89/2013).
Parasites
L. major LV 561 (MHOM/IL/67/LRC-L137 JERICHO II) was maintained in rump lesions of BALB/c females. Amastigotes were transformed to promastigotes using SNB-9 [43]. 107 promastigotes from the passage, two cultivated for 6 days were inoculated in 50 μl sterile saline s.c. into mouse rump [44].
Disease phenotype
The size of the primary skin lesion was measured weekly using a Vernier caliper gauge. The mice were killed 8 weeks after infection and inguinal lymph nodes draining the site of infection were collected for further analysis.
Histological analysis
Inguinal lymph nodes of female and male mice were fixed in 10% neutral buffered formalin (NBF; approximately 4% formaldehyde) and embedded in paraffin using automatic tissue processor. Tissue sections (5–7 μm) were stained with hematoxylin, differentiated into 1% acid alcohol, stained with 1% alcoholic eosin, dehydrated, assembled with permanent mounting medium, and analyzed under a light microscope (Olympus BX51; Olympus Optical Co. (EUROPA) GMBH., Hamburg, Germany).
Eosinophil infiltration in the experiment with parental strains BALB/c, STS, and 12 RC strains was assessed using a semi-quantitative scoring system: 0, no eosinophil; 0.25, 1 eosinophil; 0.5, 2 eosinophils; 0.75, 3–4 eosinophils; 1, 5 eosinophils; 1.5, 6 eosinophils; 2, 7 eosinophils; 2.5, 8–9 eosinophils; 3, 10–15 eosinophils; and 4, more than 15 eosinophils per lymph node section (one section was used in experiment with parental strains BALB/c, STS and 12 RC strains).
In F2 mice, as well as the parental strains BALB, STS, and CcS-9, eosinophil numbers were determined quantitatively. The total number of eosinophils was counted in the node section and each lymph node was assessed in four independent sections. The mean value of these four counts was used to calculate the role of genetic factors in control of eosinophil infiltration. Sixty slides from 15 mice were blindly recounted by an independent investigator with concordant results (R = 0.913, P value = 5.66 × 10−29).
Genotyping of F2 mice by PCR
DNA was isolated from tails using a standard proteinase procedure. The strain CcS-9 differs from BALB/c at STS-derived segments on eight chromosomes ([45] and unpublished results). These differential segments were typed in the F2 hybrid mice between CcS-9 and BALB/c using 18 microsatellite markers (Research Genetics, Huntsville, FL, USA): D2Mit283, D2Mit148, D4Mit172, D4Mit23, D4Mit53, D4Mit17, D5Mit24, D5Mit143, D6Mit122, D6Mit274, D9Mit15, D11Mit141, D11Mit242, D11Nds18, D11Nds10, D16Mit19, D17Mit120, and D17Mit122. The markers were selected because their genomic location makes them suitable to detect linkage. The maximum distance between any two markers in the chromosomal segments derived from the strain STS or from the nearest BALB/c derived markers was 12.46 cM, and mean distance was 4.67 cM. The PCR genotyping for markers with fragment length difference more than 8 bp was performed using unlabeled primers as in [46, 47]. The PCR genotyping for markers with fragment length difference less than 8 bp was performed using [γ-32P]ATP end-labeled primers as described elsewhere [48].
Measurement of parasite load in lymph nodes
Total DNA was isolated from the frozen lymph nodes, and parasite load was measured using PCR-ELISA according to the previously published protocol [49]. Briefly, for detection of Leishmania parasite DNA, in total DNA, PCR was performed using two primers (digoxigenin-labeled F 5′-ATT TTA CAC CAA CCC CCA GTT-3′ and biotin-labeled R 5′-GTG GGG GAG GGG CGT TCT-3′ (VBC Genomics Biosciences Research, Austria). The 120-bp fragment within the conserved region of the kinetoplast minicircle of Leishmania parasite was amplified. In each PCR reaction, 50 ng of extracted total DNA was used. As a positive control, 20 ng of L. major DNA per reaction was amplified as a highest concentration of the standard. A 26-cycle PCR reaction was used for quantification of parasites. Parasite load was determined by measurement of the PCR product with the modified ELISA protocol (Pharmingen, San Diego, USA). The concentration of Leishmania DNA was measured at the ELISA Reader Tecan with the curve fitter program KIM-E (Schoeller Pharma, Prague, Czech Republic) using least squares-based linear regression analysis [24, 49].
Statistical analysis
The differences among BALB/c, STS, and CcS/Dem strains in eosinophil numbers in lymph nodes were evaluated by the analysis of variance (ANOVA) and Newman-Keuls multiple comparison test at 95% significance using the program Statistica for Windows 12.0 (StatSoft, Inc., Tulsa, OK, USA).
Differences between sexes in BALB/c, STS, and CcS/Dem strains were calculated by ANOVA (Statistica for Windows 12.0; StatSoft, Inc., Tulsa, OK, USA).
The role of genetic factors in control of eosinophil infiltration in F2 hybrids was examined by ANOVA (Statistica for Windows 12.0; StatSoft, Inc., Tulsa, OK, USA). In order to obtain normal distribution of the analyzed parameter required for ANOVA, the obtained values were transformed as shown in the legends of tables. Markers and interactions with P < 0.05 were combined in a single comparison. In all ANOVA analyses strain or genotype, sex, and age were fixed factors, and the experiment was considered a random parameter.
For each independent variable, the partial R 2 was computed in the usual way by subtracting the regression sums of squares of the model without the variable (SS(b1,b2,b3,b4|b0)) of interest from the regression sums of squares of the full model (SS(b1,b2,b3,b4,b5|b0)); this difference divided by total regression sums of squares ((SS(b1,b2,b3,b4,b5|b0)):
indicated the contribution of the independent variable.
To obtain whole-genome significance values (corrected P values) the observed P values (aT) were adjusted according to Lander and Schork [50] using the formula:
where G = 1.75 Morgan (the length of the segregating part of the genome: 12.5% of 14 M); C = 8 (number of chromosomes segregating in cross between CcS-9 and BALB/c); ρ = 1.5 for F2 hybrids; h(T) = the observed statistics (F ratio).
The Spearman correlation coefficients between parasite numbers and eosinophil infiltration in the lymph nodes of F2 hybrid mice were computed using the program Statistica for Windows 12.0 (StatSoft, Inc., Tulsa, OK, USA).
Results
Infiltration of eosinophils into the inguinal lymph nodes in parental strains BALB/c and STS and selected RC strains
We infected with L. major both females and males of the strains BALB/c, STS, and RC strains CcS-1, CcS-3, CcS-4, CcS-5, CcS-7, CcS-9, CcS-11, CcS-12, CcS-15, CcS-16, CcS-18, and CcS-20 and used semi-quantitative scoring system to assess eosinophil infiltration (Table 1).
Table 1.
Eosinophil numbers in inguinal lymph nodes of L. major-infected mice
| % of mice with number of eosinophils (graded as 0–4) in section of inguinal lymph node | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 0.75 | 1 | 1.5 | 2 | 2.5 | 3 | 4 | ||
| Strain | Sex | 0 | 1 | 2 | 3–4 | 5 | 6 | 7 | 8–9 | 10–15 | >15 |
| BALB/c | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 81.48 | 0 | 3.70 | 0 | 3.70 | 3.70 | 7.41 | 0 | 0 | 0 | |
| STS | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CcS-1 | Females | 80.00 | 0 | 0 | 0 | 10.00 | 0 | 10.00 | 0 | 0 | 0 |
| Males | 76.92 | 7.69 | 0 | 0 | 15.38 | 0 | 0 | 0 | 0 | 0 | |
| CcS-3 | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 90.00 | 0 | 0 | 0 | 10.00 | 0 | 0 | 0 | 0 | 0 | |
| CcS-4 | Females | 92.31 | 0 | 7.69 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 91.67 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8.33 | 0 | |
| CcS-5 | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 96.30 | 0 | 0 | 0 | 3.70 | 0 | 0 | 0 | 0 | 0 | |
| CcS-7 | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 75.00 | 0 | 0 | 0 | 25.00 | 0 | 0 | 0 | 0 | 0 | |
| CcS-9 | Females | 73.33 | 0 | 0 | 0 | 20.00 | 0 | 6.67 | 0 | 0 | 0 |
| Males | 20.00 | 0 | 10.00 | 0 | 20.00 | 0 | 10.00 | 20.00 | 20.00 | 0 | |
| CcS-11 | Females | 84.62 | 0 | 7.69 | 7.69 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 61.54 | 0 | 23.08 | 0 | 7.69 | 0 | 7.69 | 0 | 0 | 0 | |
| CcS-12 | Females | 56.25 | 0 | 0 | 0 | 31.25 | 6.25 | 0 | 0 | 6.25 | 0 |
| Males | 50.00 | 0 | 8.33 | 0 | 16.67 | 0 | 16.67 | 0 | 0 | 8.33 | |
| CcS-15 | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 91.67 | 0 | 8.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CcS-16 | Females | 90.00 | 0 | 10.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CcS-18 | Females | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Males | 33.33 | 0 | 66.67 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CcS-20 | Females | 92.31 | 0 | 0 | 0 | 7.69 | 0 | 0 | 0 | 0 | 0 |
| Males | 95.00 | 0 | 0 | 0 | 5.00 | 0 | 0 | 0 | 0 | 0 | |
Eosinophil numbers in lymph nodes depending on genotype and sex. Eosinophil infiltration was evaluated as described in the “Methods” section. Numbers higher than 75% are shown in italics
These studies showed mild and no infiltration into the lymph nodes of parental strains BALB/c (Fig. 1a, b) and STS (Fig. 1c, d), respectively. Strains CcS-9 (P = 0.00020) (Fig. 1e, f) and CcS-12 (P = 0.0024) exhibit significantly higher eosinophil infiltration in their lymph nodes than the background parental strain BALB/c. BALB/c and CcS-9 males presented higher eosinophil infiltration than females of these strains P = 0.0089 and P = 0.016, respectively. 80% of examined CcS-9 males in comparison with 26.67% of CcS-9 females contained infiltrating eosinophils, 50% of males having 7 and more eosinophils in their lymph nodes (Table 1). Sex difference in strains CcS-7,-11, and -18 was not significant. Strain CcS-9 with the highest eosinophil infiltration (Table 1) was selected for further genetic studies.
Fig. 1.
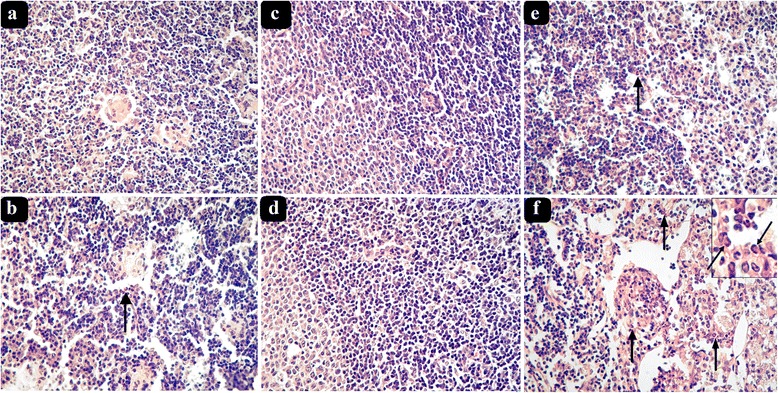
Eosinophils in hematoxylin-eosin-stained inguinal lymph node sections of L. major-infected female and male mice. a BALB/c female, b BALB/c male, c STS female, d STS male, e CcS-9 female, and f CcS-9 male with detail of eosinophils. Arrows show positions of eosinophils
Four novel loci control eosinophil infiltration in leishmaniasis
We examined eosinophil numbers in lymph nodes in 254 F2 hybrids between the strains BALB/c and CcS-9. The strain CcS-9 differs from BALB/c at STS-derived genetic regions located at eight chromosomes ([45], Šíma unpublished data). These differential STS-derived segments were genotyped in the F2 hybrid mice using 18 microsatellite markers. A statistical analysis of linkage revealed four genetic loci that influence eosinophil infiltration into the inguinal lymph nodes after L. major infection.
The effects of Lmr14 (L. major response 14) linked to D2Mit283 (corrected P value = 0.0081) and Lmr25 linked to D5Mit143 (corrected P value = 0.044) were detectable and significantly independent of each other or other genes (the main effects) (Table 2). Lmr14 operated only in males (corr. P value of marker and sex interaction =0.0085)(Table 2, Fig. 2), higher numbers of eosinophils were associated with presence of BALB/c (C) allele (Fig. 2c). The P value for Lmr14 was significant only in cross (CcS-9 × BALB/c)F2 (where the mother of the F1 hybrids was CcS-9 and the father was BALB/c) (Fig. 2c), but not in cross (BALB/c × CcS-9)F2 (where the mother was BALB/c and the father was CcS-9) (Fig. 2d). However, interaction between the cross and marker D2Mit283 was not significant (corr. P = 0.6). The effect of Lmr25 was not influenced by sex, and higher numbers of eosinophils were observed in heterozygotes (Table 2).
Table 2.
Main effect of loci that control eosinophil numbers in the inguinal lymph nodes of L. major-infected F2 hybrids between CcS-9 and BALB/c
| Locus | Group | Marker | Genotype | P value | Corr. P value | % of expl. var. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CS | SS | ||||||||||||
| Lmr14 | Both sexes | D2Mit283 | 2.62 | 1.229 | ±0.003 | 3.62 | 1.234 | ±0.002 | 2.67 | 1.229 | ±0.002 | NS | NS | NA |
| (n = 57) | (n = 111) | (n = 74) | ||||||||||||
| Females | 1.24 | 1.217 | ±0.003 | 1.45 | 1.220 | ±0.002 | 1.62 | 1.221 | ±0.003 | NS | NS | NA | ||
| (n = 35) | (n = 61) | (n = 32) | ||||||||||||
| Males both crosses | 5.15 | 1.239 | ±0.005 | 8.94 | 1.247 | ±0.003 | 4.02 | 1.236 | ±0.004 | 5.5 × 10−2 | NS | NA | ||
| (n = 22) | (n = 50) | (n = 41) | ||||||||||||
| Males (BALB/c × CcS-9)F2 | 3.37 | 1.233 | ±0.006 | 9.09 | 1.242 | ±0.004 | 3.78 | 1.235 | ±0.005 | NS | NS | NA | ||
| (n = 15) | (n = 32) | (n = 27) | ||||||||||||
| Males (CcS-9 × BALB/c)F2 | 13.63 | 1.253 | ±0.005 | 18.34 | 1.257 | ±0.004 | 4.51 | 1.237 | ±0.005 | 1.08 × 10−4 | 8.11 × 10 −3 | 36.22 | ||
| (n = 7) | (n = 18) | (n = 14) | ||||||||||||
| Lmr25 | Both sexes | D5Mit143 | 2.07 | 1.225 | ±0.003 | 4.33 | 1.237 | ±0.002 | 1.96 | 1.225 | ±0.003 | 9.53 × 10−4 | 4.36 × 10 −2 | 5.02 |
| (n = 66) | (n = 107) | (n = 67) | ||||||||||||
| Females | 1.09 | 1.215 | ±0.003 | 2.04 | 1.225 | ±0.002 | 1.20 | 1.216 | ±0.003 | 6.3 × 10−3 | NS | NA | ||
| (n = 37) | (n = 56) | (n = 36) | ||||||||||||
| Males | 5.71 | 1.241 | ±0.004 | 7.40 | 1.244 | ±0.003 | 4.41 | 1.237 | ±0.004 | NS | NS | NA | ||
| (n = 31) | (n = 51) | (n = 31) | ||||||||||||
Mean and SE values were obtained by analysis of variance. In order to obtain normal distribution required for analysis of variance, the value of eosinophil numbers in the inguinal lymph nodes was transformed by using the 0.1th power of natural logarithm of the (observed value ×1000). The numbers in bold give the average non-transformed values. C and S indicate the presence of BALB/c and STS allele, respectively
n number of mice
Fig. 2.

Effects of genotype and sex on eosinophil infiltration at Lmr14 (D2Mit283) a females (corr. P = NS), b males (corr. P = NS), c males (CcS-9 × BALB/c)F2 cross (corr. P = 8.11 × 10−3), and d males (BALB/c × CcS-9)F2 cross (corr. P = NS).These data are shown for sex and genotype CC—BALB/c homozygotes, CS—heterozygotes, SS—STS homozygotes as mean ± SD. NS not significant
In contrast to the main effects of Lmr14 and Lmr25, Lmr15 (linked to D11Nds10) and Lmr26 (linked to D9Mit15) operated in cooperation with each other (non-additive, epistatic, interaction) (corrected P = 0.010). F2 male mice of the cross (BALB/c × CcS-9)F2 with homozygous BALB/c (CC) alleles at both Lmr26 and Lmr15 had nearly nine times higher numbers of eosinophils in the lymph nodes than mice with homozygous STS (SS) alleles at both these loci, and nearly 90 times higher than mice with homozygous CC alleles at Lmr26 and CS alleles at Lmr15 (Table 3). The linkage was detected only in males, but the interaction between sex and marker was not significant (corr. P = 0.19).
Table 3.
Interaction between loci controlling eosinophil numbers in the inguinal lymph nodes in L. major-infected F2 hybrids between CcS-9 and BALB/c
| D9Mit15 (Lmr26) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CS | SS | ||||||||
| P = 3.7 × 10−2 | Corr. P = NS | % of expl. var. = NA | ||||||||
| D11Nds10 (Lmr15) | CC | 4.24 | 1.236 | ±0.006 | 3.03 | 1.231 | ±0.004 | 2.19 | 1.226 | ±0.005 |
| Both sexes | (n = 13) | (n = 26) | (n = 17) | |||||||
| CS | 1.45 | 1.220 | ±0.004 | 3.76 | 1.235 | ±0.003 | 3.88 | 1.235 | ±0.003 | |
| (n = 21) | (n = 63) | (n = 39) | ||||||||
| SS | 2.56 | 1.229 | ±0.005 | 2.37 | 1.228 | ±0.004 | 3.36 | 1.233 | ±0.005 | |
| (n = 17) | (n = 30) | (n = 16) | ||||||||
| P = 0.67 | Corr. P = NS | % of expl. var. = NA | ||||||||
| D11Nds10 (Lmr15) | CC | 1.89 | 1.224 | ±0.007 | 1.60 | 1.221 | ±0.004 | 1.42 | 1.219 | ±0.005 |
| Females | (n = 6) | (n = 18) | (n = 10) | |||||||
| Both crosses | CS | 1.37 | 1.219 | ±0.005 | 1.53 | 1.220 | ±0.003 | 2.28 | 1.227 | ±0.005 |
| (n = 11) | (n = 38) | (n = 17) | ||||||||
| SS | 1.67 | 1.222 | ±0.007 | 1.46 | 1.220 | ±0.004 | 1.06 | 1.214 | ±0.007 | |
| (n = 7) | (n = 16) | (n = 6) | ||||||||
| P = 2.63 × 10−4 | Corr. P = 1.037 × 10 −2 | % of expl. var. = 15.35 | ||||||||
| D11Nds10 (Lmr15) | CC | 11.71 | 1.251 | ±0.010 | 12.05 | 1.251 | ±0.008 | 3.49 | 1.234 | ±0.008 |
| Males | (n = 7) | (n = 8) | (n = 7) | |||||||
| Both crosses | CS | 1.06 | 1.214 | ±0.007 | 23.15 | 1.260 | ±0.005 | 12.22 | 1.251 | ±0.004 |
| (n = 10) | (n = 25) | (n = 22) | ||||||||
| SS | 6.55 | 1.243 | ±0.008 | 5.18 | 1.239 | ±0.005 | 7.27 | 1.244 | ±0.007 | |
| (n = 10) | (n = 14) | (n = 10) | ||||||||
| P = 0.41 | Corr. P = NS | % of expl. var. = NA | ||||||||
| D11Nds10 (Lmr15) | CC | 10.37 | 1.249 | ±0.008 | 12.60 | 1.252 | ±0.006 | 6.78 | 1.243 | ±0.008 |
| Males | (n = 2) | (n = 2) | (n = 3) | |||||||
| Cross CcS-9 × BALB | CS | 8.49 | 1.246 | ±0.022 | 21.20 | 1.258 | ±0.004 | 9.85 | 1.248 | ±0.007 |
| (n = 2) | (n = 10) | (n = 10) | ||||||||
| SS | 6.92 | 1.244 | ±0.012 | 3.75 | 1.235 | ±0.007 | 13.88 | 1.253 | ±0.022 | |
| (n = 4) | (n = 4) | (n = 2) | ||||||||
| P = 3.97 × 10−4 | Corr. P = 1.629 × 10 -2 | % of expl. var. = 21.19 | ||||||||
| D11Nds10 (Lmr15) | CC | 44.01 | 1.267 | ±0.012 | 32.78 | 1.264 | ±0.010 | 8.88 | 1.247 | ±0.016 |
| Males | (n = 5) | (n = 6) | (n = 4) | |||||||
| Cross BALB × CcS-9 | CS | 0.47 | 1.199 | ±0.010 | 13.04 | 1.252 | ±0.006 | 12.40 | 1.252 | ±0.006 |
| (n = 8) | (n = 15) | (n = 12) | ||||||||
| SS | 9.73 | 1.248 | ±0.011 | 6.78 | 1.243 | ±0.007 | 4.84 | 1.238 | ±0.008 | |
| (n = 6) | (n = 10) | (n = 8) | ||||||||
Mean and SE values were obtained by analysis of variance. In order to obtain normal distribution required for analysis of variance value of eosinophil numbers in serum inguinal lymph nodes was transformed by using the 0.1th power of natural logarithm of the (observed value ×1000). The numbers in bold give the average non-transformed values. C and S indicate the presence of BALB/c and STS allele, respectively
n number of mice
Positive correlation between parasite numbers and eosinophils in the inguinal lymph nodes
We have determined parasite load in the lymph nodes of the F2 hybrids between BALB/c and CcS-9 and analyzed the relationship between parasite numbers in lymph nodes and eosinophil infiltration to this organ. In both sexes pooled, there was a positive correlation between parasite numbers and eosinophil infiltration R = 0.39, P = 1.3 × 10−10, and the correlation was significant in males R = 0.29, P = 0.0017, but not in females R = 0.14, P = 0.10. This correlation is at least partly controlled by Lmr loci, because in F2 hybrid mice, this correlation was positive in male homozygous for the Lmr14 (D2Mit283) BALB/c allele (CC) (R = 0.51, P = 0.016) and STS allele (SS) (R = 0.50, P = 0.00088), but no correlation was observed in heterozygotes (R = −0.013, P = 0.92).
Discussion
Eosinophil infiltration in strain CcS-9 exceeds that of both parents
Strain CcS-9 that contains a set of approximately 12.5% genes of the donor strain STS and 87.5% genes of the background strain BALB/c exhibited numbers of infiltrating eosinophils (Fig. 1, Table 1) exceeding those in both parental strains BALB/c and STS. The observations of progeny having a phenotype, which is beyond the range of the phenotype of its parents, are not rare in traits controlled by multiple genes. It was detected in different tests of immune responses of RC strains in vitro [51–56] and in vivo [21, 57–60], and in analysis of expression quantitative trait loci (QTLs) from the livers of chromosome substitution strains [61]. These observations are due to multiple gene-gene interactions of QTLs, which in new combinations of these genes in RC or chromosomal substitution strains can lead to the appearance of new phenotypes that exceed their range in parental strains. In addition, with traits controlled by multiple loci, parental strains often contain eosinophil high infiltration alleles at some of them and eosinophil low infiltration alleles at others, and some progeny may receive predominantly eosinophil high infiltration alleles from both parents.
Sex influence on eosinophil infiltration
Our data show a sex influence on eosinophil numbers in the inguinal lymph nodes. Differences between the immune system of females and males have been well documented [62–64] and could result in differences in susceptibility to diseases with immune component. Immune responses including those involving eosinophils might be modulated by steroid hormones [65, 66]. Moreover, some of the differences between females and males might be due to sex-specific genetic architecture, characterized by profound gene-sex interactions [67, 68]. This would mean that some genes controlling response to L. major might operate differently in the two sexes. Indeed, locus Lmr14 controls eosinophil infiltration only in males. Genes controlling infections that appear to be sex dependent have been observed also with other infectious agents such as viruses [69–71], bacteria [72], parasites [58], and fungi [73] and helminths [74]. Some of sex-dependent QTLs exhibit a higher or exclusive influence on susceptibility in females [58, 69, 71–73] or males [69, 71–74], phenotypic effect of other genes is present in both sexes, but with opposite direction of effect [69, 70]. All these reported loci are situated on autosomal chromosomes. In contrast to the sex chromosomes, the autosomal genome is shared by both sexes. However, although the DNA sequence, gene structure, and frequency of polymorphism on the autosomes do not differ between males and females, the regulatory genome is sexually dimorphic [68].
Future genetic and functional studies will help to establish the mechanistic basis of the observed gene-sex interactions.
Loci controlling eosinophil infiltration and other immune traits
The Lmr loci influencing eosinophil infiltration may be related to QTLs that determine certain immunologically relevant traits, because they co-map with other immunological functional polymorphisms.
Interestingly, two of the eosinophil controlling loci, Lmr15 and Lmr26 co-localize with loci that determine hemopoietic cell cycling measured by cobblestone area-forming cell (CAFC) assay using cells from the bone marrow [75]. Lmr15 encompasses the mouse ortholog of human gene IL5, whose polymorphism was found to be associated with eosinophil counts in the blood [76], and Lmr26 co-localizes also with locus Tria5 that modifies in vitro proliferation of mouse splenocytes stimulated by soluble anti-CD3 [77].
The four described loci comprise several genes (Fig. 3), whose biological function is compatible with the effects on eosinophil infiltration [78–90] and their potential role can now be investigated. However, the effects of these Lmr loci might be also caused by genes that are at the present not considered as candidates. The issue of identity of eosinophil controlling genes and their possible relationship to other immune traits will be resolved by a recombinational analysis.
Fig. 3.

Loci controlling eosinophil numbers in CcS-9. The regions of STS and BALB/c origins are represented as dark and white, respectively; the boundary regions of undetermined origin are shaded. C and S indicate the presence of BALB/c and STS allele, respectively. Only the markers and SNPs defining the boundaries the STS-derived segment and the markers that were tested for linkage are shown. The markers that exhibit significant P values (genome-wide corrected) are shown in bold. Lmr loci on chromosomes 2 and 11 detected in a cross between CcS-9 and BALB/c overlap with loci Lmr14 [91–93] and Lmr15 [91, 93] detected in cross of CcS-16 with BALB/c, and were therefore given the same name. Abbreviations show genes that have been reported to be involved in eosinophil functions: Bcl2l1 (BCL2-like 1) [78], Ccl11 (chemokine (C-C motif) ligand 11) [79] Ccl3 (chemokine (C-C motif) ligand 3) [79], Ccl5 (chemokine (C-C motif) ligand 5) [79], Ccl7 (chemokine (C-C motif) ligand 7) [79], Ccl8 (chemokine (C-C motif) ligand 8) [80], Ccrl2 (chemokine (C-C motif) receptor-like 2) [81], Cish (cytokine inducible SH2-containing protein) [82], Gnai2 (guanine nucleotide binding protein (G protein), alpha inhibiting 2) [83], Hck (hemopoietic cell kinase) [84], Hmgb1 (high mobility group box 1) [85], Il13 (interleukin 13) [86], Il3 (interleukin 3) [87], Il4 (interleukin 4) [88], Il5 (interleukin 5) [89], and Nlrp3 (NLR family, pyrin domain containing 3) [90]. However, the effects of eosinophil controlling loci might be caused by genes that are at the present not considered as candidates
The positive correlation between eosinophil infiltration and parasite load suggests that the observed eosinophilic infiltration reflects ineffective inflammation. This is in agreement with kinetic studies showing that parasite presence preceded presence of infiltrating cells including eosinophils. This infiltration was higher in mice that were unable to control infection [41].
We have found positive correlation between eosinophil infiltration and parasite numbers in Lmr14 in homozygous (CC or SS), but not in heterozygous (CS) F2 hybrid males. The lack of positive correlation between eosinophil infiltration and parasite load in Lmr14 heterozygotes (CS) may reflect a more effective inflammation process, perhaps facilitated by other phenotypic effects of Lmr14 that include circulating levels of IFNγ, TNF, IgE, and IL-12 [91] and possibly other as yet undetected regulatory effects. This possibility has to be tested in future experiments.
Conclusions
This is the first demonstration of genetic loci and sex influence controlling infiltration of eosinophils into the lymph nodes and its relationship with parasite load. Some of these loci comprise genes with broader biological and immunological effects, so they might be relevant also in control of other diseases and symptoms mediated by eosinophils.
Our data also suggest that ignoring sex in gene mapping might prevent detection of sex-dependent QTLs.
Acknowledgements
M. Slapničková is a PhD student of Faculty of Science, The University of South Bohemia, České Budějovice, Czech Republic. M. Čepičková and M. Šíma are PhD students of Faculty of Sciences, Charles University, Prague, Czech Republic.
Funding
This investigation was supported by Czech Science Foundation (Grants GACR 14-30186S, GACR 16-22346S, and GACR 13-41002P), by the Ministry of Education, Youth and Sports of the Czech Republic (Grant Kontakt LH 12049) and The Czech Academy of Sciences (Project grant RVO: 68378050). This article is also based upon work from the COST Action BM1404 Mye-EUNITER (www.mye-euniter.eu), supported by COST (European Cooperation in Science and Technology). COST is part of the EU Framework Program Horizon 2020.
Availability of data and materials
The datasets collected and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MSl helped to conceive the study, performed animal experiments with RC strains, carried out all histological analyses, and contributed to the writing of the manuscript. VV performed statistical analyses and contributed to the writing of the manuscript. MC carried out animal experiments with F2 hybrids, typed F2 hybrids, and contributed to the writing of the manuscript. TK estimated parasite load in lymph nodes and re-counted eosinophils in number of mice. MŠí helped with typing of F2 hybrids, defined precisely STS-derived segments of strain CcS-9, and contributed to the writing of the manuscript. MSv cultivated parasites and helped with parasitology experiments. PD helped to analyze the data and contributed to the writing of the manuscript. ML conceived the study, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable. This study did not include human subjects.
Ethics approval
Not applicable. This study did not include human subjects.
Abbreviations
- CcS/Dem
Series of recombinant congenic strains derived from the mouse donor strain STS/A (STS) and the background strain BALB/cHeA (BALB/c)
- QTL
Quantitative trait locus
- RCS
Recombinant congenic strains
Contributor Information
Martina Slapničková, Email: martina.slapnickova@img.cas.cz.
Valeriya Volkova, Email: volkova@img.cas.cz.
Marie Čepičková, Email: maru.cepickova@seznam.cz.
Tatyana Kobets, Email: kobets@img.cas.cz.
Matyáš Šíma, Email: simam@img.cas.cz.
Milena Svobodová, Email: milena@natur.cuni.cz.
Peter Demant, Email: peter.demant@roswellpark.org.
Marie Lipoldová, Email: lipoldova@img.cas.cz.
References
- 1.Vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhopadhyay D, Mukherjee S, Ghosh S, Roy S, Saha B, Das NK, Chatterjee M. A male preponderance in patients with Indian post kala-azar dermal leishmaniasis is associated with increased circulating levels of testosterone. Int J Dermatol. 2016;55:250–5. doi: 10.1111/ijd.13048. [DOI] [PubMed] [Google Scholar]
- 3.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2013 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipoldová M, Demant P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet. 2006;7:294–305. doi: 10.1038/nrg1832. [DOI] [PubMed] [Google Scholar]
- 6.Noronha FS, Cruz JS, Beirão PS, Horta MF. Macrophage damage by Leishmania amazonensis cytolysin: evidence of pore formation on cell membrane. Infect Immun. 2000;68:4578–84. doi: 10.1128/IAI.68.8.4578-4584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez NE, Wilson ME. Eosinophils and mast cells in leishmaniasis. Immunol Res. 2014;59:129–41. doi: 10.1007/s12026-014-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–64. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 9.Snider H, Lezama-Davila C, Alexander J, Satoskar AR. Sex hormones and modulation of immunity against leishmaniasis. Neuroimmunomodulation. 2009;16:106–13. doi: 10.1159/000180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander J, Irving K, Snider H, Satoskar A. Sex hormones of host responses against parasites. In: Klein SL, Roberts CW, editors. Sex hormons and immunity to infection. Dordrecht, London, New York: Springer Heildelberg; 2010. pp. 147–86. [Google Scholar]
- 11.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–88. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernin H, Lotter H. Sex bias in the outcome of human tropical infectious diseases: influence of steroid hormones. J Infect Dis. 2014;15(209 Suppl 3):S107–13. doi: 10.1093/infdis/jit610. [DOI] [PubMed] [Google Scholar]
- 13.Al-Jawabreh A, Dumaidi K, Ereqat S, Al-Jawabreh H, Nasereddin A, Azmi K, Barghuthy F, Sawalha S, Salah I, Abdeen Z. Molecular epidemiology of human cutaneous leishmaniasis in Jericho and its vicinity in Palestine from 1994 to 2015. Infect Genet Evol. 2016. doi: 10.1016/j.meegid.2016.06.007. [Epub ahead of print]. [DOI] [PubMed]
- 14.Armijos RX, Weigel MM, Izurieta R, Racines J, Zurita C, Herrera W, Vega M. The epidemiology of cutaneous leishmaniasis in subtropical Ecuador. Trop Med Int Health. 1997;2:140–52. doi: 10.1046/j.1365-3156.1997.d01-236.x. [DOI] [PubMed] [Google Scholar]
- 15.Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8:e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosravani M, Moemenbellah-Fard MD, Sharafi M, Rafat-Panah A. Epidemiologic profile of oriental sore caused by Leishmania parasites in a new endemic focus of cutaneous leishmaniasis, southern Iran. J Parasit Dis. 2016;40:1077–81. doi: 10.1007/s12639-014-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun. 2002;70:2288–96. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mock BA, Nacy CA. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect Immun. 1988;56:3316–9. doi: 10.1128/iai.56.12.3316-3319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mock BA, Fortier AH, Potter M, Nacy CA. Genetic control of systemic Leishmania major infections: dissociation of intrahepatic amastigote replication from control by the Lsh gene. Infect Immun. 1985;50:588–91. doi: 10.1128/iai.50.2.588-591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology. 1988;96:297–302. doi: 10.1017/S0031182000058303. [DOI] [PubMed] [Google Scholar]
- 21.Kobets T, Havelková H, Grekov I, Volkova V, Vojtíšková J, Slapničková M, Kurey I, Sohrabi Y, Svobodová M, Demant P, Lipoldová M. Genetics of host response to Leishmania tropica in mice—different control of skin pathology, chemokine reaction, and invasion into spleen and liver. PLoS Negl Trop Dis. 2012;6:e1667. doi: 10.1371/journal.pntd.0001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannini MSH. Sex-influenced response in the pathogenesis of cutaneous leishmaniasis in mice. Parasite Immunol. 1986;8:31–7. doi: 10.1111/j.1365-3024.1986.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobets T, Grekov I, Lipoldová M. Leishmaniasis: prevention, parasite detection and treatment. Curr Med Chem. 2012;19:1443–74. doi: 10.2174/092986712799828300. [DOI] [PubMed] [Google Scholar]
- 24.Kurey I, Kobets T, Havelková H, Slapničková M, Quan L, Trtková K, Grekov I, Svobodová M, Stassen AP, Hutson A, Demant P, Lipoldová M. Distinct genetic control of parasite elimination, dissemination, and disease after Leishmania major infection. Immunogenetics. 2009;61:619–33. doi: 10.1007/s00251-009-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–63. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akuthota P, Weller PF. Spectrum of eosinophilic end-organ manifestations. Immunol Allergy Clin North Am. 2015;35:403–11. doi: 10.1016/j.iac.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusareva ES, Kurey I, Grekov I, Lipoldová M. Genetic regulation of immunoglobulin E level in different pathological states: integration of mouse and human genetics. Biol Rev Camb Philos Soc. 2014;89:375–405. doi: 10.1111/brv.12059. [DOI] [PubMed] [Google Scholar]
- 30.Raap U, Wardlaw AJ. A new paradigm of eosinophil granulocytes: neuroimmune interactions. Exp Dermatol. 2008;17:731–8. doi: 10.1111/j.1600-0625.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 31.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–52. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–54. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 33.Shin EH, Osada Y, Chai JY, Matsumoto N, Takatsu K, Kojima S. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Immunol. 1997;114(Suppl 1):45–50. doi: 10.1159/000237717. [DOI] [PubMed] [Google Scholar]
- 34.Martin C, Le Goff L, Ungeheuer MN, Vuong PN, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect Immun. 2000;68:3651–6. doi: 10.1128/IAI.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalingam T, Porte P, Lee J, Rajan TV. Eosinophils, but not eosinophil peroxidase or major basic protein, are important for host protection in experimental Brugia pahangi infection. Infect Immun. 2005;73:8442–3. doi: 10.1128/IAI.73.12.8442-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–7. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–83. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L, Gebreselassie NG, Gagliardo LF1, Ruyechan MC, Luber KL, Lee NA, Lee JJ, Appleton JA. Eosinophils mediate protective immunity against secondary nematode infection. J Immunol. 2015;194:283–90. doi: 10.4049/jimmunol.1402219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira SH, Fonseca SG, Romão PR, Figueiredo F, Ferreira SH, Cunha FQ. Microbicidal activity of eosinophils is associated with activation of the arginine-NO pathway. Parasite Immunol. 1998;20:405–12. doi: 10.1046/j.1365-3024.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 40.Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–42. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–77. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- 42.Demant P, Hart AAM. Recombinant congenic strains—a new tool for analysing genetic traits determined by more than one gene. Immunogenetics. 1996;24:416–22. doi: 10.1007/BF00377961. [DOI] [PubMed] [Google Scholar]
- 43.Grekov I, Svobodová M, Nohýnková E, Lipoldová M. Preparation of highly infective Leishmania promastigotes by cultivation on SNB-9 biphasic medium. J Microbiol Meth. 2011;87:273–7. doi: 10.1016/j.mimet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Lipoldová M, Svobodová M, Krulová M, Havelková H, Badalová J, Nohýnková E, Holáň V, Hart AAM, Volf P, Demant P. Susceptibility to Leishmania major infection in mice: multiple loci and heterogeneity of immunopathological phenotypes. Genes Immun. 2000;1:200–6. doi: 10.1038/sj.gene.6363660. [DOI] [PubMed] [Google Scholar]
- 45.Stassen AP, Groot PC, Eppig JT, Demant P. Genetic composition of the recombinant congenic strains. Mamm Genome. 1996;7:55–8. doi: 10.1007/s003359900013. [DOI] [PubMed] [Google Scholar]
- 46.Sohrabi Y, Havelková H, Kobets T, Šíma M, Volkova V, Grekov I, Jarošíková T, Kurey I, Vojtíšková J, Svobodová M, Demant P, Lipoldová M. Mapping the genes for susceptibility and response to Leishmania tropica in mouse. PLoS Negl Trop Dis. 2013;7:e2282. doi: 10.1371/journal.pntd.0002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Šíma M, Kocandová L, Lipoldová M. Genotyping of short tandem repeats (STRs) markers with 6 bp or higher length difference using PCR and high resolution agarose electrophoresis. Protoc Exch. 2015. doi:10.1038/protex.2015.054.
- 48.Krulová M, Havelková H, Kosařová M, Holáň V, Hart AA, Demant P, et al. IL-2-induced proliferative response is controlled by loci Cinda1 and Cinda2 on mouse chromosomes 11 and 12: a distinct control of the response induced by different IL-2 concentration. Genomics. 1997;42:11–5. doi: 10.1006/geno.1997.4694. [DOI] [PubMed] [Google Scholar]
- 49.Kobets T, Badalová J, Grekov I, Havelková H, Svobodová M, Lipoldová M. Leishmania parasite detection and quantification using PCR-ELISA. Nat Protoc. 2010;5:1074–80. doi: 10.1038/nprot.2010.68. [DOI] [PubMed] [Google Scholar]
- 50.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–48. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 51.Lipoldová M, Kosařová M, Zajícová A, Holáň V, Hart AA, et al. Separation of multiple genes controlling the T-cell proliferative response to IL-2 and anti-CD3 using recombinant congenic strains. Immunogenetics. 1995;41:301–11. doi: 10.1007/BF00172155. [DOI] [PubMed] [Google Scholar]
- 52.Holáň V, Lipoldová M, Demant P. Identical genetic control of MLC reactivity to different MHC incompatibilities, independent of production of and response to IL-2. Immunogenetics. 1996;44:27–35. doi: 10.1007/BF02602654. [DOI] [PubMed] [Google Scholar]
- 53.Havelková H, Badalová J, Demant P, Lipoldová M. A new type of genetic regulation of allogeneic response. A novel locus on mouse chromosome 4, Alan2 controls MLC reactivity to three different alloantigens: C57BL/10, BALB/c and CBA. Genes Immun. 2000;1:483–7. doi: 10.1038/sj.gene.6363711. [DOI] [PubMed] [Google Scholar]
- 54.Lipoldová M, Havelková H, Badalová J, Demant P. Novel loci controlling lymphocyte proliferative response to cytokines and their clustering with loci controlling autoimmune reactions, macrophage function and lung tumor susceptibility. Int J Cancer. 2005;114:394–9. doi: 10.1002/ijc.20731. [DOI] [PubMed] [Google Scholar]
- 55.Havelková H, Holáň V, Kárník I, Lipoldová M. Mouse model for analysis of non-MHC genes that influence allogeneic response: recombinant congenic strains of OcB/Dem series that carry identical H2 locus. Cent Eur J Biol. 2006;1:16–28. [Google Scholar]
- 56.Lipoldová M, Havelková H, Badalová J, Vojtíšková J, Quan L, Krulová M, Sohrabi Y, Stassen AP, Demant P. Loci controlling lymphocyte production of interferon γ after alloantigen stimulation in vitro and their co-localization with genes controlling lymphocyte infiltration of tumors and tumor susceptibility. Cancer Immunol Immunother. 2010;59:203–13. [DOI] [PMC free article] [PubMed]
- 57.Lipoldová M, Svobodová M, Havelková H, Krulová M, Badalová J, et al. Mouse genetic model for clinical and immunological heterogeneity of leishmaniasis. Immunogenetics. 2002;54:174–83. doi: 10.1007/s00251-002-0439-7. [DOI] [PubMed] [Google Scholar]
- 58.Šíma M, Havelková H, Quan L, Svobodová M, Jarošíková T, Vojtíšková J, Stassen APM, Demant P, Lipoldová M. Genetic control of resistance to Trypanosoma brucei bruceiinfection in mice. PLoS Negl Trop Dis. 2011;5:e1173. [DOI] [PMC free article] [PubMed]
- 59.Szymanska H, Sitarz M, Krysiak E, Piskorowska J, Czarnomska A, Skurzak H, Hart AA, de Jong D, Demant P. Genetics of susceptibility to radiation-induced lymphomas, leukemias and lung tumors studied in recombinant congenic strains. Int J Cancer. 1999;83:674–8. doi: 10.1002/(SICI)1097-0215(19991126)83:5<674::AID-IJC18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 60.Palus M, Vojtíšková J, Salát J, Kopecký J, Grubhoffer L, Lipoldová M, Demant P, Růžek D. Mice with different susceptibility to tick-borne encephalitis virus infection show selective neutralizing antibody response and inflammatory reaction in the central nervous system. J Neuroinflamm. 2013;10:77. doi: 10.1186/1742-2094-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shockley KR, Churchill GA. Gene expression analysis of mouse chromosome substitution strains. Mamm Genome. 2006;17:598–614. doi: 10.1007/s00335-005-0176-y. [DOI] [PubMed] [Google Scholar]
- 62.Halberg F, Hamerston O, Bittner JJ. Sex difference in eosinophil counts in tall blood of mature B1 mice. Science. 1957;125:73. doi: 10.1126/science.125.3237.73. [DOI] [PubMed] [Google Scholar]
- 63.Madalli S, Beyrau M, Whiteford J, Duchene J, Singh Nandhra I, Patel NS, Motwani MP, Gilroy DW, Thiemermann C, Nourshargh S, Scotland RS. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol Sex Differ. 2015;6:27. doi: 10.1186/s13293-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Case LK, Teuscher C. Y genetic variation and phenotypic diversity in health and disease. Biol Sex Differ. 2015;6:6. doi: 10.1186/s13293-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamano N, Terada N, Maesako K, Numata T, Konno A. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc. 1998;19:263–9. doi: 10.2500/108854198778557773. [DOI] [PubMed] [Google Scholar]
- 66.Keselman A, Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front Immunol. 2015;6:568. doi: 10.3389/fimmu.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhasin JM, Chakrabarti E, Peng DQ, Kulkarni A, Chen X, Smith JD. Sex specific gene regulation and expression QTLs in mouse macrophages from a strain intercross. PLoS One. 2008;3:e1435. doi: 10.1371/journal.pone.0001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butterfield RJ, Roper RJ, Rhein DM, Melvold RW, Haynes L, et al. Sex-specific quantitative trait loci govern susceptibility to Theiler’s murine encephalomyelitis virus-induced demyelination. Genetics. 2003;163:1041–6. doi: 10.1093/genetics/163.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuurhof A, Bont L, Siezen CL, Hodemaekers H, van Houwelingen HC, et al. Interleukin-9 polymorphism in infants with respiratory syncytial virus infection: an opposite effect in boys and girls. Pediatr Pulmonol. 2010;45:608–13. doi: 10.1002/ppul.21229. [DOI] [PubMed] [Google Scholar]
- 71.Boivin GA, Pothlichet J, Skamene E, Brown EG, Loredo-Osti JC, Sladek R, Vidal SM. Mapping of clinical and expression quantitative trait loci in a sex-dependent effect of host susceptibility to mouse-adapted influenza H3N2/HK/1/68. J Immunol. 2012;188:3949–60. doi: 10.4049/jimmunol.1103320. [DOI] [PubMed] [Google Scholar]
- 72.Min-Oo G, Lindqvist L, Vaglenov A, Wang C, Fortin P, et al. Genetic control of susceptibility to pulmonary infection with Chlamydia pneumoniae in the mouse. Genes Immun. 2008;9:383–8. doi: 10.1038/sj.gene.6364450. [DOI] [PubMed] [Google Scholar]
- 73.Carroll SF, Loredo Osti JC, Guillot L, Morgan K, Qureshi ST. Sex differences in the genetic architecture of susceptibility to Cryptococcus neoformans pulmonary infection. Genes Immun. 2008;9:536–45. doi: 10.1038/gene.2008.48. [DOI] [PubMed] [Google Scholar]
- 74.Hayes KS, Hager R, Grencis RK. Sex-dependent genetic effects on immune responses to a parasitic nematode. BMC Genomics. 2014;15:193. doi: 10.1186/1471-2164-15-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Haan G, Van Zant G. Genetic analysis of hemopoietic cell cycling in mice suggests its involvement in organismal life span. FASEB J. 1999;13:707–13. doi: 10.1096/fasebj.13.6.707. [DOI] [PubMed] [Google Scholar]
- 76.Gudbjartsson DF, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 77.Havelková H, Kosařová M, Krulová M, Demant P, Lipoldová M. T-cell proliferative response is controlled by loci Tria4 and Tria5 on mouse chromosomes 7 and 9. Mamm Genome. 1999;10:670–4. doi: 10.1007/s003359901069. [DOI] [PubMed] [Google Scholar]
- 78.Dibbert B, Daigle I, Braun D, Schranz C, Weber M, Blaser K, Zangemeister-Wittke U, Akbar AN, Simon HU. Role for Bcl-xL in delayed eosinophil apoptosis mediated by granulocyte-macrophage colony-stimulating factor and interleukin-5. Blood. 1998;92:778–83. [PubMed] [Google Scholar]
- 79.Simson L, Foster PS. Chemokine and cytokine cooperativity: eosinophil migration in the asthmatic response. Immunol Cell Biol. 2000;78:415–22. doi: 10.1046/j.1440-1711.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 80.Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol. 2011;12:167–77. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otero K, Vecchi A, Hirsch E, Kearley J, Vermi W, Del Prete A, Gonzalvo-Feo S, Garlanda C, Azzolino O, Salogni L, Lloyd CM, Facchetti F, Mantovani A, Sozzani S. Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood. 2010;116:2942–9. doi: 10.1182/blood-2009-12-259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y, Kerketta R, Lee YH, Chang SH, Corry DB, Wang D, Watowich SS, Dong C. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14:732–40. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O'Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci U S A. 2007;104:4371–6. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El-Shazly A, Yamaguchi N, Masuyama K, Suda T, Ishikawa T. Novel association of the src family kinases, hck and c-fgr, with CCR3 receptor stimulation: a possible mechanism for eotaxin-induced human eosinophil chemotaxis. Biochem Biophys Res Commun. 1999;264:163–70. doi: 10.1006/bbrc.1999.1379. [DOI] [PubMed] [Google Scholar]
- 85.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 86.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 87.Håkansson L, Venge P. Priming of eosinophil and neutrophil migratory responses by interleukin 3 and interleukin 5. APMIS. 1994;102:308–16. doi: 10.1111/j.1699-0463.1994.tb04880.x. [DOI] [PubMed] [Google Scholar]
- 88.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 89.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Köhler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/S1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 90.Kvarnhammar AM, Petterson T, Cardell LO. NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists. Immunology. 2011;134:314–25. doi: 10.1111/j.1365-2567.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Havelková H, Badalová J, Svobodová M, Vojtíšková J, Kurey I, Vladimirov V, Demant P, Lipoldová M. Genetics of susceptibility to leishmaniasis in mice: four novel loci and functional heterogeneity of gene effects. Genes Immun. 2006;7:220–33. doi: 10.1038/sj.gene.6364290. [DOI] [PubMed] [Google Scholar]
- 92.Badalová J, Svobodová M, Havelková H, Vladimirov V, Vojtíšková J, Engová J, Pilčík T, Volf P, Demant P, Lipoldová M. Separation and mapping of multiple genes that control IgE level in Leishmania major infected mice. Genes Immun. 2002;3:187–95. doi: 10.1038/sj.gene.6363838. [DOI] [PubMed] [Google Scholar]
- 93.Vladimirov V, Badalová J, Svobodová M, Havelková H, Hart AAM, Blažková H, Demant P, Lipoldová M. Different genetic control of cutaneous and visceral disease after Leishmania major infection in mice. Infect Immun. 2003;71:2041–6. doi: 10.1128/IAI.71.4.2041-2046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets collected and analyzed during the current study are available from the corresponding author on reasonable request.


