Abstract
Background
Current pediatric septic shock resuscitation guidelines from the American College of Critical Care Medicine focus on the early and goal-directed administration of intravascular fluid followed by vasoactive medication infusions for persistent and fluid-refractory shock. However, accumulating adult and pediatric data suggest that excessive fluid administration is associated with worse patient outcomes and even increased risk of death. The optimal amount of intravascular fluid required in early pediatric septic shock resuscitation prior to the initiation of vasoactive support remains unanswered.
Methods/design
The SQUEEZE Pilot Trial is a pragmatic, two-arm, parallel-group, open-label, prospective pilot randomized controlled trial. Participants are children aged 29 days to under 18 years with suspected or confirmed septic shock and a need for ongoing resuscitation. Eligible participants are enrolled under an exception to consent process and randomly assigned via concealed allocation to either the Usual Care (control) or Fluid Sparing (intervention) resuscitation strategy. The primary objective of this pilot trial is to determine feasibility, based on the ability to enroll participants and to adhere to the study protocol. The primary outcome measure by which success will be determined is participant enrollment rate ("pass" defined as at least two participants/site/month, recognizing that enrollment may be slower during the run-in phase). Secondary objectives include assessing (1) appropriateness of eligibility criteria, and (2) completeness of clinical outcomes to inform the endpoints for the planned multisite trial. To support the nested translational study, SQUEEZE-D, we will also evaluate the feasibility of describing cell-free DNA (a procoagulant molecule with prognostic utility) in blood samples obtained from children enrolled into the SQUEEZE Pilot Trial at baseline and at 24 h.
Discussion
The optimal degree of fluid resuscitation and the timing of initiation of vasoactive support in order to achieve recommended therapeutic targets in children with septic shock remains unanswered. No prospective study to date has examined this important question for children in developed countries including Canada. Recruitment for the SQUEEZE Pilot Trial opened on 6 January 2014. Findings will inform the feasibility of the planned multicenter trial to answer our overall research question.
Trial registration
ClinicalTrials.gov Identifier NCT01973907, registered on 23 October 2013.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-016-1689-2) contains supplementary material, which is available to authorized users.
Keywords: Fluid therapy, Resuscitation, Shock, Sepsis, Pediatrics
Background
Rationale
Septic shock remains one of the most significant and potentially preventable causes of death in children worldwide, with pediatric mortality rates ranging from 15 to 70% [1, 2]. Current pediatric Surviving Sepsis Guidelines [3, 4] from the American College of Critical Care Medicine (ACCM) emphasize an early and goal-directed approach to resuscitation [5–7]. These guidelines suggest that fluid resuscitation should be aggressive with repeated intravenously (IV) administered fluid boluses of 20 mL/kg, such that some children may require as much as 200 mL/kg of fluid to achieve therapeutic endpoints [2]. The guidelines also recommend initiation of vasoactive agents at the stage of “fluid-refractory shock,” i.e., when there is persistent hypoperfusion despite at least 60 mL/kg IV fluid [3]. While evidence suggests that adhering to the resuscitation goals and guidelines of the ACCM may improve mortality and functional morbidity [8, 9], fluid resuscitation guidelines from the ACCM were derived primarily from observational studies and expert opinion [3].
Aggressive fluid administration in septic shock has recently been called into question [10]. Accumulating adult [11–14] and pediatric data [15–18] suggest that excessive fluid resuscitation in patients with septic shock is associated with increased morbidity and mortality. This has sparked a furious debate in both the adult and pediatric medical literature on how “aggressive” fluid resuscitation should be, given that the main morbidity and mortality in septic shock is due not to refractory hypotension, but to end-organ failure due in part to massive fluid overload [19]. The overall objective of our research program is, therefore, to evaluate whether a fluid-sparing strategy, that involves earlier initiation and preferential escalation of vasoactive medications to achieve ACCM goal-directed targets, results in improved clinical outcomes for children experiencing septic shock.
Relevant medical literature
Mortality in pediatric septic shock has significantly improved since the introduction of rapid fluid resuscitation in the first “golden” hour of resuscitation [6–9, 20]. Subsequent improvements in pediatric septic shock survival have been attributed to adherence to the first iteration of the ACCM septic shock guidelines, and the use of goal-directed targets [21, 22]. However, the largest and most publicized pediatric trial of fluid resuscitation in children with suspected septic shock (FEAST trial), published in the New England Journal of Medicine in 2011, demonstrated an increased mortality among children treated with aggressive fluid resuscitation in comparison to the conservative fluid resuscitation arm [15]. These results sparked a flurry of commentaries and attempts to explain these unexpected findings [23–25]. The FEAST trial was conducted in sub-Saharan Africa, and enrolled a significant proportion of children with malaria. As a result, the pediatric critical care community clearly acknowledges that these results, while important, are not necessarily generalizable to developed countries such as Canada. These results did, however, fuel further discussion and debate regarding the optimum fluid resuscitation in the course of goal-directed therapy in septic shock.
Emerging publications in the Intensive Care Unit (ICU) medical literature suggest that excessive, compared to conservative, fluid administration in adults with septic shock worsens outcomes such as duration of mechanical ventilation [17, 26], complications related to the third-spacing of fluids [27, 28], length of ICU stay [17, 26], and mortality [11–14]. Recent systematic reviews reveal a paucity of randomized controlled trial (RCT) evidence, other than the FEAST trial, examining the impact of fluid resuscitation on mortality in children with septic shock [29, 30]. This raises the important question of whether children in developed countries would also benefit from fluid-sparing resuscitation. A goal-directed fluid-sparing strategy would, by default, require earlier initiation and preferential escalation of vasoactive medications to target ACCM hemodynamic goals [3]. There are potential adverse effects attributable to the earlier initiation of vasoactive medications that may outweigh those resulting from an aggressive fluid administration strategy, providing further justification for this study [27, 28, 31–33]. The optimal degree of fluid resuscitation and the timing of initiation of vasoactive support in order to achieve therapeutic targets in children with septic shock remain unanswered. No prospective study to date has examined this important question for children in developed countries including Canada.
Overall research question
In pediatric patients with septic shock, does a fluid-sparing strategy to achieve ACCM therapeutic goals result in improved clinical outcomes without an increased risk of adverse events compared to the usual care of aggressive fluid resuscitation, as currently recommended by the ACCM guidelines?
Pilot trial primary research question
Is it feasible to conduct a multicentre trial using this protocol?
Explanation for choice of comparators
There is currently not clinical equipoise to randomize children with septic shock to a “No bolus” intervention. For this reason, the only sensible way to test emerging concepts while producing high-quality evidence is to investigate comparators of a “fluid-sparing” strategy versus “usual care,” where the latter is fluid liberal.
Methods/design
The SQUEEZE Pilot Trial is a pragmatic two-arm, parallel-group, open-label, prospective pilot RCT. We will use this pilot RCT to determine the feasibility and inform the appropriate methodological design of the larger definitive trial [34, 35].
The primary objective of the SQUEEZE Pilot Trial is to determine the feasibility of a large multicentre RCT to answer our overall research question. Secondary objectives are to assess the (1) appropriateness of the eligibility criteria, and (2) completeness of clinical outcomes of interest for the main study to inform the design of the full-scale trial. Clinical outcomes under consideration for the definitive trial include clinical endpoints related to:
Clinical course and procedures, e.g., Pediatric Intensive Care Unit (PICU) admission rate, Length of Stay, measures of organ dysfunction, i.e., PELOD-2 score, ventilator-free days, mortality, invasive lines and procedures, laboratory and microbiological findings
Short-term hemodynamic outcomes, e.g., time to shock-reversal, cardiovascular indices
Adverse events: (i) complications potentially attributable to fluid overload, e.g., pulmonary edema, pleural effusion requiring drainage, abdominal compartment syndrome, and (ii) complications potentially attributable to inotrope/vasopressor use, e.g., signs of digital soft tissue ischemia, need for revision amputation
The pilot trial primary and secondary study objectives will be evaluated according to the outcomes listed in Table 1. We will also collect additional data related to study process, resource, and management aspects of feasibility to inform conduct of the definitive multicentre RCT. The proposed primary objective for the definitive SQUEEZE Trial is to determine whether time to shock-reversal is quicker in pediatric patients with septic shock treated with a Fluid Sparing resuscitation strategy versus Usual Care.
Table 1.
Summary of Pilot Trial outcomes
| Pilot Trial outcomes | Analysis | Pass threshold |
|---|---|---|
| SQUEEZE | ||
| Primary outcomes | ||
| 1. Participant enrollment ratea
Consent rate for continued participation Missed eligible patients |
Simple proportion Simple proportion Simple proportion |
≥2/month(/site) Not applicable Not applicable |
| 2. Protocol adherence: ability to initiate study procedures within 1 h of randomization | Simple proportion | Not applicable |
| Secondary outcomes | ||
| 1. Appropriateness of eligibility criteria as evidenced by the ability to identify and enroll participants in a timely manner | Descriptive | Not applicable |
| 2. Completeness of the clinical outcomes of interest to inform the design of the future multicenter trial | Descriptive | Not applicable |
| 3. We will also assess considerations related to study process, resource, and management aspects of feasibility | Descriptive | Not applicable |
| SQUEEZE-D | ||
| Primary outcome | ||
| 1. The proportion of SQUEEZE participants for whom cell-free deoxyribonucleic acid (cfDNA) can be described | Simple proportion | Not applicable |
| Secondary outcomes | ||
| 1. The availability of the required samples from patients enrolled into SQUEEZE and | Simple proportion | Not applicable |
| 2. We will also assess considerations related to study process, resource, and management aspects of feasibility which impact upon the ability to process and test samples to inform the design of a larger-scale study | Descriptive | Not applicable |
aRecognizing that enrollment may be slower during the initial run-in phase
The SQUEEZE Pilot Trial includes a nested translational study, SQUEEZE-D. The overall objective of SQUEEZE-D is to describe the levels of plasma cell-free deoxyribonucleic acid (cfDNA) in pediatric septic shock. cfDNA is released by activated neutrophils and is a potent trigger of blood coagulation. In adult patients with severe sepsis, cfDNA has high discriminative power to predict ICU mortality [36]. The primary objective of SQUEEZE-D in the context of the pilot trial is to evaluate the feasibility of describing cfDNA in blood samples obtained for clinical purposes at baseline and 24 h in children enrolled in the SQUEEZE Pilot Trial. The secondary objectives of SQUEEZE-D are to determine (1) the availability of the required samples from patients enrolled in SQUEEZE and (2) the process, resource, and management aspects of feasibility which impact upon the ability to process and test samples to inform the design of a larger-scale study. The proposed primary objective of SQUEEZE-D in the definitive trial is to determine the predictive value of cfDNA on length of time in shock.
The detailed pilot trial protocol is organized in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines, with items corresponding to the SPIRIT 2013 checklist (Additional file 1) [37, 38]. Required items, such as the World Health Organization Trial Registration Data Set (Additional file 2), a schedule of enrollment, interventions, and assessments (Additional file 3), and a participant flow diagram (Fig. 1) are included here. The protocol was registered at ClinicalTrials.gov (NCT01973907) prior to enrollment of the first participant.
Fig. 1.
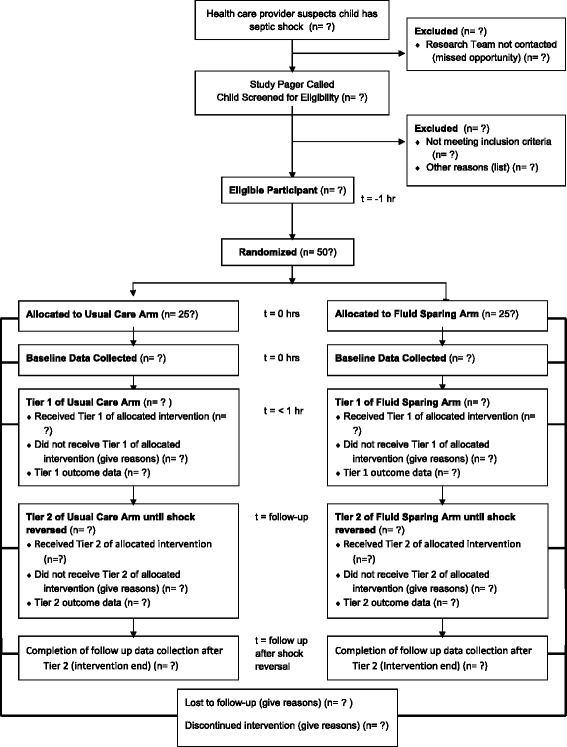
Flow of participants
Setting
The SQUEEZE Pilot Trial currently includes McMaster Children’s Hospital (Hamilton, Canada); however, we will consider adding external Canadian site(s) once the trial is established. Participating sites will be listed on the ClinicalTrials.gov trial registration page.
Participants
Patients presenting to the emergency department, or admitted to an inpatient ward (including the PICU) at participating sites who meet the following eligibility criteria:
Inclusion criteria:
Inclusion criteria for 1 and 3 must be answered yes to be eligible.
Age 29 days to under 18 years of age
Persistent signs of shock defined as one or more of the following:
Vasoactive medication dependence (need for vasoactive drug for hemodynamic support)
Hypotension (systolic blood pressure (SBP) and/or mean blood pressure (MBP) below the 5th percentile for age)
-
Abnormal perfusion, defined as the presence of two or more of the following:
abnormal capillary refill (CR) (CR <1 s (flash) or CR ≥3 s (delayed)), tachycardia (heart rate (HR) above the 95th percentile for age)
decreased level of consciousness
decreased urine output
Suspected or confirmed septic shock
Fluid resuscitation threshold met. Patient has received within the previous 6 h a minimum of:
-
40 mL/kg of isotonic crystalloid (0.9% normal saline or Ringer’s lactate), and/or colloid (5% albumin) as fluid bolus therapy administered IV for participants <50 kg
or
δ2 L of isotonic crystalloid (0.9% normal saline or Ringer’s lactate), and/or colloid (5% albumin) as IV fluid bolus therapy for participants ≥50 kg
-
3.
Fluid-refractory septic shock as defined by the presence of 2a, 2b, and 2c.
*Adapted from the International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics [1]
δBased on the adult Surviving Sepsis Guidelines initial targets for fluid resuscitation [4]
Exclusion criteria:
-
i)
Patient admitted to the Neonatal Intensive Care Unit (NICU)
-
ii)
Full active resuscitative treatment is not within the goals of care
-
iii)
Shock secondary to causes other than sepsis (i.e., obvious signs of cardiogenic shock, anaphylactic shock, hemorrhagic shock, spinal shock)
-
iv)
Patients requiring resuscitation in the operating room or Post-anesthetic Care Unit
-
v)
Previous enrollment in this trial, where known by the research team
Interventions
Patients will be randomized in a 1:1 ratio to either: (1) Usual Care, or (2) Fluid Sparing.
For all participants, care providers will be provided with a copy of the hemodynamic goals as specified in the ACCM Surviving Sepsis Guidelines and instructed that they should escalate treatment to achieve these targets according to the assigned intervention. A copy of the current ACCM Guidelines will also be provided to promote adherence to best practices in both study arms for aspects of patient care not impacted by the study intervention.
An illustration of the two study arms in ACCM Guideline format is provided in Additional file 4, while the ACCM Goal Directed Targets are summarized in Additional file 5. Table 2 provides a detailed description of the two-tiered study intervention. Each intervention tier provides direction regarding the use of bolus fluid therapy and vasoactive medications. A one-page flow diagram will be used in the clinical setting to provide simple directions regarding implementation of the intervention.
Table 2.
Detailed description of the SQUEEZE study arms
| Intervention tier | Usual Care arm | Fluid Sparing arm |
|---|---|---|
| Tier 1 | Usual Care | Early initiation of vasoactive medications to spare fluid |
| Bolus fluid therapya,b | • Following randomization, further isotonic fluid bolus therapy [crystalloid (0.9% normal saline or Ringer’s lactate) or colloid (5% albumin)] may be administered in any volume and as requested by the caring physician | • Following randomization, further isotonic fluid bolus therapy [crystalloid (0.9% normal saline or Ringer’s lactate) or colloid (5% albumin)] should be avoided and provided only if required due to: 1. delay in the ability to immediately initiate vasoactive medication(s) and/or 2. to treat intravascular hypovolemia. The reason/indication for administration of further fluid bolus therapy prior to the initiation of vasoactive medications must be documented |
| Vasoactive medicationc | • The decision to initiate vasoactive medication(s) is at the discretion of the treating physician. Vasoactive support should not be started until the participant has received a minimum of 60 mL/kg (3 L for participants ≥50 kg) of isotonic fluid as boluses (includes fluid boluses received in the 6 h prior to randomization) • The choice of initial vasoactive medication and the initial dose is to be at the discretion of the caring physician |
• Vasoactive medication(s) should be initiated immediately following randomization • The choice of initial vasoactive medication and the initial dose is to be at the discretion of the caring physician |
| Tier 2 | Usual Care | Preferential escalation of vasoactive medications |
| Bolus fluid therapya,b | • Further isotonic fluid bolus therapy may be administered at the discretion of the caring physician • The type and dose of any further isotonic fluid bolus therapy is at the discretion of the caring physician |
• Further isotonic fluid bolus therapy may be administered by the caring physician to treat documented inadequate intravascular filling/preload
• If further isotonic fluid bolus therapy is provided, the dose provided should be in 5–10-mL/kg aliquots (250-500 mL for participants ≥50 kg) with the lowest acceptable volume preferred and the indication for administration documented • Aliquots of isotonic fluid bolus therapy may be administered “back-to-back” if required to address inadequate intravascular volume status • The type of isotonic fluid bolus therapy provided is at the discretion of the caring physician |
| Vasoactive medicationc | • If initiated, vasoactive medication(s) may be titrated (increased, decreased, or discontinued) at the discretion of the caring physician • Additional vasoactive medication(s) may be initiated at the discretion of the caring physician |
• Escalation of vasoactive medications should be the first line to achieve hemodynamic goals (provided intravascular volume status is judged to be adequate) • The initiated vasoactive medication(s) may be titrated (increased, decreased, or discontinued) at the discretion of the caring physician • Additional vasoactive medication(s) may be initiated at the discretion of the caring physician |
| Intervention end | • When the patient is free from vasoactive medication support and shock is reversed or the patient is placed on mechanical circulatory support,e.g., extracorporeal membrane oxygenation (ECMO) or death occurs | • When the patient is free from vasoactive medication support and shock is reversed or the patient is placed on mechanical circulatory support, e.g., ECMO or death occurs |
aBolus: a (fluid) bolus is a discrete volume of fluid prescribed to be administered intravascularly (intravenous (IV) or intraosseous (IO)) over a defined period of time (ranging from stat, i.e., as fast as possible to typically no greater than 60 min). A fluid bolus typically ranges in size from usually not less than 5 mL/kg (250 mL for participants ≥50 kg) to 20 mL/kg (1 L for participants ≥50 kg, although some clinicians may use per kilogram dosing in larger patients). A documented medical order is required for a fluid bolus. Routine fluid replacement is not considered to be bolus(es)
bFluid therapy: isotonic crystalloid or colloid solutions which include 0.9% normal saline, Ringer’s lactate, and 5% albumin
cVasoactive medications are administered by intravascular (IV or IO) infusion and include: dobutamine, dopamine, epinephrine, norepinephrine, vasopressin, phenylephrine, milrinone
Criteria for discontinuing or modifying allocated interventions for a given trial participant:
We will allow for exit criteria from the study protocol as follows:
-
i)
Participant or their Substitute Decision Maker (SDM) withdraws consent for ongoing study participation
-
ii)
Change in the medical goals of care for a study participant, e.g., decision to limit escalation of resuscitative therapies and/or withdrawal of life-sustaining supportive measures
-
iii)
Confirmatory evidence that the participant is suffering from another form of shock other than septic shock, e.g., occult hemorrhage
-
iv)
The Most Responsible Physician (MRP) believes that ongoing patient management according to the assigned intervention will lead to patient harm
The site principal investigator (PI) or their delegate should be contacted if any of these situations arise to discuss the specific circumstances. If the PI withdraws a participant from the study, clear and objective reason(s) should be recorded.
Strategies to improve adherence to intervention protocols
For study participants, we will post an alert on the front of the medical chart and inside their room, e.g., at the head of the bed advising of trial enrollment and the assigned intervention. In the Fluid Sparing arm, a fluid bolus record requires the medical team to provide justification for any fluid boluses administered to the participant. Data will be reviewed in light of the assigned intervention and any protocol deviations will be documented. Study process feasibility includes protocol adherence [35]. We will document and report the following protocol deviations: failure to (1) implement study procedures within 1 h of randomization, (2) prescribe fluid boluses in accordance with protocol, (3) prescribe vasoactive medications in accordance with protocol, and (4) provide notification of enrollment to the participant or SDM per protocol. The importance of protocol adherence will be routinely reinforced. There will be no restrictions with respect to concomitant care and interventions.
Study outcomes
Pilot trial outcomes are summarized in Table 1. The proposed primary outcome for the full study is time to shock-reversal. We have defined time to shock-reversal as the amount of time (in hours) from allocation until shock is reversed. Shock-reversal is defined based on the ACCM therapeutic targets, when all of the following criteria have been met:
Free from all vasoactive medication support
Normalization of HR (above the 5th, and below the 95th percentile for age)
Normalization of blood pressure (SBP and MBP above the 5th percentile for age)
CR <3 s
Shock-reversal criteria will be assessed as documented in the medical record. Shock will not be determined to be reversed unless the patient has been free from vasoactive infusions for 24 h. If a participant is placed on mechanical circulatory support, such as extracorporeal membrane oxygenation (ECMO), or if death occurs during the intervention phase, shock will be coded as “never reversed.”
Measurement of cfDNA (SQUEEZE-D)
The citrated plasma tube (CPT) used for determination of the International Normalized Ratio (INR) and partial thromboplastin time (PTT) is the same collection tube used for cfDNA. We plan to use the CPT tube as the source of plasma for cfDNA determination. The cfDNA levels will be measured at baseline and at 24 h as previously described in the laboratory of Drs. Fox-Robichaud and Liaw at the Thrombosis and Atherosclerosis Research Institute [36].
Sample size
The target sample size for the SQUEEZE pilot, based on the study objective of evaluating feasibility, is 50 subjects (25 patients per arm). This is consistent with current guidelines for sample size justification for pilot trials [39]. We will consider adjusting the pilot trial sample size upward to provide an opportunity for the new site(s) to recruit sufficient subjects to allow for assessment of external feasibility.
Screening and recruitment
Patients will be screened for eligibility from the following patient care areas: emergency department, medical and surgical wards, and the Pediatric Critical Care Unit (PCCU). Posters and information sessions will be used to promote the study to physician and nursing staff, and trainees. The research coordinator and/or PI will be paged for any potentially eligible patient, e.g., suspected sepsis, receiving a fluid bolus. If/when a patient is screened and determined to meet study eligibility criteria, and provided there is no objection by the MRP or their delegate, the randomization procedures will be executed immediately and the patient enrolled. This trial will use an exception to consent (deferred consent) process (see “Ethical Considerations”).
Allocation sequence generation, allocation concealment, participant enrollment, and communication of participant assignment
The allocation sequence will be created by the Biostatistics Unit and this information will be kept secret from, and inaccessible by, the investigators. The allocation sequence will be computer-generated and utilize simple randomization, with no stratification or blocking. The allocation sequence will be implemented through a third party computer-based process accessible via telephone on a 24-h basis. A research assistant or one of the site investigators will enroll eligible participants into the study and be responsible for communicating the assigned intervention.
Blinding
The investigators, research staff, and treating health care providers will all be blinded to the allocation sequence. It will not be possible to blind the investigators, treating physicians, or bedside nursing staff from participant assignment as these individuals will need to be aware of, and implement, the intervention. We will evaluate the feasibility of blinding the research assistants (who will obtain Ultrasonic Cardiac Output Monitor (USCOM™) measurements) to participant treatment assignment. The data analysts will be blinded to treatment assignment through use of a numeric code in the trial database.
Activities to promote participant retention and follow-up
We do not anticipate difficulties with participant follow-up given that those enrolled will be receiving close monitoring and management for suspected septic shock. Data collected prior to approaching participants/SDMs for written consent to continue study participation will be retained for all participants enrolled in the trial. Where a protocol deviation occurs, this information including the reason will be noted and data collection will otherwise continue according to protocol.
Data collection methods
Participant demographic data and SQUEEZE outcome data will be collected from the hospital chart by a research assistant or one of the investigators, trained in use of the Data Collection Forms. The investigators and research assistants will be trained in the use of the noninvasive USCOM™ ultrasound device for study purposes at sites where this technology is available. At the times specified within the study protocol, and where feasible, USCOM™ measurements will be obtained by one of these individuals and recorded on the related Data Collection Form. Dr. Fox-Robichaud will be responsible for oversight of SQUEEZE-D outcome data collection.
Data collection, management, and security
Trial data will be collected by trained research staff or one of the study investigators according to study procedures. Data will be recorded on a paper-based Data Collection Form and then entered into the electronic REDCap Case Report Form (CRF) by trained research staff [40]. Paper files will be kept locked and secure in accordance with local laws and regulations. SQUEEZE-D outcome data will be stored in the Team Sepsis database, which is located at the Thrombosis and Atherosclerosis Research Institute (TaARI).
Statistical methods for analyzing primary and secondary outcomes
Baseline characteristics and outcome variables (both primary and secondary) will be summarized using descriptive summary measures. Data analysis will consist of calculating simple proportions for the feasibility outcomes. Analysis of feasibility outcomes will be descriptive expressed as an estimate with 95% confidence interval. Clinical outcomes (blinded) will be estimated for the purposes of estimating the sample size calculations for the planned definitive trial. Analysis and reporting of the results with follow the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting RCTs [41, 42]. Because we plan to include (roll-in) the pilot trial participants in the definitive trial, we do not plan to perform analyses of clinical outcomes. All analyses will be performed using SAS 9.2 (Cary, NC, USA).
Data Safety and Monitoring Board
Normally a Data Safety and Monitoring Board (DSMB) is required for studies involving vulnerable populations including children. However, we decided not to set up a DSMB for this pilot trial given the minimal risk attributable to participation. Further, the study duration is too short for the DSMB process [43].
Steering Committee
The Steering Committee will consist of Drs. Parker, Choong, Thabane, and Fox-Robichaud and will oversee the execution and conduct of the study. The PI and the Steering Committee will monitor adverse events (AEs), and report all serious adverse events (SAEs) to the Research Ethics Board (REB).
Interim analyses, stopping rules, auditing
As this is a pilot trial, there will be no interim analysis and no predetermined stopping rules. There are no planned audits. Our study may be subjected to audit by the REB(s) of participating sites. As a clinical trial, our study may also be subjected to audit by Health Canada.
Harms
The number and type of SAEs that occur will be monitored by the trial Steering Committee. There are many published complications that can normally occur as a result of septic shock and/or its treatment including multiorgan dysfunction and death. The vast majority of children in North America now survive septic shock; they may fully recover or they may be left with residual/permanent disability. The decision as to whether an AE is a SAE will be at the discretion of the PI based on proposed guiding principles for academic critical care research [44]. All SAEs along with the Steering Committee’s interpretation of attribution, will be reported to the REB. Data regarding SAEs will be collected and reported.
Research Ethics Board approval and communication of protocol modifications
Approval to conduct this pilot trial has been granted by the Hamilton Integrated Research Ethics Board (Project # 13-295). Each participating site must seek and receive full REB approval for trial participation prior to enrolling any participants.
Any modifications to trial procedures must be communicated to, reviewed by, and approved by the Hamilton Integrated REB, as well as the REBs of any other participating sites. The PI will be responsible for communicating approved changes in the trial protocol to the research coordinator.
Consent and assent procedures
Pediatric septic shock is a recognized medical emergency and, given that our study will evaluate a time-sensitive resuscitation protocol, we will use an exception to consent process (deferred consent) to achieve timely enrollment, randomization, and initiation of study procedures. The use of an exception to consent process for research evaluating treatment of emergency conditions has precedent [45–47] and is supported in Canada by the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2) (Chapter 3, Article 3.8) [48]. The use of an exception to consent process to conduct this study is ethical because some research cannot be conducted without the use of alternate consent models [48].
The participant and/or the SDM for the participant (for children who are incapable of consent) will be notified that they have been enrolled into a trial. At the earliest appropriate opportunity, the research coordinator or one of the study investigators will approach the participant or SDM to provide detailed information about the study and seek consent for ongoing participation. It will be made clear that ongoing study participation is voluntary and that any decision regarding further participation will not influence the medical care provided. Participants who are incapable of providing consent will be approached for assent as applicable. We will also monitor participants on an ongoing basis for signs of dissent.
Plans for collection and use of personal health information
Collection of participant identifiers will be limited to those determined to be necessary for trial purposes. Participants will be assigned a unique identifying code number, with participant identifiers kept separate from other trial data collected. The file linking participant code numbers to identifying information will be maintained on a password-protected computer while paper CRFs will be kept locked within a filing cabinet in a secure area.
Endpoint adjudication
We will adjudicate the time of resolution of septic shock, according to our study definitions, and post-randomization exclusions, e.g., patients for whom it is subsequently determined that shock was clearly due to another cause.
Data Management Team
The Data Management Team includes the investigators, the research coordinator, and the REDCap super administrator. The REDCap super administrator controls the setup of projects and, together with the PI, establishes user accounts and user rights for the research team.
Authorship eligibility
Criteria for authorship on any manuscript disseminating study results will be determined in accordance with the statement from the International Committee of Medical Journal Editors [49]. Medical/professional writers will not be involved in manuscript preparation.
Plans for communication of trial results and data access
Plans for communication of trial results include presentation at one or more national or international scientific meetings and publication in a peer-reviewed journal. The investigators plan to eventually make the final trial data set publicly available.
Discussion
We describe a pragmatic pilot RCT evaluating a fluid-sparing intervention in pediatric septic shock. Fluid resuscitation is a hot topic in need of rigorous study to inform optimal patient care. Our research aims to fill this gap with high-quality evidence. Resuscitation trials face unique challenges, given that interventions are frequently time-sensitive in vulnerable patients. This is certainly true of our study and supports the need for an initial pilot trial to evaluate feasibility prior to proceeding with a larger study. Pilot trials also offer an opportunity to test and refine trial processes and procedures before embarking on a more costly and complex endeavor.
Making our trial pragmatic was a priority to best reflect how the intervention would work in day-to-day practice. For this reason the trial contains very few exclusion criteria and, apart from these, aims to enroll all children with persistent signs of shock in the setting of suspected or proven infection. We recognize and accept that “usual care” for some study participants, such as those with premorbid cardiac conditions or renal impairment, may approach that of the intervention strategy. However, randomization should lead to these and other patients with analogous circumstances being evenly distributed between the study arms in any large-scale trial. Also pragmatic, we suggest that clinicians follow the ACCM guidelines for treatments apart from those impacted by the study intervention; however, we do not rigidly require guideline adherence. Some elements of the Surviving Sepsis Guidelines are currently under debate as a result of recent high-profile publications while others are in the process of being studied. Of particular note, the PROCESS, ARISE, and PROMISE trials challenge (in adults) the utility of aggressive treatment to meet ACCM goals such as central venous pressure (CVP) and hemoglobin targets [50–52]. However, we do not consider these recent publications a threat to our investigation. Rather, evidence supporting a move away from aggressively targeting measures, such as CVP, actually supports adherence to our fluid-sparing intervention. Changing attitudes towards other targets, such as transfusion thresholds, should also be evenly distributed between groups as is expected for both known and unknown confounders.
A number of considerations went into defining the study intervention. Without doubt a large volume of fluid may be administered in the initial stages of septic shock resuscitation prior to the initiation of vasoactive support. Early initiation of vasoactive support was the only acceptable way to mitigate this, with permissive hypotension the alternative. In children, hypotension is a late clinical finding and we expect that inaction in the face of hypotension would be unacceptable to any clinicians providing care for children with septic shock (including our team). An important caveat, however, is that physiologically adequate end-organ perfusion and age-based blood pressure targets are not necessarily one in the same. Nonetheless, the ACCM currently defines goal-directed hemodynamic targets by age – at least during the early stages of resuscitation (Additional file 5) [3, 4]. While the early initiation of vasoactive medications could define the intervention alone, we felt that this was likely insufficient to result in meaningful fluid sparing among any participants but those with the mildest cases of septic shock. To be effective, a fluid-sparing intervention likely requires an ongoing strategy to limit fluid administration after vasoactive support has been initiated.
We also sought to define our control arm pragmatically while being mindful of the need to achieve between-group separation. Consistent with the ACCM guidelines, we request that vasoactive support not be initiated in participants randomized to the Usual Care arm until at least 60 mL/kg (3 L for patients >50 kg) of isotonic fluid boluses has been administered from the time of shock onset. We expect between-group separation to be potentially challenging to evaluate, given the small pilot trial sample size and resulting increased risk of imbalance in potentially important confounders such as illness severity. Depending on the nature of these findings, further modifications to the study inclusion criteria and/or the intervention may be required.
The planned primary outcome for the definitive trial is time to shock-reversal. We consider time to shock-reversal a clinically meaningful outcome since this measure is objective and impacts the need for critical care resources. While we will measure and report PICU Length of Stay, this outcome may be influenced by a host of factors apart from the patient’s condition, rendering it less useful. Another candidate primary outcome is organ dysfunction, as measured by PELOD-2 [53]. While we will report mortality data, mortality is not an appropriate or feasible primary outcome for many pediatric trials due to high survivorship in children compared to adults for many conditions and a comparatively smaller number of available participants.
We expect that one of the most contentious aspects of this study will be our use of an exception to consent process. Considering the rapid rate at which septic shock resuscitation unfolds, as well as the life-threatening emergency nature of the situation, it is impracticable to seek prospective informed consent and any attempt to do so would risk coercion. Early enrollment in the course of resuscitation is also required from a scientific perspective to achieve a meaningful impact of the intervention. We are genuinely interested in SDM experiences with the exception to consent process and research is also underway to evaluate this specifically [54].
The optimal degree of fluid resuscitation and the timing of initiation of vasoactive support in order to achieve therapeutic targets in children with septic shock remain unanswered. In their systematic review Ford et al. concluded “The most important direction for future research is the applicability of the findings of the FEAST trial to other populations and settings” [16]. SQUEEZE will help to answer this very question in children with access to advanced critical care in countries such as Canada. The pilot trial described herein represents the first step in achieving this aim.
Trial status
Active recruitment at the time of manuscript submission. Recruitment is now closed.
Acknowledgements
The authors would like to thank the Pediatric Emergency Department and the Pediatric Critical Care Unit staff and trainees for their support of this trial. Methodological input into the pilot trial protocol was provided by the Canadian Critical Care Trials Group (CCCTG) and Pediatric Emergency Research Canada (PERC). The ACCADEMY (Academy of Critical Care, Design, Evaluation and Methodology) Group, based at McMaster University, also provided a forum for discussion of important methodological considerations during protocol development. Emilio Aguirre has served as the primary research assistant for this study, while Josephine Baldwin has assisted with development of hospital laboratory procedures for SQUEEZE-D. Gary Foster, PhD, organized the third-party computer-based randomization process and has provided ongoing support. MP would like to thank her scientific mentors KC, LT, and Professor Stephanie Atkinson, all of whom provided critical advice and support during the early stages of planning and conduct of this work.
Funding
Hamilton Health Sciences New Investigator Fund 2013-2015 (PI: Parker MJ. Funding reference number: NIF 12307; Operating Grant: $50,000).
Hamilton Health Sciences Research Early Career Award 2013, 2014 (PI: Parker MJ. Award – Programmatic Support: $100,000).
Hamilton Health Sciences Research Strategic Initiatives 2013-2016 (PI: Fox-Robichaud A. Funding reference number: RFA-2013 Fox-Robichaud; Operating Grant: $299,453). Funding support for several translational studies, including SQUEEZE-D.
Canadian Blood Services/Canadian Institutes of Health Research New Investigator Salary Award 2014-2019 (PI: Parker MJ. Funding reference number: CIHRPA201309-MP-322414; Award: $300,000). Supports protected research time for MJP to dedicate to this program of research.
Canadian Child Health Clinician Scientist Program Career Enhancement Program Award 2015-2019 (PI: Parker MJ. Award – Programmatic Support: $25,000).
Availability of data and materials
Not applicable.
Authors’ contributions
MP conceived of the idea for this study and is primarily responsible for development of this protocol, acquisition of funding, and study oversight. KC serves as scientific mentor for MP and made significant contributions during protocol development, preparation of funding applications, and in her role as a member of the trial Steering Committee. LT serves as a scientific mentor to MP and provided important advice regarding study sample size as well as the statistical analysis plan. AFR is primarily responsible for the oversight of SQUEEZE-D. AFR developed the biological sample management plan and is responsible for communication between the hospital and basic science research laboratories. PL is responsible for conducting the analysis of SQUEEZE-D samples. The first draft of this manuscript was prepared by MP and the final version submitted for publication was approved by all authors.
Authors’ information
MP is an associate professor of pediatrics, and an associate member of clinical epidemiology and biostatistics at McMaster University. She is board certified in both pediatric critical care and pediatric emergency medicine and practices clinically in both of these areas. Her research interests focus on resuscitation interventions and algorithms and the ethical conduct of resuscitation research. MP is currently supported by a Canadian Blood Services (CBS)/ Canadian Institutes of Health Research (CIHR) New Investigator Award and a CCHCSP Career Enhancement Program Award.
LT is a professor and associate chair in the Department of Clinical Epidemiology and Biostatistics at McMaster University, Director of the Biostatistics Unit at St. Joseph’s Healthcare Hamilton, and senior scientist of the Population Health Research Institute, Hamilton Health Sciences.
AFR is an associate professor at McMaster University in the Department of Medicine (Division of Critical Care). Her research focuses on basic science research examining the role of adhesion molecules, proinflammatory mediators and endogenous anti-inflammatory molecules in systemic inflammation. She is the principal investigator/team lead of “Team Sepsis: Bench to Bedside” strategic initiative at Hamilton Health Sciences.
PL is a professor at McMaster University in the Department of Medicine (Division of Hematology and Thromboembolism). Her overall research interest is to study the mechanistic links between blood clotting, inflammation, and the host immune system.
KC is an associate professor of pediatrics, and an associate member of clinical epidemiology and biostatistics at McMaster University. Her clinical training and expertise are in pediatric critical care and neonatology. KC has clinical research expertise in studies involving children who have experienced critical illness, with major areas of interest including pediatric septic shock and acute rehabilitation.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Approval to conduct this pilot trial has been granted by the Hamilton Integrated Research Ethics Board (Project # 13-295). The protocol involves use of an exception to consent process (deferred consent).
Role of study sponsor and funders
The study sponsor and funders have not been involved in the study design, and will not be involved in the collection, management, analysis, and interpretation of data. The study sponsor and funders will not be involved in the writing of the manuscript reporting study findings nor will they have any input into the decision to submit this for publication. The authority for overseeing trial conduct, analysis, interpretation, and dissemination of findings will rest with the study investigators.
Disclaimer
The views expressed herein do not necessarily represent the views of the federal government.
Abbreviations
- ACCM
American College of Critical Care Medicine
- AE
Adverse event
- CBS
Canadian Blood Services
- CIHR
Canadian Institutes of Health Research
- CPT
Citrated plasma tube
- CR
Capillary refill
- CRF
Case Report Form
- DSMB
Data Safety and Monitoring Board
- ECMO
Extracorporeal membrane oxygenation
- HHS
Hamilton Health Sciences
- HIREB
Hamilton Integrated Research Ethics Board
- HR
Heart rate
- ICU
Intensive Care Unit
- INR
International Normalized Ratio
- IO
Intraosseous
- IV
Intravascular
- MBP
Mean blood pressure
- MCH
McMaster Children’s Hospital
- MRP
Most Responsible Physician
- PCCU
Pediatric Critical Care Unit
- PI
Principal investigator
- PICU
Pediatric Intensive Care Unit
- PTT
Partial thromboplastin time
- RCT
Randomized controlled trial
- REB
Research Ethics Board
- SAE
Serious adverse event
- SBP
Systolic blood pressure
- SDM
Substitute Decision Maker
- TaARI
Thrombosis and Atherosclerosis Research Institute
- TCPS2
Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans
- USCOMTM
Ultrasonic Cardiac Output Monitor
Additional files
Title of data: SPIRIT 2013 checklist. Description of data: completed SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (PDF 108 kb)
Title of data: items from the World Health Organization Trial Registration Data Set. Description of data: items from the World Health Organization Trial Registration Data Set. (PDF 110 kb)
Title of data: schedule of enrollment, interventions, and assessments. Description of data: schedule of enrollment, interventions, and assessments. (PDF 155 kb)
Title of data: SQUEEZE study algorithm as Illustrated in ACCM Guideline Format. Description of data: SQUEEZE study algorithm as Illustrated in ACCM Guideline Format. (PDF 1162 kb)
Title of data: ACCM Goal Directed Targets from the Surviving Sepsis Guidelines. Description of data: summary of ACCM Goal Directed Targets from the Surviving Sepsis Guidelines [3, 4]. (PDF 78 kb)
Contributor Information
Melissa J. Parker, Phone: (905) 521-2100, Email: parkermj@mcmaster.ca
Lehana Thabane, Email: ThabanL@mcmaster.ca.
Alison Fox-Robichaud, Email: foxrob@HHSC.CA.
Patricia Liaw, Email: patricia.liaw@taari.ca.
Karen Choong, Email: choongk@mcmaster.ca.
References
- 1.Goldstein B, Giroir B, Randolph A, Sepsis ICCoP International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 2.Carcillo JA, Tasker RC. Fluid resuscitation of hypovolemic shock: acute medicine’s great triumph for children. Intensive Care Med. 2006;32(7):958–61. doi: 10.1007/s00134-006-0189-3. [DOI] [PubMed] [Google Scholar]
- 3.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.Ranjit S, Kissoon N, Jayakumar I. Aggressive management of dengue shock syndrome may decrease mortality rate: a suggested protocol. Pediatr Crit Care Med. 2005;6(4):412–9. doi: 10.1097/01.PCC.0000163676.75693.BF. [DOI] [PubMed] [Google Scholar]
- 6.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112(4):793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 7.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266(9):1242–5. doi: 10.1001/jama.1991.03470090076035. [DOI] [PubMed] [Google Scholar]
- 8.Carcillo JA, Kuch BA, Han YY, Day S, Greenwald BM, McCloskey KA, et al. Mortality and functional morbidity after use of PALS/APLS by community physicians. Pediatrics. 2009;124(2):500–8. doi: 10.1542/peds.2008-1967. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira CF, de Sa FRN, Oliveira DSF, Gottschald AFC, Moura JDG, Shibata ARO, et al. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. 2008;24(12):810–5. doi: 10.1097/PEC.0b013e31818e9f3a. [DOI] [PubMed] [Google Scholar]
- 10.Hilton AK, Bellomo R. Totem and taboo: fluids in sepsis. Crit Care. 2011;15(3):164. doi: 10.1186/cc10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 14.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 16.Ford N, Hargreaves S, Shanks L. Mortality after fluid bolus in children with shock due to sepsis or severe infection: a systematic review and meta-analysis. PLoS One. 2012;7(8):e43953. doi: 10.1371/journal.pone.0043953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13(3):253–8. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 18.van Paridon BM, Sheppard C, Garcia Guerra G, Joffe AR, Alberta Sepsis N Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care. 2015;19:293. doi: 10.1186/s13054-015-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 20.Inwald DP, Tasker RC, Peters MJ, Nadel S, Paediatric Intensive Care Society Study Group Emergency management of children with severe sepsis in the United Kingdom: the results of the Paediatric Intensive Care Society sepsis audit. Arch Dis Child. 2009;94(5):348–53. doi: 10.1136/adc.2008.153064. [DOI] [PubMed] [Google Scholar]
- 21.Carcillo JA, Fields AI, American College of Critical Care Medicine Task Force Committee Members Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30(6):1365–78. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 22.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 23.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. World Federation of Pediatric Intensive Care and Critical Care Societies: Global Sepsis Initiative. Pediatr Crit Care Med. 2011;12(5):494–503. doi: 10.1097/PCC.0b013e318207096c. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro CT, Delgado AF, de Carvalho WB. Mortality after fluid bolus in African children with sepsis. N Engl J Med. 2011;365(14):1348–9. doi: 10.1056/NEJMc1108712. [DOI] [PubMed] [Google Scholar]
- 25.Scott H, Melendez E, Cruz AT, American Academy of Pediatrics SoEM, Septic Shock Collaborative Mortality after fluid bolus in African children with sepsis. N Engl J Med. 2011;365(14):1350–1. doi: 10.1056/NEJMc1108712. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 27.De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis. 2011;57(1):159–69. doi: 10.1053/j.ajkd.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Regueira T, Hasbun P, Rebolledo R, Galindo J, Aguirre M, Romero C, et al. Intraabdominal hypertension in patients with septic shock. Am Surg. 2007;73(9):865–70. [PubMed] [Google Scholar]
- 29.Ford SR, Visram A. Mortality after fluid bolus in African children with sepsis. N Engl J Med. 2011;365(14):1348. doi: 10.1056/NEJMc1108712. [DOI] [PubMed] [Google Scholar]
- 30.Gelbart B, Glassford N, Bellomo R. Fluid bolus therapy-based resuscitation for severe sepsis in hospitalized children: a systematic review. Pediatric Crit Care Med. 2015;16:e297–307. doi: 10.1097/PCC.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 31.Koerber RK, Haven GT, Cohen SM, Fleming WH, Hofschire PJ. Peripheral gangrene associated with dopamine infusion in a child. Clin Pediatr (Phila) 1984;23(2):106–7. doi: 10.1177/000992288402300209. [DOI] [PubMed] [Google Scholar]
- 32.Golbranson FL, Lurie L, Vance RM, Vandell RF. Multiple extremity amputations in hypotensive patients treated with dopamine. JAMA. 1980;243(11):1145–6. doi: 10.1001/jama.1980.03300370019018. [DOI] [PubMed] [Google Scholar]
- 33.Dünser MW, Mayr AJ, Tür A, Pajk W, Barbara F, Knotzer H, et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med. 2003;31(5):1394–8. doi: 10.1097/01.CCM.0000059722.94182.79. [DOI] [PubMed] [Google Scholar]
- 34.Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ, et al. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med. 2009;37(1 Suppl):S69–74. doi: 10.1097/CCM.0b013e3181920e33. [DOI] [PubMed] [Google Scholar]
- 35.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios L, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw KL, Weitz JI, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16(4):R151. doi: 10.1186/cc11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan AW, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–2. doi: 10.1016/S0140-6736(12)62160-6. [DOI] [PubMed] [Google Scholar]
- 38.Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cocks K, Torgerson D. Sample size calculations for pilot randomized controlled trials: a confidence interval approach. J Clin Epidemiol. 2013;66:197–201. doi: 10.1016/j.jclinepi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Hopewell S, Shulz KF, Montori V, Gotzsche PC, Deveraux PJ, et al. Consort 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):e1–e37. [DOI] [PubMed]
- 42.Shulz KF, Altman DG, Moher D, Group C. Consort 2010 Statement: updated guidelines for reporting parallel group randomized trials. J Clin Epidemiol. 2010;63(8):834–40. [DOI] [PubMed]
- 43.U.S. Department of Health Center for Biologics Evaluation and Research (CBER). Guidance for Clinical Trial Sponsors: Establishment and Operation of Clinical Trial Data Monitoring Committees, OMB Control No. 0910–0581 (2006).
- 44.Cook D, Lauzier F, Rocha MG, Sayles MJ, Finfer S. Serious adverse events in academic critical care research. CMAJ. 2008;178(9):1181–4. doi: 10.1503/cmaj.071366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntyre L, Fergusson DA, Rowe B, Cook DJ, Arabi Y, Bagshaw SM, et al. The PRECISE RCT: evolution of an early septic shock fluid resuscitation trial. Transfus Med Rev. 2012;26(4):333–41. doi: 10.1016/j.tmrv.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291(11):1350–7. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- 47.The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56. [DOI] [PubMed]
- 48.Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada, Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. 2014. Available from: http://www.pre.ethics.gc.ca/. Accessed Nov 2016.
- 49.International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals; 2015. Available from: http://www.icmje.org/recommendations/. Accessed Nov 2016. [PubMed]
- 50.Process Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. [DOI] [PMC free article] [PubMed]
- 51.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506. [DOI] [PubMed]
- 52.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 53.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, et al. PELOD-2: an update of the PEdiatric Logistic Organ Dysfunction score. Crit Care Med. 2013;41(7):1761–73. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 54.Parker MJ, de Laat S, Schwartz L. Exploring the experiences of substitute decision-makers with an exception to consent in a paediatric resuscitation randomised controlled trial: study protocol for a qualitative research study. BMJ Open. 2016;6:e012931. doi: 10.1136/bmjopen-2016-012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


