Abstract
Background
Immune cell-mediated inflammation is an essential process for mounting a repair response following myocardial infarction (MI). The sympathetic nervous system is known to regulate immune system function through β-adrenergic receptors (βAR), however their role in regulating immune cell responses to acute cardiac injury is unknown.
Methods
Wild-type (WT) mice were irradiated followed by isoform-specific βARKO or WT bone-marrow transplantation (BMT) and after full reconstitution underwent myocardial infarction (MI) surgery. Survival was monitored over time and alterations in immune cell infiltration following MI were examined using immunohistochemistry. Alterations in splenic function were identified through the investigation of altered adhesion receptor expression.
Results
β2ARKO BMT mice displayed 100% mortality resulting from cardiac rupture within 12 days post-MI compared to ~20% mortality in WT BMT mice. β2ARKO BMT mice displayed severely reduced post-MI cardiac infiltration of leukocytes with reciprocally enhanced splenic retention of the same immune cell populations. Splenic retention of the leukocytes was associated with an increase in VCAM-1 expression, which was itself regulated via β-arrestin-dependent β2AR signaling. Further, VCAM-1 expression in both mouse and human macrophages was sensitive to β2AR activity, and spleens from human tissue donors treated with β-blocker showed enhanced VCAM1 expression. The impairments in splenic retention and cardiac infiltration of leukocytes following MI were restored to WT levels via lentiviral-mediated re-expression of β2AR in β2ARKO BM prior to transplantation, which also resulted in post-MI survival rates comparable to WT BMT mice.
Conclusions
Immune cell-expressed β2AR plays an essential role in regulating the early inflammatory repair response to acute myocardial injury by facilitating cardiac leukocyte infiltration.
Keywords: β-adrenergic receptor, acute myocardial infarction, leukocyte, inflammation, immune system
Inflammation is critical for initiating reparative processes after ischemic injury1. Following myocardial infarction (MI) an intense inflammatory response is initiated leading to recruitment of pro-inflammatory leukocytes including monocytes, neutrophils and mast cells1-6. Secreted factors from these pro-inflammatory cell populations recruit and activate reparative cell populations to promote extracellular matrix (ECM) deposition and vascularization7, 8. This rapid inflammatory response is necessary for healing and preserving the structure of the left ventricle (LV) post-MI, as dysregulation of this process results in increased cardiomyocyte death and degradation of the ECM9.
Sympathetic nervous system (SNS) regulation of immune responses is well-established10 and β-adrenergic receptor (βAR) expression has been reported on virtually all immune cell-types. All three βAR subtypes are expressed on various hematopoietic cell-derived immune cell populations with βAR subtype expression varying widely between populations and immune cell activation state11. Although the role of β1AR in the immune system is not well-established, β1AR expression has been shown to be limited primarily to cells of the innate immune system where it regulates inflammatory mediator production12, 13. β2AR is the most highly and widely expressed βAR isoform10, 14, with a similar level of immune cell expression in rodents and humans10, 14, and is known to regulate a number of functions including hematopoiesis, lymphocyte homing and immune cell maturation10. However, the focus of many of these studies involved the effect of β2AR on adaptive immune responses, while its involvement in mediating early, innate immune responses and initiation of inflammation remains unclear10, 15, 16. β3AR has been shown to be important in early stages of hematopoiesis for mediating immune cell mobilization and egress from the bone marrow17-19. While βAR subtype expression and function varies in the immune system, the role of Immune cell-expressed βAR in the acute inflammatory response post-MI has yet to be elucidated.
In our current study, the impact of immune cell-specific βAR expression on cardiac inflammation and remodeling post-MI was investigated through the use of chimeric mice that lack specific βAR isoforms on cells of hematopoietic origin. We demonstrate that β2AR are essential in initiating early immune responses following acute cardiac injury and targeting immune cell-expressed β2AR may provide a novel therapeutic strategy for preventing adverse effects following MI.
Methods
Rationale and Study Design
The purpose of this study was to investigate the impact of βAR in regulating immune responses following MI. To differentiate the effects of immune cell-expressed βAR from cardiac-expressed βAR, we generated chimeric mice using a BMT approach in which WT recipient mice received WT control or βAR subtype-specific KO BM to produce immune cell- and βAR isoform-specific KO mice. These mice were subjected to sham or MI surgery and survival outcome and immune responses were examined along with the mechanisms of observed changes.
Bone Marrow Transplant
WT C57BL/6 recipient mice (male, 8 wk) were lethally irradiated with 950 rads using x-ray irradiation to remove endogenous BM cells. Donor BM isolated from the femurs of β1ARKO, β2ARKO, β3ARKO or WT C57BL/6 mice was introduced by retro-orbital injection (1×107 cells) within 24 h of irradiation. BM was allowed to reconstitute for 1 month prior to MI surgery. Reconstitution was confirmed at the conclusion of the study for each mouse using RT-qPCR analysis for β1AR, β2AR and β3AR expression on recipient BM. All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at Temple University and in accordance to the NIH Guidelines on the Use of Laboratory Animals.
Coronary Artery Occlusion Surgery
Myocardial infarction was induced as previously described20. Mice were anesthetized with 2% isoflurane inhalation. A small skin incision was made and the pectoral muscles were retracted to expose the fourth intercostal space. A small hole was made and the heart popped out. The left coronary artery was sutured ~3 mm from its origin and the heart was placed back into the intrathoracic space followed by closure of muscle and skin. Animals received a single dose (0.3 mg/kg) of buprenorphine immediately following surgery.
Splenectomy Surgery
Mice were anesthetized as above and a small incision was made in the left subcostal abdominal wall. Sutures were placed around the splenic vasculature and the spleen was removed. The incision was closed in two layers, peritoneum and skin, using suture. Animals received a single dose (0.3 mg/kg) of buprenorphine immediately following surgery.
Echocardiography
Cardiac function was assessed via transthoracic two-dimensional echocardiography performed at baseline and at weekly intervals post MI using a 12-mHz probe on mice anesthetized with isoflurane (1.5%). M-mode echocardiography was performed in the parasternal short-axis view to assess several cardiac parameters including left ventricular (LV) end-diastolic dimension, wall thickness, LV fractional shortening and ejection fraction. Percent fractional shortening was calculated using the equation ((LVID;d-LVID;s)/LVID;s)*100%. Percent ejection fraction was calculated using the equation ((LV vol;d-LV vol;s)/LV vol;d)*100%.
Lentivirus Infection of Bone Marrow
BM isolated from the femurs of mice was transduced with lentiviral vectors for 3XFlag-β2AR-RFP or GFP using and MOI of 100. Transductions were performed in MEM+10%FBS in the presence of 5 μg/mL Polybrene (Sigma-Aldrich). For in vitro experiments, media was changed 24 h following infection to complete media (MEM+10% FBS) and incubated an additional 24 h prior experiments. For generation of bone marrow derived macrophages, isolated BM was cultured in 10% L929 conditioned MEM+10% FBS for 1 wk prior to lentiviral infection with GFP control, WT β2AR, β2ARTYY or β2ARGRK- constructs21, 22. For in vivo experiments, BM was rinsed 1 h following infection and transplanted into irradiated mice via retro-orbital injection. BM was allowed to reconstitute for 1 month.
Human Macrophage Cell Culture
THP-1 cells (American Type Culture Collection, Manassas, VA), a human monocytic cell line, were cultured in modified RPMI-1640 media containing 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose and 10 mM HEPES and 1 mM sodium pyruvate supplemented with 10% fetal bovine serum under standard cell culture growth conditions (37°C/5% CO2/95% humidified air). THP-1 cells were differentiated into macrophages using 200 nM PMA 48h prior to all experiments. Cells were washed with complete media and treated 24h with vehicle (PBS), 0.1 μM salbutamol or 0.1 μM ICI-118,551.
Human Spleen Samples
Spleen samples from deceased human tissue donors that had been chronically administered metoprolol, or age- and sex-matched subjects not treated with metoprolol, were procured by the National Disease Research Interchange (NDRI) with support from NIH grant 2 U42 OD011158. Control subjects: n=5, 74.6±15.5 y.o. (mean ± standard deviation), 1 male, 4 females; Metoprolol subjects: n=6, 77.5±8.4 y.o., 1 male, 5 females.
Reverse Transcription Quantitative PCR
cDNA was synthesized from the total RNA of BM and spleen using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Reverse transcription quantitative PCR (RT-qPCR) was performed with SYBR® Select Master Mix (Applied Biosystems) in triplicate for each sample using primers listed in Supplemental Table 1 at an annealing temperature of 60.1°C. RT-qPCR data was analyzed using Applied Biosystems Comparative CT Method (ΔΔCT), using GAPDH, TPT-1 and 18s rRNA to normalize expression of genes of interest and calculate relative quantitation (RQ) and RQmin/max values for each.
Immunoblot
BM and spleen samples were homogenized in RIPA buffer containing 1X HALT protease inhibitor cocktail (78437; Thermo Scientific; Rockford, IL) and phosphatase inhibitor cocktail set IV (524628; Calbiochem, USA). Equal amounts of lysates were resolved by SDS-polyacrylamide gel electrophoresis (10% gels) and transferred to Immobilon-PSQ polyvinylidene fluoride 0.2μm pore size membranes (Millipore; Billerica, MA). Odyssey Blocking Buffer (LI-COR Biosciences; Lincoln, NE) was used to prevent non-specific binding. Immunoblotting was performed overnight at 4°C with diluted antibodies against Flag M2 (1:10,000; Sigma-Aldrich; St. Louis, MO), GFP (1:1000; Cell Signaling; Danvers, MA), VCAM-1 (1:1000; Santa Cruz Biotechnologies; Santa Cruz, CA), β-tubulin (1:1000; Cell Signaling), β-actin (1:1000; Santa Cruz) or GAPDH (1:1000; Cell Signaling). After washing with TBS-T, membranes were incubated at room temperature for 60 min with the appropriate diluted secondary antibody (IRDye680 Donkey anti-rabbit IgG (H + L) at 1:20,000; IRDye800CW Goat anti-mouse IgG (H + L) at 1:15,000; LI-COR Biosciences; IRDye680 Donkey anti-goat IgG (H+L) at 1:20,000). Bound antibody was detected using the LI-COR Biosciences Odyssey System (LI-COR Biosciences). Intensities were normalized to corresponding GAPDH, β-tubulin and β-actin intensities.
Histological Analysis
Excised hearts were fixed in 4% paraformaldehyde, paraffin embedded and sectioned at 5 μm thickness. Deparaffinized sections were stained for hematoxylin-eosin (H&E; Sigma-Aldrich). NIS Elements software was used to measure infarct size and visualize cell infiltration and morphology.
Immunohistochemistry was performed on deparaffinized sections to examine infiltration of various immune cell types. Antigens were retrieved using a citrate-based antigen unmasking solution (Vector Laboratories; Burlingame, CA). Hearts were blocked (10% FBS/PBS) and a 0.3% H2O2 solution was used to block endogenous peroxide activity in sections used for immunohistochemical staining. Hearts were incubated with antibodies against CD3 (1:100; Abcam), CD68 (1:100; Abcam), major basic protein (MBP; Obtained from Nancy and Jamie Lee Laboratories; Mayo Clinic, Scottsdale, AZ), mast cell tryptase (1:100; Abcam), myeloperoxidase (MPO; 1:100, Santa Cruz, Dallas, TX) or VCAM-1 (1:100; Santa Cruz). Washed slides were incubated with the appropriate secondary antibodies, anti-mouse-HRP (1:1000; GE Healthcare; Piscataway, NJ), anti-goat-HRP (1:1000; Santa Cruz), anti-goat Alexa Fluor® 647 (1:1000; Invitrogen; Grand Island, NY) and anti-rabbit Alexa Fluor® 647 (1:1000; Invitrogen), followed by staining with DAPI for immunofluorescence or hematoxylin for immunohistochemical staining. Immunofluorescent stained hearts were mounted using Prolong® Gold Antifade Reagent (Invitrogen). Immunohistochemical stained hearts were developed using a DAB Substrate Kit (Vector Laboratories) and mounted using Permount™ Mounting Media (Thermo Scientific). Staining was visualized on a Nikon Eclipse microscope at 20X magnification and NIS Elements software was used for recording images and image analysis. Images were quantified as the number of positive cells per area.
Flow Cytometry
Flow cytometry analysis of immune cell populations were performed on cells isolated from blood and BM. Immune cells were separated using an antibody against CD45-FITC (BD Biosciences; San Jose, CA) and sorted on an LSRII flow cytometer for size and granularity by Forward Scatter (FSC) and Side Scatter (SSC). Analysis was performed using Flowjo software.
Statistical Analysis
Data presented is expressed as mean ± standard deviation (SD) for continuous variables and the count and/or percentage for categorical variables. Comparisons of a continuous variable between different treatment groups were performed using the nonparametric Kruskal-Wallis test for three or more groups and the exact Wilcoxon rank-sum test for two groups due to the small group sizes to guard against possibly non-normally distributed data. Comparisons of a survival endpoint between treatment groups were performed using the log-rank test. When data were collected over time on the same set of animals such as the fractional shortening in Fig 1B, they were analyzed using a mixed-effects model in order to take into account the correlation among repeated measures as well as the potential non-constant variability over time across different groups. Multiple pairwise comparison adjustments were made with Bonferroni or Dunnett correction as appropriate. P-value < 0.05 was considered statistically significant. P-values and n (group size) values are reported in the figure legends. All statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC).
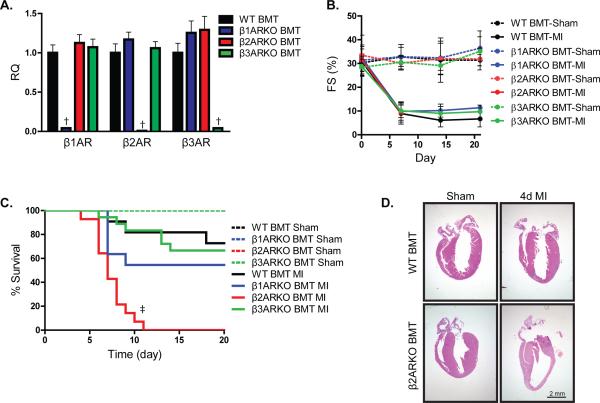
Figure 1.
Effects of hematopoietically expressed βAR subtypes on cardiac survival and function following MI. (A) C57BL/6 mice receiving WT, β1ARKO, β2ARKO or β3ARKO BMT were subjected to sham or MI surgery. Expression of β1AR, β2AR and β3AR was assessed by RT-qPCR on reconstituted WT, β1ARKO, β2ARKO and β3ARKO BM and presented as RQ+RQmax. n=6 for all groups, Exact Wilcoxon rank-sum tests with multiple comparison adjustment (3 comparisons), † p < 0.01 vs WT. (B) Left ventricular fractional shortening (FS) was measured at the short axis from M mode using Visual Sonic Analysis software. Mixed-effects modeling for repeated measures data with multiple comparison adjustments was performed indicating no significant differences compared to WT BMT. (C) WT (n=9 for sham, n=11 for MI), β1ARKO (n=7 for sham, n=11 for MI), β2ARKO (n=10 for sham, n=14 for MI) or β3ARKO (n=7 for sham, n=18 for MI) BMT mice were monitored daily for survival. Log-rank tests with multiple comparison adjustment (3 comparisons), ‡ p < 0.001 vs WT BMT MI. All sham groups had 100% survival following surgery. (D) H&E staining for sham and 4d post-MI hearts from WT and β2ARKO BMT mice.
Results
Lack of immune cell-expressed β2AR increases mortality following acute myocardial injury
To examine the contribution of immune cell-specific βAR subtype expression on survival and cardiac function post-MI, chimeric animals were generated via WT, β1ARKO, β2ARKO or β3ARKO bone marrow transplant (BMT) into irradiated recipient WT mice. β1AR, β2AR and β3AR expression was examined by RT-qPCR on reconstituted BM from all animals (Figure 1A), confirming that β1AR, β2AR and β3AR were knocked out in their respective BM with no differences in the other two βAR subtypes. Following reconstitution, the BMT mice underwent sham or MI surgery and cardiac function was monitored via echocardiography (Supplemental Figure 1A, Supplemental Table 2). LV contractility, wall thickness and cardiac dimensions were significantly altered in each MI group as shown by decreased % fractional shortening (Figure 1B), increased LV hypertrophy and increased LV dilation relative to sham animals, although no differences were evident between WT, β1ARKO, β2ARKO and β3ARKO BMT groups. However, mortality rates among the BMT mice differed significantly post-MI. All sham BMT mice displayed 100% survival, and WT BMT mice exhibited ~20% mortality by two weeks post- MI (Figure 1C), consistent with prior studies in non-BMT mice20. β1ARKO and β3ARKO BMT animals had a small, but non-significant, increase in mortality following MI when compared to WT BMT animals. Strikingly, β2AR BMT mice displayed 100% mortality following MI due to cardiac rupture with death observed 4-12 days post-MI. Although infarct size one day post-MI was not different between WT and β2ARKO chimeric mice (Supplemental Figure 1B and 1C), H&E staining revealed wall thinning and weakening in β2ARKO BMT mice 4d post-MI compared to WT BMT hearts (Figure 1D), suggesting an impairment in early repair mechanisms.
Lack of immune cell β2AR expression impairs leukocyte infiltration following acute myocardial injury
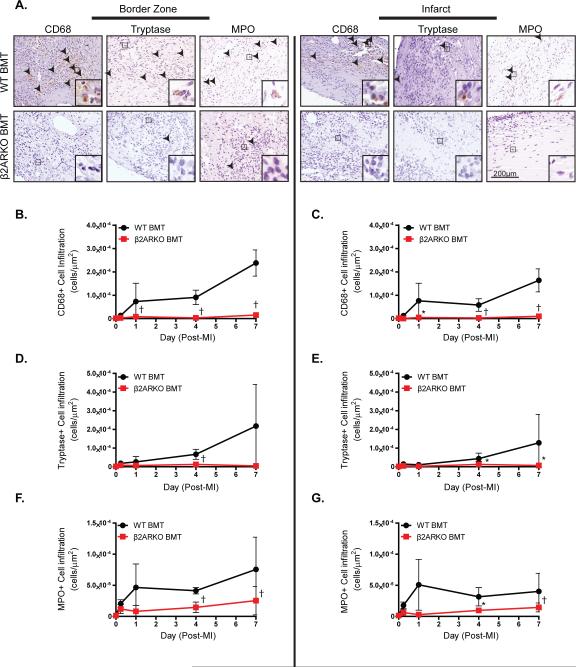
A number of immune cell populations including monocytes/macrophages, neutrophils, mast cells and T cells are known to be important for the initiation of wound healing and cardiac remodeling post-MI. Due to the high mortality via cardiac rupture observed in β2AR BMT mice following MI, which could reflect decreased immune cell-initiated repair, we assessed whether WT and β2ARKO BMT mice display differences in MI-induced cardiac immune cell population infiltration. Immunostaining was performed to identify cells of monocyte/macrophage lineage (CD68), mast cells (tryptase), neutrophils (MPO), eosinophils (MBP) and T-cells (CD3) in sham BMT hearts and in the remote, border and infarct zones of BMT hearts following MI (Figure 2, Supplemental Figures 2 and 3). Compared to WT BMT hearts, β2ARKO BMT hearts had significantly less monocyte/macrophage, mast cell and neutrophil infiltration into both the border and infarct zones (Figure 2A). Quantification of the staining demonstrated that decreased monocyte/macrophage (Figure 2B, 2C), mast cell (Figure 2D, 2E) and neutrophil (Figure 2F, 2G) recruitment to β2ARKO BMT mouse hearts was maintained over time post-MI as compared to WT BMT mice. Not all immune cell populations were affected, however, as eosinophil and T-cell infiltration into the hearts of both WT BMT and β2ARKO BMT mouse hearts were not different (Supplemental Figure 3). Flow cytometric comparative analysis of immune cell populations in the BM or blood of WT and β2ARKO BMT animals showed there was no difference in granulocyte, monocyte or lymphocyte populations (Supplemental Figure 4). Therefore, despite having similar levels of hematopoietic-derived cells as compared to WT BMT mice, those with immune cell-specific deletion of β2AR have impaired leukocyte recruitment to the heart following acute injury.
Figure 2.
Effect of hematopoietic β2AR expression on immune cell infiltration following MI. A. Representative CD68, tryptase, and MPO staining of the border or infarct zones of hearts following MI surgery in WT BMT or β2ARKO BMT mice. Quantification of CD68 (B, C), tryptase (D, E) and MPO (F, G) staining in the border and infarct zones of WT and β2ARKO BMT mouse hearts. n=4 for WT BMT sham, n=5 for β2ARKO BMT sham, n=3 for WT BMT 6 h, n=3 for β2ARKO BMT 6 h, n=4 for WT BMT 1d, n=6 for β2ARKO BMT 1d, n=5 for WT BMT 4d, n=5 for β2ARKO BMT 4d, n=6 for WT BMT 7d, n=6 for β2ARKO BMT 7d. Exact Wilcoxon rank-sum tests, * p < 0.05, † p < 0.01 vs WT BMT.
Mice lacking immune cell-expressed β2AR have increased splenic retention of leukocyte populations
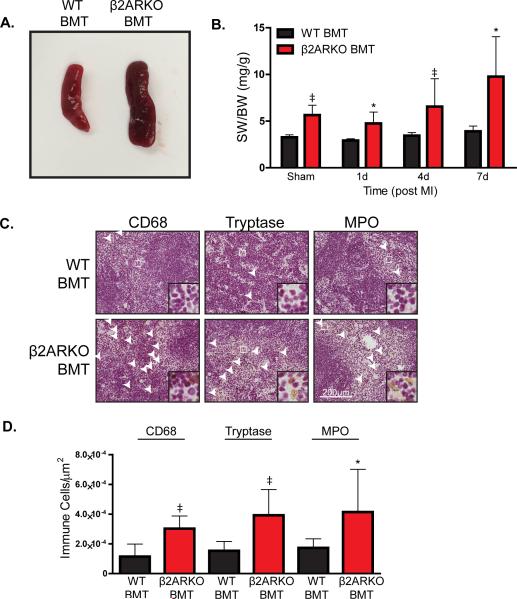
Since overall immune populations are similar between WT and β2ARKO BMT mice, but decreased leukocyte populations are observed in β2ARKO BMT hearts post-MI, we aimed to determine whether splenic retention of leukocytes could play role in this phenotype. Sham β2ARKO BMT mice had an increased spleen size compared to their WT BMT counterparts (Figure 3A), which was maintained post-MI (Figure 3B). Leukocyte levels in spleen sections from WT or β2ARKO BMT mice were examined to determine if an increase in leukocytes within the β2ARKO BMT spleens could account for the splenomegaly observed between the two groups in sham animals (Supplemental Figure 5) and 4d following MI (Figure 3C). Increased levels of monocyte/macrophages, mast cells and neutrophils (Figure 3D) were observed in β2ARKO BMT spleens compared to WT BMT mice, suggesting that β2ARKO leukocytes have an impaired ability to mobilize from the spleen to the heart following injury.
Figure 3.
β2ARKO mice have splenomegaly and retention of leukocyte populations. (A) Representative images of spleens from WT and β2ARKO BMT animals. (B) Gravimetric analysis of spleen weight to body weight (SW/BW) of spleens from WT and β2ARKO BMT sham and MI animals. n=6 for WT BMT sham, n=8 for β2ARKO BMT sham, n=4 for WT BMT 1d, n=4 for β2ARKO BMT 1d, n=10 for WT BMT 4d, n=7 for β2ARKO BMT 4d, n=7 for WT BMT 7d, n=5 for β2ARKO BMT 7d. Exact Wilcoxon rank-sum tests, * p < 0.05, ‡ p < 0.001 vs WT BMT. (C) Representative CD68, tryptase and MPO staining from 4d post-MI spleens of WT or β2ARKO BMT mice. (D) Quantification of CD68, tryptase and MPO staining from WT and β2ARKO BMT spleens 4d following MI surgery. n=10 for WT BMT, n=8 for β2ARKO BMT, Exact Wilcoxon rank-sum tests, * p < 0.05, ‡ p < 0.001 vs WT BMT.
Splenic and macrophage VCAM1 expression is sensitive to β2AR activity and expression in mice and humans
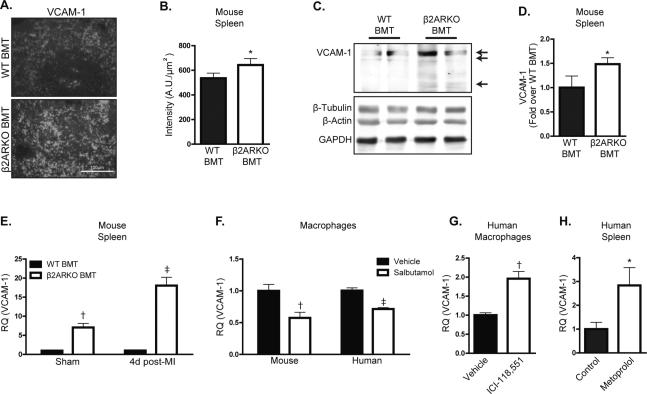
VCAM-1 expression on splenic macrophages has recently been identified as a hematopoietic stem cell retention factor important for splenic myelopoiesis23. To determine if VCAM-1 levels were increased in the spleens of β2ARKO BMT mice, leading to the retention of myeloid populations, its expression was assessed in the spleens of WT or β2ARKO BMT mice. Immunostaining indicated increased splenic VCAM-1 expression with localization in the red pulp, where macrophages reside (Figure 4A and B). Protein levels of VCAM-1 were confirmed to be elevated in β2ARKO chimeric mouse spleens when compared to WT BMT mice via immunoblotting analysis (Figure 4C and D). Further, transcript expression of VCAM-1 was increased in β2ARKO BMT spleens both basally and 4d post-MI (Figure 4E).
Figure 4.
VCAM-1 is increased in β2ARKO BMT spleens. (A) Immunohistochemistry for VCAM-1 (white) showing levels and localization of VCAM-1 expression in WT and β2ARKO BMT spleens. (B) Quantification of the intensity of VCAM-1 staining. n=5 for WT BMT, n=5 for β2ARKO BMT, Exact Wilcoxon rank-sum test, * p < 0.05 vs WT BMT. (C) Representative immunoblot showing VCAM-1 expression in WT and β2ARKO BMT spleens. Arrows indicate the three isoforms of VCAM-1. Β-tubulin, β-actin and GAPDH are shown as loading controls. (D) Quantification of VCAM-1 immunoblot expression from. n=12 for WT BMT, n=12 for β2ARKO BMT, Exact Wilcoxon rank-sum test, ‡ p < 0.001. (E) RT-qPCR was used to measure VCAM-1 expression in WT or β2ARKO BMT spleens and presented as RQ+RQmax. n=8 for WT BMT sham, n=6 for WT BMT MI, n=6 for β2ARKO BMT sham, n=8 for β2ARKO BMT MI, Exact Wilcoxon rank-sum tests, † p < 0.01, ‡ p < 0.001 vs WT BMT. (F) RT-qPCR was used to measure VCAM-1 expression in mouse (BMDM) or human (THP-1 derived) macrophages and presented as RQ+RQmax. n=7 for mouse vehicle, n=10 for mouse salbutamol, n=9 for human vehicle, n=10 for human salbutamol, Exact Wilcoxon rank-sum tests, † p < 0.01, ‡ p < 0.001 vs Veh. (G) VCAM-1 expression in human macrophages treated with vehicle or ICI-118,551 was quantified by RT-qPCR and presented as RQ+RQmax, Exact Wilcoxon rank-sum test, † p < 0.01 vs Veh. (H) RT-qPCR was used to measure VCAM-1 expression in human spleens from control or metoprolol-treated patients and presented as RQ+RQmax. n=5 for control, n=6 for metoprolol, Exact Wilcoxon rank-sum test, * p < 0.05 vs control.
To determine if β2AR stimulation alters VCAM-1 expression at a cellular level, WT bone marrow-derived macrophages (BMDM) were treated with the β2AR-selective agonist salbutamol, which decreased VCAM-1 expression (Figure 4F). Strikingly, salbutamol also decreased VCAM-1 expression in a human macrophage cell line (Figure 4F), confirming that β2AR-mediated alterations in VCAM-1 are translatable between species. Since VCAM-1 was decreased by β2AR stimulation, we next tested whether pharmacological inhibition of β2AR could reciprocally increase VCAM-1 expression. Indeed, treatment of human macrophages with the β2AR-selective antagonist, ICI-118,551 (Figure 4G) increased VCAM-1 expression. Further, VCAM-1 expression was significantly increased in the spleens of human subjects treated with the β-blocker metoprolol versus age- and sex-matched subjects that had not taken a β-blocker (Figure 4H), demonstrating the clinical relevance of our findings.
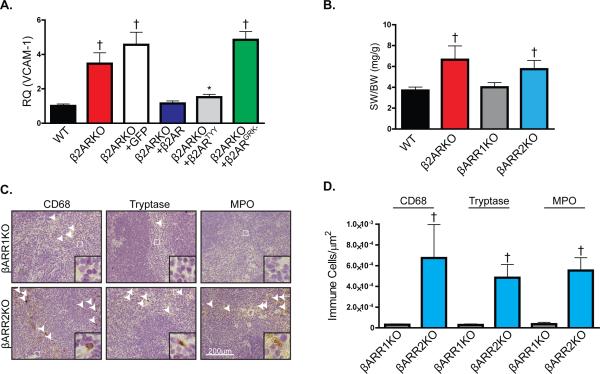
Proximal β2AR signaling through either G protein- or β-arrestin-dependent pathways have been shown to exert distinct cellular effects21, 22. Thus, to determine the proximal mechanism through which β2AR controls VCAM-1 expression, lentiviral constructs were generated containing either β2ARTYY, which is unable to couple to Gαs22, or β2ARGRK-, which cannot be phosphorylated by GRK21 thereby preventing the recruitment of β-arrestins (βARR). Using BMDM from WT or β2ARKO mice, VCAM-1 transcript expression was shown to be increased in β2ARKO macrophages (Figure 5A). Lentivirus-mediated restoration of β2AR expression in β2ARKO macrophages (Supplemental Figure 5E) decreased VCAM-1 expression to that in WT macrophages (Figure 5A), while a GFP control lentivirus had no effect on VCAM-1 expression. Mechanistically, β2ARKO BMDM transduced with β2ARGRK- had elevated expression of VCAM-1 where as β2ARTYY had decreased VCAM-1 similar to WT levels (Figure 5A), indicating that GRK-dependent β2AR signaling is required for regulation of VCAM-1 expression in macrophages. In support of this observation, βARR2KO mice had splenomegaly similar to β2ARKO mice (Figure 5B) with retention of monocytes/macrophages, mast cells and neutrophils (Figure 5C and D). Interestingly, βARR1KO mice had normal splenic size and leukocyte levels, indicating that β2AR regulates VCAM-1 expression selectively via βARR2 signaling.
Figure 5.
β2AR regulates VCAM-1 through β-arrestin dependent mechanisms. (A) RT-qPCR was used to measure VCAM-1 expression in BMDM from WT or β2ARKO mice and β2ARKO BMDM transduced with GFP, β2AR, β2ARTYY or β2ARGRK- lentivirus and presented as RQ+RQmax. n=9 for WT, n=6 for β2ARKO, n=6 for β2ARKO+β2AR, n=6 for β2ARKO+GFP, n=6 for β2ARKO+β2ARTYY, n=6 for β2ARKO+β2ARGRK-, Exact Wilcoxon rank-sum tests with multiple comparison adjustment (5 comparisons), * p < 0.05, † p < 0.01 vs WT. (B) Gravimetric analysis of spleen weight to body weight (SW/BW) of spleens from WT, β2ARKO, βARR1KO and βARR2KO animals. n=7 for WT, n=6 for β2ARKO, n=6 for βARR1KO, n=6 for βARR2KO, Exact Wilcoxon rank-sum tests with multiple comparison adjustment (3 comparisons), † p < 0.01 vs WT. (C) Representative CD68, tryptase, and MPO staining of spleens from βARR1KO and βARR2KO mice. (D) Quantification of CD68, tryptase and MPO staining from βARR1KO and βARR2KO spleens. n=6 for βARR1KO, n=4 for βARR2KO, Exact Wilcoxon rank-sum tests, † p < 0.01 vs βARR1KO.
Splenectomized WT and β2ARKO BMT mice have similar levels of leukocyte infiltration following acute myocardial injury
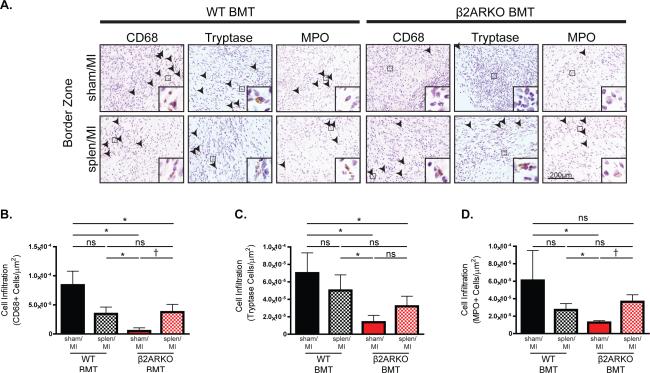
To confirm whether splenic retention of β2ARKO leukocytes is primarily responsible for their decreased infiltration into the heart following MI, we examined leukocyte recruitment to the heart in splenectomized WT and β2ARKO BMT animals receiving sham or MI surgery (Figure 6A, Supplemental Figure 6). Splenectomy in WT BMT animals decreased MI-induced infiltration of monocytes/macrophages (Figure 6B) and neutrophils (Figure 6D) into the border zone by about 50%, with less impact on mast cells (Figure 6C). Conversely, splenectomy of β2ARKO BMT animals increased cardiac infiltration of monocytes/macrophages, neutrophils and mast cells to levels not different from those observed in splenectomized WT BMT mice. Altogether, these results confirm that β2AR-deficient leukocytes accumulate in the spleen where they remain after MI, but do have the capacity to infiltrate the heart following injury in the absence of the spleen, similar to spleen-independent leukocyte infiltration levels attained in WT BMT mice.
Figure 6.
Splenectomy restores β2ARKO leukocyte infiltration into the heart following MI. (A) Representative CD68, tryptase, and MPO staining of the border or infarct zones of hearts 4d following MI surgery in WT or β2ARKO BMT that received sham and splenectomy surgery. Quantification of CD68 (B), tryptase (C) and MPO (D) staining in the border and infarct zones of 4d post-MI hearts from sham and splenectomy WT and β2ARKO BMT mice. n=6 for WT BMT Sham/MI, n=5 for WT BMT Splen/MI, n=6 for β2ARKO BMT Sham/MI, n=7 for β2ARKO BMT Splen/MI, Exact Wilcoxon rank-sum tests with multiple comparison adjustment (6 comparisons), * p < 0.05, † p < 0.01, ns = not significant.
Restoration of β2AR expression reverses leukocyte dysfunction and restores survival rates following MI
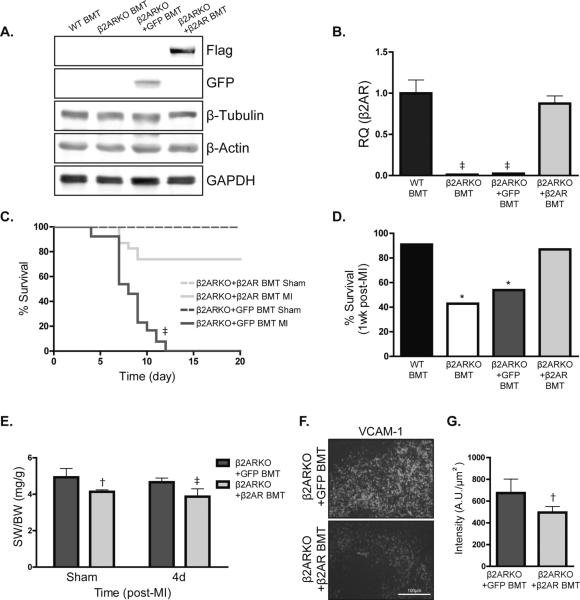
To determine if restoration of β2AR expression in β2ARKO BM could revert the β2AR BMT phenotype toward the WT BMT phenotype post-MI, β2ARKO BM was transduced with the WT β2AR lentivirus construct, or GFP control lentivirus, prior to transplantation. Immunoblotting was used to confirm protein expression of GFP in control and Flag-tagged β2AR expression for lentivirus-transduced reconstituted BM (Figure 7A) and β2AR expression in β2ARKO BM following reconstitution was approximately 95% of endogenous levels (Figure 7B). Similar to β2ARKO BMT mice, mice receiving β2ARKO BM transduced with GFP control lentivirus displayed 100% mortality post-MI with all mice dying between day 4 and 14 from cardiac rupture (Figure 7C), however restoration of β2AR expression in β2ARKO BM increased survival following MI to near WT BMT levels (Figure 7D). Reconstitution with β2AR-infected β2ARKO BM also reduced both spleen size (Figure 7E) and VCAM-1 expression (Figure 7F, 7G) compared with GFP-transduced β2ARKO BMT mice.
Figure 7.
Restoration of β2AR expression in β2ARKO BM restores survival following MI. (A) Representative immunoblot showing protein expression of GFP and Flag in reconstituted BM from β2ARKO, β2ARKO+GFP and β2ARKO+β2AR BMT mice. β-tubulin, β-actin and GAPDH are shown as loading controls. (B) β2AR expression was measured by RT-qPCR in reconstituted BM from WT and β2ARKO mice and reconstituted BM from mice that had GFP or β2AR transduced into β2ARKO BM by lentivirus prior to transplantation. Values are presented as RQ+RQmax expressed relative to WT BMT. n=10 for WT, n=10 for β2ARKO BMT, n=9 for β2ARKO+GFP BMT, n=11 for β2ARKO+β2AR BMT. Exact Wilcoxon rank-sum tests with multiple comparison adjustment (3 comparisons), ‡ p < 0.001. (C) β2ARKO+GFP and β2ARKO+β2AR BMT were subjected to sham or MI surgery and monitored daily for survival. All sham groups had 100% survival following surgery. n=6 for β2ARKO+GFP and n=7 β2ARKO+β2AR BMT sham, n=27 for β2ARKO+GFP MI, n=26 for β2ARKO+β2AR BMT MI. Log-Rank test, ‡ p < 0.001 vs β2ARKO+β2AR BMT. (D) % survival of β2ARKO+GFP and β2ARKO+β2AR BMT mice 1 week post-MI. Log-Rank tests with multiple comparison adjustment (3 comparisons), * p < 0.05 vs WT BMT MI. (E) Gravimetric analysis of spleen weight to body weight (SW/BW) of spleens from β2ARKO+GFP and β2ARKO+ β2AR BMT mice. n=7 for β2ARKO+GFP sham, n=6 for β2ARKO+ β2AR sham, 4d post n=8 for β2ARKO+GFP MI and n=10 for β2ARKO+ β2AR MI. Exact Wilcoxon rank-sum tests, † p < 0.01, ‡ p < 0.001 vs β2ARKO+GFP BMT. (F) Representative VCAM-1 staining for β2ARKO+GFP and β2ARKO+β2AR BMT spleens 4d post-MI. (G) Quantification of VCAM-1 intensity from immunohistochemistry of β2ARKO+GFP (n=6) and β2ARKO+β2AR BMT (n=7) spleens. Exact Wilcoxon rank-sum test, † p < 0.01vs β2ARKO+GFP BMT.
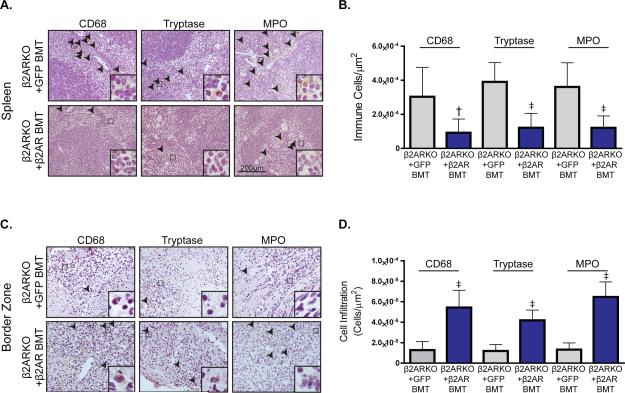
Accordingly, restoration of β2AR in β2ARKO BM also reduced levels of the leukocyte populations in the spleen (Figure 8A and 8B) to those not different from WT BMT mice (Supplemental Table 3). Conversely, immunohistochemistry for leukocyte infiltration in sham (Supplemental Figure 7) and injured myocardium of β2AR-rescued β2ARKO BMT mice revealed the reciprocal results. Thus, leukocyte infiltration was increased in the border (Figure 8C) and infarct (Supplemental Figure 8) zones of the heart in β2ARKO BMT mice transduced with β2AR versus GFP lentivirus, including monocytes/macrophages, mast cells and neutrophils (Figure 8D), which were restored to WT BMT levels (Supplemental Table 3).
Figure 8.
β2AR re-expression on reconstituted β2ARKO BM reverses splenic retention of leukocytes. (A) Representative CD68, tryptase and MPO staining of spleens from β2ARKO+GFP and β2ARKO+β2AR BMT mice 4d post-MI. (B) Quantification of CD68, tryptase and MPO staining from the spleens from (A). n=8 β2ARKO+GFP BMT and n=8 β2ARKO+β2AR BMT, Exact Wilcoxon rank-sum tests, † p < 0.01, ‡ p < 0.001 vs β2ARKO+GFP BMT. (C) Representative CD68, tryptase and MPO staining of the border zone of hearts from β2ARKO+GFP and β2ARKO+β2AR BMT animals 4d following MI surgery. (D) Quantification of CD68, tryptase and MPO staining from the border zone of 4d post-MI hearts from β2ARKO+GFP (n=8) and β2ARKO+β2AR BMT (n=6) mice. Exact Wilcoxon rank-sum tests, ‡ p < 0.001 vs β2ARKO+GFP BMT.
Discussion
Inflammatory responses are critical for wound-healing following MI1. All three βAR isoforms have been shown to mediate a number of effects in the immune system, including hematopoiesis, lymphocyte homing and cytokine/chemokine production, however little is known about how they regulate immune cell responses following acute cardiac injury10, 14. To investigate the immune cell-specific impact of βARs on cardiac survival and remodeling following MI, we generated chimeric mice lacking β1AR, β2AR or β3AR expression on cells of hematopoietic origin. The most striking outcome was observed with β2ARKO BMT animals, which displayed 100% mortality due to cardiac rupture, in contrast to their WT counterparts that had ~20% death. β2AR chimeric mice had decreased infiltration of leukocyte populations compared to their WT counterparts demonstrating impaired innate immune responses. Recent findings have shown the importance of pro-inflammatory monocytes in initiating early immune cell-dependent reparative responses following MI2. Thus, it is likely that the inability of leukocyte populations to traffic to the heart acutely following MI in β2ARKO chimeric mice impairs early repair processes, contributing to scar instability, cardiac rupture and death.
Of great importance, the β2ARKO BMT mice had decreased leukocyte infiltration into the heart following MI with a reciprocal increase in spleen size and leukocyte retention, suggesting an impairment in immune cell egress from the spleen to the heart following acute cardiac injury. As such, splenectomy of the β2ARKO BMT mice restored cardiac leukocyte infiltration responses to those of splenectomized WT BMT mice. Recently, the spleen has been shown to be an important monocyte reservoir, holding active monocytes for release upon inflammatory injury6, 24, and has been demonstrated to be of particular importance following MI and during heart failure where there is increased antigen processing and adaptive immune system activation25. These processes are regulated through a variety of signals6. One molecular mechanism of leukocyte egress from the spleen through macrophage expression of VCAM-1 has recently been identified23. Our results demonstrate an increase in VCAM-1 in the macrophage-containing red pulp region of spleens from β2ARKO BMT animals, resulting in the retention of leukocyte populations in the spleens of these animals. VCAM-1 was also increased with a β2AR antagonist in human macrophages, with a reciprocal decrease in expression following β2AR stimulation. Interestingly, macrophage VCAM-1 expression appears to be regulated within this context in a β2AR/βARR2-dependent manner.
A common limitation of studies in mice is that they do not always translate toward human pathophysiology. However, βAR activation has been implicated in reducing spleen size and release of certain immune cell populations in a number of different species including murine and swine models and humans26-30. These changes were independent of alterations in blood flow, although the mechanism was never identified. Furthermore, β-blocker administration was shown to prevent the splenic release of immune populations, similar to our current study using β2ARKO chimeric animals, and this response was amplified when combined with an inflammatory stimulus31. Mechanistically, our study demonstrates increased levels of leukocyte populations in the spleens of β2ARKO BMT mice both before and after MI, effects that are clearly independent of vascular or splenic β2AR expression. Importantly, inhibition of leukocyte egress from the spleen and decreased infiltration of these populations into the heart can be reversed by restoring β2AR expression in the bone marrow prior to transplantation using a lentiviral construct, confirming the specificity of the response and demonstrating the ability to modulate hematopoietic cell receptor expression using gene therapy approaches. In addition, our data in human macrophages are consistent with those in mouse macrophages and we also observed increased VCAM-1 expression in the spleens of human donors that had taken the β-blocker metoprolol. While metoprolol has preference for β1AR, it loses its selectivity at the higher doses often used clinically32 that would also antagonize β2AR. This could account for the retention of immune cell populations observed in other studies and confirmed in our chimeric mouse model, providing further clinical relevance. Interestingly, increases in circulating immune cells with epinephrine or isoproterenol is greatly diminished in splenectomized patients33, 34 and multiple long-term studies examining the effects of splenectomy in humans have demonstrated an increased incidence in MI and HF with worsened prognosis following such events35, 36.
While many of the benefits of β-blockers are thought to be mediated through their actions on β1AR in cardiomyocytes37, immune cell-expressed βAR and β-blocker therapy have been suggested to play roles in the regulation of immune responses during HF38-42. Our findings suggest administration of β-blockers with selectivity toward β2AR around the time of MI could diminish leukocyte egress from the spleen and subsequent cardiac immune cell-dependent remodeling. The impact of such a process on overall cardiac remodeling would likely depend on the severity of β2AR inhibition, where a short-term decrease in activity may simply dampen the inflammatory response, but not ultimately prevent it, whereas chronic inhibition of leukocyte-expressed β2AR could negatively impact post-MI repair processes. Interestingly, it has recently been suggested that peri-operative use of β-blockers may actually increase cardiovascular events, including MI43 and, according to the American Heart Association/American College of Cardiology, continues to have an uncertain mortality risk44. A link between β2AR inhibition in leukocytes and these clinical observations has not been demonstrated, but warrants further investigation.
Converse to inhibition, short-term β2AR agonist administration during the inflammatory phase following MI in combination with chronic β1AR antagonist administration may provide an improved therapeutic strategy to prevent detrimental remodeling and preserve cardiac function following cardiac injury. Several studies investigating the use of β2AR agonists in the treatment of heart failure have found beneficial effects, which was attributed to the promotion of cardiomyocyte survival, however the long term benefits of βAR blockade in HF has contraindicated the use of β2AR agonists45-50. In many of these studies β2AR agonist administration commenced at later time points, missing the acute inflammatory phase. Regardless, in animal models β2AR agonists have been shown to improve cardiac remodeling following MI or ischemia/reperfusion to a greater extent than that achieved by β1AR antagonists46, 49, 50, while cardiac function and survival were further improved with a combined β1AR blocker/β2AR agonist strategy46-48. Remarkably, a single dose of the β2AR agonist clenbuterol prior to ischemic insult was shown to decrease the resulting cardiac injury45. However, these studies did not assess the contribution of the early immune cell responses to their outcomes.
In summary, using a chimeric mouse approach, we identified a critical role for hematopoietic cell-expressed β2AR in the regulation of acute cardiac inflammation and remodeling following MI. β2AR-deficient immune cells displayed impaired recruitment to the injured myocardium following MI, with reciprocal leukocyte retention within the spleen that was maintained following MI. Lentiviral-mediated re-expression of β2AR in β2ARKO BM prior to transplantation restored BM migration, splenic retention levels of leukocyte populations and leukocyte infiltration into the heart following injury. Altogether, our results highlight an immunomodulatory role for β2AR that could be targeted to promote early leukocyte-dependent reparative processes following MI, with negligible or even beneficial effects on cardiomyocytes, while avoiding issues inherent to the promotion of prolonged inflammatory events.
Supplementary Material
Clinical Perspective.
What is new?
Using chimeric mice, we demonstrate that immune cell-specific β2-adrenergic receptor (β2AR) expression is essential to the repair process following myocardial infarction. In the absence of β2AR, vascular cell adhesion molecule 1 (VCAM-1) expression is increased in leukocytes, inducing their splenic retention following injury and leading to impaired scar formation followed by rupture and death.
VCAM-1 expression is regulated dynamically by βAR ligands, including β-blockers, in both mouse and human tissues. Splenectomy partially restores β2AR-deficient leukocyte infiltration into the heart following injury, and gene therapy to rescue leukocyte β2AR expression completely restored all injury responses to that observed in normal mice.
What are the clinical implications?
βARs regulate cardiac function and remodeling following injury, classically through their effects in cardiomyocytes, and are targeted by β-blockers to help prevent detrimental myocardial remodeling. However, our findings indicate that inhibition/deletion of immune cell-expressed β2AR causes leukocyte dysfunction and altered immunomodulatory responses to acute injury.
These results have important clinical implications since β-blockers are used frequently in patients around the time of myocardial infarction, as well as peri-operatively for non-cardiac surgeries with uncertain mortality risk.
Thus, understanding the essential role for β2AR in mediating immune cell responses will inform strategies for β-blocker, or βAR agonist, administration following acute injury.
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL105414 (to D.G.T.), HL091799 and HL085503 (to W.J.K.), HL098481 (to J.W.C.), and an AHA postdoctoral fellowship (to L.A.G.).
Footnotes
Disclosures
DGT and WJK have equity in Renovacor, Inc., which has neither funded this study nor has a relevant product related to this study.
References
- 1.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nature reviews. Cardiology. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed tnf-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-kappab and induces neutrophil infiltration via lipopolysaccharide-induced cxc chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 6.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 8.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following st-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Seminars in immunology. 2014;26:357–368. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacological reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- 12.Grisanti LA, Talarico JA, Carter RL, Yu JE, Repas AA, Radcliffe SW, Tang HA, Makarewich CA, Houser SR, Tilley DG. Beta-adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of erk1/2 and akt. J Mol Cell Cardiol. 2014;72:39–51. doi: 10.1016/j.yjmcc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speidl WS, Toller WG, Kaun C, Weiss TW, Pfaffenberger S, Kastl SP, Furnkranz A, Maurer G, Huber K, Metzler H, Wojta J. Catecholamines potentiate lps-induced expression of mmp-1 and mmp-9 in human monocytes and in the human monocytic cell line u937: Possible implications for peri-operative plaque instability. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:603–605. doi: 10.1096/fj.03-0454fje. [DOI] [PubMed] [Google Scholar]
- 14.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain, behavior, and immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. The Journal of experimental medicine. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubahn CL, Lorton D, Schaller JA, Sweeney SJ, Bellinger DL. Targeting alpha- and beta-adrenergic receptors differentially shifts th1, th2, and inflammatory cytokine profiles in immune organs to attenuate adjuvant arthritis. Frontiers in immunology. 2014;5:346. doi: 10.3389/fimmu.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Annals of the New York Academy of Sciences. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 19.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nature medicine. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liggett SB, Bouvier M, Hausdorff WP, O'Dowd B, Caron MG, Lefkowitz RJ. Altered patterns of agonist-stimulated camp accumulation in cells expressing mutant beta 2-adrenergic receptors lacking phosphorylation sites. Molecular pharmacology. 1989;36:641–646. [PubMed] [Google Scholar]
- 22.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. Beta-arrestin-dependent, g protein-independent erk1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 23.Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Macrophages retain hematopoietic stem cells in the spleen via vcam-1. The Journal of experimental medicine. 2015;212:497–512. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. European heart journal. 2014;35:376–385. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernstrom U, Sandberg G. Effects of adrenergic alpha- and beta-receptor stimulation on the release of lymphocytes and granulocytes from the spleen. Scandinavian journal of haematology. 1973;11:275–286. doi: 10.1111/j.1600-0609.1973.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Modin A, Pernow J, Lundberg JM. Comparison of the acute influence of neuropeptide y and sympathetic stimulation on the composition of blood cells in the splenic vein in vivo. Regulatory peptides. 1993;47:159–169. doi: 10.1016/0167-0115(93)90420-d. [DOI] [PubMed] [Google Scholar]
- 28.Moerman EJ, Scapagnini U, De Schaepdryver AF. Adrenergic receptors in the isolated perfused dog spleen. European journal of pharmacology. 1969;5:279–285. doi: 10.1016/0014-2999(69)90149-6. [DOI] [PubMed] [Google Scholar]
- 29.Harris TJ, Waltman TJ, Carter SM, Maisel AS. Effect of prolonged catecholamine infusion on immunoregulatory function: Implications in congestive heart failure. J Am Coll Cardiol. 1995;26:102–109. doi: 10.1016/0735-1097(95)00123-h. [DOI] [PubMed] [Google Scholar]
- 30.Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z. Spleen volume changes during adrenergic stimulation with low doses of epinephrine. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2013;64:649–655. [PubMed] [Google Scholar]
- 31.Rogausch H, del Rey A, Oertel J, Besedovsky HO. Norepinephrine stimulates lymphoid cell mobilization from the perfused rat spleen via beta-adrenergic receptors. The American journal of physiology. 1999;276:R724–730. doi: 10.1152/ajpregu.1999.276.3.R724. [DOI] [PubMed] [Google Scholar]
- 32.Zebrack JS, Munger M, Macgregor J, Lombardi WL, Stoddard GP, Gilbert EM. Beta-receptor selectivity of carvedilol and metoprolol succinate in patients with heart failure (select trial): A randomized dose-ranging trial. Pharmacotherapy. 2009;29:883–890. doi: 10.1592/phco.29.8.883. [DOI] [PubMed] [Google Scholar]
- 33.Landmann R, Durig M, Gudat F, Wesp M, Harder F. Beta-adrenergic regulation of the blood lymphocyte phenotype distribution in normal subjects and splenectomized patients. Advances in experimental medicine and biology. 1985;186:1051–1062. doi: 10.1007/978-1-4613-2463-8_127. [DOI] [PubMed] [Google Scholar]
- 34.Van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler FW, Soliman A, Brodde OE. Catecholamines increase lymphocyte beta 2-adrenergic receptors via a beta 2-adrenergic, spleen-dependent process. The American journal of physiology. 1990;258:E191–202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- 35.Tsai MS, Chou SE, Lai HS, Jeng LB, Lin CL, Kao CH. Long-term risk of acute coronary syndrome in splenectomized patients due to splenic injury. Medicine. 2015;94:e610. doi: 10.1097/MD.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinette CD, Fraumeni JF., Jr. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, Chen X. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res. 2013;112:498–509. doi: 10.1161/CIRCRESAHA.112.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maisel AS. Beneficial effects of metoprolol treatment in congestive heart failure. Reversal of sympathetic-induced alterations of immunologic function. Circulation. 1994;90:1774–1780. doi: 10.1161/01.cir.90.4.1774. [DOI] [PubMed] [Google Scholar]
- 39.Maisel AS, Harris T, Rearden CA, Michel MC. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation. 1990;82:2003–2010. doi: 10.1161/01.cir.82.6.2003. [DOI] [PubMed] [Google Scholar]
- 40.Mayer B, Holmer SR, Hengstenberg C, Lieb W, Pfeifer M, Schunkert H. Functional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levels. International journal of cardiology. 2005;103:182–186. doi: 10.1016/j.ijcard.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 41.von Haehling S, Schefold JC, Jankowska E, Doehner W, Springer J, Strohschein K, Genth-Zotz S, Volk HD, Poole-Wilson P, Anker SD. Leukocyte redistribution: Effects of beta blockers in patients with chronic heart failure. PloS one. 2009;4:e6411. doi: 10.1371/journal.pone.0006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw SM, Coppinger T, Waywell C, Dunne L, Archer LD, Critchley WR, Yonan N, Fildes JE, Williams SG. The effect of beta-blockers on the adaptive immune system in chronic heart failure. Cardiovascular therapeutics. 2009;27:181–186. doi: 10.1111/j.1755-5922.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- 43.Scali S, Patel V, Neal D, Bertges D, Ho K, Jorgensen JE, Cronenwett J, Beck A. Preoperative beta-blockers do not improve cardiac outcomes after major elective vascular surgery and may be harmful. Journal of vascular surgery. 2015;62:166–176. e162. doi: 10.1016/j.jvs.2015.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr., Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, Members AATF, Society for Cardiovascular A Interventions, the Society of Thoracic S. 2014 aha/acc guideline for the management of patients with non-st-elevation acute coronary syndromes: Executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Xiang J, Wang X, Liu H, Hu B, Feng M, Fu Q. Beta(2)-adrenoceptor agonist clenbuterol reduces infarct size and myocardial apoptosis after myocardial ischaemia/reperfusion in anaesthetized rats. British journal of pharmacology. 2010;160:1561–1572. doi: 10.1111/j.1476-5381.2010.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 47.Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, Xiao RP, Lakatta EG, Talan MI. Cardioprotective and survival benefits of long-term combined therapy with beta2 adrenoreceptor (ar) agonist and beta1 ar blocker in dilated cardiomyopathy postmyocardial infarction. The Journal of pharmacology and experimental therapeutics. 2008;325:491–499. doi: 10.1124/jpet.107.135335. [DOI] [PubMed] [Google Scholar]
- 48.Ahmet I, Morrell C, Lakatta EG, Talan MI. Therapeutic efficacy of a combination of a beta1-adrenoreceptor (ar) blocker and beta2-ar agonist in a rat model of postmyocardial infarction dilated heart failure exceeds that of a beta1-ar blocker plus angiotensin-converting enzyme inhibitor. The Journal of pharmacology and experimental therapeutics. 2009;331:178–185. doi: 10.1124/jpet.109.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaldi B, Donniacuo M, Sodano L, Gritti G, Martuscelli E, Orlandi A, Rafaniello C, Rossi F, Calzetta L, Capuano A, Matera MG. Effects of the new ultra-long-acting beta -ar agonist indacaterol in chronic treatment alone or in combination with the beta -ar blocker metoprolol on cardiac remodelling. British journal of pharmacology. 2015;172:3627–37. doi: 10.1111/bph.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhushan S, Kondo K, Predmore BL, Zlatopolsky M, King AL, Pearce C, Huang H, Tao YX, Condit ME, Lefer DJ. Selective beta2-adrenoreceptor stimulation attenuates myocardial cell death and preserves cardiac function after ischemia-reperfusion injury. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1865–1874. doi: 10.1161/ATVBAHA.112.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.