Abstract
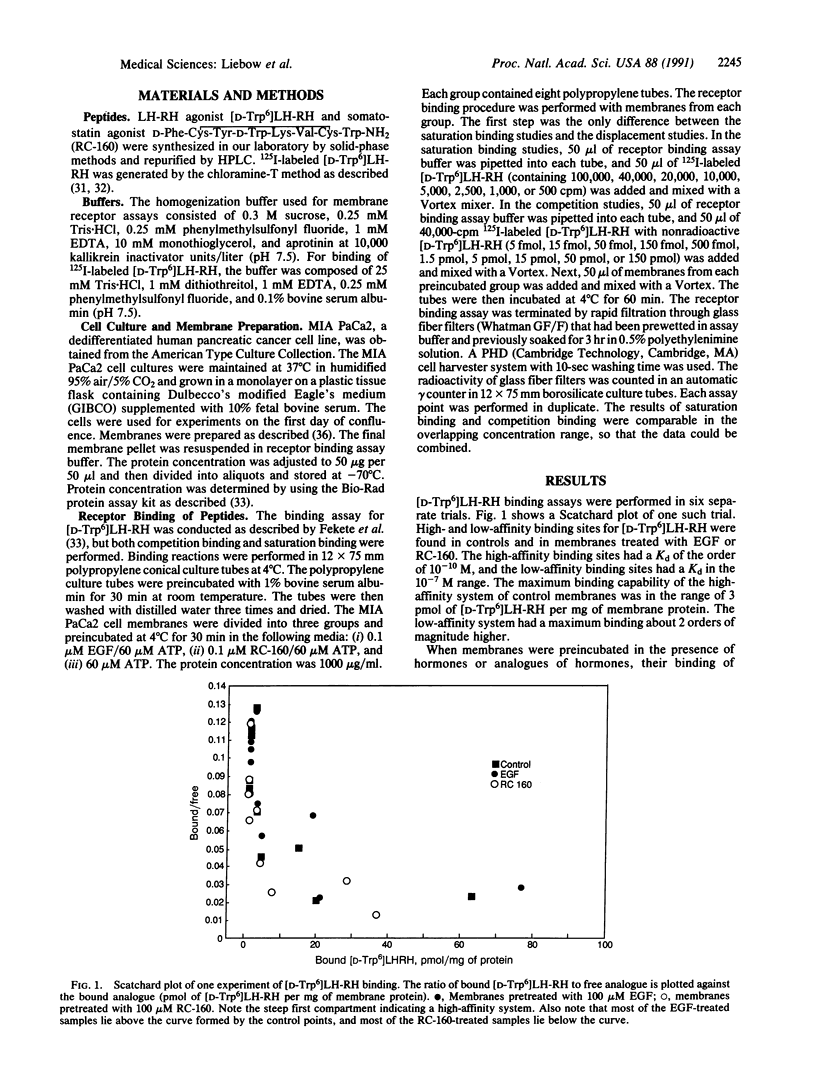
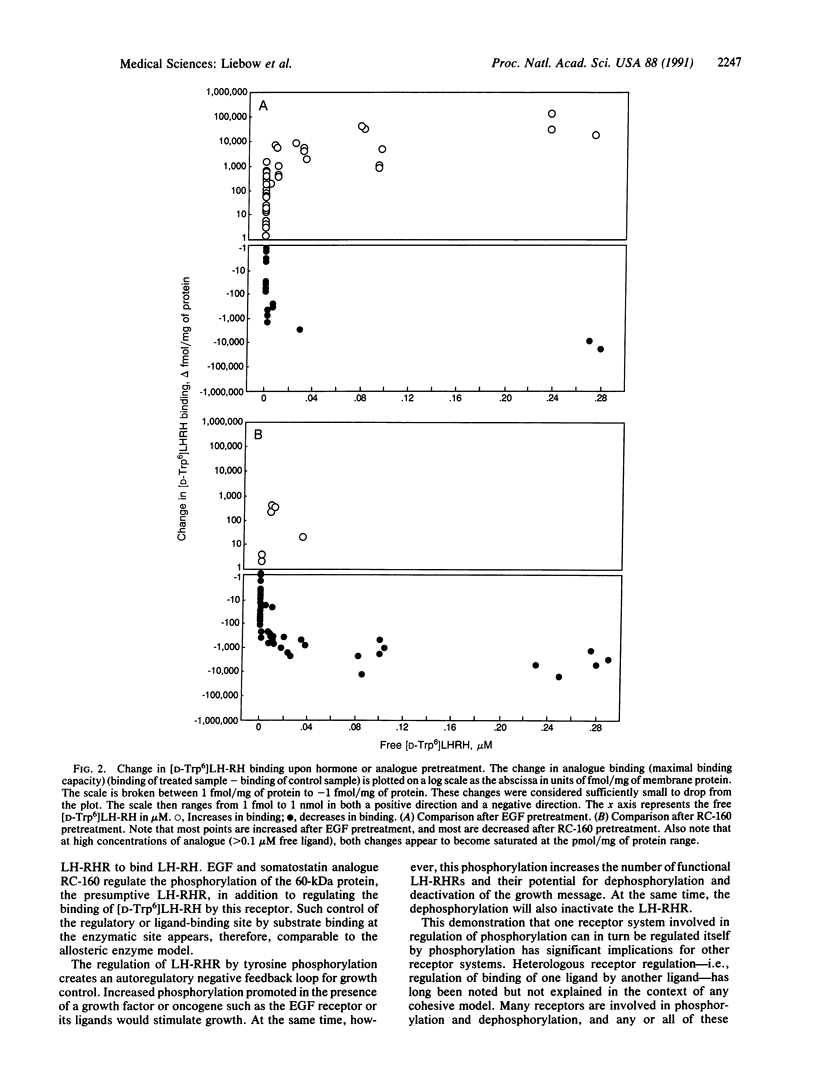
Pancreatic cancers overexpress tyrosine kinase and luteinizing hormone-releasing hormone (LH-RH) receptor (LH-RHR)-mediated tyrosine phosphatase. LH-RHR is a 60-kDa protein. One of the substrates of epidermal growth factor (EGF)-stimulated tyrosine kinase activity and LH-RH- and somatostatin-stimulated tyrosine phosphatase activity is also a 60-kDa protein. This suggests the possibility that LH-RHR regulation by tyrosine phosphatase and tyrosine kinase is mediated by (de)phosphorylation of existing LH-RHR. To test this hypothesis, membranes of MIA PaCa-2 cells, a human dedifferentiated pancreatic cancer cell line, were incubated without hormone (control) or with 0.1 microM EGF or somatostatin analogue RC-160 for 1 hr at 4 degrees C to phosphorylate the 60-kDa protein. Competition binding experiments with I125-labeled [D-Trp6]LH-RH by displacement with a nonradioactive ligand showed that the LH-RH binding in 69% of the points was increased by EGF and 85% was decreased by RC-160 compared with controls (n = 61; both significant, P less than 0.001). The specific binding was altered, increasing 50-150% after preincubation with EGF and decreasing 60-70% after RC-160. No change was seen in the binding affinity constant after pretreatment with EGF or RC-160. This shows that phosphorylation regulates binding of LH-RH and may explain the up-regulation by EGF and down-regulation by RC-160 and by LH-RH of the LH-RH response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Cicirelli M. F., Tonks N. K., Diltz C. D., Weiel J. E., Fischer E. H., Krebs E. G. Microinjection of a protein-tyrosine-phosphatase inhibits insulin action in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5514–5518. doi: 10.1073/pnas.87.14.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Eidne K. A., Hendricks D. T., Millar R. P. Demonstration of a 60K molecular weight luteinizing hormone-releasing hormone receptor in solubilized adrenal membranes by a ligand-immunoblotting technique. Endocrinology. 1985 May;116(5):1792–1795. doi: 10.1210/endo-116-5-1792. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fekete M., Zalatnai A., Comaru-Schally A. M., Schally A. V. Membrane receptors for peptides in experimental and human pancreatic cancers. Pancreas. 1989;4(5):521–528. doi: 10.1097/00006676-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Fekete M., Zalatnai A., Schally A. V. Presence of membrane binding sites for [D-TRP6]-luteinizing hormone-releasing hormone in experimental pancreatic cancer. Cancer Lett. 1989 May;45(2):87–91. doi: 10.1016/0304-3835(89)90141-9. [DOI] [PubMed] [Google Scholar]
- Ferluga J. Potential role of anti-oncogenes in aging. Mech Ageing Dev. 1990 Apr 30;53(3):267–275. doi: 10.1016/0047-6374(90)90044-g. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Tonks N. K., Charbonneau H., Cicirelli M. F., Cool D. E., Diltz C. D., Krebs E. G., Walsh K. A. Protein tyrosine phosphatases: a novel family of enzymes involved in transmembrane signalling. Adv Second Messenger Phosphoprotein Res. 1990;24:273–279. [PubMed] [Google Scholar]
- Gill G. N. Regulation of EGF receptor expression and function. Mol Reprod Dev. 1990 Sep;27(1):46–53. doi: 10.1002/mrd.1080270110. [DOI] [PubMed] [Google Scholar]
- Hazum E. Purification of gonadotropin releasing hormone receptors using the avidin-biotin technique. J Chromatogr. 1990 Jun 27;510:233–238. doi: 10.1016/s0021-9673(01)93757-4. [DOI] [PubMed] [Google Scholar]
- Hierowski M. T., Liebow C., du Sapin K., Schally A. V. Stimulation by somatostatin of dephosphorylation of membrane proteins in pancreatic cancer MIA PaCa-2 cell line. FEBS Lett. 1985 Jan 7;179(2):252–256. doi: 10.1016/0014-5793(85)80529-9. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Lindberg R. A., Middlemas D. S. Novel receptor protein-tyrosine kinases. Adv Second Messenger Phosphoprotein Res. 1990;24:260–265. [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Kadar T., Redding T. W., Ben-David M., Schally A. V. Receptors for prolactin, somatostatin, and luteinizing hormone-releasing hormone in experimental prostate cancer after treatment with analogs of luteinizing hormone-releasing hormone and somatostatin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):890–894. doi: 10.1073/pnas.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers R., Van Obberghen E., Ballotti R., Schlessinger J., Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990 Oct 5;265(28):16886–16890. [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl protein phosphatases. Biochem J. 1989 Jan 1;257(1):23–36. doi: 10.1042/bj2570023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. T., Liebow C., Kamer A. R., Schally A. V. Effects of epidermal growth factor and analogues of luteinizing hormone-releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyrosine residues of specific protein substrates in various tumors. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1656–1660. doi: 10.1073/pnas.88.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow C., Lee M. T., Schally A. Antitumor effects of somatostatin mediated by the stimulation of tyrosine phosphatase. Metabolism. 1990 Sep;39(9 Suppl 2):163–166. doi: 10.1016/0026-0495(90)90237-7. [DOI] [PubMed] [Google Scholar]
- Liebow C., Reilly C., Serrano M., Schally A. V. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. E., Khew-Goodall Y., Stone S. R., Hemmings B. A. Expression and organization of protein phosphatase 2A catalytic subunit genes. Adv Second Messenger Phosphoprotein Res. 1990;24:236–241. [PubMed] [Google Scholar]
- McCarley D. J., Parsons S. J. Reduced tyrosine kinase specific activity is associated with hypophosphorylation of pp60c-src in cells infected with avian erythroblastosis virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5793–5797. doi: 10.1073/pnas.84.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Chapdelaine A., Chevalier S. Prostatic acid phosphatase in serum of patients with prostatic cancer is a specific phosphotyrosine acid phosphatase. Clin Chem. 1990 Aug;36(8 Pt 1):1450–1455. [PubMed] [Google Scholar]
- Ogier S. A., Mitchell R., Fink G. Solubilization of a large molecular weight form of the rat LHRH receptor. J Endocrinol. 1987 Oct;115(1):151–159. doi: 10.1677/joe.0.1150151. [DOI] [PubMed] [Google Scholar]
- Srkalovic G., Cai R. Z., Schally A. V. Evaluation of receptors for somatostatin in various tumors using different analogs. J Clin Endocrinol Metab. 1990 Mar;70(3):661–669. doi: 10.1210/jcem-70-3-661. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Identifying tumor suppressor genes in human colorectal cancer. Science. 1990 Jan 5;247(4938):12–13. doi: 10.1126/science.2403692. [DOI] [PubMed] [Google Scholar]
- Szende B., Srkalovic G., Schally A. V., Lapis K., Groot K. Inhibitory effects of analogs of luteinizing hormone-releasing hormone and somatostatin on pancreatic cancers in hamsters. Events that accompany tumor regression. Cancer. 1990 May 15;65(10):2279–2290. doi: 10.1002/1097-0142(19900515)65:10<2279::aid-cncr2820651020>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]


