Chronic metabolic insult to the liver by alcohol and other nutritional abuse results in alcoholic liver disease (ALD) and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH), both of which are well-recognized risk factors for hepatocellular carcinoma (HCC). ALD is a leading HCC etiology in several European countries, whilst the epidemic of obesity and associated metabolic syndrome, e.g., type 2 diabetes (T2D), has led to an increased recognition of NAFLD/NASH as a rapidly increasing HCC risk factor globally, particularly in Western countries such as the US. Given the disproportionally high population attributable fraction (PAF, the proportion of cases attributable to a given risk factor) of ALD (24%) and obesity/T2D (37%) for HCC in the US 1 and still incompletely understood risk factors, further studies are clearly needed to establish strategies for clinical management of the patients. In this commentary article, we overview shared or unique clinical and molecular factors linked to ALD and NAFLD/NASH-related HCC, and highlight unmet needs to be addressed in future studies (Figure 1).
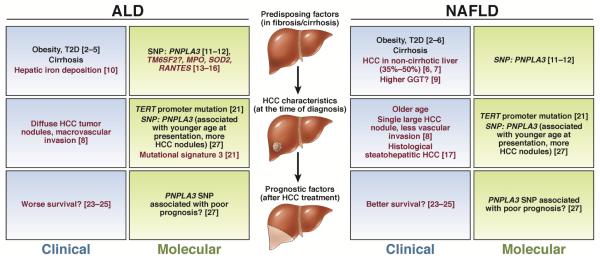
Figure 1.
Common (black text) and unique (red text) features of ALD and NAFLD in HCC predisposing factors, HCC characteristics and prognostic factors after therapy. Molecular factors are described in green boxes and clinical characteristics in blue boxes. References supporting each association are indicated. GGT, γ-glutamyl transferase; HCC, hepatocellular carcinoma; SNP, single nucleotide polymorphism; T2D, type 2 diabetes.
Clinical predisposing factors for ALD- or NAFLD-related HCC
Elucidation of HCC risk factors specific to ALD and NAFLD/NASH is critical for the establishment of rational and accurate monitoring of cancer development and potential preventive interventions (Table 1). A population-based US study of nearly 7,000 cases of HCC and more than 250,000 controls confirmed that the odds ratio (OR) for developing HCC in ALD is higher than that in NAFLD-associated T2D and/or obesity (OR 4.1 and 2.5 respectively) 1. Features of the metabolic syndrome, often accompanying NAFLD/NASH, have been reported as independent risk factors for HCC development. A systematic review of 11 cohort studies observed that the risk of developing HCC was 17% higher in overweight and 89% higher in obese individuals 2. Obesity was also associated with increased HCC risk in alcoholic cirrhosis (OR 3.2), although the magnitude was higher in cryptogenic cirrhosis (assumed to be enriched with NAFLD/NASH; OR 11.1), but not viral hepatitis 3. Insulin resistance or T2D have been recurrently reported as a risk factor for the development of cancer, in particular HCC. A recent retrospective study of 480 subjects with ALD or NAFLD showed that diabetes was associated with an increased cumulative incidence of HCC in both ALD and NAFLD with higher absolute HCC incidence in ALD 4. In a multicenter cohort study of 741 patients with ALD- or NAFLD-related HCC, diabetes, hypertension, insulin resistance, and hypertriglyceridemia were more frequent in NAFLD 5. These reports collectively indicate that features of the metabolic syndrome are shared risk factors associated with elevated HCC risk in both ALD and NAFLD, but more prominent in the latter.
Table 1.
Clinical and molecular factors associated with ALD and NAFLD-related HCC.
| Group / Author |
Total n | Study type | Tissue assessed |
Parameter assessed |
Liver histology | ALD (n, % total) |
Characteristics associated with ALD-HCC |
NAFLD (n, % total) |
Characteristics associated with NAFLD-HCC |
Ref |
|---|---|---|---|---|---|---|---|---|---|---|
|
Predisposing
factors to HCC |
||||||||||
| Ascha et al | 510 | Retrospective cohort |
Clinical | 100% cirrhosis | 0 (0%) | NA | 195 (38%) | Age and any consumption of alcohol were risk factors for HCC |
32 | |
| Loomba et al | 23712 | Prospective cohort |
Clinical | NA | 2401 alcohol use (10%) |
BMI ≥30 was associated with increased risk of HCC |
NA | 33 | ||
| Tokushige et al | 14530 | Cross- sectional |
Clinical | NAFLD HCC: 62% cirrhosis ALD HCC: 78% cirrhosis |
991 (7%) | ALD HCC was associated with younger age and lower proportion of women. |
292 (2%) | Associated with metabolic syndrome and lower rates of cirrhosis. |
34 | |
| Raff et al | 480 | Retrospective cohort |
Clinical | NAFLD: 12% cirrhosis ALD: 46% cirrhosis |
165 (34%) | Diabetes associated with HCC development in ALD and NAFLD |
315 (66%) | See alcohol characteristics |
4 | |
| Mittal et al | 1500 | Retrospective cohort |
Clinical | NAFLD-HCC: 70% cirrhosis ALD-HCC: 89% cirrhosis |
1209 (81%) | Two times more ALD-HCC without cirrhosis than HCV- HCC. |
120 (8%) | Five times more NAFLD-HCC without cirrhosis than HCV-HCC. |
6 | |
| Kodama et al | 157 | Retrospective- prospective cohort |
Clinical | 100% cirrhosis | 85 (54%) | Diabetes. | 72 (46%) | Age, GGT and Child-Pugh score. |
9 | |
| Nahon et al | 301 | Prospective cohort |
Non- tumoral liver in subjects with HCC |
Liver iron deposition HFE mutations |
100% cirrhosis | 162 (54%) | Liver iron and HFE
C282Y mutations. |
NA | NA | 10 |
| Trepo et al | 2503 | Meta-analysis of individual participant data |
Blood |
PNPLA3 SNP (rs738409) |
100% cirrhosis | 1374 (55%) | rs738409 GG genotype |
2 (0.1%) | NA | 11 |
| Singal et al | 2937 | Meta-analysis | Blood |
PNPLA3 SNP (rs738409) |
NA | NA | rs738409 GG genotype |
NA | rs738409 GG genotype |
12 |
| Nischalke et al | 482 with ALD cirrhosis 382 controls Validation: 229 ALD cirrhosis |
Case-control | Blood |
PNPLA3 SNP (rs738409) NCAN (rs228603) |
100% cirrhosis | 356 (100%) | rs2228603 risk variant (CT/TT) and rs738409 risk variant (IM/MM) |
0 (0%) | 14 | |
| Ueyama et al | 389 | Retrospective cohort |
Blood | Multiple SNPs | 13% cirrhosis | 223 (57%) | rs738409 (PNPLA3) GG genotype |
35 | ||
| Charni et al | 496 | Cohort | Blood |
RANTES
promoter SNP (rs2107538) |
253 (51%) | rs2107538 risk variant associated with HCC occurrence MPO (rs2333227) and SOD2 (rs4880) SNP associated with risk of HCC and death |
0 (0%) | 16 | ||
| Nahon et al | 190 | Prospective cohort |
Blood | Multiple SNP | 100% cirrhosis | 191 (100%) | 0 (0%) | 15 | ||
|
Clinical and
Molecular Features of HCC |
||||||||||
| Lee et al | 512 | Retrospective cohort |
Clinical | 55 (11%) | 35 (7%) cryptogenic HCC |
Single nodule HCC, less portal vein invasion. No difference in survival. |
8 | |||
| Jeong et al | 91 HCC | Cohort | HCC | Gene expression |
50% cirrhosis | 7 (8%) | ALD-HCC enriched in gene expression cluster B |
NA | 18 | |
| Boyault et al | Derivation: 57 HCC Validation: 63 HCC |
Cohort | HCC | Gene expression |
NA | 41 (33%) | ALD-HCC enriched in HCC subtypes G3, G4 and G6 |
NA | NA | 19 |
| Schulze et al | 243 | Cohort | HCC | Mutational signatures based on exome sequencing |
49% cirrhosis | 100 (41%) | Mutational signature 3. CTNNB1 mutations. |
44 (18%) | NA | 21 |
|
Prognostic
factors after HCC diagnosis/ treatment |
||||||||||
| Siriwardana et al |
150 | Prospective cohort |
Clinical | 61 (41%) | Diffuse tumor nodules and macrovascular invasion more common. Worse survival of advanced HCC. |
89 (59%) cryptogenic HCC |
Single HCC more common. Improved survival in advanced HCC compared to ALD-HCC. |
24 | ||
| Takeuchi et al | 638 | Retrospective cohort |
Blood |
PNPLA3 SNP (rs738409) |
NA | 89 (14%) heavy drinkers |
Worse survival in ALD subjects with low BMI and rs738409 GG genotype rs738409 GG genotype associated with higher number of HCC lesions, higher HCC grade and worse survival in ALD and NAFLD subjects compared to other etiologies. |
70 (11%) | No association | 26 |
| Valenti et al | 460 HCC | Retrospective- prospective cohort |
Blood |
PNPLA3 SNP (rs738409) |
96% cirrhosis | 80 (17%) | 28 (6%) | See alcohol characteristics |
27 |
Studies with more than 100 ALD and/or NAFLD subjects were included.
Advanced liver fibrosis or cirrhosis is a well-established HCC risk factor and is a primary feature that justifies enrollment for regular HCC surveillance. However, a growing number of recent epidemiological studies have consistently shown that established cirrhosis is less frequent in NAFLD-related HCC (50-65%) compared to ALD-related HCC (69-89%) 5, 6. Prevalence of cirrhosis is lower in older subjects with ALD and HCC, whereas NAFLD exhibited an opposite trend, suggesting distinct mechanisms of carcinogenesis between the two conditions. Of note, there is a striking sex difference in prevalence of non-cirrhotic HCC when comparing males (62%) to females (27%) which may be linked to genetic and/or environmental factors 5. Clarification of HCC predisposing factors, especially in NAFLD patients without cirrhosis, is an urgent unmet need because there is no strategy of HCC surveillance targeting this highly prevalent group of patients in current practice guidelines.
Older age, male sex, and severe impairment of liver function are common HCC risk factors shared by ALD and NAFLD, although subjects with NAFLD tend to be older and possibly more often female 7, 8. High serum γ-glutamyl transferase (GGT) and a higher Child-Pugh score were reported as risk factors for HCC in NAFLD 9. Excess iron deposition in hepatocytes and the C282Y HFE mutation frequent in subjects of European descent, were associated with elevated HCC risk in ALD patients, but not in HCV-infected patients 10.
Molecular predisposing factors to ALD- or NAFLD-related HCC
Several germline DNA variants have been identified as potential risk factors for ALD- and/or NAFLD-related HCC. Two systematic reviews reported that a single-nucleotide polymorphism (SNP) in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene (rs738409, I148M) was associated with ALD-and NASH-related HCC (OR = 1.3 to 2.2) as well as fibrosis severity 11, 12. Although the mechanism by which the SNP leads to HCC development in ALD and NAFLD is yet to be elucidated, several reports have underlined that the variant could cause lipid accumulation in hepatocytes through increased triglyceride synthesis and impaired hydrolysis. Recently, a SNP in the transmembrane 6 superfamily member 2 (TM6SF2) gene (rs58542926), a regulator of liver fat metabolism associated with presence of NAFLD and liver fibrosis, was found to be associated with NAFLD-related HCC in univariable, but not in multivariable analysis adjusting for age, sex, body mass index (BMI), T2D, and cirrhosis in a sub-cohort of 99 Caucasian patients 13. A SNP in the neurocan (NCAN) gene (rs228603) previously found to be associated with ALD-related HCC 14 was in fact in strong linkage disequilibrium with the TM6SF2 SNP 13. Other SNP implicated in HCC development in ALD patients include genes implicated in reactive oxygen species formation (MPO, SOD) and in inflammation (RANTES) 15, 16.
Clinical demographics at the time of HCC diagnosis
Clinical cohort or case series studies have elucidated several distinct clinical demographic features of ALD- and NAFLD-related HCC (Table 1). These associations may arise from unique etiology-specific mechanisms of carcinogenesis or from the clinical context at diagnosis, i.e., incidental diagnosis or during the course of regular follow-up for liver or non-liver diseases. Recurrently reported clinical characteristics of NAFLD-related HCC include a single and relatively large tumor nodule with less vascular invasion as well as older age at presentation when compared to ALD- or viral hepatitis-related HCC 8. These findings may suggest a generally indolent nature, i.e., slow-growing and less-disseminative, of NAFLD-related HCC tumors incidentally found at an older age. Frequent co-existence of the metabolic syndrome is a key feature of NAFLD-related HCC or alternatively HCC in the context of cryptogenic cirrhosis, thought to be closely associated to NAFLD or a previous history of NAFLD 8. Histologically, tumors are similar, although better tumor differentiation compared to other etiologies has been reported in NAFLD-HCC and alpha-fetoprotein serum levels have been reported to be lower 7. In addition, a recently recognized histological variant of HCC, steatohepatitic HCC, has been associated with features of NAFLD and NASH 17.
Molecular features of ALD- and NAFLD-related HCC
Molecular, especially genomic, features of ALD- and NAFLD-related HCC are less well characterized. In early genome-wide transcriptome profiling studies of approximately 80 to 90 HCC cases, aiming at depicting functional molecular pathway dysregulation, several ALD-related HCC samples (up to 8% of the cohort) showed a trend or no association with a less-aggressive molecular subclass, better post-surgical survival, low serum AFP level, and well differentiated histology 18. Another transcriptome study of 57 HCC tumors, including a larger fraction of ALD-related HCC (33%), reported somewhat contradictory finding: ALD-related HCC was distributed across both aggressive and less-aggressive molecular subclasses 19. In a recent study combining one of the human datasets with a genetic mouse model of NAFLD-related HCC (MAT1A knock-out mouse), the murine HCC tumors co-clustered with the less-aggressive subtype of human HCC 20.
A more recent study of somatic DNA mutations in 243 cases, including 41% ALD- and 18% NAFLD-related HCC, showed that prevalence of recurrently mutated genes such as TERT was generally comparable to other etiologies 21. A mutational signature (i.e., specific pattern of nucleotide sequence surrounding mutated site) no.3 was associated with alcohol (and tobacco) exposure and ALD-related HCC tumors were associated with mutations in CTNNB1, TERT, CDKN2A, SMARCA2 and HGF genes. No genetic aberration specific to NAFLD-related HCC was identified potentially due to insufficient sample size. More studies are clearly needed to fully characterize the HCC tumors with metabolic etiologies and (dis)similarity to viral hepatitis-related HCC to elucidate etiology-specific therapeutic strategies.
Prognostic factors after HCC diagnosis/treatment.
Prognostic factors are similarly understudied in the metabolic etiologies especially NAFLD. Although a recent Brazilian study demonstrated that the current American Association for the Study of Liver Diseases (or Barcelona Clinic Liver Cancer) prognostic staging system could be applicable to NAFLD-related HCC patients 22, refinement of HCC management guidelines with ALD and NAFLD-specific recommendations will be required to account for the difference in clinical presentation at the time of diagnosis. For example, the older age of NAFLD-related HCC patients and increased comorbidities leads to increased postoperative complications and 30-day mortality compared to HCV-related HCC, although post-surgical long-term outcomes are generally more favorable 23. A Sri Lankan study including 150 consecutive HCC patients with cryptogenic (assumed to be enriched for NAFLD) or ALD found that cryptogenic HCC was associated with single HCC nodules and better survival, whilst ALD-related HCC was associated with worse liver function at presentation, diffuse tumors with vascular invasion, and worse survival 24. In a tertiary center in the UK, NAFLD-related HCC showed similar survival to other etiologies despite older age and later incidental detection outside the regular surveillance due to absence of cirrhosis 25.
Prognostic relevance of the genetic polymorphism in the PNPLA3 gene (rs738409) has been evaluated in ALD- and NAFLD-related HCC. In a Japanese study of 638 consecutive HCCs, the subgroup of ALD with the GG genotype and low BMI had a worse survival than those with a BMI over 25kg/m2, however the result was not statistically significant, and no prognostic association was observed in NAFLD subjects 26. In an Italian study of 460 subjects, ALD- or NAFLD-HCC subjects with the PNPLA3 GG genotype were younger, had less advanced cirrhosis at presentation, a higher number of HCC lesions and worse survival compared to other ALD- and NAFLD-HCC subjects 27. This prognostic association was not found in subjects with non-ALD or NAFLD etiologies of HCC, however, these findings are based on limited patient series and should be confirmed in future studies covering a wider range of clinical and racial/ethnic diversities.
Potential HCC-preventive intervention
Despite intensive efforts, a specific HCC preventive intervention has yet to be endorsed by international guidelines. Although a systematic review showed that alcohol abstinence reduced the risk of developing HCC in ALD, it also indicated uncertainty in clinically meaningful risk reduction 28. Similarly, it remains unclear whether treatment of NAFLD, or features of the metabolic syndrome associated with NAFLD, reduces the risk of developing HCC although one case-control study involving patients with HCC from multiple etiologies found that treatment of diabetes with biguanides or thiazolidinediones was associated with a 70% HCC risk reduction among diabetics 29. In another large population-based cohort study, aspirin use was associated with a reduced incidence of HCC (risk ratio 0.59), in which 20.6% and 7.5% of subjects had a BMI over 30kg/m2 and consumed more than 3 alcoholic drinks per day, respectively 30. Animal experiments in a PTEN knock-out model of mice developing spontaneous steatohepatitis and HCC found a reduction of HCC development in mice undergoing regular exercise for 32 weeks compared to non-exercised controls although there was no improvement in steatosis or histological activity score 31. More research is evidently needed to establish HCC-preventive interventions, in particular for subjects with ALD and NAFLD.
Conclusions
As outlined in this commentary, there are still multiple gaps in our knowledge of the natural history of ALD- and NAFLD-related HCC. Given the growing epidemic of obesity and metabolic disorders accompanied with NAFLD and the elevated HCC risk in non-cirrhotic NAFLD, future studies should focus on identification of clinical and/or molecular predisposing factors to specify target populations for HCC surveillance and preventive intervention. Further clarification of clinical demographics such as older age and more frequent comorbidities in NAFLD-related HCC will enable the design of cost-effective implementations of surveillance, treatment, and follow-up strategies applicable in clinical practice.
Acknowledgments
Grant support:
This work was supported by the FLAGS foundation, the Nuovo-Soldati Cancer Research Foundation and an advanced training grant from Geneva University Hospital to NG and NIH/NIDDK R01 DK099558 and the Irma T. Hirschl Trust to YH.
Glossary
- ALD
alcoholic liver disease
- BMI
body mass index
- CTP
Child-Turcotte-Pugh
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OR
odds ratio
- PAF
population attributable fraction
- SNP
single nucleotide polymorphism
- T2D
type 2 diabetes
Biographies


Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Nothing to disclose.
Author contributions:
NG and YH contributed to conception, drafting, critical revision for important intellectual content and final approval of the version to be published.
References
- 1.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–21. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolk SCL. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. British Journal of Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair S, Mason A, Eason J, et al. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–5. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 4.Raff EJ, Kakati D, Bloomer JR, et al. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol. 2015;3:9–16. doi: 10.14218/JCTH.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokushige K, Hyogo H, Nakajima T, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. Journal of gastroenterology. 2015:1–11. doi: 10.1007/s00535-015-1129-1. [DOI] [PubMed] [Google Scholar]
- 6.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Jeong SH, Byoun YS, et al. Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer. 2013;13:335. doi: 10.1186/1471-2407-13-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodama K, Tokushige K, Hashimoto E, et al. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(Suppl 1):E247–52. doi: 10.1111/j.1530-0277.2012.01900.x. [DOI] [PubMed] [Google Scholar]
- 10.Nahon P, Sutton A, Rufat P, et al. Liver Iron, HFE Gene Mutations, and Hepatocellular Carcinoma Occurrence in Patients With Cirrhosis. Gastroenterology. 2008;134:102–110. doi: 10.1053/j.gastro.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Trepo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology. 2014;59:2170–2177. doi: 10.1002/hep.26767. [DOI] [PubMed] [Google Scholar]
- 12.Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. The American journal of gastroenterology. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nischalke HD, Lutz P, Kramer B, et al. A common polymorphism in the NCAN gene is associated with hepatocellular carcinoma in alcoholic liver disease. J Hepatol. 2014;61:1073–9. doi: 10.1016/j.jhep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Nahon P, Sutton A, Rufat P, et al. Myeloperoxidase and superoxide dismutase 2 polymorphisms comodulate the risk of hepatocellular carcinoma and death in alcoholic cirrhosis. Hepatology. 2009;50:1484–93. doi: 10.1002/hep.23187. [DOI] [PubMed] [Google Scholar]
- 16.Charni F, Sutton A, Rufat P, et al. Chemokine RANTES Promoter Dimorphisms and Hepatocellular Carcinoma Occurrence in Patients with Alcoholic or Hepatitis C Virus–Related Cirrhosis. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1439–1446. doi: 10.1158/1055-9965.EPI-11-0341. [DOI] [PubMed] [Google Scholar]
- 17.Shibahara J, Ando S, Sakamoto Y, et al. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology. 2014;64:951–962. doi: 10.1111/his.12343. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Chu IS, Heo J, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 19.Boyault S, Rickman DS, de Reynies A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 20.Frades I, Andreasson E, Mato JM, et al. Integrative genomic signatures of hepatocellular carcinoma derived from nonalcoholic Fatty liver disease. PLoS One. 2015;10:e0124544. doi: 10.1371/journal.pone.0124544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nature genetics. 2015 doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi L, Oliveira CP, Alvares-da-Silva MR, et al. Hepatocellular Carcinoma Management in Nonalcoholic Fatty Liver Disease Patients: Applicability of the BCLC Staging System. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pocha C, Kolly P, Dufour JF. Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: A Problem of Growing Magnitude. Semin Liver Dis. 2015;35:304–17. doi: 10.1055/s-0035-1562949. [DOI] [PubMed] [Google Scholar]
- 24.Siriwardana RC, Niriella MA, Dassanayake AS, et al. Clinical characteristics and outcome of hepatocellular carcinoma in alcohol related and cryptogenic cirrhosis: a prospective study. Hepatobiliary Pancreat Dis Int. 2015;14:401–5. doi: 10.1016/s1499-3872(15)60343-5. [DOI] [PubMed] [Google Scholar]
- 25.Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2013;60:110–7. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi Y, Ikeda F, Moritou Y, et al. The impact of patatin-like phospholipase domain-containing protein 3 polymorphism on hepatocellular carcinoma prognosis. J Gastroenterol. 2013;48:405–12. doi: 10.1007/s00535-012-0647-3. [DOI] [PubMed] [Google Scholar]
- 27.Valenti L, Motta BM, Soardo G, et al. PNPLA3 I148M polymorphism, clinical presentation, and survival in patients with hepatocellular carcinoma. PloS one. 2013;8:e75982. doi: 10.1371/journal.pone.0075982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heckley GA, Jarl J, Asamoah BO, et al. How the risk of liver cancer changes after alcohol cessation: A review and meta-analysis of the current literature. BMC Cancer. 2011;11:446. doi: 10.1186/1471-2407-11-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–46. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. Journal of the National Cancer Institute. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piguet A-C, Saran U, Simillion C, et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. Journal of hepatology. 2015;62:1296–1303. doi: 10.1016/j.jhep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 33.Loomba R, Yang H-I, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. American journal of epidemiology. 2013:kws252. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokushige K, Hashimoto E, Horie Y, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230–7. doi: 10.1007/s00535-011-0431-9. [DOI] [PubMed] [Google Scholar]
- 35.Ueyama M, Nishida N, Korenaga M, et al. The impact of PNPLA3 and JAZF1 on hepatocellular carcinoma in non-viral hepatitis patients with type 2 diabetes mellitus. J Gastroenterol. 2015 doi: 10.1007/s00535-015-1116-6. [DOI] [PubMed] [Google Scholar]