Abstract
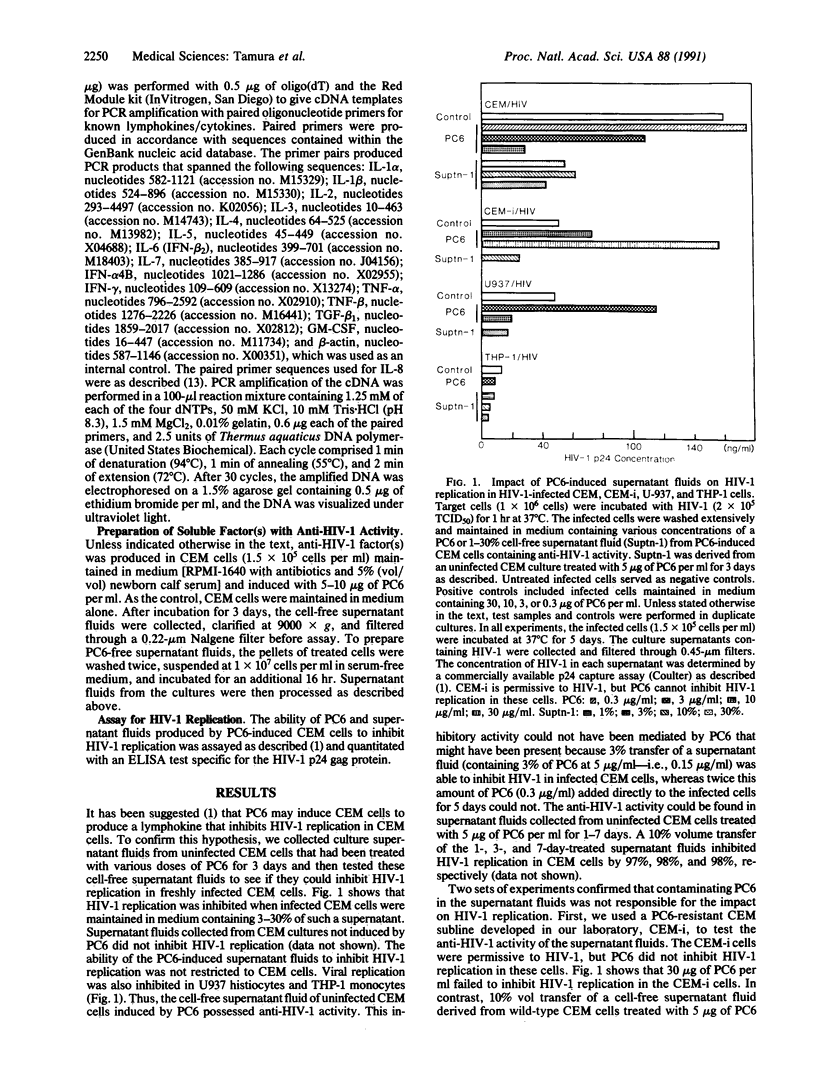
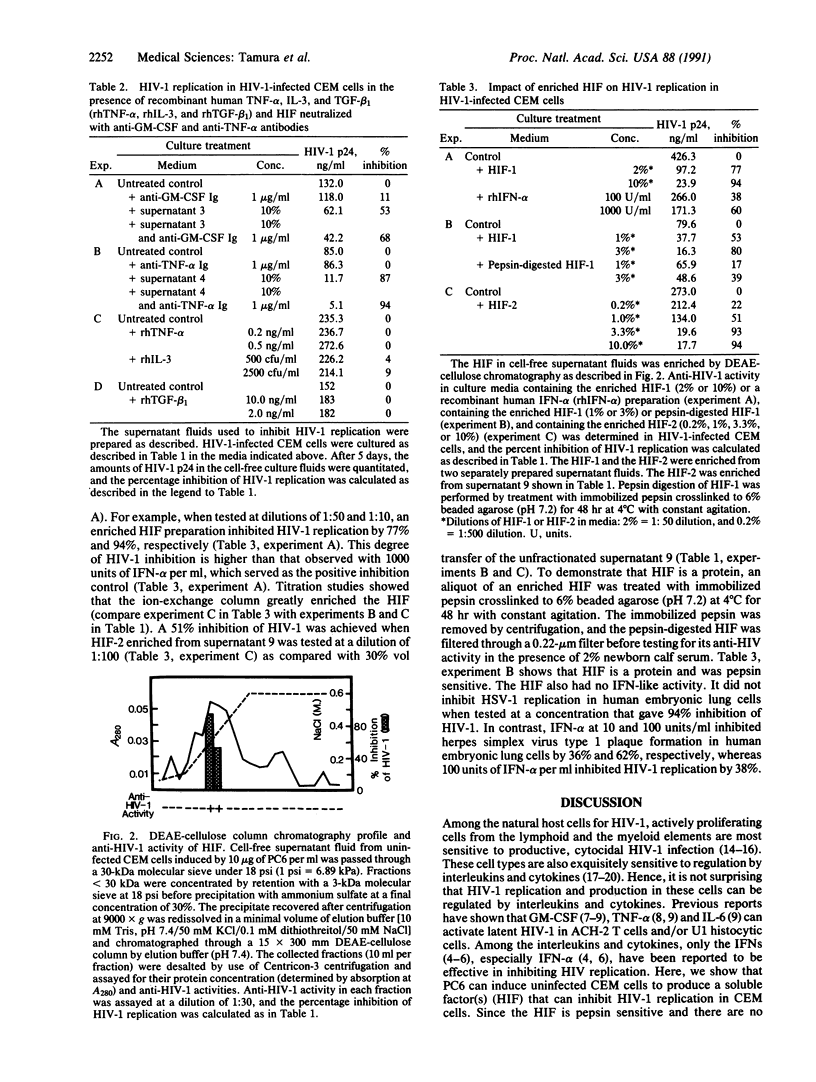
We showed that an extract (PC6) from cones of Pinus parviflora Sieb et Zucc induced the human T-cell line CEM to produce a pepsin-sensitive soluble factor(s) that could inhibit the replication of the type 1 human immunodeficiency virus (HIV-1) in CEM T cells, in U-937 histocytes, in THP-1 monocytes, and in mitogen-activated human tonsillar mononuclear cells. Indirect immunofluorescence staining and polymerase chain reaction analysis of the PC6-induced CEM cells revealed the absence of known lymphokines/cytokines except granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), transforming growth factor beta 1 (TGF-beta 1), and tumor necrosis factor alpha (TNF-alpha). However, functional studies with recombinant IL-3, TNF-alpha, and TGF-beta 1 showed that these three factors did not inhibit HIV-1 replication in CEM cells. Neutralization of the PC6-induced HIV-1-inhibiting factor(s) with commercially available neutralizing antibodies to GM-CSF and TNF-alpha also did not abrogate the anti-HIV-1 impact. Thus, the anti-HIV-1 factor induced by PC6 may be novel. Molecular sieve separation showed that the anti-HIV-1 factor(s) is smaller than 30 kDa. Affinity chromatography using a DEAE-cellulose column enriched the factor that inhibited HIV-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Brenner C. A., Tam A. W., Nelson P. A., Engleman E. G., Suzuki N., Fry K. E., Larrick J. W. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989 Nov-Dec;7(10):1096–1103. [PubMed] [Google Scholar]
- Casareale D., Dewhurst S., Sonnabend J., Sinangil F., Purtilo D. T., Volsky D. J. Prevalence of AIDS-associated retrovirus and antibodies among male homosexuals at risk for AIDS in Greenwich Village. AIDS Res. 1984;1(6):407–421. doi: 10.1089/aid.1.1983.1.407. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Harada H., Sakagami H., Konno K., Sato T., Osawa N., Fujimaki M., Komatsu N. Induction of antimicrobial activity by antitumor substances from pine cone extract of Pinus parviflora Sieb. et Zucc. Anticancer Res. 1988 Jul-Aug;8(4):581–587. [PubMed] [Google Scholar]
- Ho D. D., Hartshorn K. L., Rota T. R., Andrews C. A., Kaplan J. C., Schooley R. T., Hirsch M. S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985 Mar 16;1(8429):602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Pietrangeli C. E., Hayashi S., Gimble J. M. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 1989;7:111–143. doi: 10.1146/annurev.iy.07.040189.000551. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Lai P. K., Donovan J., Takayama H., Sakagami H., Tanaka A., Konno K., Nonoyama M. Modification of human immunodeficiency viral replication by pine cone extracts. AIDS Res Hum Retroviruses. 1990 Feb;6(2):205–217. doi: 10.1089/aid.1990.6.205. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Mitchell W. M. Antiviral activity of mismatched double-stranded RNA against human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1987 May;84(9):2985–2989. doi: 10.1073/pnas.84.9.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Yoshida T., Harada S., Yamamoto N. Recombinant human interferon gamma suppresses HTLV-III replication in vitro. Int J Cancer. 1986 Sep 15;38(3):433–436. doi: 10.1002/ijc.2910380320. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Sakagami H., Ikeda M., Unten S., Takeda K., Murayama J., Hamada A., Kimura K., Komatsu N., Konno K. Antitumor activity of polysaccharide fractions from pine cone extract of Pinus parviflora Sieb. et Zucc. Anticancer Res. 1987 Nov-Dec;7(6):1153–1159. [PubMed] [Google Scholar]
- Sakagami H., Takeda K., Makino Y., Konno K. Partial purification of novel differentiation-inducing substances(s) from hot water extract of Japanese pine cone. Jpn J Cancer Res. 1986 Jan;77(1):59–64. [PubMed] [Google Scholar]
- Takayama H., Bradley G., Lai P. K., Tamura Y., Sakagami H., Tanaka A., Nonoyama M. Inhibition of human immunodeficiency virus forward and reverse transcription by PC6, a natural product from cones of pine trees. AIDS Res Hum Retroviruses. 1991 Mar;7(3):349–357. doi: 10.1089/aid.1991.7.349. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]



