Abstract
This paper describes the development of cyclic peptides for G protein coupled receptors to enable structure-function knowledge and the design of novel therapeutics. One important property of cyclic peptides is that they tend to be resistant to the digestion, enabling them to survive in the human digestive tract. This trait makes them very important as drug leads or as scaffolds which, in theory, can be engineered to incorporate a peptide domain of medicinal value. This is especially important for delivery of peptides that would be destroyed without such implementation. The melanocortin system is the focus of this article, and includes melanotropin ligands and melanocortin receptors (MCRs). We examine two strategies to constrain the melanotropin peptide backbone. The first is based on global constraint of peptides by cyclization using various kinds of linkers. In the second approach we describe the use of a natural cyclized template, the cyclotide, to graft the melanotropin phamacophore, -His-Phe-Arg-Trp-, to obtain selective drug leads. In these examples the conserved melanocyte stimulating hormone (MSH) pharmacophore is examined and the modified peptides were synthesized by solid phase methodology. Biological studies confirmed the production of selective, potent and in some cases orally available ligands.
Keywords: GPCR, Melanocortin System, Melanotropin, Cyclized Peptide, Cyclotide, Selectivity
Introduction
Peptides and proteins participate in the control of all biological processes in living systems. In recent years there has been an increasing effort to design and synthesize highly potent and selective analogues of biologically active peptides.1–3 in particular, the development of new ligands for cell surface receptors is a principal focus in biological science. Selective receptor ligands are very important pharmacological tools for in vitro and in vivo studies as well as for drug candidates, but the design of a small peptide that selectively binds to a protein receptor is a very challenging task. The protein receptors that are the subject of this review belong to the group of G-protein coupled receptors (GPCRs), a large family of integral membrane proteins that act as cell surface receptors. GPCRs are very important targets in pharmaceutical research and the discovery of drugs acting at GPCRs has been extremely successful. Indeed, 50% of all recently produced drugs are targeted at GPCRs (Table 1).4 However, some of these drugs have efficacy problems and side-effects because they do not differentiate between the receptor subtypes. Thus there is a huge open research need for finding ligands for single receptor subtypes that affect a particular physiology.
Table 1.
Examples of drugs targeted at GPCRs.
| Name | Disease | Receptor and mode of action |
|---|---|---|
| Claritin, Allegra | allergies | H1 antagonist |
| Zyprexa | schizophrenia | D1, D2, 5-HT2 antagonist |
| Risperdal | psychosis | D2, 5-HT2A antagonist |
| Imigran | migraine | 5-HT1 agonist |
| Buspar | depression | 5-HT1a agonist |
| Cozaar, Diovan | hypertension | AT1 antagonist |
| Lupron a, Zoladex a | cancer | LH-RH agonist |
| Neurontin | neurogenic pain | GABA B agonist |
| Serevent | asthma | β2 agonist |
| Plavix | stroke | P2Y12 antagonist |
| Zantac, Pepcidine | ulcers | H2 antagonist |
| Oxytocin a | uterus contraction | OTR agonist |
| Insulin a | diabetes | IR agonist |
Peptides
The human genome project has revealed several hundred members of the GPCRs,5 of which only approximately 30 represent targets of currently produced drugs. Endogenous ligands are known for more than 200 GPCRs but another 160 receptors, so-called “orphan receptors”, have unknown ligands and undetermined physiological function.6 Structurally, GPCRs share seven predicted transmembrane helices connected by three extracellular loops and three intracellular loops. However, only a few GPCR X-ray structures have been identified and most of the three-dimensional structures of GPCRs still remain to be determined. The main reason for a lack of structural information is the difficult purification and crystallization of membrane proteins compared with water-soluble proteins.
Alternative strategies for structural studies of GPCRs have been derived from biochemical and biophysical methods and from site-directed mutagenesis.7–12 In addition, homology modeling plays a very important role in GPCR research. This computer-assisted method to obtain a three-dimensional model of a protein with an unknown structure is based on the structures of related protein templates. Since the three-dimensional structures of most GPCRs are not available the use of structure-based receptor design is limited, but classical ligand-based design is still very powerful even if the structure of the protein receptor is not defined. In many of our studies we applied a ligand-based approach for the synthesis of new molecules that are selectively recognized by a group of closely related proteins, the melanocortin receptors (MCRs).
The melanocortin system includes five receptor isoforms known as MC1R– MC5R. All five MCRs belong to the superfamily of GPCRs.13 Receptor activation induced by agonist ligand binding leads to the activation of the cyclic AMP signal transduction pathway. These five MCRs are involved in a large variety of physiological functions and are distributed in several different tissues. MC1R is primarily distributed in mammalian skin, melanocytes, microphtalmia, and in melanoma.14 The primary functions of the MC1R are the regulation of melanocyte pigmentation, hair color, and pain.15, 16 MC2R is found within the adrenal cortex, human skin, and murine adipocytes. The major functions of MC2R include the production of glucocorticoids as well as the fear/flight response.17, 18 MC3R is expressed in the brain, placenta, gut, heart, septum, hippocampus, and the thalamus.19–21 MC3R is involved in obesity, regulation of energy (homeostasis), inflammation, as well as sexuality.22–24 MC4R can be found in the majority of the brain, including cortex, thalamus, hypothalamus, brainstem, and spinal cord. MC4R has important roles in feeding behavior (obesity, anorexia) as well as sexual behavior.22, 25, 26 MC5R is located in peripheral tissue and the brain, and is involved in thermoregulation and sebaceous gland secretion.27
The endogenous ligands, the melanotropins, which bind to these receptors are linear peptides, α-, β-, γ-MSH and ACTH, with 12, 13, 22 and 39 amino acids respectively.28 The selectivity of these peptide ligands is low and therefore the physiological function of each receptor subtype cannot be easily delineated. Thus there is an urgent need for the synthesis of potent and highly selective molecules, which would be pharmacological tools for further receptor investigation. The objectives of our research are the design and synthesis of new, selective and potent melanotropins for the melanocortin receptor subtypes to help elucidate their biological functions. Since the three-dimensional structures of the melanocortin receptors and their endogenous ligands are unknown, receptor structure-based design has not been applied. We have chosen endogenous peptide-based design with the primary structure of the endogenous ligands, α-, β-, γ-MSH, as starting points. Our design strategy is to gain highly selective, potent and bioavailable melanotropins by fixing the spatial structure of the very flexible peptide ligand in the hope that we can induce the bioactive conformation. We have successfully developed several strategies to constrain the peptide backbone and side chain. The first is based on global constraint of the peptides by cyclization using various kinds of linker. In the second strategy we have introduced a local constraint into a peptide backbone similar to a reverse turn structure. (Not discussed in this paper). We have also made very radical changes in ligand conformation by peptide complexation with a transition metal in different positions of the sequence.
The goal of our research is the design and synthesis of a selective melanocortin ligand that binds to a receptor subtype at nanomolar concentration. The starting point in the ligand-based design is a primary structure of an endogenous ligand (α-, β-, γ-MSH). The main strategy to obtain a selective ligand is restriction of conformational freedom of very flexible peptide. This approach has been successfully developed in the Hruby research group and many others.29, 30 Two methods of constrained peptides have been designed. The first series contains cyclic peptides. The use of different ring sizes from 15- to 30-membered rings allows us to study the influence of a global constraint of peptide backbone on biological activity. In the second method, the core sequence of MSH, His-Phe/DPhe-Arg-Trp, is grafted into the different loops of cyclotides such as kalata B1. A very powerful synthetic method for obtaining peptide ligands is the solid-phase strategy, which has been used for the preparation of all new compounds. So far, we have successfully developed all MCRs selective agonists, antagonists; orthostatic and allosteric melanotropins.
Design of Cyclized Melanotropins
Linear peptides are characteristically highly flexible molecules and their structures are strongly influenced by their environment. The conformational mobility of peptides in solution complicates the determination of their bioactive structures. Constraint of the peptide backbone by cyclization can significantly limit the number of possible conformations and hopefully stabilize a bioactive conformation. This restriction of conformation allows us to study the interaction of the ligand with a receptor.
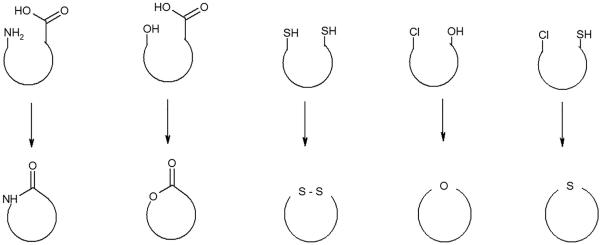
Cyclic peptides can be classified as either homodetic or heterodetic compounds. Homodetic peptides contain only Nα amide bonds. Heterodetic peptides may also contain ester, disulfide, ether or thioether bonds. Cyclization requires two reactive groups within the same peptide, which react intramolecularly to produce the cyclic compound. Figure 1 shows the common groups that have been used for cyclization. The ring size can be adjusted based on the different GPCR binding pockets. However, the peptides might also react intermolecularly to produce dimers or polymers. Dimerization and polymerization can be prevented by applying the dilution principal. In dilute solutions the rate of dimerization, a bimolecular reaction, is sufficiently reduced to minimize the intermolecular reaction, whereas the rate of cyclization, a unimolecular reaction, should be unaffected. It is recommended that cyclization should be carried out at concentrations below 10−3 M. The advantage of cyclization on solid phase is due to a direct pseudo-dilution effect on the reaction so that polymerization is minimized.
Figure 1.
Different types of intramolecular reactions that can form cyclic peptides. The cyclisation can occur via amide, ester, disulfide, ether or thioether linkages.
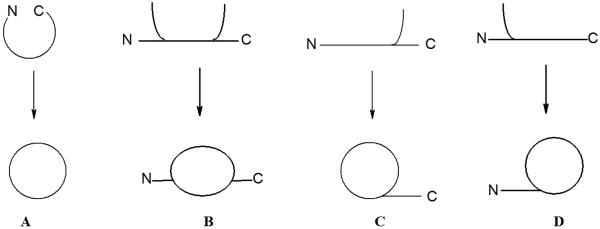
General topologies for peptide cyclization are depicted in Figure 2. Head-to-tail cyclization requires reaction between the N-terminal and C-terminal residues of a linear peptide. In principle there are three other general approaches. Side chain-to-side chain ring closure may be achieved by reaction between the functional groups of peptide side chains. Head-to-side chain or side chain-to-tail cyclization are performed in a similar manner. The latest cyclization strategies have demonstrated that using aromatic linkers or bulky linkers can modulate the backbone conformation to obtain selectivity for GPCRs.
Figure 2.
General topologies for peptide cyclization: A) head-to-tail, B) side chain-to-side chain, C) head-to-side chain, D) side chain-to-tail.
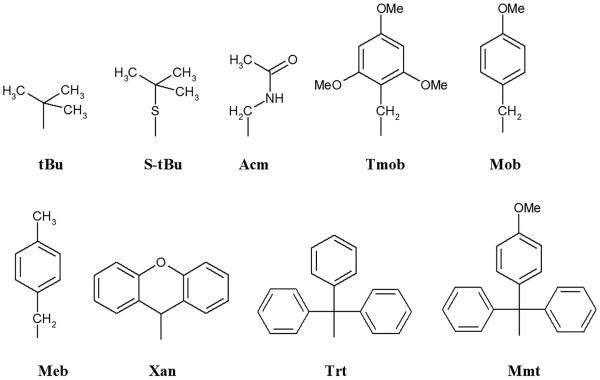
The most frequently used methods for peptide cyclization involve lactam bond formation and disulfide bridges. Lactam synthesis can be achieved by activation of a carboxylic group as HOBt (or superior HOAt) active esters using a number of reagents. Carbodiimide and phosphonium activation have been used for cyclization with minimal racemization. Successful peptide cyclization is sequence dependent. A general method for the synthesis of disulfide bridges is the oxidation of the corresponding linear peptide with two free thiol groups. Simultaneous deprotection and oxidation of two protected thiols can be applied as well. The most convenient oxidation is carried out in the presence of atmospheric oxygen and under slightly alkaline conditions. The addition of dimethylsulphoxide is often useful to promote oxidation of free thiols to disulfides. A very common oxidation is by potassium ferricyanide, K3Fe(CN)6, a relatively mild oxidation reagent.31–34 Disulfide bridges can also be formed directly from thiols with iodine, thallium trifluoroacetate, Tl(Tfa)3,35 or methyltrichlorosilane in the presence of sulphoxide. Oxidation by iodine is compatible with thiol protecting groups such as acetamidomethyl (Acm), triphenylmethyl (Trt), 2,4,6-trimethoxybenzyl (Tmob) and 9H-xanthen-9-yl (Xan) (Figure 3). Thallium trifluoroacetate is a mild oxidant and is used with the Acm thiol protecting group. A mixture of methyltrichlorosilane and sulphoxide can cleave several thiol protecting groups (acetamidomethyl, tert-butylthio, p-methoxybenzyl, p-methylbenzyl) resulting in the direct formation of disulfides.
Figure 3.
Protecting groups for thiol groups of cysteine.
Structure-activity relationships
Many GPCR endogenous ligands tend to have a β-turn like structure. To build upon the concept of a β-turn conformation, a new generation of cyclic peptide melanocortin ligands were advanced to further explore MCR selectivity and functional properties. The first breakthrough cyclic peptide to be made was the disulfide-bridged cyclo[Cys4,Cys10]-α-MSH.36 This cyclic peptide had potent bioactivity in a frog skin bioassay, but did not possess the prolonged activity as seen in NDP-α-MSH.37 To improve upon the bio-stability of cyclo[Cys4,Cys10]-α-MSH, new cyclic peptides incorporating d-Phe7 were developed, including lactam bridging analogs as exemplified by the following generic sequences: Ac-Nle4-cyclo[Xxx5,d-Phe7,Yyy10]-α-MSH4-10-NH2 and Ac-Nle4-cyclo[Xxx5,d-Phe7,Yyy10]-Gly11-α-MSH4–13-NH2 (note: lactam-bridging between Xxx [Asp or Glu] and Yyy [Lys, Orn, Dab, or Dpr]).38, 39 From these studies, Ac-Nle4-cyclo[Asp5,d-Phe7,Lys10]-α-MSH4-10-NH2, also known as Melanotan-II (MT-II), was shown to exhibit nearly 100-fold more potency than α-MSH in the lizard skin bioassay. Nevertheless, MT-II did not exhibit high selectivity for the individual MCRs. To address this hurdle, a series of d-amino acids and bulky amino acid scans were performed, and by introducing a bulkier amino acid d-Nal(2')7 into the template of MTII, the first selective antagonist of MC3R and MC4R, SHU9119, Ac-Nle4-cyclo[Asp5,d-Nal(2')7,Lys10]-α-MSH4-10-NH2 was discovered.40 To stabilize the β-turn-like structure in SHU9119, Grieco et al. incorporated Pro6 into the SHU9119 template to get PG901, Ac-Nle4-cyclo[Asp5, Pro6,d-Nal(2')7,Lys10]-α-MSH4-10-NH2. This peptide has an order of magnitude higher binding affinity than SHU9119, but the functional selectivity is similar to that of SHU9119.41
Recent studies of peptide backbone modulation using N-methylation have been very successful. We systematically performed multiple N-methylation of the MTII template and discovered the most selective MC1R agonists. Peptide 1: Ac-Nle-cyclo[(NMe)His-d-Phe-(NMe)Arg-(NMe)Trp-(NMe)Lys]- NH2 in particular is a very potent (nM) and selective MC1R agonist.42 We also applied a thio-ether bridge to cyclize γ-MSH and got a selective MC1R antagonist, Peptide 2: cyclo[(CH2)3-CO-Gly-His-d-Phe-Arg-d-Trp-Cys(S)]-Asp-Arg-Phe-Gly-NH2.43 Moreover, to achieve improved selectivity of MCR ligands, Mayorov et al.42 designed a series of novel cyclic MSH analogues with the following general sequence: c[Nle4-Xaa5-d-Phe6/d-Nal(2')6-Arg7-Trp8-Glu]-NH2, which features an amide bond between the ε-carboxyl group of the C-terminal glutamic acid amide residue and the alpha-amino group of the N-terminal norleucine residue as the global constraint. We introduced a bulky hydrophobic residue (Nle4) close to the pharmacophore (Xaa-d-Phe/d-Nal(2')-Arg-Trp) to investigate the impact of steric hindrance on receptor selectivity. In addition, a variety of amino acids with a broad range of hydrophobic/hydrophilic properties was placed in position 5 to further explore their complementary role in receptor selectivity. We also developed a series of selective MC3R agonists and antagonists. Peptides 3 and 4 are examples for this series.44 Recently, another group developed the disulfide-bridged cyclic peptide setmelanotide (RM-493, Peptide 5) which is now in phase II clinical trials for obesity and Prader-Willi syndrome. The sequence of Peptide 5 is Ac-Arg-cyclo[Cys4, d-Ala5, d-Phe7, Cys10]-α-MSH3–10-NH2 and it is said to be a selective agonist towards MC4R.45
Although Peptide 5 is involved in a clinical trial, the MC4R selective ligands we discovered earlier appear to be more stable. These peptides involve the use of linker arms and a backbone-to-side chain cyclization strategy such as cyclic peptide, Peptide 6, (O)C-CH2CH2-C(O)-c-[His6-d-Phe7-Arg8-Trp9-Lys10]-NH2. It was found to be a highly selective and potent MC4R agonist.46 Structure-activity studies have also shown that replacing the succinyl linker arm of Peptide 6 by an o-phthalic acid group and substituting a d-Nal(2′)7 residue in place of d-Phe7 results in a potent MC4R antagonist, Peptide 7, (o)C-C6H4-C(O)-c[His6-d-Nal (2′)7-Arg8-Trp9-Lys10]NH246. Grieco et al. also used the PG 901 template to introduce a group of bulky amino acids in the position 6 of SHU9119 to produce a series selective antagonists of MC3R and MC4R.47 One of the replacements, Cpe, achieved highly selective antagonist at the MC4R, Peptide 8. In addition, based on the study of the NMR structure of MTII and SHU9119 Ying et al developed several MC4R allosteric antagonists. Peptide 9: Ac-c[Cys-His-d-Phe-Cys]-NR-guanidinylbutyl-d-Trp-NH2 is an example of an allosteric antagonist of MC4R.48 Finally, we systematically synthesized N-methylated SHU9119 and discovered multiple selective melanotropin ligands for MCRs. One of the MC5R selective agonists, peptide 10: Ac-Nle-cyclo[His-d-Nal(2')-Arg-(NMe)Trp-Lys]- NH249 and two antagonists of MCRs, Peptide 11: Ac-Nle-cyclo[His-(NMe)d-Nal(2')-Arg-Trp-(NMe)Lys]-NH249 and Peptide 12: Ac-Nle-cyclo[(NMe)His-dNal(2')-Arg-(NMe)Trp-(NMe)Lys]-NH2 are shown in Table 3.49 To sum up, we have discovered a wide range of ligands for all subtypes of MCRs, including agonists, antagonists, orthostatic and allosteric ligands, and used them to explore novel functions of melanocortin system.
Table 3.
Selective melanotropins for different subtypes of melanocortin receptors (MCRs).
| Selectivity | Cyclized Melanotropins | |
|---|---|---|
| hMC1R | 1. Ac-Nle-cyclo[(NMe)His-d-Phe-(NMe)Arg-(NMe)Trp-(NMe)Lys]-NH2 | Agonist |
| 2. cyclo[(CH2)3-CO-Gly-His-d-Phe-Arg-d-Trp-Cys(S)]-Asp-Arg-Phe-Gly-NH2 | Antagonist | |
|
| ||
| hMC3R | 3. cyclo[Nle-Arg-d-Phe-Arg-Trp-Glu]-NH2 | Agonist |
| 4. cyclo[Nle-Val-d-Nal(2')-Arg-Trp-Glu]-NH2 | MC3,5R Antagonist | |
|
| ||
| hMC4R | 5. Ac-Arg-cyclo[Cys4, d-Ala5, d-Phe7, Cys10]-α-MSH3–10-NH2 | Agonist |
| 6. (O)C-CH2CH2-C(O)-cyclo[His6-d-Phe7-Arg8-Trp9-Lys10]-NH2 | Agonist | |
| 7. (o)C-C6H4-C(O)-cyclo[His6-d-Nal (2')7-Arg8-Trp9-Lys10]NH2 | Antagonist | |
| 8. Ac-Nle-cyclo[Asp-Cpe-d-Nal(2')-Arg-Trp-Lys]-NH2 | Antagonist | |
| 9. Ac-c[Cys-His-d-Phe-Cys]-NR-guanidinylbutyl-d-Trp-NH2 | Allosteric Antagonist | |
|
| ||
| hMC5R | 10. Ac-Nle-cyclo[His-d-Nal(2')-Arg-(NMe)Trp-Lys]- NH2 | Agonist |
|
| ||
| hMCRs | 11. Ac-Nle-cyclo[His-(NMe)dNal(2')-Arg-Trp-(NMe)Lys]- NH2 | Universal Antagonist |
| 12. Ac-Nle-cyclo[(NMe)His-d-Nal(2')-Arg-(NMe)Trp-(NMe)Lys]- NH2 | Universal Antagonist | |
|
| ||
| MTII SHU9119 PG901 | Ac-Nle-cyclo[Asp-His-d-Phe-Arg-Trp-Lys]-NH2 | Universal Agonist |
| Ac-Nle-cyclo[Asp-His-d-Nal(2')-Arg-Trp-Lys]-NH2 | MC3,4R Antagonist, MC1,5R Agonist | |
| Ac-Nle-cyclo[Asp-Pro-d-Nal(2')-Arg-Trp-Lys]-NH2 | MC3,4R Antagonist, MC5R Antagonist | |
Cyclized Melanotropins Using a Cyclotide Template
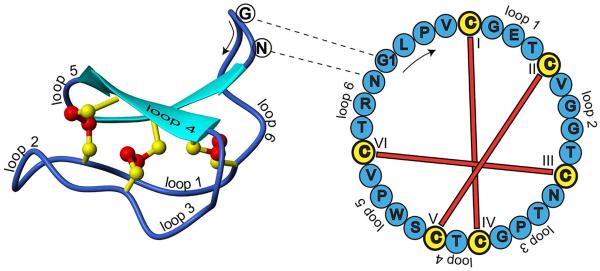
Cyclotides are small disulfide-rich peptides isolated from plants. Typically containing 28–37 amino acids, they are characterized by their head-to-tail cyclized peptide backbone and an interlocking arrangement of their three disulfide bonds. These combined features have been termed the cyclic cystine knot (CCK) motif.50 To date, over 300 cyclotides have been isolated and characterized from species of the Rubiaceae, Violaceae, Solanaceae, Fabaceae and Cucurbitaceae plant families.51 Due to their characteristic high thermal and enzymatic stability, and uses in indigenous medicines, drug designers have been using their structural template to develop stable and in some cases orally active drugs. Figure 4 shows the structure of the prototypic cyclotide, kalata B1.52
Figure 4.
Structure and sequence of the prototypic cyclotide kalata B1. (Reproduced from Ireland et. al., J Nat Prod 2010, 73, 1610–1622)52
Cyclotides have been suggested as scaffolds for the insertion and stabilization of pharmaceutically active peptides. To develop novel melanotropins, the Craik group53 explored the development of appetite-reducing peptides by synthesizing MC4R agonists based on the insertion of the His-Phe/dPhe-Arg-Trp sequence into loop 6 of the cyclotide kalata B1. In that study the ability of the analogs to fold in a manner similar to that of kalata B1, while displaying MC4R activity, was investigated. Four peptides were synthesized using t-butoxycarbonyl peptide chemistry with a C-terminal thioester to facilitate backbone cyclization. The structures of the peptides were found to be similar to kalata B1, as evaluated by Hα NMR chemical shifts. Kalata B1(GHFRWG;23–28) had a Ki of 29 nM at the MC4R and was 100 or 300 times more selective over this receptor than MC1R or MC5R, respectively, and had no detectable binding to MC3R. The motivation for the grafting was to place the bioactive tetrapeptide in a molecular context where it would be less susceptible to proteolysis and will have more favorable biopharmaceutical properties than the isolated peptide sequence, and this turned out to be the case, with the grafted peptide being very stable. More broadly, the approach of using cyclotides as drug design templates has proven successful and has been broadly applied in a range of other peptide-based drug design applications.54, 55
Conclusion
The lack of selectivity, potency and bioavailability in agonist or antagonist properties of melanotroptin ligands for the MCR subtypes are still the most difficult hurdles for their applications in medicine. To ameliorate the biological properties of the melanocortin ligands and to achieve selective agonists and antagonists for MCRs, conformational modulation in peptide backbone by cyclization has been advanced. One important property of cyclic peptides is that they tend to be extremely resistant to the process of digestion, enabling them to survive in the human digestive tract. This trait makes cyclic peptides very applicable as scaffolds which, in theory, could be engineered to incorporate any arbitrary protein domain of medicinal value, in order to allow those components to be delivered orally. This is especially important for delivery of peptides that would be destroyed without such implementation. Cyclic peptides are rigid compared to the corresponding linear peptides, and this attribute promotes binding by minimizing the entropic penalty. The studies of cyclized peptides described herein, in general, encourages us to foresee a bright future for peptide chemistry in the development of peptides therapeutic agents.
Table 2.
Amino acid sequences of endogenous melanocortin agonists. The highlighted HFRW is the core sequence or pharmacophore of the melanotropins
| Peptide | Sequence |
|---|---|
| α -MSH | Ac–S1Y2S3M4E5 H6F7R8W9 G10K11P12V13–NH2 |
| β –MSH | AEKKDEGPYRME H6F7R8W9 GSPPKD–OH |
| γ –MSH | YVMG H6F7R8W9 DRFG-OH |
| ACTH | SYSME H6F7R8W9 GKPVGKKRRPVKVYPNGAEDESAEAFPLEF-OH |
Acknowledgments
Supported by NIH GM108040
References
- 1.Kaspar AA, Reichert JM. Drug Discov Today. 2013;18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Albericio F, Kruger HG. Future Med Chem. 2012;4:1527–1531. doi: 10.4155/fmc.12.94. [DOI] [PubMed] [Google Scholar]
- 3.Craik DJ, Fairlie DP, Liras S, Price D. Chem Biol Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 4.Ellis C. Nat Rev Drug Discov. 2004;3:577–626. doi: 10.1038/nrd1458. [DOI] [PubMed] [Google Scholar]
- 5.Alem K, Louhichi A, Ladjama A, Rebai A. Arch Inst Pasteur Tunis. 2007;84:57–63. [PubMed] [Google Scholar]
- 6.Maurer MH, Grunewald S, Gassler N, Rossner M, Propst F, Wurz R, Weber D, Kuner T, Kuschinsky W, Schneider A. J Neurochem. 2004;91:1007–1017. doi: 10.1111/j.1471-4159.2004.02799.x. [DOI] [PubMed] [Google Scholar]
- 7.Pardo L, Ballesteros JA, Osman R, Weinstein H. Proc Natl Acad Sci U S A. 1992;89:4009–4012. doi: 10.1073/pnas.89.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kufareva I, Katritch V, Participants of, G. D. Stevens RC, Abagyan R. Structure. 2014;22:1120–1139. doi: 10.1016/j.str.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kufareva I, Rueda M, Katritch V, Stevens RC, Abagyan R, participants GD. Structure. 2011;19:1108–1126. doi: 10.1016/j.str.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Chen M, McPherson D, Mishra V, Harmon CM. Peptides. 2011;32:2377–2383. doi: 10.1016/j.peptides.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Cai M, McPherson D, Hruby V, Harmon CM, Yang Y. Biochem Pharmacol. 2009;77:114–124. doi: 10.1016/j.bcp.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. J Biol Chem. 2007;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cone RD. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 14.Lopez MN, Pereda C, Ramirez M, Mendoza-Naranjo A, Serrano A, Ferreira A, Poblete R, Kalergis AM, Kiessling R, Salazar-Onfray F. Invest Ophthalmol Vis Sci. 2007;48:1219–1227. doi: 10.1167/iovs.06-0090. [DOI] [PubMed] [Google Scholar]
- 15.Wickelgren I. Science. 2007;315:1215. doi: 10.1126/science.315.5816.1215. [DOI] [PubMed] [Google Scholar]
- 16.Wendt J, Rauscher S, Burgstaller-Muehlbacher S, et al. JAMA Dermatol 2016. 152:776–782. doi: 10.1001/jamadermatol.2016.0050. [DOI] [PubMed] [Google Scholar]
- 17.Vamvakopoulos NC, Rojas K, Overhauser J, Durkin AS, Nierman WC, Chrousos GP. Genomics. 1993;18:454–455. doi: 10.1006/geno.1993.1499. [DOI] [PubMed] [Google Scholar]
- 18.Liang L, Angleson JK, Dores RM. Gen Comp Endocrinol. 2013;181:203–210. doi: 10.1016/j.ygcen.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Hani EH, Dupont S, Durand E, Dina C, Gallina S, Gantz I, Froguel P. J Clin Endocrinol Metab. 2001;86:2895–2898. doi: 10.1210/jcem.86.6.7589. [DOI] [PubMed] [Google Scholar]
- 20.Zegers D, Beckers S, Hendrickx R, Van Camp JK, Van Hoorenbeeck K, Desager KN, Massa G, Van Gaal LF, Van Hul W. Endocrine. 2013;44:386–390. doi: 10.1007/s12020-012-9862-1. [DOI] [PubMed] [Google Scholar]
- 21.Millington GW. Nutr Metab. 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cone RD. Ann Endocrinol. 1999;60:3–9. [PubMed] [Google Scholar]
- 23.Cone RD. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 24.Barb CR, Robertson AS, Barrett JB, Kraeling RR, Houseknecht KL. J Endocrinol. 2004;181:39–52. doi: 10.1677/joe.0.1810039. [DOI] [PubMed] [Google Scholar]
- 25.Hinney A, Volckmar AL, Knoll N. Prog Mol Biol Transl Sci. 2013;114:147–191. doi: 10.1016/B978-0-12-386933-3.00005-4. [DOI] [PubMed] [Google Scholar]
- 26.Krashes MJ, Lowell BB, Garfield AS. Nat Neurosci. 2016;19:206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 28.Rudman D, Chawla RK, Khatra BS, Yodaiken RE. Ann N Y Acad Sci. 1975;248:324–335. doi: 10.1111/j.1749-6632.1975.tb34195.x. [DOI] [PubMed] [Google Scholar]
- 29.Hruby VJ. Nat Rev Drug Discovery. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 30.Hruby VJ, Cai M. Annu Rev Pharmacol Toxicol. 2013;53:557–580. doi: 10.1146/annurev-pharmtox-010510-100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreu D, Albericio F, Sole NA, Munson MC, Ferrer M, Barany G. Methods Mol Biol. 1994;35:91–169. doi: 10.1385/0-89603-273-6:91. [DOI] [PubMed] [Google Scholar]
- 32.Kiso Y. Adv Exp Med Biol. 1995;362:413–423. doi: 10.1007/978-1-4615-1871-6_54. [DOI] [PubMed] [Google Scholar]
- 33.Annis I, Hargittai B, Barany G. Methods Enzymol. 1997;289:198–221. doi: 10.1016/s0076-6879(97)89049-0. [DOI] [PubMed] [Google Scholar]
- 34.Kamber B. Helv Chim Acta. 1971;54:927–930. doi: 10.1002/hlca.19710540319. [DOI] [PubMed] [Google Scholar]
- 35.Fujii N, Otaka A, Funakoshi S, Bessho K, Watanabe T, Akaji K, Yajima H. Chem Pharm Bull. 1987;35:2339–2347. doi: 10.1248/cpb.35.2339. [DOI] [PubMed] [Google Scholar]
- 36.Sawyer TK, Hruby VJ, Darman PS, Hadley ME. Proc Natl Acad Sci U S A. 1982;79:1751–1755. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. Proc Natl Acad Sci U S A. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. J Med Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 39.Cody WL, Mahoney M, Knittel JJ, Hruby VJ, Castrucci AM, Hadley ME. J Med Chem. 1985;28:583–588. doi: 10.1021/jm50001a008. [DOI] [PubMed] [Google Scholar]
- 40.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 41.Grieco P, Han G, Weinberg D, Van der Ploeg LH, Hruby VJ. Biochem Biophys Res Commun. 2002;292:1075–1080. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 42.Doedens L, Opperer F, Cai M, Beck JG, Dedek M, Palmer E, Hruby VJ, Kessler H. J Am Chem Soc. 2010;132:8115–8128. doi: 10.1021/ja101428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai M, Stankova M, Muthu D, Mayorov A, Yang Z, Trivedi D, Cabello C, Hruby VJ. Biochemistry. 2013;52:752–764. doi: 10.1021/bi300723f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. J Med Chem. 2006;49:1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, Panaro BL, Gottesdiener KM, Van der Ploeg LH, Reitman ML, Skarulis MC. J Clin Endocrinol Metab. 2015;100:1639–1645. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kavarana MJ, Trivedi D, Cai MY, Ying JF, Hammer M, Cabello C, Grieco P, Han GX, Hruby VJ. J Med Chem. 2002;45:2644–2650. doi: 10.1021/jm020021z. [DOI] [PubMed] [Google Scholar]
- 47.Grieco P, Lavecchia A, Cai M, Trivedi D, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. J Med Chem. 2002;45:5287–5294. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- 48.Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi DB, Hruby VJ. J Med Chem. 2006;49:6888–6896. doi: 10.1021/jm060768f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai M, Marelli UK, Bao J, Beck JG, Opperer F, Rechenmacher F, McLeod KR, Zingsheim MR, Doedens L, Kessler H, Hruby VJ. J Med Chem. 2015;58:6359–6367. doi: 10.1021/acs.jmedchem.5b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craik DJ, Daly NL, Bond T, Waine C. J Mol Biol. 1999;294:1327–1336. doi: 10.1006/jmbi.1999.3383. [DOI] [PubMed] [Google Scholar]
- 51.Mulvenna JP, Wang C, Craik DJ. Nucleic Acids Res. 2006;34:D192–D194. doi: 10.1093/nar/gkj005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ireland DC, Clark RJ, Daly NL, Craik DJ. J Nat Prod. 2010;73:1610–1622. doi: 10.1021/np1000413. [DOI] [PubMed] [Google Scholar]
- 53.Eliasen R, Daly NL, Wulff BS, Andresen TL, Conde-Frieboes KW, Craik DJ. J Biol Chem. 2012;287:40493–40501. doi: 10.1074/jbc.M112.395442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henriques ST, Craik DJ. Drug Discov Today. 2010;15:57–64. doi: 10.1016/j.drudis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Poth AG, Chan LY, Craik DJ. Biopolymers: Peptide Science. 2013;100:480–491. doi: 10.1002/bip.22284. [DOI] [PubMed] [Google Scholar]