Abstract
Background
Psychosocial risk for cardiovascular disease (CVD) may be especially deleterious in persons with low socioeconomic status. Most work has focused on psychosocial factors individually, but emerging research suggests that the confluence of psychosocial risk may be particularly harmful. Using data from the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study, we examined associations among depressive symptoms and stress, alone and in combination, and incident CVD and all‐cause mortality as a function of socioeconomic status.
Methods and Results
At baseline, 22 658 participants without a history of CVD (58.8% female, 41.7% black, mean age 63.9±9.3 years) reported on depressive symptoms, stress, annual household income, and education. Participants were classified into 1 of 3 psychosocial risk groups at baseline: (1) neither depressive symptoms nor stress, (2) either depressive symptoms or stress, or (3) both depressive symptoms and stress. Cox proportional hazards models were used to predict physician‐adjudicated incident total CVD events (nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death) and all‐cause mortality over a median of 7.0 years (interquartile range 5.4–8.3 years) of follow‐up. In fully adjusted models, participants with both depressive symptoms and stress had the greatest elevation in risk of developing total CVD (hazard ratio 1.48, 95% CI 1.21–1.81) and all‐cause mortality (hazard ratio 1.33, 95% CI 1.13–1.56) but only for those with low income (<$35 000) and not high (≥$35 000) income. This pattern of results was not observed in models stratified by education.
Conclusions
Findings suggest that screening for a combination of elevated depressive symptoms and stress in low‐income persons may help identify those at increased risk of incident CVD and mortality.
Keywords: cardiovascular diseases, depression, mortality, socioeconomic status, stress
Subject Categories: Epidemiology, Mental Health, Cardiovascular Disease, Risk Factors
Introduction
In the United States, >85 million adults—>1 in 3—have at least 1 type of cardiovascular disease (CVD), including hypertension, coronary heart disease (CHD), and stroke.1 Furthermore, CVD remains the leading cause of death in the United States and accounts for 1 death every 40 seconds.1 Despite advances in detection, prevention, and intervention, continued efforts are needed to improve cardiovascular health.
Emphasis has been placed on targeting health factors (eg, cholesterol, blood pressure) and health behaviors (eg, physical activity, diet) at both the individual and population levels to reduce the burden of cardiovascular morbidity and mortality.2 Substantial evidence, however, has demonstrated that psychosocial factors also contribute to the development of CVD.3 In particular, depression4, 5, 6 and perceived psychosocial stress7, 8, 9 have been linked to increased CVD risk, and some research has demonstrated that the confluence of these 2 factors may be particularly deleterious for cardiovascular health.10, 11, 12 This latter finding is consistent with a recently proposed psychosocial “perfect storm13” model13 of CVD risk that suggests an underlying vulnerability (eg, depression) is associated with greater risk of cardiac events and mortality, particularly in the presence of perceived stress. Although examinations of the association between depressive symptoms or stress and CVD are increasingly common,4, 6, 7, 8, 9, 14, 15, 16, 17, 18, 19 fewer studies have examined the combined effects of depressive symptoms and stress.10, 11 The perfect storm model emphasizes the importance of considering the convergence of a variety of factors that contribute to CVD risk rather than focusing on 1 risk factor in isolation. It provides a useful framework for studying the effects of depressive symptoms and perceived stress alone and in combination, ultimately elucidating the vulnerability and trigger mechanisms through which depression and/or stress prompt CVD events.
The importance of social determinants of CVD risk has also been increasingly appreciated.3 Socioeconomic status (SES) has been emphasized as a key social factor with relevance to differential CVD risk, with low income and limited educational attainment exhibiting robust links to poor cardiovascular outcomes.3 Furthermore, researchers have postulated that persons with low SES may be particularly vulnerable to the effects of psychosocial risk factors for poor physical health, suggesting that such persons may have fewer economic, social, and psychological resources for dealing with challenges compared with those with higher SES.20, 21, 22
Growing evidence indicates that psychosocial factors have important physical health implications for persons with low SES in particular. In the Health Survey for England cohort study, participants with both high general psychological distress and low SES were at the greatest risk of mortality from CHD and stroke23 and of all‐cause mortality.21 In addition, in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, elevated stress24 and depressive symptoms25 were each separately associated with increased risk of incident CVD events and death only for those with annual household income <$35 000 and not with higher income. Other findings from the REGARDS study suggested that the joint influence of psychosocial risk factors on the occurrence of CVD events and/or mortality may be more pronounced for certain subgroups. Paralleling prior REGARDS findings with respect to risk of CHD recurrence,10 Cummings et al12 found that the combination of elevated stress and depressive symptoms was most deleterious for risk of cardiovascular death and that this was true for persons with, but not without, diabetes mellitus. It is noteworthy that persons with diabetes mellitus disproportionately live in poverty.26 As population health management increasingly becomes integrated into health care reform, better understanding is needed of which subgroups should be targeted for which types of interventions. These results suggest that among persons with low SES, psychosocial factors may be particularly important contributors to increased rates of CVD events and mortality.
Using data from the REGARDS national longitudinal cohort study, we examined the association among depressive symptoms and perceived stress, alone and in combination, and risk of incident CVD and all‐cause mortality stratified as a function of SES. Given prior findings in the REGARDS study suggesting that individual psychosocial factors are particularly associated with CVD risk in those with low versus high income,24, 25 we examined annual household income as our primary SES indicator. Education was examined in a supplemental analysis. We hypothesized that the joint presence of elevated depressive symptoms and perceived stress would be associated with the greatest increase in risk of incident CVD events and all‐cause mortality in persons with low, but not high, SES.
Methods
Study Design and Cohort Description
The REGARDS study is a prospective longitudinal observational cohort study of stroke risk in black and white adults aged ≥45 years.27 The overarching aims of the investigation are to better understand the causes of elevated stroke morbidity and mortality observed in the southeastern United States (the stroke “belt” and “buckle”) relative to the rest of the nation and among black participants relative to white participants. Between January 2003 and October 2007, 30 239 adults were recruited from the community in the continental United States (42% black, 55% female, 55% from the stroke belt) using a targeted recruitment strategy for specific age, race, and geographic strata. The current study used data from REGARDS‐MI, an ancillary study that adjudicated all heart‐related events and causes of death and incorporated them into the larger REGARDS data set.28
At baseline, participants were administered a computer‐assisted telephone interview assessing demographics, medical history, functional status, health behaviors, and psychosocial factors. In addition, participants completed an in‐home physical examination 3 to 4 weeks after the telephone interview. At these examinations, trained health care professionals used standardized quality‐controlled protocols to collect anthropometric data (height and weight, waist circumference), blood pressure, ECGs, blood and urine samples, and medication use by pill bottle review. Participants provided written informed consent during the in‐home examination. This study was approved by the Institutional Review Board for Human Subjects at the University of Alabama at Birmingham and by all other participating institutions. ECGs were analyzed by trained cardiologists at Wake Forest University. Blood and urine samples were analyzed at the University of Vermont. Every 6 months, participants were contacted by telephone to query new‐onset CVD events, hospitalizations, and mortality, followed by retrieval of medical records that formed the basis of adjudication of end points. Proxies for deceased participants were queried on 1 occasion to gather parallel information for the deceased participant.
Psychosocial Measures
At baseline, participants completed measures of depressive symptoms and perceived stress. Depressive symptoms were assessed with the 4‐item Center for Epidemiologic Studies Depression (CES‐D) questionnaire,29 a previously validated version of the CES‐D30 that has been found to correlate highly with the original 20‐item questionnaire (r=0.87) and to have sensitivity of 79.2% and specificity of 81.2% compared with the original version.29 Participants indicated the extent to which they experienced each of 4 depressive symptoms (felt depressed, felt lonely, had crying spells, felt sad) in the past week on a scale from 1 (<1 day) to 4 (5–7 days). Responses to the 4 items were summed (Cronbach α=0.80). Consistent with previous research,10, 12, 18, 25 we classified participants as having elevated depressive symptoms if they had a CES‐D score ≥4, a validated cut point suggestive of clinically significant levels of depressive symptoms.29
Perceived stress was measured with a 4‐item version of the Perceived Stress Scale (PSS),31 a well‐validated instrument for assessing perceptions of personal stress. The 4‐item version has been found to have adequate psychometric properties and good predictive validity.32 Items measure the degree to which participants view their lives as unpredictable, uncontrollable, overloaded, or unable to be handled; responses were summed to create a total score (Cronbach α=0.72). There is no established cut point for high stress on the PSS. In the current investigation, participants in the upper tertile of total PSS score (≥5) were classified as having elevated levels of perceived stress.
SES Indicators
At the baseline interview, participants reported their annual household income. Consistent with previous research in the REGARDS study,24, 25 we categorized participants as low income (<$35 000/year) or high income (≥$35 000/year), given that this level of income was found to modify associations between perceived stress and CHD in a spline analysis.24 In addition, participants reported their highest grade or year of school completed. As in other REGARDS research,33 we classified participants’ educational level as low (some high school or less) or high (high school graduate or more).
Cardiovascular and Death Outcomes
For the current study, the primary outcome of interest was a composite total CVD outcome comprising acute CHD, nonfatal stroke, and cardiovascular death (CHD death, stroke death, heart failure death, sudden cardiac death, other cardiovascular‐related death). Secondary analyses examined acute CHD and cardiovascular death separately. We also examined all‐cause mortality as an outcome. At each 6‐month follow‐up assessment, trained interviewers administered a standardized questionnaire assessing whether participants had been hospitalized for stroke or CHD since the last follow‐up. If hospitalizations were reported, the date and time of each event was recorded and medical records were retrieved.
Acute CHD events comprised nonfatal myocardial infarction and CHD death events. Medical records were reviewed by a physician‐led team, and events were adjudicated following established guidelines.34, 35 Specifically, medical records were examined for signs or symptoms of ischemia, a rising and/or falling pattern in cardiac troponin or creatinine phosphokinase‐MB over ≥6 hours with a peak value greater than or equal to twice the upper limit of normal (diagnostic cardiac enzymes), and ECG changes consistent with ischemia or myocardial infarction. Review was guided by the Minnesota code, and events were classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia.36, 37
Each stroke event was adjudicated by medical record review conducted by a neurologist‐led team. World Health Organization criteria38 were used to define stroke events, although the study also included (1) events with symptoms lasting <24 hours with neuroimaging consistent with acute ischemia or hemorrhage and (2) cases with incomplete information for World Health Organization or clinical classification but for which adjudicators agreed that the event was likely a stroke or stroke‐related death.
Deaths were detected through next‐of‐kin report, online repositories (eg, Social Security Death Index), or the National Death Index. Proxies or next of kin were interviewed to obtain information about the circumstances of the death. Proxy interviews, medical history, medical records in the final year of life, death certificates, and autopsy reports were gathered and reviewed by physician‐led adjudicators to determine whether the death was caused by CVD.34, 35
Covariates
Data on sociodemographics, physiological and medical CVD risk factors, and health behaviors were included in models as potential confounders. The following sociodemographic measures were assessed at baseline: race (black, white), age, sex, number of persons (adults and children) living in the household, and geographic region of residence (stroke belt, stroke buckle, non–stroke belt or buckle). Several physiological CVD risk factors were also measured from data collected during the at‐home examination: waist circumference (in cm), systolic blood pressure (in mm Hg), total cholesterol (in mg/dL), high‐density lipoprotein (in mg/dL), estimated glomerular filtration rate, high‐sensitivity C‐reactive protein (in mg/L), and albumin‐to‐creatinine ratio (in mg/g). Antihypertensive medication use and statin use were also assessed at the at‐home examination and included as CVD risk factors. History of diabetes mellitus was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or self‐reported oral hypoglycemic or insulin use. Furthermore, the Physical Component Summary score of the 12‐Item Short Form Health Survey (SF‐12)39 was used as an indicator of overall physical health. The following health behaviors were also assessed at the baseline interview and included in analyses: cigarette smoking (current, past, never), alcohol use (none, moderate, heavy, based on National Institute on Drug Abuse categories for sex‐specific high‐risk drinking), physical activity (never, at least once a week), and medication adherence (perfect versus not perfect adherence, as measured by the Morisky Medication Adherence Scale40).
Analytic Approach
The end of follow‐up for this study was December 31, 2012. Follow‐up time for each participant was calculated from the date of his or her study in‐home visit to the date of the first CVD event, death, last telephone follow‐up, or end of follow‐up. Consistent with prior research,12 participants were classified into 3 groups reflecting psychosocial risk at baseline: (1) those reporting no depressive symptoms (CES‐D <4) and without elevated levels of perceived stress (PSS <5), (2) those reporting either elevated depressive symptoms (CES‐D ≥4) or perceived stress (PSS ≥5), and (3) those reporting both elevated depressive symptoms and perceived stress. Because of small sample sizes for those with elevated depressive symptoms but without elevated perceived stress (income ≥$35 000, n=230; income <$35 000, n=357), we combined those with either elevated depressive symptoms or stress into 1 group, as in previous research in the REGARDS study.12 Sample sizes for those with elevated perceived stress but without elevated depressive symptoms were n=2004 with income ≥$35 000 and n=2143 with income <$35 000. Primary analyses were stratified separately by annual household income at baseline (<$35 000 or ≥$35 000). A post hoc supplemental analysis was also stratified by education (graduating from high school versus not graduating).
Baseline characteristics of participants with and without elevated psychosocial risk at baseline were compared using chi‐square tests, ANOVA (for normally distributed characteristics), and Wilcoxon rank sum tests (for variables that were not normally distributed). Using Poisson regression, we calculated age‐adjusted incidence rates separately for total CVD, all‐cause mortality, acute CHD, and cardiovascular death events for each group of psychosocial risk within the strata of SES per 1000 years of person‐time. Sequentially adjusted Cox proportional hazards regression models were constructed to separately estimate the hazard ratios (HRs) for the association between psychosocial risk groups and incident total CVD, all‐cause mortality, incident acute CHD, and cardiovascular death. The initial model estimated the HRs of end points for psychosocial risk groups, adjusted for age. Model 1 adjusted for sex, race, geographic region of residence, and number of people in the household. Model 2 adjusted for model 1 covariates plus systolic blood pressure, self‐reported antihypertensive medication use, total cholesterol, high‐density lipoprotein cholesterol, statin use, log‐transformed albumin:creatinine ratio, log‐transformed high‐sensitivity C‐reactive protein, estimated glomerular filtration rate, waist circumference, and diabetes mellitus. Model 3 added adjustments for cigarette smoking, alcohol use, physical activity, and medication adherence. The final model for all‐cause mortality adjusted for model 3 covariates plus the Physical Component Summary score of the SF‐12. We also conducted formal tests for interaction between psychosocial risk group and income and education (separately) in the fully adjusted models in the overall sample for each of the examined end points. The assumptions of proportionality were tested by assessing psychosocial risk group by log of follow‐up time interactions in the fully adjusted models and were met for all analyses. Missing data in covariates were multiply imputed using chained equations and sample bootstrapping in 10 data sets. Analyses were conducted using SAS software version 9.4 (SAS Institute) and Stata version 12 (StataCorp).
Results
Participant Characteristics
After excluding participants who were missing follow‐up data (n=489), baseline depressive symptom data (n=208), or baseline perceived stress data (n=2), or who had prevalent CVD at baseline (history of CHD, stroke, peripheral arterial disease, or aortic aneurism; n=6826), a total of 22 658 participants composed the analytic sample for the current study. At baseline, 45.4% (n=9020) had an annual household income <$35 000. Overall, 12% of the REGARDS sample (n=2768) declined to report annual income. We used multiple imputation to replace missing data for these participants to preserve the original sample. In addition, 6.9% (n=1567) of participants reported both elevated depressive symptoms and stress, 24.0% (n=5441) reported either elevated depressive symptoms or stress, and 69.1% (n=15 650) reported neither elevated depressive symptoms nor stress. Furthermore, those with low income were more likely to report both elevated depressive symptoms and stress (10.9%) compared with those with high income (3.5%; P<0.001).
Baseline characteristics for the 3 psychosocial risk groups, stratified as a function of low versus high income (our primary SES indicator), are presented in Table 1. Compared with those with neither elevated depressive symptoms nor stress, participants in the group with elevated depressive symptoms and stress were more likely to be female, black, residents of the stroke belt or buckle, and to have less than a high school education; these differences were more pronounced for the low‐ versus high‐income group. Across both income groups, participants with both elevated depressive symptoms and stress were younger than those with neither psychosocial risk factor, although those with low income had an older mean age than those with high income in each psychosocial risk group. Participants with low income had a greater cardiovascular risk burden (as indicated by physiological, medical, and behavioral risk factors) than those with high income. Furthermore, those with elevated depressive symptoms and stress, in general, had a worse physiological and behavioral risk profile than those with neither elevated depressive symptoms nor stress.
Table 1.
Baseline Characteristics of REGARDS Participants Free of CVD With Neither, Either, or Concurrent Elevated Depressive Symptoms and Perceived Stress Shown Separately for High and Low Income
| Characteristic | Income ≥$35 000 | Income <$35 000 | ||||||
|---|---|---|---|---|---|---|---|---|
| No Depressive Symptoms and No/Low Stress (n=8256) | Elevated Depressive Symptoms or Stress (n=2234) | Elevated Depressive Symptoms and Stress (n=380) | P Value | No Depressive Symptoms and No/Low Stress (n=5536) | Elevated Depressive Symptoms or Stress (n=2500) | Elevated Depressive Symptoms and Stress (n=984) | P Value | |
| Sociodemographics | ||||||||
| Age, y, mean (SD) | 62.1±8.5 | 60.6±8.9 | 58.0±8.2 | <0.001 | 66.9±9.0 | 65.4±9.7 | 62.2±9.6 | <0.001 |
| Female, n (%) | 3740 (45.3) | 1338 (59.9) | 260 (68.4) | <0.001 | 3535 (63.9) | 1777 (71.1) | 750 (76.2) | <0.001 |
| Black, n (%) | 2529 (30.6) | 800 (35.8) | 134 (35.3) | <0.001 | 2786 (50.3) | 1445 (57.8) | 600 (61.0) | <0.001 |
| Did not graduate from high school, n (%) | 187 (2.3) | 64 (2.9) | 17 (4.5) | 0.01 | 921 (16.6) | 544 (21.8) | 280 (28.5) | <0.001 |
| Number of people in the household, median (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) | <0.001 | 2 (1–2) | 2 (1–2) | 2 (1–3) | <0.001 |
| Lives alone, n (%) | 1296 (15.7) | 372 (16.7) | 83 (21.8) | 0.005 | 2270 (41.0) | 990 (39.6) | 371 (37.7) | 0.11 |
| Region | 0.09 | <0.001 | ||||||
| Stroke belt, n (%) | 2599 (31.5) | 750 (33.6) | 121 (31.8) | 2052 (37.1) | 939 (37.6) | 407 (41.4) | ||
| Stroke buckle, n (%) | 1738 (21.1) | 472 (21.1) | 95 (25.0) | 1074 (19.4) | 545 (21.8) | 230 (23.4) | ||
| Non–stroke belt or buckle, n (%) | 3919 (47.5) | 1012 (45.3) | 164 (43.2) | 2410 (43.5) | 1016 (40.6) | 347 (35.3) | ||
| Physiological risk factors | ||||||||
| Waist circumference, cm, mean (SD) | 94.9±14.8 | 94.6±15.8 | 96.0±16.4 | 0.22 | 95.9±16.0 | 96.6±16.1 | 98.8±17.0 | <0.001 |
| Diabetes mellitus, n (%) | 1077 (13.5) | 321 (14.9) | 78 (21.1) | <0.001 | 1172 (22.1) | 624 (26.1) | 274 (28.9) | <0.001 |
| Physical Component Summary score of SF‐12, mean (SD) | 50.6±7.8 | 47.8±10.4 | 45.5±12.1 | <0.001 | 47.0±9.9 | 43.1±11.2 | 39.3±11.9 | <0.001 |
| Systolic blood pressure, mm Hg, mean (SD) | 125.0±15.1 | 123.6±15.6 | 122.5±15.9 | <0.001 | 129.6±16.9 | 129.1±17.5 | 129.3±18.1 | 0.55 |
| Total cholesterol, mg/dL, mean (SD) | 193.9±37.1 | 195.5±37.6 | 196.5±36.5 | 0.10 | 196.1±40.1 | 197.5±42.1 | 199.1±43.3 | 0.08 |
| High‐density lipoprotein, mg/dL, mean (SD) | 52.0±16.3 | 53.5±16.4 | 54.1±17.6 | 0.0002 | 52.9±16.1 | 53.4±15.9 | 53.2±16.0 | 0.50 |
| Estimated glomerular filtration rate <60, n (%) | 482 (6.0) | 124 (5.8) | 19 (5.2) | 0.75 | 633 (12.0) | 299 (12.6) | 72 (7.7) | 0.002 |
| High‐sensitivity C‐reactive protein, mg/L, median (IQR) | 1.7 (0.8–4.0) | 2.1 (0.9–4.6) | 2.5 (1.2–6.2) | <0.001 | 2.5 (1.1–5.5) | 2.8 (1.2–6.5) | 3.5 (1.3–7.8) | <0.001 |
| Albumin:creatinine ratio >30 mg/g, n (%) | 775 (9.5) | 224 (10.4) | 32 (8.8) | 0.37 | 811 (15.4) | 406 (17.2) | 161 (17.5) | 0.06 |
| Medications | ||||||||
| Antihypertensive medication use, n (%) | 3391 (41.3) | 920 (41.7) | 164 (43.6) | 0.67 | 2863 (52.3) | 1388 (56.1) | 576 (59.3) | <0.001 |
| Statin use, n (%) | 2114 (25.7) | 517 (23.1) | 91 (24.1) | 0.04 | 1319 (23.9) | 639 (25.6) | 238 (24.3) | 0.24 |
| Behavioral risk factors | ||||||||
| Smoking | <0.001 | <0.001 | ||||||
| Current, n (%) | 851 (10.3) | 265 (11.9) | 88 (23.3) | 856 (15.5) | 455 (18.2) | 276 (28.1) | ||
| Never, n (%) | 4003 (48.6) | 1140 (51.3) | 163 (43.1) | 2545 (46.1) | 1149 (46.1) | 450 (45.9) | ||
| Past, n (%) | 3381 (41.1) | 818 (36.8) | 127 (33.6) | 2116 (38.4) | 890 (35.7) | 255 (26.0) | ||
| Alcohol use | 0.001 | 0.62 | ||||||
| Heavy, n (%) | 439 (5.4) | 105 (4.8) | 21 (5.6) | 181 (3.3) | 67 (2.8) | 31 (3.3) | ||
| Moderate, n (%) | 3622 (44.5) | 895 (40.7) | 141 (37.9) | 1371 (25.2) | 595 (24.4) | 233 (24.4) | ||
| None, n (%) | 4074 (50.1) | 1197 (54.5) | 210 (56.5) | 3895 (71.5) | 1774 (72.8) | 689 (72.3) | ||
| Physical inactivity, n (%) | 2125 (26.0) | 716 (32.5) | 153 (40.8) | <0.001 | 1896 (34.8) | 995 (40.3) | 458 (47.1) | <0.001 |
| Medication nonadherence, n (%) | 1950 (26.8) | 632 (31.7) | 141 (40.6) | <0.001 | 1342 (26.9) | 741 (33.1) | 367 (40.6) | <0.001 |
Elevated depressive symptoms were defined as a score ≥4 on the Center for Epidemiologic Studies–Depression scale, and elevated perceived stress was defined as a score ≥5 on the Perceived Stress Scale. P values from chi square, ANOVA tests. The stroke belt was defined as the states of Alabama, Arkansas, Louisiana, Mississippi, Tennessee, and the noncoastal regions within the states of North Carolina, South Carolina and Georgia. The stroke buckle was defined as coastal regions within the states of North Carolina, South Carolina, and Georgia. Diabetes mellitus was defined as fasting blood glucose ≥126 or random glucose >200 mL/dL, or oral hypoglycemic or insulin use. CVD was defined as baseline coronary heart disease, stroke, periphery artery disease, or aortic aneurism. CVD indicates cardiovascular disease; IQR, interquartile range; REGARDS, Reasons for Geographical and Racial Differences in Stroke; SF‐12, 12‐Item Short Form Health Survey.
Income, Psychosocial Risk, and Risks for Incident Total CVD, Incident Acute CHD, Cardiovascular Death, and All‐Cause Mortality
Over a median of 7.0 years (interquartile range 5.4–8.3 years) of follow‐up, there were 1753 total CVD events. Even though there were fewer participants in the low‐income group than in the high‐income group, the low‐income group accounted for a greater share of events (1071 versus 682, respectively). Participants with low income had higher cumulative incidence of total CVD than those with high income, and those with low income and both elevated depressive symptoms and stress had the highest cumulative incidence of total CVD (Kaplan–Meier survival curves are shown in Figure 1). Table 2 presents age‐adjusted incidence rates per 1000 person‐years of follow‐up for the incident total CVD outcome for the 3 psychosocial risk groups as a function of low and high income. Age‐adjusted incidence rates for those with low income were substantially higher than for those with high income, and greater psychosocial risk was associated with greater elevations in total CVD incidence only among those with low income. Specifically, for participants with low income, those with concurrent elevated depressive symptoms and stress had the highest age‐adjusted incidence rate per 1000 person‐years of follow‐up (21.2), followed by those with either elevated depressive symptoms or stress (16.2) and with neither psychosocial risk factor (13.4).
Figure 1.
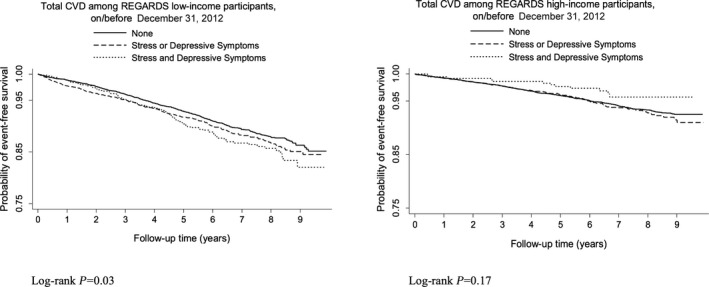
Kaplan–Meier survival curves for incident total cardiovascular disease (CVD) in participants with low and high income. Incident CVD includes nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. REGARDS indicates Reasons for Geographical and Racial Differences in Stroke.
Table 2.
Association of Concurrent Depressive Symptoms and Perceived Stress With Incident Total CVD, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Income
| Variable | Income ≥$35 000 | Income <$35 000 | ||||
|---|---|---|---|---|---|---|
| No Depressive Symptoms and No/Low Stress (n=9265) | Elevated Depressive Symptoms or Stress (n=2504) | Elevated Depressive Symptoms and Stress (n=430) | No Depressive Symptoms and No/Low Stress (n=6385) | Elevated Depressive Symptoms or Stress (n=2937) | Elevated Depressive Symptoms and Stress (n=1137) | |
| Total CVD (incident nonfatal MI, nonfatal stroke, or cardiovascular death) | ||||||
| Number of events | 524 | 144 | 14 | 636 | 312 | 123 |
| Age‐adjusted IR/1000 person‐years (95% CI) | 6.8 (6.1–7.5) | 7.9 (6.6–9.4) | 5.2 (3.0–9.2) | 13.4 (12.3–14.7) | 16.2 (14.3–18.3)a , d | 21.2 (17.6–25.7)c , d |
| Age‐adjusted HR (95% CI) | Ref | 1.16 (0.96–1.41) | 0.77 (0.43–1.37) | Ref | 1.20 (1.05–1.38)d | 1.53 (1.05–1.39)d |
| Model 1 HR (95% CI) | Ref | 1.24 (1.03–1.51)d | 0.90 (0.50–1.60) | Ref | 1.23 (1.07–1.42)d | 1.58 (1.29–1.93)d |
| Model 2 HR (95% CI) | Ref | 1.18 (0.97–1.42) | 0.85 (0.48–1.51) | Ref | 1.21 (1.05–1.39)d | 1.54 (1.26–1.88)d |
| Model 3 HR (95% CI) | Ref | 1.17 (0.97–1.42) | 0.78 (0.44–1.39) | Ref | 1.20 (1.04–1.38)d | 1.48 (1.21–1.81)d |
| Psychosocial risk and income interaction, P valuee | 0.11d | |||||
| Acute coronary heart disease | ||||||
| Number of events | 298 | 77 | 7 | 302 | 151 | 59 |
| Age‐adjusted IR/1000 person‐years (95% CI) | 4.0 (3.5–5.6) | 4.4 (3.5–5.6) | 2.6 (1.2–5.8) | 6.9 (6.1–7.8) | 8.3 (7.0–9.9) | 9.9 (7.6–13.0)a , d |
| Age‐adjusted HR (95% CI) | Ref | 1.08 (0.84–1.40) | 0.66 (0.30–1.46) | Ref | 1.20 (0.99–1.47) | 1.45 (1.09–1.93)d |
| Model 1 HR (95% CI) | Ref | 1.19 (0.91–1.54) | 0.80 (0.36–1.77) | Ref | 1.24 (1.02–1.52)d | 1.50 (1.12–1.99)d |
| Model 2 HR (95% CI) | Ref | 1.11 (0.86–1.43) | 0.74 (0.33–1.64) | Ref | 1.21 (1.00–1.48) | 1.44 (1.08–1.92)d |
| Model 3 HR (95% CI) | Ref | 1.10 (0.85–1.43) | 0.68 (0.31–1.52) | Ref | 1.21 (0.99–1.47) | 1.37 (1.03–1.83)d |
| Psychosocial risk and income interaction, P valuee | 0.33 | |||||
| Cardiovascular death | ||||||
| Number of events | 160 | 48 | 4 | 289 | 147 | 54 |
| Age‐adjusted IR/1000 person‐years (95% CI) | 1.7 (1.4–2.2) | 2.3 (1.7–3.2) | 1.2 (0.4–3.7) | 4.9 (4.2–5.6) | 6.2 (5.2–7.5)a , d | 7.4 (5.5–7.4)a , d |
| Age‐adjusted HR (95% CI) | Ref | 1.30 (0.94–1.81) | 0.78 (0.23–2.63) | Ref | 1.26 (1.03–1.54)d | 1.59 (1.18–2.14)d |
| Model 1 HR (95% CI) | Ref | 1.34 (0.96–1.87) | 0.87 (0.26–2.91) | Ref | 1.27 (1.04–1.56)d | 1.59 (1.18–2.15)d |
| Model 2 HR (95% CI) | Ref | 1.23 (0.88–1.72) | 0.77 (0.24–2.53) | Ref | 1.23 (1.002–1.50)d | 1.61 (1.19–2.17)d |
| Model 3 HR (95% CI) | Ref | 1.21 (0.86–1.69) | 0.71 (0.21–2.36) | Ref | 1.22 (0.99–1.49) | 1.54 (1.13–2.08)d |
| Psychosocial risk and income interaction, P valuee | 0.54 | |||||
| All‐cause mortality | ||||||
| Number of events | 618 | 183 | 27 | 1043 | 506 | 191 |
| Age‐adjusted IR/1000 person‐years (95% CI) | 6.4 (5.8–7.2) | 8.2 (7.0–9.7)a , d | 8.7 (5.8–13.1) | 19.2 (17.8–20.7) | 22.8 (20.6–25.3)b , d | 28.4 (24.3–33.3)c , d |
| Age‐adjusted HR (95% CI) | Ref | 1.31 (1.10–1.55)d | 1.47 (0.98–2.21) | Ref | 1.21 (1.09–1.35)d | 1.54 (1.32–1.80)d |
| Model 1 HR (95% CI) | Ref | 1.34 (1.13–1.59)d | 1.64 (1.09–2.48)d | Ref | 1.24 (1.12–1.39)d | 1.61 (1.37–1.88)d |
| Model 2 HR (95% CI) | Ref | 1.26 (1.06–1.50)d | 1.56 (1.03–2.26)d | Ref | 1.22 (1.09–1.36)d | 1.61 (1.37–1.89)d |
| Model 3 HR (95% CI) | Ref | 1.25 (1.05–1.48)d | 1.40 (0.92–2.12) | Ref | 1.21 (1.08–1.35)d | 1.55 (1.31–1.82)d |
| +SF‐12 PCS HR (95% CI) | Ref | 1.15 (0.96–1.37) | 1.27 (0.83–1.92) | Ref | 1.11 (0.99–1.24) | 1.33 (1.13–1.56)d |
| Psychosocial risk and income interaction, P valuee | 0.53 | |||||
IRs and HRs for the psychosocial risk groups. Model 1 adjusts for age, sex, race, geographic region, and number of people in the household. Model 2 adjusts for model 1 covariates plus systolic blood pressure, self‐reported antihypertensive medication use, total cholesterol, high‐density lipoprotein cholesterol, statin use, log‐transformed albumin:creatinine ratio, log‐transformed high‐sensitivity C‐reactive protein, estimated glomerular filtration rate, waist circumference, and diabetes mellitus. Model 3 adjusts for model 2 covariates plus cigarette smoking, alcohol use, physical activity, and medication adherence. The final model for all‐cause mortality adjusts for model 3 covariates plus the PCS of the SF‐12. Missing data in covariates were imputed using chain equations in 10 data sets with sample bootstrapping. CVD indicates cardiovascular disease; HR, hazard ratio; IR, incidence rate; MI, myocardial infarction; PCS, Physical Component Summary score; Ref, reference value; REGARDS, Reasons for Geographical and Racial Differences in Stroke; SF‐12, 12‐Item Short Form Health Survey.
Incidence rate is significantly different compared with the group with no depression and no stress, P<0.05.
Incidence rate is significantly different compared with the group with no depression and no stress, P≤0.01.
Incidence rate is significantly different compared with the group with no depression and no stress, P≤0.001.
Significant at P<0.05.
Interaction term P value from the overall (not stratified) final model.
Table 2 also presents the results of a series of increasingly adjusted Cox proportional hazards regression models demonstrating the associations between the different levels of psychosocial risk with incident total CVD stratified by income. For participants with low income, the co‐occurrence of elevated depressive symptoms and stress at baseline was associated with significantly heightened risk of developing total CVD compared with those with neither elevated depressive symptoms nor stress in models that increasingly adjusted for age (hazard ratio [HR] 1.53, 95% CI 1.05–1.39), sociodemographics (model 1: HR 1.58, 95% CI 1.29–1.93), physiological and medical CVD risk factors (model 2: HR 1.54, 95% CI 1.26–1.88), and health behaviors (model 3: HR 1.48, 95% CI 1.21–1.81). In other words, even when accounting for a variety of potential confounders, participants with low income and concurrent depressive symptoms and stress had a nearly 50% higher risk of incident CVD events compared with those with low income and neither elevated depressive symptoms nor stress. Among those with annual household income <$35 000, participants with either elevated depressive symptoms or stress at baseline also had significantly heightened risk of incident total CVD over follow‐up compared with those with neither psychosocial risk factor, although HRs were not as high as those for participants with both elevated depressive symptoms and stress. In contrast, for the high‐income group, the risks for participants with 1 psychosocial risk factor versus both were not significantly different than those for participants with neither psychosocial risk factor. The psychosocial risk group by income interaction term had a P value of 0.11.
Similar patterns emerged from separate models examining income‐stratified associations of psychosocial risk with incident acute CHD and cardiovascular death (Table 2). Even in models adjusting for sociodemographics, physiological and medical CVD risk factors, and health behaviors, low‐income participants with elevated depressive symptoms and stress had significantly elevated risk of acute CHD (model 3: HR 1.37, 95% CI 1.03–1.83) and cardiovascular death (model 3: HR 1.54, 95% CI 1.13–2.08) compared with low‐income participants with no psychosocial risk factors at baseline. This pattern of results was observed only for those with low income and not for those with high income.
Over the course of follow‐up, there were 2568 deaths due to all causes. Cumulative incidence of all‐cause mortality was higher for those with low (versus high) income and highest for those with low income and 1 or both psychosocial risk factors (Kaplan–Meier survival curves shown in Figure 2). The age‐adjusted incidence rates per 1000 person‐years of follow‐up for all‐cause mortality for participants with low income were staggering, and they were progressively higher as a function of greater psychosocial risk: 19.2 for those with neither elevated depressive symptoms nor stress, 22.8 for those with either elevated depressive symptoms or stress, and 28.4 for those with both elevated depressive symptoms and stress (Table 2). Similar to the total CVD analysis, low‐income participants with elevated depressive symptoms and stress had increased risk of all‐cause mortality compared with low‐income participants with neither elevated depressive symptoms nor stress (HR 1.33 [95% CI 1.13–1.56] in the final model adjusting for sociodemographics, physiological and medical CVD risk factors, health behaviors, and the Physical Health Component score of the SF‐12). HRs for all‐cause mortality for low‐income participants with either elevated depressive symptoms or stress compared with low‐income participants with neither psychosocial risk factor were also elevated, although the HR was not significantly different from 1 in the final model (Table 2). Unlike the total CVD analysis, there was some evidence that psychosocial risk factors were also associated with increased risk of all‐cause mortality for high‐income participants. High‐income participants with 1 or both psychosocial risk factors had significantly elevated risk of all‐cause mortality compared with high‐income participants with neither psychosocial risk factor in models adjusting for sociodemographics and physiological and medical CVD risk factors (models 1 and 2 are described in Table 2; participants with one psychosocial risk factor also had significantly elevated risk in model 3). However, the HRs for the groups with 1 or both psychosocial risk factors were not significantly >1 in the final model that additionally adjusted for the Physical Component Summary score of the SF‐12 (Table 2).
Figure 2.
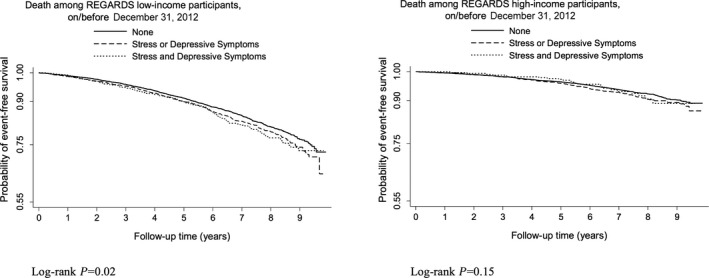
Kaplan–Meier survival curves for all‐cause mortality in participants with low and high income. REGARDS indicates Reasons for Geographical and Racial Differences in Stroke.
Education, Psychosocial Risk, and Risks for Incident Total CVD, Incident Acute CHD, Cardiovascular Death, and All‐Cause Mortality
Table S1 shows the results of analyses examining the association of depressive symptoms and perceived stress with incident total CVD, incident acute CHD, cardiovascular death, and all‐cause mortality separately for those with low and high education (our secondary SES indicator). Notably, the same pattern of associations between the combination of elevated depressive symptoms and stress and increased risk of incident CVD and mortality primarily for those with low (and not high) SES was not observed when we used education rather than income as the SES indicator. In general, psychosocial risk factors were associated with increased risk of incident total CVD and all‐cause mortality in those with both low and high education.
Sensitivity Analyses for Definition of Elevated Perceived Stress
Results of sensitivity analyses using a PSS score ≥8 (corresponding to the 95th percentile) to indicate elevated levels of perceived stress are presented in Tables S2 and S3. The pattern of results was highly similar when using this higher cutoff for elevated stress to create the psychosocial risk groups.
Discussion
In a major national cohort study using population‐based sampling methods, we demonstrated that the combination of elevated depressive symptoms and perceived stress was associated with increased risk of first‐onset CVD events, including nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death in low‐ but not high‐income participants. Our findings add to a growing body of work indicating that psychosocial factors related to CVD risk are most deleterious for those with low income.21, 24, 25 Furthermore, our results suggest that it is the combination of psychosocial risk factors that is associated with the greatest vulnerability to poor cardiovascular health in low‐income persons. The comorbidity of elevated depressive symptoms and stress in those with low income was associated with higher incidence of CVD than either psychosocial risk factor alone, although those with 1 risk factor had higher incidence of CVD than those with neither elevated depressive symptoms nor stress. Notably, these findings were observed even when adjusting for a variety of potential confounders, including sociodemographics, physiological and medical CVD risk factors, and health behaviors.
SES and psychosocial factors, including depressive symptoms, psychological distress, and perceived stress, have been increasingly recognized as risk factors for CVD.3, 4, 7 Only recently have researchers begun to appreciate the information to be learned by considering both sets of factors simultaneously to better identify persons at elevated risk of poor cardiovascular outcomes and mortality.21, 23, 24, 25, 41 Whereas most prior work in this area has focused on the joint contributions of SES and a single psychosocial risk factor (eg, depression, stress), our research emphasized the importance of considering a confluence of psychosocial risk factors, in addition to SES, to identify those who are most vulnerable to CVD. Our findings extend results from persons with a history of CHD10 and diabetes mellitus,12 suggesting that the comorbidity of elevated depressive symptoms and stress is most pernicious for cardiovascular risk. Interestingly, although the combination of elevated depressive symptoms and stress was associated with significantly elevated risk of all‐cause mortality in fully adjusted models only for those with low income, effect sizes for the psychosocial risk groups were relatively similar in both income groups. Differences across income groups might emerge in an analysis with more cases of all‐cause mortality and greater statistical power, thereby precluding definitive conclusions about no effect of income with respect to the relationship of psychosocial risk and all‐cause mortality. Nevertheless, our results suggest that among persons with no history of CVD at baseline, elevated depressive symptoms and stress may be particularly associated with risk of incident CVD in those with low income but that the confluence of psychosocial risk factors may be linked to all‐cause mortality regardless of individual income. Additional research is needed to replicate these findings and to probe mechanisms underlying this apparent discrepancy.
Why might the combination of elevated depressive symptoms and stress be particularly associated with CVD risk in those with low but not high income? Consistent with the reserve capacity model,20, 22 persons with low (versus high) income may have fewer resources on which to draw and may be exposed to taxing situations that require resources for coping more frequently as a function of their position in society. When faced with a psychosocial perfect storm13 of both elevated depressive symptoms and stress, persons with low income may be less able to cope effectively with concurrent psychosocial challenges, and this may negatively affect cardiovascular health by both behavioral and physiological pathways. As observed in our sample (Table 1), persons with low income may be more likely to engage in poor health behaviors that may contribute to risk for CVD (eg, smoking, physical inactivity, overeating, and poor adherence to medical treatment regimens).8, 42 In addition, those with low income have been characterized by a lack of physiological reserve.20, 43 Consequently, when faced with psychosocial stress, those with low income may have exaggerated physiological responses (eg, elevated sympathetic nervous system activity and hypothalamic–pituitary–adrenal axis dysregulation43, 44) that can have negative downstream effects for cardiovascular health, particularly when activated in a chronic manner.
The nature of stress may differ for persons with low versus high income. Elevated perceived stress for those with low income may reflect stress related to concerns with significant implications for one's livelihood (eg, not making rent or mortgage payments, food insecurity), whereas elevated perceived stress for those with high income may reflect challenges that have less impact on quality of life (eg, stress related to interactions with a coworker rather than stress over being unemployed). In addition, compared with those with high income, persons with low income may be more likely to be exposed to environmental conditions (eg, crime, high population density) that contribute to a state of chronic stress, which takes a toll on physical health.42 Furthermore, the stress faced by those with low (versus high) income may be more likely to be perceived as unpredictable, uncontrollable, and unmanageable. In both animal and human research, the experience of uncontrollable stress has been shown to induce physiological changes (eg, hypothalamic–pituitary–adrenal axis activation) that can have negative downstream consequences for cardiovascular health; in contrast, stress that is perceived as being controllable does not trigger such physiological responses to the same degree.45 Consequently, the types of stress faced by those with low income may be particularly detrimental for health, especially for persons who are depressed. It is of interest to examine this notion empirically in future research.
Interestingly, we found that the combination of elevated depressive symptoms and stress was associated with increased risk of CVD incidence and all‐cause mortality primarily for those with low SES when we stratified analyses based on income and not on education. When stratifying on low versus high education, elevated depressive symptoms and stress were associated with the greatest elevation in risk for incident total CVD and all‐cause mortality in both education groups. A possible explanation for these findings is that income may be a better indicator of life quality and resources than education. Indeed, researchers have highlighted how educational attainment does not necessarily translate into earnings potential,20 and one can imagine a situation in which a person is educated (eg, with a high school or even a college degree) and yet not financially comfortable. This scenario is disproportionately true for black persons (particularly black women). Black women earn the least among other college degree holders,46 and compared with white applicants, black applicants are less likely to be offered jobs, even when they have college degrees from prestigious universities.47 These findings highlight an important disparity between educational attainment and earning potential for certain groups of people. These results are also consistent with the recent finding from the REGARDS study that high education was not able to offset risk for CHD associated with low income in adults aged <65 years.33 Although education and income are indicators of SES that are robustly associated with CVD outcomes,3 income may be a better factor for identifying persons who may be most susceptible to the effects of psychosocial risk factors on CVD health. Additional research is needed to determine whether this finding can be generalized beyond the REGARDS study.
Demonstrating a differential impact of concurrent psychosocial risk factors on the incidence of CVD events and mortality for certain persons has the potential to inform targeted efforts for allocating resources to offset risk. Both the American Heart Association 2020 Impact Goals2 and the US Department of Health and Human Services “Million Hearts” initiative48 identified reducing CVD incidence and mortality as critical health goals for the United States. We believe that our findings have several implications for policy and for directing health care prevention resources in an age of targeted medicine. First, our finding that the combination of elevated depressive symptoms and stress was associated with the greatest elevation in risk for developing total CVD and all‐cause mortality compared with having 1 or no psychosocial risk factors demonstrates the importance of taking a number of psychosocial factors into consideration when assessing cardiovascular risk, most markedly among those with low income. Screening for psychosocial risk factors can be accomplished easily and quickly in health care and community settings with short screening questionnaires.29, 32 Our results suggest the value of screening for both elevated depressive symptoms and stress—not just one or the other—to identify persons at the greatest cardiovascular risk, and our findings indicate that persons with low income may predominantly benefit from such psychosocial screening. Second, our findings suggest that low‐income persons do not need to be at or below the poverty level to experience the deleterious health effects of a confluence of psychosocial risk factors. Notably, our definition of low income was an annual household income <$35 000, which was derived from the data and is significantly higher than the poverty level. The Federal Poverty Level for a family of 4 was $18 400 in 2003 and $20 650 in 2007 (the years of the baseline period for the REGARDS study). Although this finding warrants replication in other modern samples, it provides preliminary evidence that persons with low household income well above the Federal Poverty Level may be particularly vulnerable to the effects of psychosocial risk factors for poor physical health and may benefit from screening and prevention efforts. More research is needed to better understand the mechanisms by which concurrent psychosocial risk factors contribute to poor health outcomes in low‐income persons to best tailor prevention and intervention efforts for this population (eg, described by Wells and Miranda49). In the meantime, our study provides important information for population health managers by supporting targeted screening of the population with low income.
Despite these implications for health policy, our study has several limitations that merit acknowledgement. First, the REGARDS study included an observational cohort and was not designed to derive causal links. Nevertheless, by using psychosocial and SES data from the baseline assessment and by limiting our sample to those without a history of CVD at baseline, we were able to ensure that our predictors occurred prior to our outcomes of interest. Second, we did not adjust for the competing risk of mortality in the incident CVD analyses, and that approach could have introduced potential bias, particularly for low‐income participants. However, prior studies of incident CVD in the REGARDS study obtained similar results with and without a competing risk approach.50 Third, depressive symptoms, perceived stress, annual household income, education, and health behaviors were assessed by self‐report at only 1 point in time (the baseline assessment), and depressive symptoms and perceived stress were measured with short screening questionnaires. Consequently, we were unable to assess participants’ psychosocial risk immediately preceding CVD incidence or mortality or to investigate how changes in psychosocial risk or SES over time relate to risk of incident CVD and mortality. Furthermore, by using these brief measures, we were not able to comprehensively assess the complex constructs of depression, stress, and SES. Additional research that incorporates multiple measures of psychosocial risk and SES over time and that includes methods that go beyond self‐report (eg, objective measures of SES, diagnostic interviews for depression, life stress interviews) will add greater precision to our understanding of how a confluence of psychosocial risk factors and SES contributes to risk of CVD and mortality. Moreover, assessing potential underlying mechanisms in the context of a combination of elevated depressive symptoms and stress may shed light on how cardiovascular risk unfolds. Fourth, because of the design of the REGARDS study, only white and black participants were included in our sample. Additional research is needed to examine whether findings generalize to participants of other racial and ethnic groups. Because of low statistical power, we did not investigate whether the associations of the psychosocial risk groups with CVD risk among those with low (versus high) SES differed for black and white participants. This topic is important for future study in larger samples, particularly given racial differences in depressive symptoms, perceived stress, SES, and CVD outcomes.
Even with these limitations, we believe our study has a number of strengths and makes a unique and notable contribution to the literature. We used longitudinal data from a large national cohort with population‐based sampling methods, and we predicted CVD and mortality events that were adjudicated by physician‐led teams. Furthermore, we accounted for a number of potential confounders in our analyses, several of which were assessed with an in‐home examination.
Conclusions
Our study suggests that taking a confluence of psychosocial risk factors and SES into consideration may hold promise for working to offset CVD risk. In an age of precision medicine and population health management, screening for a combination of elevated depressive symptoms and stress in low‐income persons may help identify those at increased risk of incident CVD and mortality. Going forward, it would be of interest to identify these persons not only in health care settings but also in community settings and to pinpoint the most effective interventions to offset risk in this population.
Sources of Funding
The REGARDS study is supported by cooperative agreement U01NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and Department of Health and Human Service and the National Heart, Lung, and Blood Institute R01HL080477. This work was additionally supported by grants K01HL130650 (to Dr Sumner), R01 HL115941 (to Dr Davidson), HL084034 (to Dr Davidson), and HL088117 (to Dr Davidson) from the National Heart, Lung, and Blood Institute and K12HS02300 (to Dr Redmond) from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosures
Dr Davidson is a member of the United States Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF. Dr Davidson is the co‐owner of MJBK, a small business that provides mhealth technology solutions to consumers. She is also the co‐owner of IOHealthWorks, a small consulting services company. Dr Davidson has disclosed those interests fully to Columbia University Medical Center, and has in place an approved plan for managing any potential conflicts arising from this arrangement.
Supporting information
Table S1. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥5) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Education, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups
Table S2. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥8) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Income, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups
Table S3. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥8) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Education, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Dr Redmond contributed to this work as an employee of the University of Alabama at Birmingham (UAB). Since submission of this manuscript, Dr Redmond has changed affiliation and is now with the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH). The views expressed are her own and do not necessarily represent the views of the NIH or the United States Government.
(J Am Heart Assoc. 2016;5: e003930 doi: 10.1161/JAHA.116.003930)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ERI, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF. Defining and setting national goals for cardiovascular health promotion and disease reduction the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 3. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 4. Nemeroff CB, Goldschmidt‐Clermont PJ. Heartache and heartbreak—the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–539. [DOI] [PubMed] [Google Scholar]
- 5. Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. [DOI] [PubMed] [Google Scholar]
- 6. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. [DOI] [PubMed] [Google Scholar]
- 7. Steptoe A, Kivimäki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–354. [DOI] [PubMed] [Google Scholar]
- 8. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. [DOI] [PubMed] [Google Scholar]
- 9. Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta‐analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110:1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alcántara C, Muntner P, Edmondson D, Safford MM, Redmond N, Colantonio LD, Davidson KW. Perfect storm: concurrent stress and depressive symptoms increase risk of myocardial infarction or death. Circ Cardiovasc Qual Outcomes. 2015;8:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 12. Cummings DM, Kirian K, Howard G, Howard V, Yuan Y, Muntner P, Kissela B, Redmond N, Judd SE, Safford MM. Consequences of comorbidity of elevated stress and/or depressive symptoms and incident cardiovascular outcomes in diabetes: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2016;39:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burg MM, Edmondson D, Shimbo D, Shaffer J, Kronish IM, Whang W, Alcántara C, Schwartz JE, Muntner P, Davidson KW. The ‘perfect storm'and acute coronary syndrome onset: do psychosocial factors play a role? Prog Cardiovasc Dis. 2013;55:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kivimäki M, Nyberg ST, Batty GD, Fransson EI, Heikkilä K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A. Job strain as a risk factor for coronary heart disease: a collaborative meta‐analysis of individual participant data. Lancet. 2012;380:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, Vorchheimer D, Clemow L, Schwartz JE, Rieckmann N. Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Arch Gen Psychiatry. 2010;67:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edmondson D, Newman JD, Whang W, Davidson KW. Emotional triggers in myocardial infarction: do they matter? Eur Heart J. 2012;34:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long‐term mortality and health status outcomes. J Am Coll Cardiol. 2012;60:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye S, Muntner P, Shimbo D, Judd SE, Richman J, Davidson KW, Safford MM. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2013;61:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman MA. Risk of acute myocardial infarction after the death of a significant person in one's life: the Determinants of Myocardial Infarction Onset Study. Circulation. 2012;125:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazzarino AI, Hamer M, Stamatakis E, Steptoe A. The combined association of psychological distress and socioeconomic status with all‐cause mortality: a national cohort study. JAMA Intern Med. 2013;173:22–27. [DOI] [PubMed] [Google Scholar]
- 22. Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. [DOI] [PubMed] [Google Scholar]
- 23. Lazzarino AI, Hamer M, Stamatakis E, Steptoe A. Low socioeconomic status and psychological distress as synergistic predictors of mortality from stroke and coronary heart disease. Psychosom Med. 2013;75:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Redmond N, Richman J, Gamboa CM, Albert MA, Sims M, Durant RW, Glasser SP, Safford MM. Perceived stress is associated with incident coronary heart disease and all‐cause mortality in low‐ but not high‐income participants in the Reasons for Geographic and Racial Differences in Stroke study. J Am Heart Assoc. 2013;2:e000447 doi: 10.1161/JAHA.113.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sims M, Redmond N, Khodneva Y, Durant RW, Halanych J, Safford MM. Depressive symptoms are associated with incident coronary heart disease or revascularization among blacks but not among whites in the Reasons for Geographical and Racial Differences in Stroke study. Ann Epidemiol. 2015;25:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaskin DJ, Thorpe RJ Jr, McGinty EE, Bower K, Rohde C, Young JH, LaVeist TA, Dubay L. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104:2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 28. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melchior LA, Huba G, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53:1117–1125. [Google Scholar]
- 30. Radloff LS. The CES‐D scale a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 32. Cohen S, Williamson G. Perceived stress in a probability sample of the United States In: Spacapan S, Oskamp S, eds. The Social Psychology of Health. Newbury Park, CA: Sage; 1988:31–67. [Google Scholar]
- 33. Lewis MW, Khodneva Y, Redmond N, Durant RW, Judd SE, Wilkinson LL, Howard VJ, Safford MM. The impact of the combination of income and education on the incidence of coronary heart disease in the prospective Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. BMC Public Health. 2015;15:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. [DOI] [PubMed] [Google Scholar]
- 35. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall‐Pedoe H; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 36. Prineas RJ, Crow RS, Zhang Z‐M. The Minnesota Code Manual of Electrocardiographic Findings. London: Springer Science & Business Media; 2009. [Google Scholar]
- 37. Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: Wright‐OSG; 1982. [Google Scholar]
- 38. World Health Organization . Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 39. Ware J Jr, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 40. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 41. Poole L, Leigh E, Kidd T, Ronaldson A, Jahangiri M, Steptoe A. The combined association of depression and socioeconomic status with length of post‐operative hospital stay following coronary artery bypass graft surgery: data from a prospective cohort study. J Psychosom Res. 2014;76:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baum A, Garofalo J, Yali AM. Socioeconomic status and chronic stress: does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–144. [DOI] [PubMed] [Google Scholar]
- 43. Janicki‐Deverts D, Cohen S, Adler NE, Schwartz JE, Matthews KA, Seeman TE. Socioeconomic status is related to urinary catecholamines in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2007;69:514–520. [DOI] [PubMed] [Google Scholar]
- 44. Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. [DOI] [PubMed] [Google Scholar]
- 45. Kemeny ME. The psychobiology of stress. Curr Dir Psychol Sci. 2003;12:124–129. [Google Scholar]
- 46. Black Women's Roundtable Public Policy Network . Black women in the United States. 2015. Available at: http://ncbcp.org/news/releases/BWRReport.BlackWomeninU.S.2015.3.26.15FINAL.pdf. Accessed January 26, 2016.
- 47. Gaddis SM. Discrimination in the credential society: an audit study of race and college selectivity in the labor market. Soc Forces. 2015;93:1451–1479. [Google Scholar]
- 48. Frieden TR, Berwick DM. The “Million Hearts” initiative—preventing heart attacks and strokes. N Engl J Med. 2011;365:e27. [DOI] [PubMed] [Google Scholar]
- 49. Wells KB, Miranda J. Differential mortality for persons with psychological distress and low socioeconomic status: what does it mean and what can be done? Comment on “The combined association of psychological distress and socioeconomic status with all‐cause mortality”. JAMA Intern Med. 2013;173:27–28. [DOI] [PubMed] [Google Scholar]
- 50. Gutiérrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, Warnock DG, Safford MM; REGARDS Investigators . Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥5) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Education, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups
Table S2. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥8) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Income, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups
Table S3. Association of Concurrent Depressive Symptoms (Center for Epidemiologic Studies–Depression scale ≥4) and Perceived Stress (Perceived Stress Scale ≥8) With Incident Total Cardiovascular Disease, Acute Coronary Heart Disease, Cardiovascular Death, and All‐Cause Mortality Shown Separately for REGARDS Participants With High and Low Education, With Incidence Rates and Hazard Ratios for the Psychosocial Risk Groups


