Abstract
Integrins play key roles in the developing and mature nervous system from promoting neuronal process outgrowth to facilitating synaptic plasticity. Recently, in hippocampal pyramidal neurons, β3 integrin (ITGβ3) was shown to stabilise synaptic AMPA receptors (AMPARs) and be required for homeostatic scaling of AMPARs elicited by chronic activity suppression. To probe the physiological function for ITGβ3-dependent processes in the brain, we examined if the loss of ITGβ3 affected fear-related behaviours in mice. ITGβ3 knockout (KO) mice showed normal conditioned fear responses that were comparable to control wild type mice. However, anxiety-like behaviour appeared substantially compromised, which could be reversed to control levels by lentivirus-mediated re-expression of ITGβ3 bilaterally in the ventral hippocampus. In hippocampal slices, the loss of ITGβ3 activity did not compromise Hebbian forms of plasticity: neither acute pharmacological disruption of ITGβ3 ligand interactions nor genetic deletion of ITGβ3 altered LTP or LTD. Moreover, we did not detect any changes in short-term synaptic plasticity upon loss of ITGβ3 activity. In contrast, acutely disrupting ITGβ1-ligand interactions or genetic deletion of ITGβ1 selectively interfered with LTP stabilisation whereas LTD remained unaltered. These findings indicate a lack of requirement for ITGβ3 in the two robust forms of hippocampal long-term synaptic plasticity, LTP and LTD, and suggest differential roles for ITGβ1 and ITGβ3 in supporting hippocampal circuit functions.
Keywords: synapse adhesion, hippocampal pyramidal neuron, LTP, LTD, mouse
Introduction
Integrins are α/β heterodimeric adhesion receptors that regulate a large variety of cellular processes by coupling the extracellular environment to intracellular signalling pathways and the actin cytoskeleton. Several α and β integrins are expressed in the adult mammalian central nervous system, where they have been implicated in controlling short and long-term synaptic plasticity but also basal synaptic transmission (McGeachie et al., 2011). Peptides containing the Arg-Gly-Asp (RGD) sequence, which is present in many extracellular matrix (ECM) proteins such as fibronectin, are recognised by many integrins, and early studies using RGD peptides have demonstrated an involvement of integrins in stabilising hippocampal long-term potentiation (LTP; e.g. Staubli et al., 1998). In the presence of RGD peptides, robust potentiation elicited upon LTP induction gradually decayed back to baseline levels. Recent studies using mice deficient in integrin subtypes have confirmed a requirement of specific integrin subtypes in LTP maintenance, possibly through stabilising newly acquired synaptic morphology and function (Chan et al., 2006; Huang et al., 2006; McGeachie et al., 2011). Notably, mice conditionally deleted for ITGβ1 in the forebrain showed reduced basal synaptic transmission and LTP in the hippocampus. Moreover, the loss of ITGβ1 expression compromised some hippocampus dependent behaviour such as the working memory but not others, including spatial reference memory and contextual memory in a fear conditioning paradigm. Therefore, integrins affect synaptic circuits in the hippocampus to modulate behaviour in subtle ways.
Recently, ITGβ3 was shown to regulate synaptic AMPARs in cultured hippocampal pyramidal neurons (Cingolani et al., 2008). Disrupting ITGβ3-ECM ligand interactions reduced excitatory synaptic currents by promoting the endocytosis of GluA2-containing AMPARs. Moreover, loss of ITGβ3 prevented compensatory, homeostatic scaling up of AMPARs upon chronic activity suppression (Cingolani and Goda, 2008; Cingolani et al., 2008). Whether such ITGβ3-dependent regulation of glutamatergic synaptic strength modulates synaptic circuits underlying particular types of behaviour is not known. Upon exploring the potential contribution of ITGβ3 in controlling fear-related responses, we find that mice lacking ITGβ3 show an apparently reduced anxiety-like behaviour whereas conditioned fear responses remain normal, and that expressing ITGβ3 in the ventral hippocampus of ITGβ3 KO animals rescues the anxiolytic phenotype. Moreover, hippocampal LTP and LTD remain unchanged in ITGβ3 KO mice. These observations are in contrast to mice lacking ITGβ1 expression that show normal anxiety-like behaviour but impaired LTP. Collectively, our findings highlight distinct roles played by the two integrin subtypes ITGβ3 and ITGβ1 in supporting hippocampal circuit functions.
Materials and Methods
Animals
Animal care and use protocols were approved by the UK Home Office. Integrin β3 KO mice (B6;129S2-Itgb3tm1Hyn/J) were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). The mice had been interbred for at least 10 generations and backcrossed once to C57BL/6 background followed by 16 generations of filial breeding. After purchasing the mice were backcrossed at least once more to C57BL/6 background, and the colony was maintained using a heterozygote × heterozygote mating system. Mice were bred and housed in our animal facility with 12h/12h light dark cycles and ad libitum access to food and water. Mice were genotyped using the following primers: 5′-CCTGCCTGAGGCTGAGTG-3′, 5′-CTTAGACACCTGCTACGGGC-3′, and 5′-CACGAGACTAGTGAGACGTG-3′. Floxed integrin β1 mice (B6;129-Itgb1tm1Efu/J) were purchased from the Jackson Laboratory and CaMKII-cre mice (Camkcre4) were kindly provided by Seth Grant (University of Cambridge). Floxed integrin β1 mice and control littermates were obtained by crossing Cre +ve; β1 fl/+ with +/+; β1 fl/+ or Cre +ve; β1 fl/+ with +/+; β1 fl/fl. Mice were genotyped using the following primers: floxed integrin β1; 5′-CGCAGAACAATAGGTGCTGAAATTAC-3′, 5′-CTGACACTGAGAACCACAAACGGC-3′, CaMKII-cre; 5′-GCGGTCTGGCAGTAAAAACTATC-3′, 5′-GTGAAACAGCATTGCTGTCACTT-3′. Recombination was determined using the following primers: 5′-CGCAGAACAATAGGTGCTGAAATTAC-3′, 5′-CCACAACTTTCCCAGTTAGCTCTC-3′.
Slice electrophysiology
Mice were decapitated, and subsequently the skull was removed from the brain using small scissors by making a midline cut as far as possible in the caudal-rostral direction while submerged in ice cold ACSF containing (in mM): 120 NaCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 3.5 KCl, 1.3 MgCl2, 2.5 CaCl2. The cerebellum and rostral 1/4 - 1/3 portion of the brain were removed with a scalpel. When removing the cerebellum a small angle of 20-30° was introduced (in a dorsal-ventral/caudal-rostral direction), and this cut surface was used to glue the trimmed brain on a pre-cooled vibratome plate. Sections (350 μm; dorsal-ventral direction) were cut using a Pelco Vibratome Series 1000, and maintained in ACSF at room temperature for 1.5-2 h before recording at 30°C (flow rate of 3 ml/min). This method yielded optimal slices for recordings primarily from the mid-ventral regions of the hippocampus. Mice were P9-17 for LTD experiments and P20-35 for LTP experiments, and age-matched animals were used for comparisons between genotypes. Recordings were performed with an Axopatch 200B amplifier (Axon Instruments, USA). Signals were filtered at 2 kHz and digitised at 10 kHz using Clampex (Axon Instruments, USA), and data analysed using Clampfit (Axon Instruments, USA). Synaptic responses were evoked by stimulating Schaffer collaterals with 0.1 ms pulses using concentric bipolar electrodes (FHM). fEPSPs were recorded in the stratum radiatum of the CA1 region using glass microelectrodes filled with ACSF (1-1.5 MΩ). Input-output relations were determined and the stimulation set to elicit a fEPSP slope 50% of maximum (for LTP experiments) or 70% of maximum (for LTD experiments). Baseline measurements were recorded at 0.033 Hz for 20 min before application of drugs or conditioning protocols. LTP was induced by applying a tetanus (4 × 100 Hz for 1 s, 20 s interval) or TBS (consisting of 5 trains which each contained 10 bursts (5 Hz) of 5 pulses (100 Hz)). LTD was induced by applying 900 pulses at 1 Hz or 50 μM 3,5-(S)-DHPG for 20 min. Slices were perfused for 30 min with aCSF containing cilengitide (EMD121974: Merck Sereno, Darmstadt, Germany) before applying conditioning protocols. Cilengitide was perfused throughout the experiment. For some experiments GRGDSP and GRADSP control peptides (Calbiochem, Merck Biosciences LTD., Beeston, UK) were locally perfused via a second micropipette (20-30 μm tip diameter) containing aCSF +1 mg/ml phenol red +/− 500 μM GRGDSP or control peptide and placed within 150 μm of the recording electrode at the same depth using pressure ejection (Picospritzer, General Valve, Fairfield, NJ, USA) throughout the experiment. PPF was determined by averaging 10 measurements (separated by 15 s) for inter-pulse intervals of 300, 200, 100, 50, 20 and 10 ms. All ACSF solutions were saturated with 95% O2 and 5% CO2. For experiments using floxed integrin β1 and CaMKII-Cre mice acute slices were prepared from P78-P129 mice. Kynureic acid (1mM) was included in the cutting solution and for 30 min during recovery. Picrotoxin (100 μM) was included in the recording solution. LTP was induced by applying a tetanus (2 × 100 Hz for 1 s, 20 s interval). LTD was induced by applying 1200 pulses at 2 Hz.
Western blot analysis
Hippocampi were homogenised in cold 4 mM HEPES (pH 7.4) containing 0.32 M sucrose and protease inhibitors (Complete Mini, Roche). Homogenates (H) were cleared at 1000 × g for 10 min, and the supernatant collected and centrifuged at 16,000 × g for 15 min. The pellet referred to as a crude synaptosome fraction (P2), was then resuspended in homogenisation buffer and protein quantified using a BCA protein assay kit (Thermo, Rockford, IL, USA). Samples were boiled at 95°C for 5 min, separated by 7% SDS-PAGE, transferred and membranes probed using the following antibodies: ITGβ3 rabbit polyclonal (Cell Signaling Technology, Danvers, MA, USA #4702), ITGβ1 rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA #SC-8978), ITGαV mouse monoclonal (BD Biosciences, San Jose, CA, USA #611012), ITGβ5 rabbit polyclonal (Cell Signaling Technology #3629), ITGβ8 rabbit polyclonal (Santa Cruz Biotechnology #SC-25714), ITGα5 rabbit polyclonal (Millipore, Billerica, MA, USA #AB1928). HRP-conjugated secondary antibodies were from Sigma (St Louis, MA, USA), and ECL reagent from Thermo. All blots were normalised relative to actin (α-actin mouse monoclonal, Millipore #MAB1501) or tubulin (α-tubulin mouse monoclonal, Sigma #T6199), and quantified by densitometry analysis.
Dissociated hipppcampal culture and mEPSC recordings
All procedures were essentially as described (Cingolani et al., 2008) with the following modifications. In experiments examining the effects of cilengitide on NMDA/glycine induced reduction in mEPSC amplitudes, neurons were plated on coverslips pre-coated with 100 μl of a 1:50 solution of Matrigel in neuronal media (1:50 B27 supplement, 6 mM glutamax, prepared in Neurobasal Medium (Invitrogen, 21103-049)) for 1 h at RT before aspiration leaving a thin, wet film. Cells (15,000) were seeded and placed in 37°C incubator for 6-8 h to settle before addition of 500 μl of neuronal media. At DIV5 200 μl of media was exchanged for fresh neuronal media containing AraC to give a final concentration of 2 μM. Hippocampal cultures were used between DIV10-DIV12. The recording chamber was continuously perfused with an aCSF containing (in mM) 130 NaCl, 2.5 KCl, 2.2 CaCl2, 1.5 MgCl2, 10 D-glucose, 10 HEPES, 0.1 picrotoxin (pH 7.35, osmolarity adjusted to 290 mOsm). 0.5mM TTX was added to block sodium channels when recording mEPSCs. The intracellular solution contained (in mM) 100 Kgluconate, 17 KCl, 5 NaCl, 5 MgCl2, 10 HEPES, 0.5 EGTA, 4 ATPK2, 0.5 GTPNa (pH 7.3, osmolarity adjusted to 280 mOsm). Recorded neurons were held under voltage clamp at −70 mV and series resistance was left uncompensated. Pipette resistance was 3-5 MΩ and only cells with stable resting potential < −50mV and series resistance <20 MΩ were analysed. mEPSCs were filtered at 2kHz and sampled at 10kHz using the pClamp software (Axon Instruments). Chemical LTD (chem-LTD) was elicited by co-applying 20 μM of both NMDA and glycine in MgCl2-free aCSF for 3 min.
Elevated Plus Maze
The elevated-plus maze test was performed as previously described (Pawlak et al., 2003). The apparatus consisted of four non-transparent white Plexiglas arms: two enclosed arms (50×10×30 cm) that formed a cross shape with the two open arms (50×10 cm) opposite each other. The maze was 55 cm above the floor and dimly illuminated. Mice were placed individually on the central platform, facing an open arm, and allowed to explore the apparatus for 5 min. Behaviour was recorded by an overhead camera. The number of entries of the animal from the central platform (10×10 cm) to closed or open arms was counted. The maze was cleaned with 70% alcohol after each session to avoid any odorant cues.
Fear Conditioning
Mice were individually placed in the conditioning chamber (Coulbourn Instruments) for 2 min before they received three conditioned stimulus-unconditioned stimulus (CS-US) pairings. The last 2 s of the tone (CS: 30 s, 2.8 kH, 85 dB) were paired with the footshock (US: 2 s, 0.4 mA) delivered through a grid floor. After training was completed mice remained in the conditioning chamber for one more minute and were then moved to their home cage. The next day the mice were placed back in the training chamber and freezing was monitored for 3 min to assess context-dependent learning. Cued-conditioning was evaluated 48 h after training. The mouse was placed in a novel context (chamber with flat plastic floor and walls) for 2 min, after which the CS was delivered (2 min, 2.8 kHz, 85 dB) and freezing was monitored. Data were analysed using FreezeView software (Coulbourn Instruments).
Pain threshold
Mice were subjected to a series of mild footshocks of increasing intensities (in 0.05 mA increments) and behavioural reaction was measured as previously described (Bourtchuladze et al., 1994). The lowest current intensity to elicit flinching, jumping and vocalisation was determined.
Open field testing
Mice were placed in a 50 × 50 × 50 cm plexiglass box and were left free to move for 5 min. The box was cleaned with 70% alcohol after each session to avoid any odorant cues. An overhead camera placed above the box recorded the session. Locomotor parameters were analysed with the ANY-MAZE software (Stoelting).
Lentivirus production
Full length human ITGβ3-GFP and GFP alone were cloned into the lentiviral vector FUW. To produce viral vectors, these constructs were transiently transfected into HEK293T cells along with VSV-G and Δ8.9 constructs using calcium phosphate. The supernatant was collected 36-48 h after transfection, cleared, passed through 0.45 μm filters and concentrated by ultracentrifugation (110,000 × g for 90 min at 4°C). The viral pellet was resuspended in PBS and the transduction unit (TU) titre was determined on dissociated hippocampal neurons.
Surgery/lentivirus injection
ITGβ3 KO mice were intraperitoneally anaesthetised with ketamine/xylazine (100 and 10 mg/kg, respectively) and placed in a stereotaxic apparatus. 0.7 μl of the lentivirus encoding ITGβ3-GFP or GFP was injected into the ventral hippocampus at point 3.0 mm posterior from bregma, 3.0 mm lateral from the midline and 3 mm ventral at 200 nl/min using the Nanofil syringe with a 33G needle through UMP-3.1 micropump (all from World Precision Instruments, USA) mounted on Stoelting stereotaxic frame. After 5 min the needle was lowered to 4 mm ventral and additional 0.7 μl of the virus injected. The needle remained in place for another 5 min to prevent the backflow, slowly removed and the skin closed with Vetbond (3M, USA). The virus was injected bilaterally. After two-weeks of recovery the animals were behaviourally tested. Hippocampi were then dissected to determine ITGβ3 expression levels.
RT-PCR
Mice were anaesthetised with an intraperitoneal injection of 50 mg/kg sodium pentobarbital and perfused transcardially with ice cold PBS. Hippocampi were dissected from a coronal slice −2.7 to −3.5 mm relative to Bregma and stored in “RNA later” (QIAgen) at 4°C. RNA was extracted using QIAzol lysis reagent (QIAgen) and Mini Spin Columns according to the manufacturers’ instructions (RNeasy Lipid tissue mini kit, QIAgen). RNA (2 μg) was converted to cDNA using Superscript III (Invitrogen) and oligo (dT) primers according to manufacturer’s instructions. PCR was carried out using nested primers: external primers; 5′- TGGGGGCGCTGGCGGGCGTTG-3′ and 5′-GTTAGCGTCAGCACGTGTTTGTAGCC-3′, internal primers; 5′-TGTGTGCCTGGTGCTCWGATGAG-3′ and 5′-TAATCCTCCACCTGCCGCACTTG-3′.
Statistical analyses
All data were analysed using Prism (Graphpad software Inc., USA). LTP/LTD data were analysed with two-way repeated-measures ANOVA with treatment/genotype as the between subject factor and time as the within-subject factor. The LTP/LTD data were analysed for the whole period recorded after conditioning, as well as specified time windows where indicated. mEPSC and behavioural data were analysed with either unpaired or paired t test as indicated.
Results
β3 integrin KO mice exhibit abnormal anxiety-like behaviour
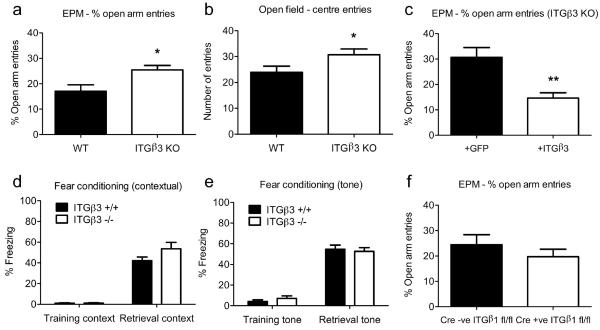
To begin to explore the physiological function for ITGβ3, we focused on fear-related behaviours and made use of mice deficient for ITGβ3. ITGβ3 KO mice were originally developed as a mouse model for a human disorder Glanzmann thrombasthenia based on the implicated roles for platelet ITGβ3 in hemostasis and thrombosis (Hodivala-Dilke et al., 1999). Despite some tendency to bleed, ITGβ3 KO mice were viable, bred normally, and showed no overt changes in overall brain development and morphology (Hodivala-Dilke et al., 1999; Cingolani et al., 2008; data not shown). We first tested the mice for unconditioned fear or anxiety-like behaviour using the elevated-plus maze (EPM; Walf and Frye, 2007). Interestingly, ITGβ3 KO mice entered the open arm significantly more frequently compared to wild type mice (expressed as % of total arm entries, ITGβ3 +/+: 17.1 ± 2.5 %, n = 16; ITGβ3 −/−: 25.5 ± 1.7 %, n = 12; P=0.0176; Fig. 1a, see also Fig. S1a). Such a reduced bias for avoidance of height and open spaces suggested of a possible decrease in anxiety level upon loss of ITGβ3 expression. The number of entries into closed arms (ITGβ3 +/+: 9.6 ± 0.96, n = 16; ITGβ3 −/−: 10.6 ± 1.1, n = 12; P=0.51; Fig. S1b) and total arm entries (ITGβ3 +/+: 11.8 ± 1.2, n = 16; ITGβ3 −/−: 14.2 ± 1.3, n = 12; P=0.20; Fig. S1c) were however not different from wild type control mice, indicating that general locomotor activity was not altered by the loss of ITGβ3. To test the effects of ITGβ3 loss in another behavioural measure of anxiety, we subjected wild type and ITGβ3 KO mice to the open field test. In agreement with reduced anxiety-like behaviour in the EPM test, ITGβ3 KO mice showed an increase in the number of centre field entries compared to wild type mice (ITGβ3 +/+: 23.9 ± 2.3, n = 13; ITGβ3 −/−: 30.7 ± 2.2, n = 11; P=0.049; Fig. 1b). We did not observe any significant difference between wild type and ITGβ3 KO mice in either time spent in the centre (ITGβ3 +/+: 40.7 ± 6.8, n = 13; ITGβ3 −/−: 36.3 ± 2.9, n = 11; P=0.58; Fig. S1d) or periphery (ITGβ3 +/+: 259 ± 6.8, n = 13; ITGβ3 −/−: 263.7 ± 2.9, n = 11; P=0.56; Fig. S1e).
Figure 1.
(a) ITGβ3 KO mice show decreased levels of anxiety-like behaviour, as judged by the % of entries into open arms of the elevated-plus maze, * unpaired t test, P = 0.0176, and by the number of centre entries in the open field test (b), * unpaired t test, P = 0.0493. (c) Expression of ITGβ3 in the ventral hippocampus is able to rescue the anxiolytic phenotype in ITGβ3 KO mice, ** unpaired t test, P = 0.0063. (d-e) Fear conditioning (both tone and contextual) are normal. (f) ITGβ1 KO mice show no difference in the % of entries into open arms of the elevated-plus maze, unpaired t test, P = 0.358. Error bars are ±s.e.m.
Next we asked whether the loss of ITGβ3 also compromised conditioned fear responses. To this end, wild type and ITGβ3 KO mice were assessed in a paradigm in which animals were trained with tone-footshock pairings in a closed chamber. Both wild type and ITGβ3 KO mice exhibited comparable pain threshold (Fig. S2). Context-dependent memory was measured by freezing of animals when placed in the same chamber where the training took place. This hippocampus and amygdala-dependent fear memory was not different between wild type and ITGβ3 KO mice (ITGβ3 +/+: 42.2 ± 3.5, n = 16; ITGβ3 −/−: 53.6 ± 6.1, n = 12; P=0.098; Fig. 1d). Cue-dependent fear memory was measured by placing mice in a different cage and monitoring their freezing upon presenting the tone that was previously coupled to the footshock. This amygdala-dependent fear memory was also not significantly affected upon loss of ITGβ3 (ITGβ3 +/+: 54.7 ± 4.0, n = 16; ITGβ3 −/−: 52.6 ± 3.6, n = 12; P=0.72; Fig. 1e). Therefore, the loss of ITGβ3 appears to selectively compromise unconditioned over conditioned fear responses.
In order to test the specificity of the apparently altered anxiety behaviour to ITGβ3 and to address the possibility that the observed behavioural change could have resulted from developmental effects of ITGβ3 deficiency, we carried out a rescue experiment in adult ITGβ3 KO mice. We also confined the area of exogenous expression of ITGβ3 to help define the brain region where ITGβ3 potentially played a role in modulating anxiety-like behaviour. Lentivirus encoding either ITGβ3-GFP or GFP was bilaterally injected into the ventral hippocampus, a region implicated in innate fear reactions (McEown and Treit, 2009, 2010; Adhikari et al., 2010, 2011). RT-PCR of isolated hippocampal tissue confirmed the exogenous ITGβ3 expression in KO mice injected with the ITGβ3-GFP lentivirus but not the control GFP lentivirus (Fig. S3). When mice were tested for anxiolytic behaviour using the EPM test, ITGβ3 KO mice injected with ITGβ3-GFP lentivirus showed a decrease in the percentage number of open arm entries compared to KO animals injected with control GFP lentivirus (Fig. 1c: +ITGβ3: 14.6 ± 0.2 %, n = 5; +GFP: 30.7 ± 3.8 %, n = 5; P=0.0063; see also Fig. S1f). Importantly, the relative number of open arm entries of rescued ITGβ3 KO mice was comparable to that of wild type control mice (Fig. 1a). Closed arm and total arm entries were not different between ITGβ3 KO mice injected with ITGβ3-GFP lentivirus and control mice (Fig. S1 g,h, closed arm entries: +ITGβ3: 12.0 ± 1.9, n = 5; +GFP: 10.4 ± 1.5, n = 5; P=0.52; total arm entries: +ITGβ3: 14.0 ± 2.0, n = 5; +GFP: 14.8 ± 1.7, n = 5; P=0.76). ITGβ3 expression in ventral hippocampus is therefore apparently necessary and sufficient for mice to display normal level of anxiety in the EPM test.
In order to determine if another β integrin subtype could also be involved in controlling anxiety, we assessed the behaviour of mice lacking ITGβ1 expression in the EPM test. Because of the early embryonic lethality of ITGβ1 KO mice (Stephens et al., 1995), conditional ITGβ1 KO mice were obtained by crossing mice carrying a homozygous floxed allele of ITGβ1 with a mouse line that expressed Cre under a CaMKII promoter (Mantamadiotis et al., 2002). PCR analysis of DNA isolated from ventral hippocampal slices confirmed recombination of the ITGβ1 sequence in Cre +ve; ITGβ1 fl/fl mice compared to controls (Fig. S4). In contrast to ITGβ3 KO mice, mice lacking ITGβ1 expression in the forebrain entered the open arm at a frequency comparable to that of control mice (Fig. 1f, % of total entries: Cre −ve, ITGβ1 fl/fl: 24.5 ± 3.9 %, n = 6; Cre +ve, ITGβ1 fl/fl: 19.7 ± 2.9 %, n = 6; P=0.358; see also Fig. S1i). Closed arm and total arm entries were also not different between ITGβ1 KO and control mice (Fig. S1 j-k, closed arm entries: Cre −ve, ITGβ1 fl/fl: 9.2 ± 1.1, n = 6; Cre +ve, ITGβ1 fl/fl: 9.8 ± 0.91, n = 6; P=0.65; total arm entries: Cre −ve, ITGβ1 fl/fl: 12.0 ± 1.1, n = 6; Cre +ve, ITGβ1 fl/fl: 12.3 ± 1.2, n = 6; P=0.84). Therefore, unlike ITGβ3, ITGβ1 does not appear to be required for anxiety related behaviour as monitored by the EPM test.
RGD peptides suggest differential involvement for ITGβ3 and ITGβ1 in Hebbian plasticity
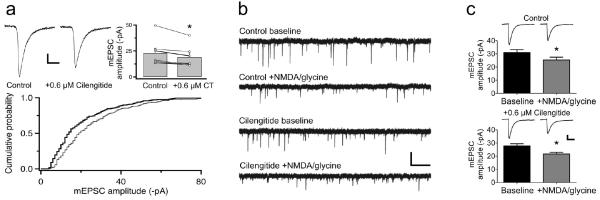
Our observations thus far suggest the requirement for ITGβ3 but not ITGβ1 in anxiety related behaviour, and furthermore, raise the possibility that the underlying mechanism might involve ITGβ3-dependent components of hippocampal circuit function. We therefore examined the potential contribution of ITGβ3 in two highly studied forms of synaptic plasticity that are robustly expressed in the hippocampus: LTP and LTD. We first used a pharmacological approach to disrupt integrin-ECM interactions with RGD peptides. Amongst a variety of RGD peptides available, echistatin and cilengitide show specificity for ITGβ3 in that the former preferentially targets ITGβ1/β3-ECM interactions (Pfaff et al., 1994) whereas the latter targets ITGβ3/β5-ECM interactions (Dechantsreiter et al., 1999; Nisato et al., 2003). To help discriminate between the effects of interfering with ITGβ3 from that of blocking ITGβ1, whose role in LTP is well established, we have mostly used cilengitide in our experiments. The potency of cilengitide on ITGβ3 activity was first confirmed by testing its ability to inhibit ITGβ3-dependent stabilization of synaptic AMPARs in cultured hippocampal neurons under basal conditions (Cingolani et al., 2008). Bath application of cilengitide (0.6 μM) decreased miniature excitatory postsynaptic current (mEPSC) amplitudes (15.2 ± 3.4%; Fig. 2a) as had been found for echistatin (Cingolani et al., 2008). This suggested that cilengitide could be effectively used to block ITGβ3-dependent activity. We then tested a role for ITGβ3 in Hebbian plasticity in cultured hippocampal neurons by eliciting chemical LTD (chem-LTD) of mEPSC amplitudes by co-applying NMDA and glycine (Lu et al., 2001). In the absence of cilengitide, chem-LTD induction reduced the mean mEPSC amplitude relative to baseline by 18.1% (before NMDA/glycine = −31.0 pA ± 2.2, after = −25.4 pA ± 2.1; Fig. 2b-c). In the presence of cilengitide, chem-LTD induction also produced a comparable extent of decrease in mean mEPSC amplitude (22.0%: before NMDA/glycine = −27.8 pA ± 1.6, after = −21.7 pA ± 1.2; Fig. 2b-c). Thus, chem-LTD is not dependent on ITGβ3 or ITGβ5-ECM interactions.
Figure 2.
Cilengitide does not affect chemical-LTD. (a) Disrupting ITGβ3/β5-ECM interactions in dissociated hippocampal neurons by applying cilengitide (0.6 μM), reduces mEPSC amplitudes. Average traces (top left) and a cumulative probability plot (bottom: control, thin line; cilengitide, thick line) from a representative cell. Summary of all recordings [top right: control (n = 7); cilengitide (n = 7), paired t test, P = 0.02]. (b-c) Cilengitide (0.6 μM) does not affect chem-LTD of mEPSC amplitudes in dissociated hippocampal neurons [control (n = 6), paired t test, P = 0.0061; +cilengitide (n = 6), P = 0.0041]. Scale bars: (a) = 5 ms, 5 pA; (b) = 1 s, 50 pA; (c) = 5 ms, 20 pA. Error bars are ±s.e.m.
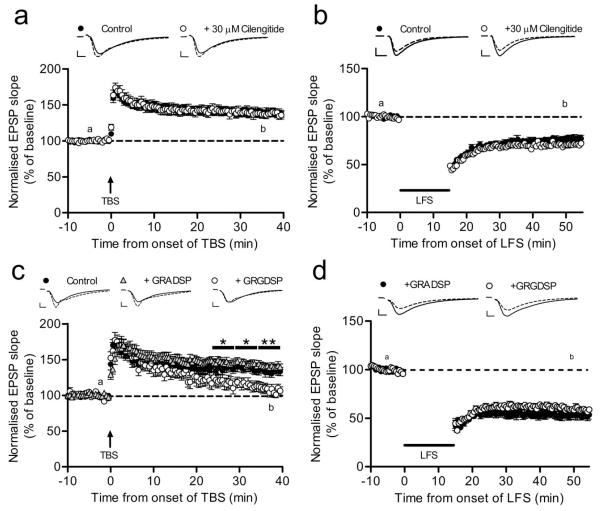
We next tested in acute hippocampal slices whether cilengitide-sensitive integrin interactions are involved in NMDAR-dependent LTP and LTD (Fig. S5). The method of hippocampal slice preparation we used yielded optimal slices for recordings mostly from mid- ventral sections. LTP induction produced a robust increase in field EPSP responses both in control and in the presence of cilengitide to similar extents, while LTD could also be elicited to comparable extents in control and cilengitide treated slices (Fig. 3a-b). Altogether, the extent changes in mean synaptic responses relative to baseline upon LTP or LTD induction in the presence of cilengitide were not significantly different from control conditions for two different concentrations of cilengitide tested: 0.6 μM (two-way repeated-measures ANOVA; LTP: F1,6 = 1.31; P=0.296; LTD: F1,10 = 0.39; P=0.547; Fig. S6) and 30 μM (two-way repeated-measures ANOVA LTP: F1,19 = 2.4; P=0.138; LTD: F1,22 = 2.47; P=0.130; Fig. 3a-b). In contrast, when LTP was induced in the presence of the short synthetic peptide containing the RGD sequence, GRGDSP (500 μM), although the early potentiation was spared, synaptic responses gradually declined to the baseline level as previously reported (Staubli et al., 1998; Fig. 3c). Given that GRGDSP would block ITGβ1-ligand interactions, the observed decline in potentiation was consistent with the reported role for ITGβ1 in LTP maintenance (Chan et al., 2006; Huang et al., 2006). When LTD induction protocol was applied in the presence of GRGDSP, LTD appeared normal (two-way repeated-measures ANOVA; F1,22 = 0.35; P=0.56; Fig. 3d). Altogether, these observations indicate that integrin- dependence of LTP does not involve ITGβ3 or ITGβ5 subtypes, and that the RGD-sensitive integrin- ECM interactions, including those engaging ITGβ1, are required for LTP but dispensable for the induction and maintenance of LTD.
Figure 3.
Cilengitide does not affect LTP or LTD whereas synthetic RGD peptide affects LTP stabilization. (a, b) Cilengitide (30 μM) has no effect on the induction and maintenance of TBS-induced LTP or LFS-induced LTD in acute hippocampal slices [LTP: cilengitide (n = 6, 11) vs. control (n = 6, 11); LTD: cilengitide (n = 5, 14) vs. control (n = 5, 12)]. (c) Disrupting RGD-sensitive integrin-ECM interactions in acute hippocampal slices by 500 μM GRGDSP slowly reverses potentiation to baseline [control (n = 7, 10), GRADSP (n = 4, 12), GRGDSP (n = 7, 8), two-way repeated-measures ANOVA; (control vs GRGDSP): t25-29.5: F1,16 = 4.87; P = 0.0423; t30-34.5: F1,16 = 7.73; P = 0.0134; t35-39.5: F1,16 = 9.95; P = 0.0061]. (d) GRGDSP (500 μM) has no effect on LTD in acute hippocampal slices [GRADSP (n = 8, 12), GRGDSP (n = 8, 12)]. Representative traces are from time points “a” (solid) and “b” (dashed). Scale bars: (a-d) = 5 ms, 0.5 mV. Error bars are ±s.e.m.
β3 integrin KO mice display normal LTP and LTD
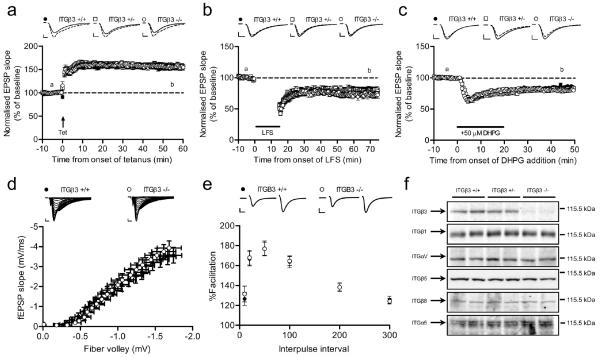
To complement the RGD peptide experiments that supported a lack of involvement for ITGβ3 in Hebbian plasticity, we compared LTP and LTD in acute hippocampal slices from ITGβ3 KO, heterozygote mutant and control wild type mice, again using a method of slice preparation that mostly yielded sections from mid to ventral hippocampus. LTP was robustly induced and maintained in ITGβ3 KO and heterozygote slices, whose time course and the extent increase of synaptic responses were not significantly different from LTP elicited in control slices (two-way repeated-measures ANOVA; ITGβ3 −/−: F1,36 = 1.25; P=0.271; ITGβ3 +/−: F1,38 = 0.34; P=0.564; Fig. 4a). Moreover, two different forms of LTD expressed at CA3-CA1 hippocampal synapses, NMDAR-LTD and mGluR-LTD that both involved AMPAR endocytosis (Citri and Malenka, 2008), remained unchanged in ITGβ3 KO and heterozygote neurons compared to controls (two-way repeated-measures ANOVA; ITGβ3 −/− NMDAR-LTD: F1,22 = 2.38; P=0.137; ITGβ3 +/− NMDAR-LTD: F1,22 = 3.61; P=0.071; ITGβ3 −/− mGluR-LTD: F1,28 = 3.71; P=0.064; ITGβ3 +/− mGluR-LTD: F1,28 = 0.1; P=0.751; Fig. 4b,c). We also monitored basal synaptic transmission, which appeared unaltered in ITGβ3 KO slices compared to controls as supported by the lack of change in the input-output curve of fEPSP slope as function of the presynaptic fibre volley amplitude across a range of stimulation intensities tested (Fig. 4d). Paired-pulse facilitation was also unchanged in ITGβ3 KO slices at all inter-pulse intervals examined, suggesting a lack of change in presynaptic release probability (Zucker and Regehr, 2002) upon loss of ITGβ3 (Fig. 4e). In order to determine whether these observed lack of change in synaptic properties were due to functional redundancy by the other integrin family members, particularly of RGD receptor subtypes that share the same integrin αV subunit as β3 integrin (Hynes, 2002), we compared protein expression levels of such related family members in synaptosome enriched fractions from ITGβ3 KO versus wild type mice brains. Western blots revealed no overt upregulation of ITGβ1, ITGαV, ITGβ5, ITGβ8 and ITGα5 in P2 synaptosome samples from KO and heterozygote mice compared to those from wild type mice (Fig. 4f). Together, these results indicate that distinct from the previously reported requirement for ITGβ3 in hippocampal neurons for modulating synaptic AMPARs during homeostatic synaptic scaling, ITGβ3 is not required for expressing hippocampal LTP or LTD.
Figure 4.
Loss of ITGβ3 does not affect LTP or LTD. (a-c) Tetanus-induced LTP, LFS-induced LTD or mGluR-LTD induced by 3,5-(S)-DHPG are not changed in acute hippocampal slices from ITGβ3 KO mice [LTP: +/+ (n = 6, 20), +/− (n = 7, 20), −/− (n = 6, 18); LFS-LTD: +/+ (n = 7, 14), +/− (n = 5, 10), −/− (n = 7, 10); mGluR-LTD: +/+ (n = 7, 16), +/− (n = 5, 14), −/− (n = 6, 14)]. Representative traces are from time points “a” (solid) and “b” (dashed). (d) I/O curve is normal in ITGβ3 KO slices (n = 3, 25) compared to wild type littermate slices (n = 3, 20). (e) PPF (slope 2/slope 1) is normal in ITGβ3 KO slices (n = 13, 3) compared to wild type controls (n = 13, 3). Inter-pulse intervals are 10 ms, 20 ms, 50 ms, 100 ms, 200 ms and 300 ms. Example traces are from 50 ms inter-pulse interval. Scale bars = 5 ms, 0.5 mV. (f) Loss of ITGβ3 does not affect expression of other integrin subunits. Western blot analysis of P2 synaptosome samples prepared from ITGβ3 +/+, ITGβ3 +/− and ITGβ3 KO mice. The expected size and position of indicated integrin subunits are shown at right. Equal loading was confirmed by blotting for actin on the same membrane.
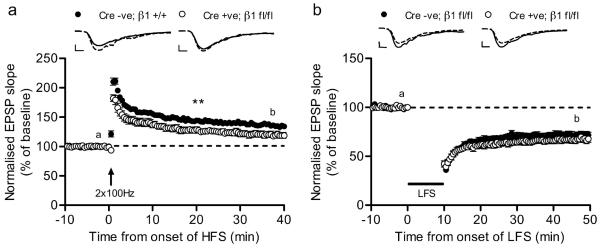
Finally, we sought to confirm the apparent lack of requirement for ITGβ1 in LTD that was based on RGD peptide experiments by using conditional ITGβ1 KO mice (see above). As reported previously, LTP induction produced a smaller LTP in mice lacking ITGβ1 where the extent potentiation of EPSP slope was 119 ± 5 % compared with 135 ± 4% in wild type controls (two-way repeated-measures ANOVA; F1,20 = 10.13; P=0.005; Fig. 5a). In contrast, LTD induction produced a robust depression of synaptic responses that were comparable in both ITGβ1 KO and control mice (two-way repeated-measures ANOVA; F1,19 = 1.18; P=0.29; Fig. 5b). Therefore, consistent with RGD peptide experiments, molecularly interfering with ITGβ1 expression also supports a specific role for ITGβ1 in LTP but not LTD.
Figure 5.
(a) LTP is specifically impaired in ITGβ1 knockout mice [control (n = 5, 11), Cre +ve; β1 fl/fl (n = 5, 11)], whereas LTD is unaffected (b) [control (n = 5, 10), Cre +ve; β1 fl/fl (n = 5, 11)]. Representative traces from time frames “a” (solid) and “b” (dashed) shown above. Scale bars = 5 ms, 0.5 mV. Error bars are ±s.e.m.
Discussion
In this study we have identified a potentially novel function for ITGβ3 in controlling anxiety-related responses in mice. Importantly, the anxiolytic phenotype of ITGβ3 KO mice in the EPM test could be rescued in adult animals by exogenously expressing ITGβ3 in the ventral hippocampus. A recent study using an optogenetic approach has indicated a major role of amygdala circuits in controlling anxiety-related behaviour (Tye et al., 2011), although previous work has also implicated the ventral hippocampus in controlling innate fear (McEown and Treit, 2009, 2010; Adhikari et al., 2010, 2011). The sufficiency of ITGβ3 expression in the ventral hippocampus for restoring anxiety-like behaviour could suggest of a more critical role played by the ventral hippocampal circuits in controlling anxiety-like behaviour or it could represent a higher demand for the level of expression of ITGβ3 or its activity in the hippocampus compared to amygdala for the circuitry underlying anxiety-like behaviour. Our finding differs from a recent study reporting of a lack of change in EPM test in ITGβ3 KO mice that used animals that have been extensively backcrossed (Carter et al., 2011). Despite the same origin of the mice, the differences in the number of generations backcrossed could have contributed to the discrepancy, and in such a case, the basis for the reversal of the anxiolytic phenotype we have observed by lentivirus-mediated expression of ITGβ3 warrants further investigation, for instance, by testing the effects of overexpressing ITGβ3 in wild type mice. Interestingly, the study by Carter et al. (2011) finds a decreased preference for social novelty accompanied by an increased repetitive behaviour in a novel environment in ITGβ3 KO mice. Whether these phenotypes, which are commonly associated with some symptoms of autism (Carter et al., 2011), are also found in our colony that exhibits altered anxiety-like behaviour, will be of interest to examine.
In order to gain insights into how hippocampal circuits that potentially modulate anxiety- like behaviour might depend on ITGβ3 function, we determined the requirement for ITGβ3 on LTP and LTD, two forms of Hebbian synaptic plasticity robustly expressed in hippocampus. However, we found little evidence for the involvement of ITGβ3 in Hebbian plasticity. Interestingly, we have previously demonstrated a role for ITGβ3 in controlling synaptic AMPARs during homeostatic synaptic plasticity in hippocampal neurons (Cingolani and Goda, 2008; Cingolani et al., 2008). This raises an intriguing possibility implicating homeostatic tuning of synaptic strengths in modulating circuits that underlie anxiety-like behaviour. We acknowledge that the loss of ITGβ3 per se does not produce overt effects on synaptic transmission in acute hippocampal slices, a preparation in which homeostatic synaptic scaling has not been extensively studied to date. We have previously found that under basal conditions the mean quantal size in ITGβ3 KO slice cultures is larger compared to that of age-matched ITGβ3 heterozygous control slice cultures (Cingolani and Goda, 2008), a difference, which is not apparent in dissociated cultures (Cingolani et al., 2008). Therefore, the observed difference in mean basal postsynaptic strength in slice cultures suggests for engagement of some compensatory mechanism upon loss of ITGβ3 in a preparation that better preserves the native hippocampal circuitry. How such an alteration in basal quantal size, if it also extends to acute hippocampal slices, relates to ITGβ3 function in controlling AMPARs during synaptic scaling and deficient anxiety-like behaviour, remains to be established.
An apparent lack of requirement for ITGβ3 in LTP contrasts with other integrin subtypes such as α3, α5, α8, and β1 that are involved in LTP (McGeachie et al., 2011). Therefore, unlike many molecules that participate in both Hebbian and homeostatic forms of synaptic plasticity (Turrigiano, 2008; Pozo and Goda, 2010), the present findings point to a selective function for ITGβ3 in homeostatic processes. Such a selective role for ITGβ3 in controlling the compensatory regulation of synaptic strength is similar to TNFα (Stellwagen and Malenka, 2006). Notably, TNFα modulates cell surface levels of ITGβ3 (Cingolani et al., 2008), and therefore, the two molecules could be involved in the same signalling pathway for regulating homeostatic synaptic scaling. A mechanism allowing independent control of synaptic scaling versus LTP or LTD expression could enable individual synapses to express two opposing forms of plasticity simultaneously, for example, by targeting different subpopulation of synaptic AMPARs.
Our study also confirmed a role for ITGβ1 in LTP (Chan et al., 2006; Huang et al., 2006; Kramar et al., 2006), unlike for ITGβ3. Whether ITGβ1 also contributes to homeostatic synaptic scaling of AMPARs or is specifically required for LTP remains to be tested. Nevertheless, we have previously found in hippocampal neurons that overexpressing an ITGβ3 mutant, which is unable to form links with the ECM, decreases synaptic AMPARs whereas a homologous ITGβ1 mutant that is also unable to bind to the ECM, has no effect on synaptic AMPARs (Cingolani et al., 2008). Furthermore, bidirectional homeostatic synaptic scaling of AMPARs accompanies a corresponding bidirectional change in the level of surface ITGβ3 but not of surface ITGβ1 (Cingolani et al., 2008). These observations suggest that ITGβ1 may not be involved in homeostatic synaptic scaling, at least not in a manner by which ITGβ3 regulates homeostatic synaptic plasticity. Moreover, at a behavioural level, ITGβ1 and ITGβ3 subtypes are also differentially required for controlling anxiety- like behaviour such that mice lacking ITGβ1 show a normal level of anxiety-like behaviour in contrast to ITGβ3 KO mice in the EPM test, albeit further work is needed following additional backcrossing of ITGβ3 KO mice. Altogether, clarifying how each integrin subtype affects the activity of synaptic circuits could help untangle the physiological basis of integrin-dependent behaviours.
Supplementary Material
Acknowledgements
We thank David Elliott and Satyam Patel for expert technical assistance and Charles Yokoyama for helpful comments on an earlier version of the manuscript. This work was supported by the Medical Research Council and the European Union Seventh Framework Program under grant agreement no. HEALTH-F2-2009-241498 (“EUROSPIN” project) (YG), and the Marie Curie Excellence Grant (MEXT-CT-2006-042265 from the European Commission) (RP). Cilengitide was a generous gift from Merck Serono (Darmstadt, Germany), which reviewed the contents of this work. The views and opinions described herein do not necessarily reflect those of Merck Serono.
Footnotes
The authors declare no conflict of interest.
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during activity. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron. 2011;71:898–910. doi: 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, Veenstra-VanderWeele J. Absence of preference for social novelty and increased grooming in integrin β3 knockout mice: initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 2006;26:223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Differential involvement of beta3 integrin in pre- and postsynaptic forms of adaptation to chronic activity deprivation. Neuron Glia Biol. 2008;4:179–187. doi: 10.1017/S1740925X0999024X. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, Goodman SL, Kessler H. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. The role of the dorsal and ventral hippocampus in fear and memory of a shock-probe experience. Brain Res. 2009;1251:185–194. doi: 10.1016/j.brainres.2008.11.041. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. Inactivation of the dorsal or ventral hippocampus with muscimol differentially affects fear and memory. Brain Res. 2010;1353:145–151. doi: 10.1016/j.brainres.2010.07.030. [DOI] [PubMed] [Google Scholar]
- McGeachie AB, Cingolani LA, Goda Y. A stabilising influence: Integrins in regulation of synaptic plasticity. Neurosci Res. 2011;70:24–29. doi: 10.1016/j.neures.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Pfaff M, McLane MA, Beviglia L, Niewiarowski S, Timpl R. Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins alpha IIb beta 3, alpha V beta 3 and alpha 5 beta 1 and in cell adhesion inhibition. Cell Adhes Commun. 1994;2:491–501. doi: 10.3109/15419069409014213. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.