Abstract
Rationale: The natural history of lung function in patients with bronchiolitis obliterans syndrome (BOS) after allogeneic hematopoietic cell transplant is poorly characterized. Understanding the trajectory of lung function is necessary for prompt clinical recognition and treatment and also for the rational design of prospective studies.
Objectives: To describe the longitudinal trajectory of lung function parameters, including FEV1, in patients with BOS after hematopoietic cell transplant.
Methods: Subjects with BOS defined by National Institutes of Health consensus guidelines criteria from a recent multicenter prospective trial of combination treatment with fluticasone, azithromycin and montelukast and a retrospective cohort from Fred Hutchinson Cancer Research Center were included. Longitudinal change in FEV1 for each patient was calculated on the basis of available pulmonary function tests in three periods: pre-BOS, from BOS diagnosis to 6 months, and 6–18 months after diagnosis. The effect of treatment on FEV1 trajectory was analyzed by univariate and multivariate linear regression. The Kaplan-Meier method was used to estimate survival.
Measurements and Main Results: The FEV1 percent predicted value at diagnosis was 46% (interquartile range, 35–57%) for trial participants and 53% (interquartile range, 41–64%) for the retrospective cohort. There was a concomitant mild reduction in FVC, as well as a marked reduction in forced expiratory flow, midexpiratory phase, at diagnosis. While there was individual heterogeneity, the overall FEV1 trajectory was characterized by a marked decline within 6 months prior to BOS diagnosis, followed by stability of FEV1 early after diagnosis and a slow rate of decline beyond 6 months. The effect of the trial medications on FEV1 trajectory after BOS diagnosis was a mean rate of change of 0.92% predicted per month (95% confidence interval, −0.53 to 2.37) compared with the retrospective cohort, but this was not statistically significant. Two-year overall survival rates were 76% and 72% for the study participants and the retrospective cohort patients, respectively. Earlier time to diagnosis after hematopoietic cell transplant and severity of FVC at diagnosis were significantly associated with reduced survival.
Conclusions: The FEV1 trajectory in patients with BOS after hematopoietic cell transplant in a contemporary era of management follows a predominant pattern of rapid FEV1 decline in the 6 months prior to diagnosis, followed by FEV1 stabilization after diagnosis.
Keywords: bronchiolitis obliterans syndrome, hematopoietic cell transplantation, pulmonary complications, FEV1 trajectory, outcomes
Bronchiolitis obliterans syndrome (BOS) is a devastating complication of allogeneic hematopoietic cell transplant characterized by new-onset fixed airflow obstruction, first recognized over 40 years ago in association with chronic graft-versus-host disease (GVHD) (1). BOS was also recognized in recipients of lung transplants (2), suggesting an alloimmune mechanism for the development of the common pathologic lesion of obliterative bronchiolitis (3). In lung transplantation, the trajectory of lung function decline after BOS diagnosis is variable and is influenced by sex, type of allograft, and underlying transplant indication (4). Although the pathologic lesion is histologically identical, the natural history of the disease for allogeneic hematopoietic cell transplant recipients may not be the same as lung transplant recipients.
While progress has been made in standardizing the diagnosis of BOS after allogeneic hematopoietic cell transplant through spirometric criteria (5) and clinical features, there remains a paucity of information on the clinical course and factors that influence lung function. BOS is usually recognized after the onset of clinical symptoms, at which point the risk of nonrelapse mortality has already increased (6). Routine pulmonary function screening has been recommended (7), but it has not been widely adopted. An understanding of the course of BOS over time is critical for disease recognition, clinical management, and rational design of prospective trials. The prevalence of BOS is 5.5% in all allogeneic hematopoietic cell transplant recipients and 14% in those with chronic GVHD (8), and it is likely to increase with improved survivorship (9), an increasing number of hematopoietic cell transplants performed every year, and the concomitant prevalence of chronic GVHD (10).
The primary aim of this study was to describe the longitudinal trajectory of FEV1 and other spirometric parameters of patients with BOS in a contemporary era of allogeneic hematopoietic cell transplantation. Additional aims were to determine if combination treatment with fluticasone, azithromycin, and montelukast (FAM) influences FEV1 trajectory and if lung function at diagnosis is associated with outcomes. We used data derived from a recent prospective study of FAM for newly diagnosed BOS (11) with a previously described cohort that was treated per clinical preference that did not include FAM (8). FAM treatment was associated with stable lung function, reduced systemic corticosteroids, and improved quality of life at 3 months (11).
Methods
Study Cohort
Subjects were required to have a diagnosis of BOS defined by spirometric criteria according to modified National Institutes of Health (NIH) consensus guidelines: FEV1 less than 75% predicted and a greater than 10% decline from pretransplant baseline, an FEV1/FVC ratio less than 0.7, and absence of concurrent pulmonary infection (5, 12, 13). Two previously described cohorts were used.
The first cohort (FAM) included all subjects enrolled from June 2011 to June 2014 in a multicenter, prospective, single-arm trial testing combination FAM therapy for newly diagnosed BOS after allogeneic hematopoietic cell transplant (11). Oral prednisone of 1 mg/kg/d for 2 weeks followed by a taper over 5 weeks was permitted at the discretion of the provider. Clinical data were collected prospectively for the clinical trial and were supplemented via chart review outside the study window.
The second cohort (Fred Hutchinson Cancer Research Center [FHCRC]) included patients from the FHCRC who received allogeneic hematopoietic cell transplants between January 2002 and June 2006 (8). Treatment of BOS was determined at the discretion of the provider and usually included prednisone. Chart review was performed to determine if the patient would have met FAM trial eligibility criteria (11) at diagnosis. Patients were included if they met the NIH criteria for BOS and excluded if they had been treated with an inhaled corticosteroid or a leukotriene inhibitor for more than 1 month in the 3 months prior to diagnosis, or if they had received FAM therapy at diagnosis. Follow-up was defined from as the period from diagnosis of BOS to the date of last contact as of September 4, 2015.
Written informed consent was provided by all participants in the FAM clinical trial, as previously reported. Prior to undergoing hematopoietic cell transplant, all subjects in the FHCRC cohort signed informed consent forms allowing the use of their clinical data for research. Additional informed consent for this study was waived on the basis of minimal risk to the subjects. This study was approved by the institutional review board of FHCRC and participating FAM trial centers.
Lung Function Trajectory
Pulmonary function tests (PFTs) were performed within the clinical pulmonary function laboratories of participating institutions according to institutional practice. All allogeneic hematopoietic cell transplant recipients perform pretransplant PFTs as part of the pretransplant evaluation. Routine screening PFTs after transplant varied among the 10 centers: 4 had no screening protocol, 4 (including FHCRC) screened at Day 80 and at 1 year, 1 screened at Day 80 and every 6 months after hematopoietic cell transplant through 2 years, and 1 screened every 3 months if chronic GVHD was present.
The results of PFTs were collected as part of the FAM trial or abstracted from the medical record. Prebronchodilator spirometric parameters (FEV1, FVC, and forced expiratory flow, midexpiratory phase [FEF25-75]) were expressed as percent predicted values calculated according to published formulae (14). Individual spirometric measurements for each subject were plotted against time and aggregated into a spaghetti plot. Automated smoothing splines were produced using SAS/STAT version 9.4 software (SAS Institute, Inc., Cary, NC) to provide a view of the aggregate overall trajectory over time.
Changes in FEV1 and FVC for each subject were expressed as a monthly rate of absolute change in percent predicted values. Change in absolute FEV1 was also expressed in milliliters per month. These spirometric values were assessed per the following time windows: 6 months pre-BOS to BOS diagnosis (with pre-BOS defined as Day −210 to Day 0), BOS diagnosis to 6 months after diagnosis (referred to hereinafter as “early BOS,” defined as Day 0 to Day 210), and from 6 to 18 months after diagnosis (referred to hereinafter as “late BOS,” defined as Day 150 to Day 570). A minimum of two studies at least 30 days apart were required to calculate a slope.
Because the decline in FEV1 prior to BOS diagnosis appeared to occur within 6 months for those patients with available studies, that time frame was chosen to calculate the pre-BOS rate of change. Except at BOS diagnosis, a 30-day allowance was added for the nominal 6- and 12- month windows to incorporate additional PFT assessments. The date of the PFT that corresponded to clinical recognition of BOS diagnosis, or the date of enrollment into the FAM trial if this occurred after clinical recognition of BOS, was considered the date of BOS diagnosis and designated as Day 0. The FAM protocol allowed a maximum of 6 months between clinical recognition of BOS and enrollment.
Statistical Analysis
Patient characteristics were reported as means with SDs or as medians and ranges for continuous variables, and as count and percentage for categorical variables. PFT measurements were analyzed as average rates of change per month, as estimated by linear least squares for each patient (described above).
To test the hypothesis that FAM treatment was associated with reduced FEV1 decline compared with standard treatment, univariate and multivariate linear regression was used. Multivariate analysis was performed to adjust for factors that could potentially affect FEV1 trajectory, including systemic corticosteroids, which is the first-line treatment for chronic GVHD, and other factors that have previously been associated with poor prognosis, including early onset after hematopoietic cell transplant (15, 16) and severity of FEV1 at diagnosis (15, 17, 18) and FVC (19) as expressed in tertiles.
Overall survival analysis was based on a landmark time of 6 months after BOS diagnosis. The Kaplan-Meier method was used to estimate overall survival. Cox regression was used to explore the association of FEV1 trajectory and covariates, including those listed above, with survival. Additional factors that may influence survival in hematopoietic cell transplant recipients were included in the evaluation of overall survival. These factors included patient age (20), cytomegalovirus serostatus (9), pre–hematopoietic cell transplant conditioning intensity (20), and donor type (20). Statistical analyses were performed with SAS/STAT version 9.4 software (SAS Institute, Inc., Cary, NC) and R version 3.1.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Cohort Characteristics
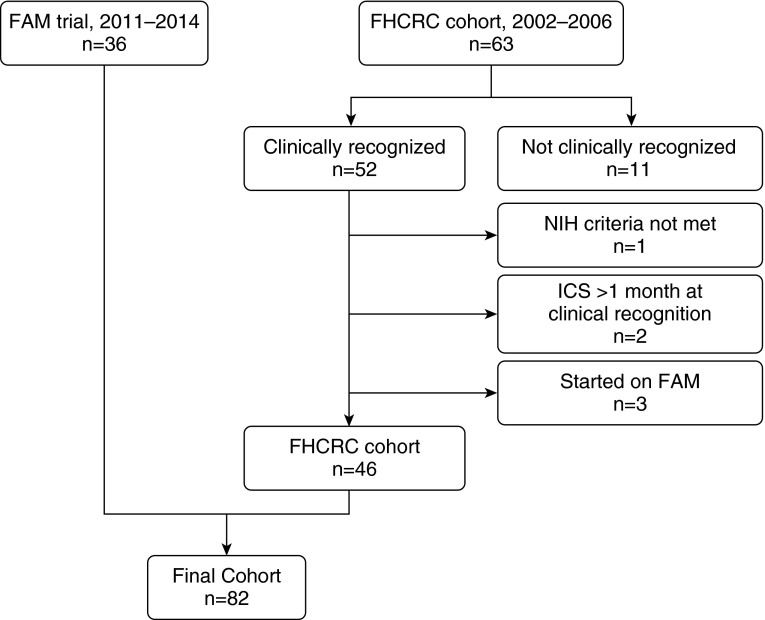
Eighty-two subjects (FAM, n = 36; FHCRC, n = 46) with NIH criteria–defined BOS were included in this study (Figure 1). Clinical characteristics are shown in Table 1. In the FHCRC cohort, 11 subjects (26%) were started on inhaled corticosteroids, 5 (13%) on azithromycin, 1 (2%) on both inhaled corticosteroids and azithromycin, and none on montelukast. The majority of patients (42 of 46 [91%]) were already on prednisone at diagnosis. None of the subjects had prednisone prescribed as a new medication, but the dosage was increased in 18 subjects (39%). In the FAM cohort, prednisone was prescribed as a new medication or increased in 25 patients (70%) at BOS diagnosis; the remaining continued prednisone without an alteration in dose.
Figure 1.
Schema of cohort of patients with bronchiolitis obliterans syndrome (BOS) as defined by updated National Institutes of Health (NIH) spirometric criteria. All 36 participants in the fluticasone, azithromycin, and montelukast (FAM) trial were included (11). Only patients with clinically recognized BOS from the Fred Hutchinson Cancer Research Center (FHCRC) retrospective cohort were included. Patients from the FHCRC cohort were excluded for factors that would confound analysis of treatment with FAM on FEV1 trajectory. ICS = inhaled corticosteroid.
Table 1.
Patient characteristics of patients in the fluticasone, azithromycin, and montelukast trial and retrospective Fred Hutchinson Cancer Research Center cohorts
| FAM Cohort (n = 36) | FHCRC Cohort (n = 46) | |
|---|---|---|
| Age at allogeneic hematopoietic cell transplant, yr, median (range) | 53 (23–70) | 47 (13–67) |
| Months to bronchiolitis obliterans syndrome, median (range) | 17.9 (5.0–131) | 13.9 (5.0–39.9) |
| Sex, n (%) | ||
| Female | 17 (47) | 16 (35) |
| Male | 19 (53) | 30 (65) |
| Cytomegalovirus, n (%) | ||
| Negative | 16 (50) | 20 (43) |
| Positive | 16 (50) | 26 (57) |
| Conditioning, n (%) | ||
| Nonmyeloablative | 14 (39) | 18 (39) |
| Myeloablative | 22 (61) | 28 (61) |
| Stem cell source, n (%) | ||
| Peripheral blood | 31 (86) | 42 (91) |
| Bone marrow | 5 (14) | 4 (9) |
| Donor, n (%) | ||
| Related | 21 (58) | 24 (52) |
| Unrelated | 15 (42) | 22 (48) |
Definition of abbreviations: FAM = fluticasone, azithromycin, montelukast; FHCRC = Fred Hutchinson Cancer Research Center.
The median durations to last follow-up among surviving patients were 115 months (range, 57–130) for the FHCRC cohort and 23 months (range, 9–48) for the FAM group. The median number of PFTs per patient used to estimate lung function trajectories is shown in Table 2. Of the entire cohort, 48% had at least one pre-BOS PFT within 6 months of diagnosis. In addition, 94% had available early BOS studies, and 62% had late BOS studies, from which an FEV1 slope could be estimated.
Table 2.
Lung function trajectory calculated as FEV1 percent predicted per month
| Time Frame | n | Median Number* (Range) | Mean Slope (SD) | Median Slope (IQR) |
|---|---|---|---|---|
| Pre-BOS | ||||
| FAM | 15 | 2 (2–4) | −6.88 (8.44) | −6.54 (−12.1 to 0.32) |
| FHCRC | 24 | 2 (2–5) | −6.71 (5.87) | −5.27 (−9.36 to −2.79) |
| Early BOS | ||||
| FAM | 35 | 5 (3–7) | 0.06 (2.24) | −0.10 (−1.43 to 0.79) |
| FHCRC | 39 | 4 (2–10) | −0.86 (3.73) | −0.45 (−1.48 to 1.13) |
| Late BOS | ||||
| FAM | 22 | 3 (2–8) | −0.04 (1.25) | 0.04 (−0.63 to 0.54) |
| FHCRC | 29 | 5 (2–14) | −0.12 (0.80) | −0.10 (−0.35 to 0.35) |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; FAM = fluticasone, azithromycin, montelukast study cohort patients; FHCRC = Fred Hutchinson Cancer Research Center retrospective cohort patients; IQR = interquartile range.
This is the median number of pulmonary function tests per subject used in the calculation of trajectory.
FEV1 Trajectory
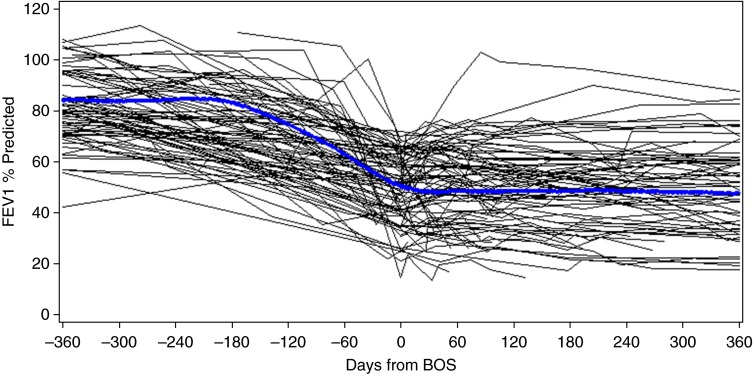
Qualitatively, individual FEV1 trajectories after BOS diagnosis to date of last contact were heterogeneous (Figure E1 in the online supplement), but a predominant pattern emerged (Figure 2). The overall trajectory of FEV1 in the pre-BOS time frame for both cohorts was notable for marked FEV1 decline within 6 months to BOS diagnosis, followed by relative stability in FEV1 (Table 2 and Figure E2). There was a concomitant mild reduction in FVC and a marked reduction in FEF25-75 at BOS diagnosis.
Figure 2.
Trajectory of FEV1 percent predicted values after allogeneic hematopoietic cell transplant. Bronchiolitis obliterans syndrome (BOS) diagnosis is designated as time 0. Individual trajectories (n = 82) are plotted with a smoothed regression line to illustrate the aggregate trajectory of the entire cohort over time.
Pretransplant lung function parameters were within normal limits for FEV1 and FVC, with some variability in FEF25-75 (Table 3). At BOS diagnosis, the median FEV1 percent predicted was 46% (interquartile range [IQR], 35–57) for the FAM cohort and 53% (IQR, 41–64) for the FHCRC group. For the entire cohort (FAM + FHCRC), FEV1 percent predicted at diagnosis expressed in tertiles was greater than 58.8% for the upper tertile, 44.0–58.8% for the middle tertile, and less than 44.0% for the lower tertile. The median FVC percent predicted at diagnosis was 72% for both cohorts (FAM IQR, 65–80; FHCRC IQR, 63–78). The FVC percent predicted values in tertiles were as follows: greater than 77.5% for the upper tertile, 67–77.5% for the middle tertile, and less than 67% for the lower tertile. FEF25-75 percent predicted at diagnosis was 17% (IQR, 12–27) for the FAM group and 25% (IQR, 15–34) for the FHCRC cohort.
Table 3.
Spirometric parameters at baseline before hematopoietic cell transplant, at bronchiolitis obliterans syndrome diagnosis, and at 6 months after bronchiolitis obliterans syndrome diagnosis
| Pretransplant |
BOS Diagnosis |
6 mo |
||||
|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | |
| FEV1, % predicted | ||||||
| FAM | 33 | 94 (85–103) | 36 | 46 (35–57) | 25 | 49 (43–60) |
| FHCRC | 45 | 87 (77–97) | 46 | 53 (41–64) | 25 | 54 (42–64) |
| FVC, % predicted | ||||||
| FAM | 33 | 99 (88–105) | 36 | 72 (65–80) | 25 | 77 (65–83) |
| FHCRC | 45 | 91 (83–100) | 46 | 72 (63–78) | 25 | 71 (63–80) |
| FEF25-75, % predicted | ||||||
| FAM | 33 | 81 (66–100) | 36 | 17 (12–27) | 25 | 21 (15–27) |
| FHCRC | 45 | 77 (63–92) | 46 | 25 (15–34) | 25 | 32 (18–38) |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; FAM = fluticasone, azithromycin, montelukast study cohort patients; FHCRC = Fred Hutchinson Cancer Research Center retrospective cohort patients; IQR = interquartile range.
Because FEV1 is the primary spirometric parameter used in clinical practice to evaluate BOS, subsequent analysis was focused on FEV1. For subjects with available PFTs pre-BOS, the FAM and FHCRC cohorts had a similar slope of FEV1 percent predicted decline to diagnosis. The mean change in absolute FEV1 percent predicted per month in the pre-BOS window was −6.88 ± 8.44% for the FAM group versus −6.71 ± 5.87% for the FHCRC cohort (Table 2).
In the early BOS period, the rate of change for FEV1 percent predicted ranged from −17.6% per month to +8.2% per month for the entire cohort. The mean changes in absolute FEV1 percent predicted per month from BOS diagnosis to 6 months were 0.06 ± 2.24% and −0.86 ± 3.73% for the FAM and FHCRC groups, respectively (Table 2). FVC remained stable during this time frame (Table 3 and Table E1).
In the late BOS period, the mean changes in FEV1 percent predicted per month were −0.04 ± 1.25% and −0.12 ± 0.80% for the FAM and FHCRC groups, respectively. To gain a perspective on lung function loss over time, the change in FEV1 was estimated in milliliters per year (Table E2). In the late BOS period, the estimated declines in FEV1 were 83 ml/yr (12 × −6.9 ml/mo) for the FAM group and 60 ml/yr (12 × −5.0 ml/mo) for the FHCRC cohort.
To evaluate factors affecting the FEV1 percent predicted trajectory early after diagnosis, FAM treatment, new or increased use of prednisone, time of onset after hematopoietic cell transplant, and FEV1 severity at diagnosis were considered. In univariate regression analysis of rate of change from BOS diagnosis to 6 months, the effect of FAM on FEV1 slope was a difference in the mean rate of change of 0.92% predicted per month (95% confidence interval [CI], −0.53 to 2.37), which means that the FAM cohort had a slightly better rate of change in FEV1 percent predicted relative to the FHCRC group, but this was not statistically significant (P = 0.21). This result did not change with the exclusion of FHCRC subjects who were started on inhaled corticosteroids or azithromycin (data not shown) or in multivariate analysis adjusted for treatment with prednisone at diagnosis, timing of diagnosis after hematopoietic cell transplant, or FEV1 percent predicted at diagnosis (0.79%; 95% CI, −0.78 to 2.34; P = 0.31).
Outcomes after BOS Diagnosis
The estimated 2-year overall survival times from BOS diagnosis were 76% (95% CI, 61–91%) for the FAM group and 72% (95% CI, 59–85%) for the FHCRC cohort. Nine patients in the FAM group and 29 patients in the FHCRC group died in the respective follow-up period for each cohort.
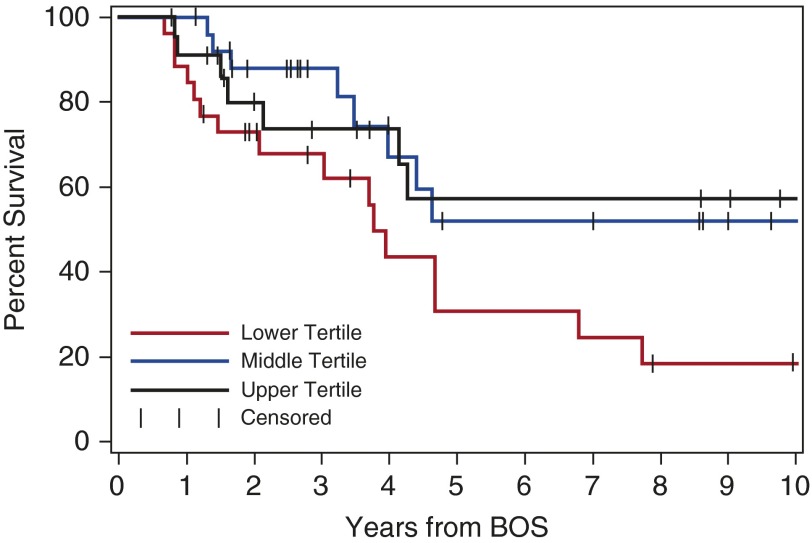
An exploratory analysis of survival was performed to determine if FEV1 trajectories and other factors were associated with worse outcomes. Survival analysis at a 6-month landmark after BOS diagnosis (n = 75) suggested that patients with a change of FEV1 percent predicted from BOS diagnosis to 6 months above the median (−0.30%/mo) had better survival than those with a change below the median, although this difference was not statistically significant (Table 4). Factors that were associated with survival in univariate analysis were included in a multivariate analysis; only diagnosis within 1 year and FVC in the lowest tertile remained significantly associated with decreased survival (Table 4 and Figure 3). The hazard ratio for an FVC less than 67% was 2.67 (95% CI, 1.2–6.0; P = 0.02). The correlation of FEV1 to FVC was r = 0.66, r2 = 43 (P < 0.0001) (Figure E3).
Table 4.
Exploratory analysis of risk factors for mortality at 6 months after onset of bronchiolitis obliterans syndrome (n = 75, with 31 deaths)
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| FAM | 1.04 (0.4–2.7) | 0.94 | —– | —– |
| Slope FEV1, % predicted greater than −0.30/mo | 0.61 (0.3–1.3) | 0.21 | —– | —– |
| Diagnosis 0–1 yr post-HCT | 1.97 (0.9–4.1) | 0.07 | 2.30 (1.0–5.1) | 0.04 |
| FEV1 <44% predicted | 2.12 (1.0–4.4) | 0.04 | 1.40 (0.6–3.1) | 0.40 |
| FVC, % <67% predicted | 2.36 (1.2–4.8) | 0.02 | 2.67 (1.2–6.0) | 0.02 |
| Myeloablative conditioning | 0.54 (0.3–1.1) | 0.09 | 0.59 (0.3–1.2) | 0.12 |
Definition of abbreviations: CI = confidence interval; FAM = fluticasone, azithromycin, montelukast study cohort; HCT = hematopoietic cell transplant; HR = hazard ratio.
Figure 3.
Kaplan-Meier survival estimates at 6-month landmark after bronchiolitis obliterans syndrome (BOS) diagnosis, stratified by tertiles of FVC percent predicted values for the entire cohort (n = 75): upper tertile, greater than 77.5% (black line); middle tertile, 67–77.5% (blue line); and lower tertile, less than 67% (red line). The comparisons are lowest versus highest, hazard ratio (HR) of 2.31 (95% confidence interval [CI], 1.0–5.7; P = 0.06); lowest versus middle, HR of 2.40 (95% CI, 1.0–5.6; P = 0.04); and lowest versus middle/highest, HR of 2.36 (95% CI, 1.2–4.8; P = 0.02).
Discussion
In two contemporary cohorts of patients with BOS, we observed that the trajectory of FEV1 in patients with BOS after hematopoietic cell transplant was variable. We observed a predominant pattern of rapid FEV1 decline in the 6 months prior to clinical recognition of BOS, followed by a period of relative FEV1 stabilization, with a concomitant mild reduction in FVC and a marked reduction in FEF25-75. FEV1 impairment was severe at diagnosis. Patients who survived beyond 6 months experienced a slow decline in FEV1 over time. The rate of FEV1 decline after BOS diagnosis was not affected by type of treatment analyzed and was not associated with survival. Overall 2-year survival rates were greater than 70%. In an exploratory analysis, diagnosis within 1 year after hematopoietic cell transplant and FVC less than 67% predicted at diagnosis were associated with reduced survival.
Our data indicate that the magnitude of FEV1 decline before diagnosis greatly exceeded the diagnostic requirement of a greater than 10% decline compared with baseline FEV1 (5) as well as the annualized rate of FEV1 percent predicted decline of 5%/yr described by Chien and colleagues (21). Given that FEV1 preceded BOS diagnosis within 6 months, and in some cases within 3–4 months, it is possible that the majority of these patients would have met the criteria for BOS weeks to months prior to formal diagnosis, offering an opportunity to intervene before significant lung function was lost (22).
The precision of FEV1 trajectory was limited by the lack of standardized intervals of PFT monitoring. Thus, the rates of FEV1 decline to diagnosis may be less severe than reported, as they do not take into account patients who may have had a more indolent course to BOS diagnosis. Conversely, the time intervals between the pre-BOS measurements used to calculate the slope may underestimate the true rate of decline for those with rapid onset of severe disease. These data are unable to pinpoint the precise onset of lung function decline; they simply indicate the time at which patients met the BOS criteria.
This limitation highlights the larger problem of a lack of an effective strategy for the early detection of BOS after allogeneic hematopoietic cell transplant. The optimal frequency for routine PFT testing has never been established, and screening protocols do not exist at many centers. At some centers in the United States, including the FHCRC, allogeneic hematopoietic cell transplant recipients undergo PFTs at Days 80–100 and at 1 year post–hematopoietic cell transplant, or more frequently as dictated by the clinical scenario.
The severe degree of lung dysfunction at diagnosis seen in our study suggests that BOS is diagnosed late in the course of the disease with current practices. The stability of FEV1 after diagnosis may reflect lung function that had already plateaued to a new baseline by the time BOS was clinically recognized in some patients. The FEV1 trajectory of our cohort is similar to that described in a cohort of lung transplant recipients (4), but the rates of decline are shifted by 6 months, again supporting the notion that BOS after hematopoietic cell transplant is diagnosed too late.
The need for early detection and treatment is well recognized within the transplant community (22); however, there are significant roadblocks to the implementation of screening protocols. BOS is rare after hematopoietic cell transplant; unlike in lung transplant recipients, relapse of malignancy, not pulmonary dysfunction, is the primary clinical concern after transplant. Performing routine PFTs is onerous for the patient in the context of other symptomatic post–hematopoietic cell transplant complications, particularly if the patient is discharged to community care far from a hematopoietic cell transplant center. Current guidelines recommend testing patients at high risk, specifically those with newly diagnosed chronic GVHD, every 3 months (7), but this is not the standard of care at most centers.
The poor prognosis associated with BOS diagnosed within 1 year of allogeneic hematopoietic cell transplant is consistent with prior observations (15, 16, 23) and suggests that close interval screening should occur well before the 1-year milestone. Home monitoring by using spirometry with a portable handheld device, as is done for lung transplant recipients, could be used in high-risk hematopoietic cell transplant recipients (24), which may alleviate the cost and inconvenience of performing frequent PFTs in a laboratory setting and help detect BOS earlier. Weekly or monthly measurements with consecutive FEV1 decline should prompt immediate evaluation.
Effective detection of early disease depends upon an understanding of the natural history of the disease and innovation of spirometric or clinical biomarkers more sensitive than current methods. In lung allograft recipients, clinically symptomatic BOS may be preceded by an isolated decline in FEF25-75, also known as “BOS 0-P” (25, 26), which may also be the case in hematopoietic cell transplant recipients (27). Analysis of FEF25-75 prior to BOS diagnosis in our study was limited owing to the lack of available spirometry, but there was a marked reduction of FEF25-75 at diagnosis, which is consistent with the pathophysiology of small airway obstruction (28, 29). The theoretical utility of this parameter is possible only if PFTs are done frequently enough to detect isolated FEF25-75 reduction prior to FEV1 decline.
While the stability of lung function after diagnosis may reflect an already fixed decline in FEV1, there remains a possibility that treatment directed at BOS could prevent further decline. The retrospective design and noncontemporaneous cohorts did not allow us demonstrate an effect of FAM compared with standard of care; however, given the likelihood that some individuals will be recognized during the steep part of FEV1 decline, the pattern of FEV1 stabilization may be due to treatments initiated for these individuals. A recent prospective trial in which patients with a median FEV1 of 55–65% predicted at BOS diagnosis were randomized to receive inhaled budesonide/formoterol versus placebo without addition of systemic corticosteroids provides evidence that reversing lung function decline is possible if BOS is diagnosed at a less severe stage of lung dysfunction (30). Again, this underscores the need for early detection to reduce long-term lung dysfunction.
The 2-year survival rates of patients with BOS after hematopoietic cell transplant of 76% and 72% in the FAM and FHCRC cohorts, respectively, are an improvement on an oft-quoted historical 2-year survival rate of 44% (15, 23). Improvement in supportive care for patients with severe lung function in the last two decades may have improved survival even for individuals with severe lung dysfunction. Our analysis did not show a significant association of FEV1 trajectory with outcomes, which may be biased if patients with declining lung function were too ill to perform PFTs. Clinical events such as respiratory infection are likely to contribute to FEV1 deterioration and subsequent mortality in patients with BOS, as is the case in other chronic airway diseases such as chronic obstructive pulmonary disease (31–33) and cystic fibrosis (34). The impact of respiratory infections was beyond the scope of this study and should be investigated prospectively.
The severity of FVC, not FEV1, was significantly associated with mortality in this cohort. FEV1 was not associated with worse outcomes in analyses by Dudek and colleagues (23) and Bergeron and coworkers (16), the latter of whom used a cutpoint of FEV1 less than 50% predicted. However, Ahn and colleagues showed that a very severe FEV1 less than 30% predicted at diagnosis was significantly associated with mortality (17), consistent with what is seen clinically in patients with end-stage disease. While there is some degree of correlation of FVC with FEV1, our analysis suggests that FVC is an independent risk factor for mortality, which is consistent with what has recently been observed in lung transplantation (19, 35). The modest reduction in FVC at diagnosis, as observed in our cohort, likely reflects reduced ventilatory capacity due to air trapping from obliterated distal small airways (13, 16). However, the effect of severe FVC reduction on mortality may be due to factors such as thoracic cage restriction from sclerotic GVHD, noninfectious pneumonia, or neuromuscular weakness. Our data suggest that the diagnostic and prognostic roles of FVC in this disease are more important than previously appreciated and should be evaluated in future studies.
Limitations
In addition to the lack of standardized PFT intervals, this study was limited in several ways. The retrospective design and noncontemporaneous comparison cohorts limited our ability to adequately control for important clinical confounding factors, including treatment with prednisone, which also has potential for slowing FEV1 decline. Prior to the use of FAM at the FHCRC in 2008 (36), the prevailing clinical practice for treatment of BOS was the use of prednisone, which was also permitted in the FAM trial.
We were unable to assess the effect of extrathoracic manifestations of chronic GVHD, which could accelerate FEV1 decline, because the FAM cohort was assessed prospectively according to an updated symptom-based scale (12, 37) that was not available for the FHCRC cohort. The survival analysis was exploratory and must be interpreted with caution. The long-term impact of treatment on lung function and survival requires prospective studies with larger numbers of patients and longer follow-up.
Conclusions
Despite the limitations of our study, this description of lung function trajectory provides insight into the clinical course of BOS after hematopoietic cell transplant in the current treatment era and in a context with other chronic lung diseases. Notably, the estimated yearly rate of FEV1 loss beyond 6 months after clinical recognition in our study exceeds FEV1 decline due to normal aging (38) and is similar to the rate of FEV1 loss in emphysematous chronic obstructive pulmonary disease (39).
Our observations suggest that any intervention should be targeted before or during the steep part of the slope of FEV1 decline. Clinical trials for the treatment of BOS must incorporate a means of identifying and treating patients early in the course of the disease. An appropriate endpoint could be stabilization of lung function above a minimal degree of FEV1 impairment. For this to be possible, more work needs to be done to define early BOS and to standardize serial lung function monitoring after allogeneic hematopoietic cell transplant in individuals with chronic GVHD.
Footnotes
Funding for this study was provided by the Chronic Graft-versus-Host Disease Consortium (grant U54 CA163438), which is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network, funded through a collaboration between NCATS and the National Cancer Institute (S.J.L., principal investigator). Additional funding was provided by a Cancer Center Support Grant New Investigator Award, funded by the Cancer Consortium of the Fred Hutchinson Cancer Research Center and the University of Washington (G.-S.C., principal investigator).
Author Contributions: G.-S.C., B.S., J.W.C., S.J.L., and K.M.W.: conceived of and designed the study; K.C. contributed additional study design; G.-S.C., J.W.C., M.J., J.J.H., L.B., V.T.H., J. Pidala, J. Palmer, L.J., S.M., I.P., S.J.L., and K.M.W.: collected the data; B.S.: performed the statistical analysis; and G.-S.C.: wrote the first draft of the manuscript. All authors analyzed and interpreted the data and reviewed, edited, and approved the final draft of the manuscript for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ralph DD, Springmeyer SC, Sullivan KM, Hackman RC, Storb R, Thomas ED. Rapidly progressive air-flow obstruction in marrow transplant recipients: possible association between obliterative bronchiolitis and chronic graft-versus-host disease. Am Rev Respir Dis. 1984;129:641–644. [PubMed] [Google Scholar]
- 2.Burke CM, Theodore J, Dawkins KD, Yousem SA, Blank N, Billingham ME, Van Kessel A, Jamieson SW, Oyer PE, Baldwin JC, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984;86:824–829. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 3.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 4.Lama VN, Murray S, Lonigro RJ, Toews GB, Chang A, Lau C, Flint A, Chan KM, Martinez FJ. Course of FEV1 after onset of bronchiolitis obliterans syndrome in lung transplant recipients. Am J Respir Crit Care Med. 2007;175:1192–1198. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer J, Williams K, Inamoto Y, Chai X, Martin PJ, Tomas LS, Cutler C, Weisdorf D, Kurland BF, Carpenter PA, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:337–344. doi: 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter PA, Kitko CL, Elad S, Flowers ME, Gea-Banacloche JC, Halter JP, Hoodin F, Johnston L, Lawitschka A, McDonald GB, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, Urbano-Ispizua A, Cutler CS, Bacigalupo AA, Battiwalla M, et al. Graft-vs-Host Disease Working Committee of the CIBMTR. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:266–274. doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT, Pidala J, Palmer J, Johnston L, Mayer S, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:710–716. doi: 10.1016/j.bbmt.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 15.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation: clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 16.Bergeron A, Godet C, Chevret S, Lorillon G, Peffault de Latour R, de Revel T, Robin M, Ribaud P, Socié G, Tazi A. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2013;48:819–824. doi: 10.1038/bmt.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn JH, Jo KW, Song JW, Shim TS, Lee SW, Lee JS, Kim DY, Lee JH, Lee JH, Choi Y, et al. Prognostic role of FEV1 for survival in bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2015;29:1133–1139. doi: 10.1111/ctr.12638. [DOI] [PubMed] [Google Scholar]
- 18.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010;182:784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belloli EA, Wang X, Murray S, Forrester G, Weyhing A, Lin J, Ojo T, Lama VN. Longitudinal forced vital capacity monitoring as a prognostic adjunct after lung transplantation. Am J Respir Crit Care Med. 2015;192:209–218. doi: 10.1164/rccm.201501-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au BK, Gooley TA, Armand P, Fang M, Madtes DK, Sorror ML, Boeckh MJ, Gibson CJ, Deeg HJ, Storb R, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2015;21:848–854. doi: 10.1016/j.bbmt.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, Clark JG. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 22.Cooke KRA. A “window of opportunity” for patients with late-onset pulmonary dysfunction after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:291–292. doi: 10.1016/j.bbmt.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 24.Cheng GS, Campbell AP, Xie H, Stednick Z, Callais C, Leisenring WM, Englund JA, Chien JW, Boeckh M. Correlation and agreement of handheld spirometry with laboratory spirometry in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2016;22:925–931. doi: 10.1016/j.bbmt.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hachem RR, Chakinala MM, Yusen RD, Lynch JP, Aloush AA, Patterson GA, Trulock EP. The predictive value of bronchiolitis obliterans syndrome stage 0-p. Am J Respir Crit Care Med. 2004;169:468–472. doi: 10.1164/rccm.200307-1018OC. [DOI] [PubMed] [Google Scholar]
- 26.Lama VN, Murray S, Mumford JA, Flaherty KR, Chang A, Toews GB, Peters-Golden M, Martinez FJ. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med. 2005;172:379–383. doi: 10.1164/rccm.200501-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abedin S, Yanik GA, Braun T, Pawarode A, Magenau J, Goldstein SC, Levine JE, Kitko CL, Couriel DR. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol Blood Marrow Transplant. 2015;21:1127–1131. doi: 10.1016/j.bbmt.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 29.Perez T, Chanez P, Dusser D, Devillier P. Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respir Med. 2013;107:1667–1674. doi: 10.1016/j.rmed.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, Peffault de Latour R, Dominique S, de Revel T, Juvin K, Maillard N, et al. Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. doi: 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez BL, Masaki K, Burchfiel C, Curb JD, Fong KO, Chyou PH, Marcus EB. Pulmonary function decline and 17-year total mortality: the Honolulu Heart Program. Am J Epidemiol. 1994;140:398–408. doi: 10.1093/oxfordjournals.aje.a117262. [DOI] [PubMed] [Google Scholar]
- 32.Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health. 1999;53:230–234. doi: 10.1136/jech.53.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donaldson GC, Law M, Kowlessar B, Singh R, Brill SE, Allinson JP, Wedzicha JA. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:943–950. doi: 10.1164/rccm.201412-2269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellaffi M, Vinsonneau C, Coste J, Hubert D, Burgel PR, Dhainaut JF, Dusser D. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:158–164. doi: 10.1164/rccm.200405-667OC. [DOI] [PubMed] [Google Scholar]
- 35.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, Palmer SM. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman BC, Jacobsohn DA, Williams KM, Au BK, Au MA, Lee SJ, Moravec CK, Chien JW. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. 2011;46:1369–1373. doi: 10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, Pidala J, Olivieri A, Martin PJ, Przepiorka D, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21:984–999. doi: 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaz Fragoso CA, McAvay G, Van Ness PH, Metter EJ, Ferrucci L, Yaggi HK, Concato J, Gill TM. Aging-related considerations when evaluating the forced expiratory volume in 1 second (FEV1) over time. J Gerontol A Biol Sci Med Sci. 2016;71:929–934. doi: 10.1093/gerona/glv201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, Shimizu K, Betsuyaku T, Ito YM, Fuke S, et al. Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]