Abstract
Patients with isolated unilateral pupil-sparing third or isolated fourth or sixth nerve palsies over 50 years are often diagnosed with “microvascular extraocular palsies”. This condition and its management provoke divergent opinions. We review the literature and describe the incidence, pathology, clinical presentation, yield of imaging, and management. A retrospective diagnosis of exclusion has little practical use. We suggest a pragmatic approach to diagnosis, investigation, and management from initial presentation.
Keywords: Abducens, cranial nerve, microvascular ischaemia, oculomotor, trochlear
Introduction
Patients aged over 50 who present with isolated unilateral pupil-sparing third or isolated fourth or sixth nerve palsies are often labelled as having “microvascular extraocular palsies”. This condition and its management provoke divergent opinions. The aim of this review is to look critically at what is actually known about what is loosely called microvascular non-arteritic extraocular palsies, clarify areas of uncertainty, and suggest practical ideas on diagnosis and management of suspected cases.
The Extent of the Clinical Problem
The annual incidence of microvascular cranial nerve palsies is unclear. A retrospective population-based case series in the United States found the incidence of sixth nerve palsy was 11.3/100,000, of these 35% were microvascular giving an incidence of ∼4/100,000.1 Isolated fourth nerve palsies are the least common ocular motor nerve palsy.2 The estimated extent of a microvascular cause varies—from 35% of all third nerve palsies, 17% of all fourth nerve palsies, and 28% of all sixth nerve palsies to 86% of isolated third, fourth, or sixth nerve palsies.3,4
Pathology
There are no post-mortem studies of microvascular sixth nerve palsy. However, there are 3 cases of diabetics with third nerve palsy compared with 17 controls.5–7
Cause of Microvascular Ischaemia
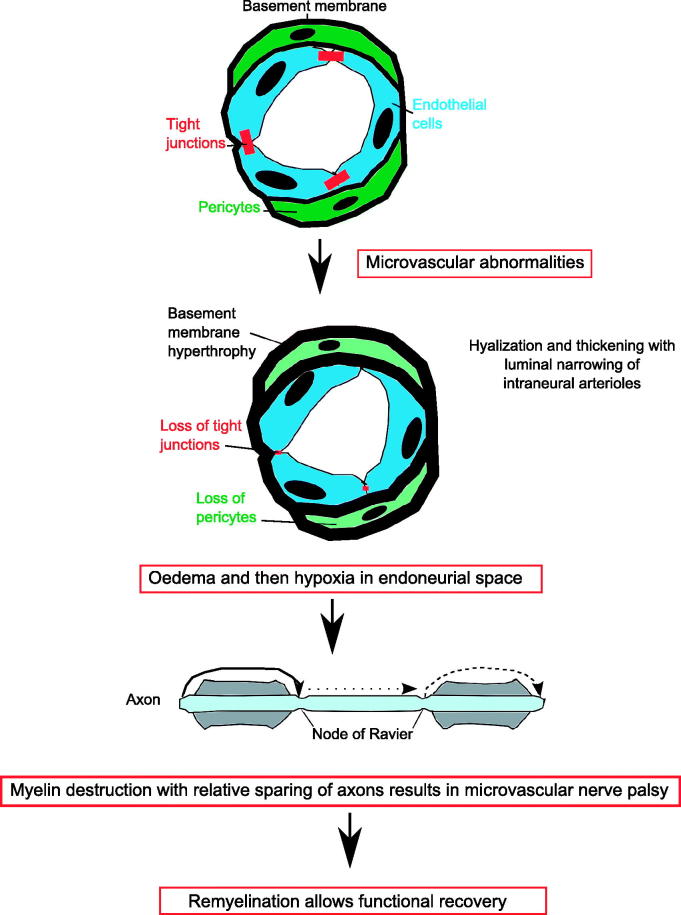
No blocked arteries were seen, but two out of three cases had hyalinisation of the arterioles. Microvascular abnormalities in diabetics occur due to alterations in the blood-nerve barrier resulting in loss of tight junctions, hypertrophy of the microvascular basement membrane, loss of microvascular pericytes producing oedema, and then hypoxia in the endoneurial space.8
Two cases showed focal ischaemia with demyelination and no axonal damage,5,6 and one showed swelling, demyelination, loss of axons, and Wallerian degeneration.7 Therefore, the deficit is likely to be caused predominantly by conduction block or demyelination rather than axonal damage, which presumably explains the good recovery (Figure 1).
FIGURE 1.
Pathophysiology of microvascular ischaemia. The blood supply to cranial nerves is via an intraneural plexus. There is a blood-nerve barrier. Microvascular abnormalities in diabetics occur due the alterations in the blood-nerve barrier resulting in loss of tight junctions, hypertrophy of the microvascular basement membrane, and loss of microvascular pericytes.8 This may underlie the hyalinzation seen in the post-mortem studies and results in oedema and then hypoxia in the endoneurial space, which causes demyelination and conduction block.5 This prevents salutatory conduction down the axon and paralysis of the extraocular muscles and microvascular nerve palsy. As remyelination occurs, there is complete functional recovery.
Site of Ischaemia
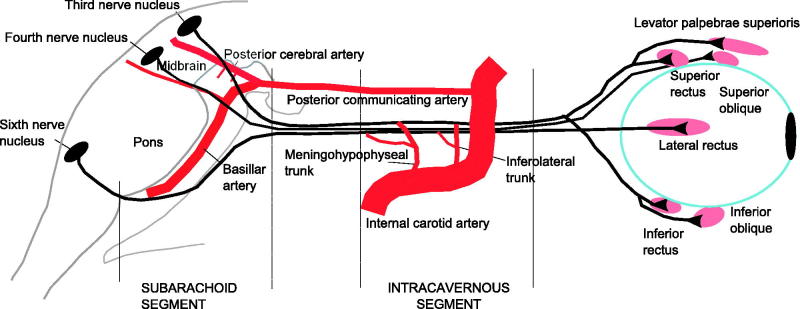
Further insight into the pathphysiology of microvascular nerve palsies comes from the site of ischaemia. The ischaemia occurred in the intracavernous (2 out of 3 cases) or subarachnoid (1 out of 3 cases) segment of the third nerve where watershed territory exists.5 Both third and sixth nerves have a plexus of intraneural arterioles and an extraneural blood supply. In the third nerve, the proximal segment is primarily supplied by thalamoperformating arteries (from the posterior cerebral artery) and supplemented from brainstem vessels. The middle section does not have an extraneural supply but depends on the intraneural plexus, and the distal (or intracavernous) section is primarily supplied by the inferior cavernous sinus artery (also called inferolateral trunk) and supplemented from the meningohypophyseal trunk—both trunks are branches of the internal carotid artery.9
In the fourth nerve, the proximal nerve received branches from the inferolateral trunk in 80% and from the tentorial artery of the meningohypophyseal trunk in 20%. The distal half was supplied by the branches from the inferolateral trunk only. In the sixth nerve, the proximal third received branches from the dorsal clival artery of the meningohypophyseal trunk. The middle and distal thirds received branches from the inferior cavernous sinus artery (Figure 2).10
FIGURE 2.
Extraneural blood supply to third, fourth, and sixth nerves. In the third nerve, the proximal segment is primarily supplied by thalamoperformating arteries (from the posterior cerebral artery) and supplemented by brainstem vessels. The middle section does not have an extraneural supply but depends on intraneural plexus and the distal (or intracavernous) section is primarily supplied by the inferior cavernous sinus artery (also called inferolateral trunk) and supplemented from the menigohypophyseal trunk (branches of the internal carotid artery). In the fourth nerve, the proximal nerve received branches from the inferolateral trunk in 80% and from the tentorial artery of the meningohypophyseal trunk in 20%. The distal half was supplied by the branches from the inferolateral trunk only. In the sixth nerve, the proximal third received branches from the dorsal clival artery of the meningohypophyseal trunk. The middle and distal thirds received branches from the inferior cavernous sinus artery (also called inferolateral trunk).
Therefore, watersheds in the blood supply to the ocular motor cranial nerves may explain their vulnerability to microvascular ischaemia when intraneural arterioles are narrowed by hyalinisation.
Risk Factors
Risk factors for microvascular nerve palsies are age (93% occur in people over 50 years11), hypertension (25%), diabetes (18%), or both hypertension and diabetes (7%).12,13
A case-control study of 65 patients 50 years of age or older with a microvascular nerve palsy showed increased risk with previously diagnosed diabetes (odds ratio [OR] = 5.75), left ventricular hypertrophy (OR = 5.20), and haematocrit (per % increase) (OR = 1.35).14
A recent study in 35 patients with diabetes associated microvascular third nerve palsy confirmed hypertension (43%), hypercholesterolemia (40%), smoking (29%), coronary artery disease (14%), and alcoholism (11%) as risk factors.15 Of note, approximately 60% of patients with non-microvascular palsies also had vascular risk factors.4,16
Clinical Features
The clinical assessment is vital. It is essential that the practitioner is confident that they are able to diagnose an isolated, complete pupil-sparing third or isolated fourth or sixth nerve palsy. This can be achieved with careful history taking and clinical examination, which should include complete neurological and ophthalmological assessment.17 Orthoptic examination including Hess chart can demonstrate a subtle second palsy and more detailed testing can aid examination.
However, as highlighted in a recent study based in the emergency department, specific neuro-ophthalmologic tests required to identify whether diplopia is caused by fourth, sixth, partial, or complete third nerve palsy is not easy.18 The poor diagnostic accuracy of neurological problems in the emergency department has been recognised—35% not arriving at the final diagnosis.19 In cases requiring eye examination, 59% of cases seen in the emergency department have significant eye examination omissions, compared with only 8% of eye casualty records.20
Pain
Sixty-one percent are associated with orbital pain, which often precedes the double vision by hours or days, usually aching, behind or above the eye, sometimes more diffuse but always ipsilateral to the palsy.12 Pain is attributed to involvement of the first division of the trigeminal nerve within the cavernous sinus, or to activation of pain-sensitive trigeminal fibres within the sheath of the third nerve as it traverses the cavernous sinus.
Pain is most common in third nerve palsies (77%), with 54% of sixth nerve palsies, and it is more common in diabetics (72%) than non-diabetics (55%).21 In a prospective study of 35 patients with diabetes associated third nerve palsy, 77% had headache and 49% periocular pain.15
Degree of Ocular Motor Dysfunction
A complete third nerve palsy results in an eye displaced outward and downward with ptosis and mydriasis. Oculomotor dysfunction is often incomplete due to partial paralysis of all the muscles or complete paralysis of some, or complete
paralysis of some and partial paralysis of others. In the two published series in microvascular third nerve palsies, Capó et al. reported 28 cases—7 had complete and 21 partial weakness12—and Sanders et al. reported 42 cases—19 were complete, 20 involved partial weakness of all muscles, and 3 had weakness of specific extraocular muscles.22
Fourth nerve palsy results in double vision (oblique vertical separation of images) due to paralysis of the superior oblique muscle.
Sixth nerve palsy results in the inability of an eye to turn outward with an inward deviation producing double vision due to paralysis of the lateral rectus muscle. Jacobson reported on 35 patients with microvascular sixth nerve palsies seen within 1 week of onset—2 were complete and 33 were partial.23
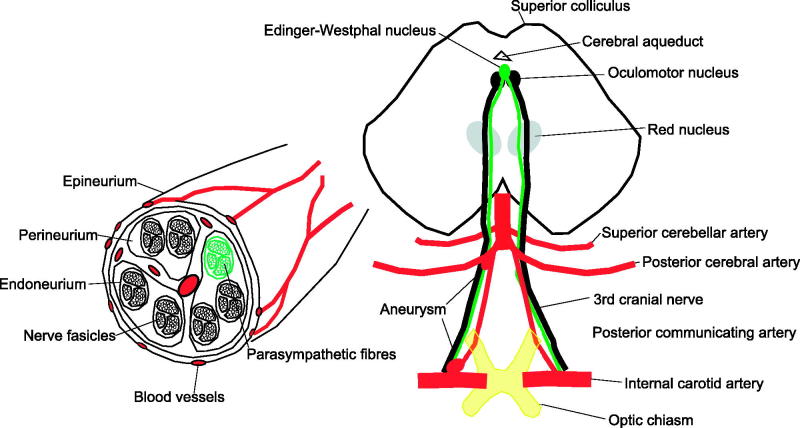
Pupil Involvement (Third Nerve Only)
The parasympathetic fibres that control pupillary constriction lie superficially in the nerve from the brainstem to the cavernous sinus. The artery to the nerve is central.24 This is the traditional explanation of why the pupil is often spared in microvascular palsies as compared with palsies secondary to external compression by aneurysms (Figure 3). Seventy-five percent of microvascular third nerve palsies have a normal pupil as opposed to 5–14% of compressive third nerve palsies.12,22
FIGURE 3.
Significance of pupil involvement. Third nerve palsies often have pupillary involvement because the parasympathetic nerves innervating the iris travel with the third nerve. Pupillary involvement is an important diagnostic sign, as usually compressive lesions involve the pupil, whereas microvascular palsies do not. The parasympathetic fibres that control pupillary constriction originate in the Edinger–Westphal nucleus in the midbrain (green/light grey). These fibres then run in the superficial medial portion of the third nerve as it travels in the subarachnoid space and therefore are susceptible to compression from aneurysms arising from the nearby posterior communicating artery aneurysm. A complete third nerve palsy that spares the pupil is not caused by compression from an aneurysm because the parasympathetics fibres are located near the surface of the nerve, and if the nerve compression is severe enough to cause complete paralysis of the extraocular muscles, then the parasympathetic fibres must also be compressed. However, the microvascular pathology affects the blood vessels inside the nerve and therefore could affect all fibres travelling to the muscles and spare the superficial parasymapthetics. If the third nerve palsy is partial and spares the pupil, it is possible it is missing the parasympathetic fibres so could be caused by an aneurysm.
There are two studies of pupillary involvement in diabetic third nerve palsies. None had major pupillary involvement. Dhume and Paul reported on 35 patients, of whom 9 (26%) had minor pupil dilatations of 1 to 2 mm, and Jacobsen found that 10 of the 26 (38%) patients had minor pupil dilatation (≤1 mm).15,25
The significance of minor degrees of anisocoria has to be questioned because 1 mm differences can be physiological. This is supported by a Japanese study of 63 isolated third nerve palsies (39 were aged ≥50 years). Ninety percent due to an aneurysm had anisocoria in comparison with 32% caused by microvascular ischaemia. The anisocoria was 1 mm or less among the seven microvascular patients with anisocoria, whereas those with aneurysms had anisocoria of >2.0 mm.2 Diabetes can also alter the iris sphincter and panretinal photocoagulation can affect pupillary efferent function.26
The interpretation of normal pupillary size and light reaction needs to take account of both the duration of the third nerve palsy and whether it is complete. Thus, a normal pupil in an incomplete palsy at 24 hours has much less significance than a normal pupil in a complete palsy after 2 weeks.
Progression
Progression of weakness in microvascular nerve palsy is common. Twenty-one out of 28 third nerve palsies progressed over 1–3 days and some worsened for up to 15 days.12 Similarly in microvascular sixth nerve palsies seen within 1 week of onset, 18 (54%) showed subsequent progression of weakness.23
Recovery
Prognosis for full recovery from isolated microvascular nerve palsies is almost invariable—typically over 8–12 weeks.12 Of microvascular third nerve palsies, 90.9% (20/22 patients) recovered completely within 12 months and 81.8% (18/22 patients) resolved within 3 months. Sixty percent (6/10 patients) of microvascular fourth nerve palsies recovered completely within 12 months.2 Eighty-six percent (51/59 patients) of microvascular sixth nerve palsy had complete recovery at 6-year follow-up—recovery was usually complete at 3 months, but was occasionally delayed for up to 1 year.13 Aberrant reinnervation is said never to occur presumably as it implies axonal damage.
Recurrence
Eighteen out of 59 patients (31%) with microvascular sixth nerve palsy had 24 recurrences involving the same or a different ocular motor nerve over a 2–13-year follow-up period.11
Risk of Death from Generalised Vascular Disease
This is not known, but in a follow-up study of 59 patients with microvascular sixth nerve palsy for 2–13 years, 8 (14%) died, 4 from cardiac causes.11 There are no prospective randomised controlled data on whether aspirin prevents microvascular palsies in at-risk patients, but it probably does not. In a retrospective case-control study of 100 patients with microvascular nerve palsy associated with diabetes and/or hypertension, 34% were using aspirin prior to the nerve palsy compared with 30.1% of the 103 diabetic/hypertensive patients without a microvascular nerve palsy.27 However, patients with microvascular cranial nerve palsy have a significantly lower rate of strokes and transient ischaemic attacks compared with the control group (6% versus 23.9%). The authors proposed that this may have been due to aspirin being started earlier in the palsy group—median 5 years in cases and 4 years in controls. There is no evidence on whether treating risk factors reduced recurrence rate. However, the risk of cardiac disease was high in both groups (23% palsy group versus 30.7% non-palsy group).
Imaging
In a recent study of 118 patients with isolated double vision, scanned at presentation, an unenhanced computed tomography (CT) brain scan did not identify any of the 13 cases with a secondary cause.18 This study also found that an unenhanced CT scan had a positive predictive value of 97% if it wasn’t truly isolated diplopia. Three prospective studies have examined the role of magnetic resonance imaging (MRI) scans in a total of 268 patients aged 50 and over with isolated ocular motor palsies.4,16,28 MRI identified neoplasms, infarcts, aneurysms, demyelination, and pituitary apoplexy in 43 cases (16.5%). Tamhankar et al. found 3/64 (4.7%) with fourth and sixth nerve palsies had other causes once giant cell arteritis was excluded—infarction, lymphoma, and meningioma.16
Chi and Bhatti suggest an algorithm for the evaluation of acute isolated sixth nerve palsy, which allows for initial expectant observation and re-consideration of obtaining neuroimaging upon follow-up if the ophthalmoplegia does not improve, progresses, or if other cranial nerves become involved.29
In the future it is possible that high-resolution MR of the nerves and muscles or diffusion tensor tractography may allow a positive imaging diagnosis of microvascular ocular motor nerve palsy but this is not currently possible.30,31
Comparison With Current Clinical Practice
The literature review demonstrates that the clinical presentation is more diverse than often thought. We have compared the accepted clinical management of microvascular nerve palsies in a standard text used by many neuro-opthalmologists in Table 1.32 There are a number of differences, including age, risk factors, progression, pain, degree of ocular motor palsy, pupil involvement, and role of MRI brain imaging. The approaches differ mainly in the degree to which they minimise the risk of missing alternative treatable diagnoses.
TABLE 1. Comparison of controversies/key diagnostic criteria for microvascular ocular motor nerve palsies (as derived from Pane et al.29) with current evidence.
| Current clinical practice: all of the following (Pane et al., 2006)32 | Evidence from literature review | Reference | ||
|---|---|---|---|---|
| History | ||||
| Age | 40 or over | 93% occur in people over 50 years. | 11 | |
| 63% acute isolated 6th nerve palsy in patients aged 2–82 years (mean 48 years) had non-microvascular cause compared with 16.5% in patients aged >50 years. | 4, 16, 25, 43 | |||
| Risk factors | One or more vasculopathic risk factors (e.g. hypertension, diabetes, smoking) | Risk factors are hypertension, diabetes, left ventricular hypertrophy, hypercholesterolemia, smoking. | 12, 13, 14, 15 | |
| 60% of patients with non-microvascular palsies also had vascular risk factors | 4, 16 | |||
| All nerves | Sudden onset of diplopia | 75% 3rd nerve palsies progressed over 1–3 days with some progression for up to 15 days. | 12 | |
| Diplopia remains stable until spontaneous improvement | 54% 6th nerve palsies showed subsequent progression. | 19 | ||
| No persisting pain | 60% microvascular palsies are associated with pain. | 12 | ||
| 77% 3rd nerve palsy and 54% 6th nerve palsy have pain. | 17 | |||
| Diabetic 3rd nerve palsy, 77% had headache and 49% had periocular pain. | 15 | |||
| No numbness or pins and needles | – | – | ||
| No other systemic neurological symptoms | – | – | ||
| Third nerve only | Sudden onset of unilateral ptosis | |||
| Examination | ||||
| Third nerve | Complete ptosis, no movement on attempted elevation, depression or adduction | 25% complete and 75% partial weakness | 12 | |
| 45% complete paralysis, 48% partial weakness of all muscles, and 7% had weakness of specific extraocular muscles. | 18 | |||
| Entirely normal pupil (same size as other side, constricts briskly to light) | 75% have a normal pupil as opposed to 5–14% of compressive third nerve palsies. | 12, 18 | ||
| 26% diabetic 3rd nerve palsies had minor pupil dilatations (1– 2 mm). | 15 | |||
| 38% diabetic 3rd nerve palsies had minor pupil dilatation (≤1 mm). | 21 | |||
| Fourth nerve | Vertical or oblique deviation in the primary position on cover test Motility testing: hypertropia in the primary position that increases on gaze toward the side of the lower eye and on tilting of the head to the side of the higher eye | Degree of horizontal deviation is useful for making a determination between microvascular anddecompensation of congenital 4th nerve palsy. | ||
| Sixth nerve | Esotropia in primary position on observation and cover test Motility testing: unilateral restriction of abduction with slow abduction saccades | At 1 week 6% were complete and 94% were partial. | 19 | |
| All nerves | Diplopia, motility (and ptosis, 3rd nerve palsy only) begins to resolve within 3 months | 90.9% 3rd nerve palsies recovered completely within 12 months; 81.8% resolved within 3 months. | 2 | |
| 60% 4th nerve palsies recovered completely within 12 months. | 2 | |||
| 86% 6th nerve palsy had complete recovery after 6 years. Recovery is usually complete by 3 months. | 13 | |||
| Investigations | ||||
| MRI brain (plus MRA or CTA brain third nerve palsy only) if the palsy has not started to resolve by 3 months after onset or new neurologic symptoms or signs develop at any stage | MRI brain in patients over 50 years with isolated ocular motor palsies identified other causes (neoplasms, infarcts, aneurysms, demyelination, pituitary apoplexy) in 43/268 (16.5%). Tamhankar et al. found (4.7%) with 4th and 6th nerve palsy had other causes.16 once giant cell arteritis was excluded (infarction, lymphoma, and meningioma). | 4, 16, 25 | ||
| Treatment | ||||
| Treat risk factors (smoking, cholesterol, blood pressure, improve diabetic control): this could prevent a heart attack or stroke in the future | 14% of patients with microvascular sixth nerve palsy died; 7% from cardiac causes in follow-up period (range 2–13 years). | 11 | ||
| Commence long term low dose aspirin (unless contraindicated) to decrease future vascular risk | Retrospective case-control study of 100 patients with microvascular nerve palsy associated with diabetes and/or hypertension found 34% were using aspirin compared with 30.1% of diabetic/hypertensive patients without a microvascular nerve palsy. Patients with microvascular cranial nerve palsy have a significantly lower rate of strokes and transient ischaemic attacks compared to the control group (6% versus. 23.9%) possibly due to earlier use of aspirin. | 23 | ||
| Botulinum toxin injections to the ipsilateral medial rectus 3 months post 6th nerve palsy | Four RCTs on the therapeutic use of botulinum toxin in strabismus (due to any cause) have shown varying responses. Complication rates for use of Botox or Dysport ranged from 24% to 55.54%. | 44 | ||
The crucial two differential diagnoses that must be considered immediately in an isolated ocular motor palsy are giant cell arteritis (GCA) and posterior communicating artery (PComA) aneurysm. Giant cell arteritis (GCA) can present as isolated oculomotor nerve palsy. In 62 patients aged 50 years or older with acute isolated sixth nerve palsy, 3 had GCA.16 In this series, there were no reports of isolated third nerve palsies caused by GCA but at least 10 acute painful third nerve palsy both pupil involving and pupil sparing due to GCA are reported in the literature.33 In one study, 2/74 biopsy proven cases of GCA presented with diplopia.34 Therefore, all patients should have GCA excluded clinically by asking for GCA symptoms, including headache, jaw and tongue claudication, loss of vision in one eye, fever, myalgia, weight loss, anorexia, and fatigue, and with erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and full blood count (FBC) as per current guidelines.35,36 If doubt exists because of intermediate elevation of ESR and/or CRP, there should be a low threshold for treating for GCA and obtaining a temporal artery biopsy. Patients with GCA recover rapidly, over days, with steroids.33
Posterior communicating artery (PComA) aneurysm can cause an isolated third nerve palsy. The “rule of the pupil” states that 97% of third nerve palsies caused by compression by an aneurysm will involve the pupil. However, to apply this rule safely it is important to note Trobe’s four constraints: (1) use with great caution under 50 years of age, (2) do not use if the extraocular palsy is incomplete, (3) do not use if relatively pupil sparing, and (4) ensure that the third nerve palsy is isolated.37 Therefore, third nerve palsy is only “pupil sparing” if there is complete ptosis and complete external ophthalmoplegia while the pupils are the same size and react equally well to light and near stimuli. If the ophthalmoplegia and ptosis are only partial, then the third nerve palsy is not considered pupil sparing. All third nerve palsies that involve the pupil with greater than a 1-mm difference require urgent brain CT and CT angiogram (CTA). Clearly typical pupil-involving third nerve palsy patients need to be admitted until a PComA aneurysm has been excluded. The more borderline cases should ideally be imaged as an outpatient with a CT and CTA within 72 hours. The possibility of missing a posterior communicating aneurysm has resulted in differing views on the need to consider imaging in pupil-sparing complete third nerve palsies. There are rare reported cases of aneurysms causing third nerve palsy without pupillary involvement.38 Kupersmith et al. suggest that there is no need for a CTA in a patient with an isolated pupil-sparing complete third nerve palsy and no subarachnoid blood on CT.39 In contrast, Trobe states clinical features of a third nerve palsy do not provide adequate criteria to exclude aneurysm and suggest every non-pregnant adult patient with an isolated third cranial nerve palsy should have an urgent CTA.40
In theory, non-invasive vascular imaging (CTA or MRA) should be sensitive enough to detect nearly all aneurysmal third nerve palsies. Most reports have maintained that a PComA aneurysm needs to be ≥4 mm to cause a third nerve palsy, within the range of highest sensitivity for both current MRA and CTA machines (CTA [64 multidetector] = 99–100%, MRA [1.5 T] = 98–98.5%, MRA [3 T] ∼100%). One study reviewed all PComA aneurysmal third nerve palsies in their institution in 10 years and found 17 isolated PComA aneuryms producing third nerve palsies. Four of 17 were detected with subarachonoid haemorrhage on plain CT, 5/17 were detected with CTA, and 8/17 were missed due to inexperience or inappropriate training of radiologist. Therefore, the reporting radiologist is more important than the method used.41 Having excluded GCA and PComA aneurysms, the possibility of brainstem stroke, multiple sclerosis, or neoplasms remains. Tamhankar et al. found 10 such cases out of 109 (9%) (1 midbrain infarction, 1 inflammation, 2 pituitary apoplexy, and 6 tumours).16
Problems in Agreeing Management Guidelines
At one extreme is the probably correct view that overall this is a relatively easy condition to diagnose with a high probability of full recovery and a low chance of missing major treatable alternative diagnoses. This points at least in theory to a fairly “hands off” approach.
The opposite often happens in practice. Patients themselves can be extremely worried, they present to relatively inexperienced doctors in eye unit or hospital emergency department. Some are admitted to hospital and over-investigated because the diagnosis is treated as one of “exclusion” at the end of a lengthy process.
Is it possible to bridge these two contradictory realities with the patient expectation for investigation, fear of missing major diagnoses of medicolegal significance, the widely varied levels of experience of doctors involved and differences in ease of access to imaging?
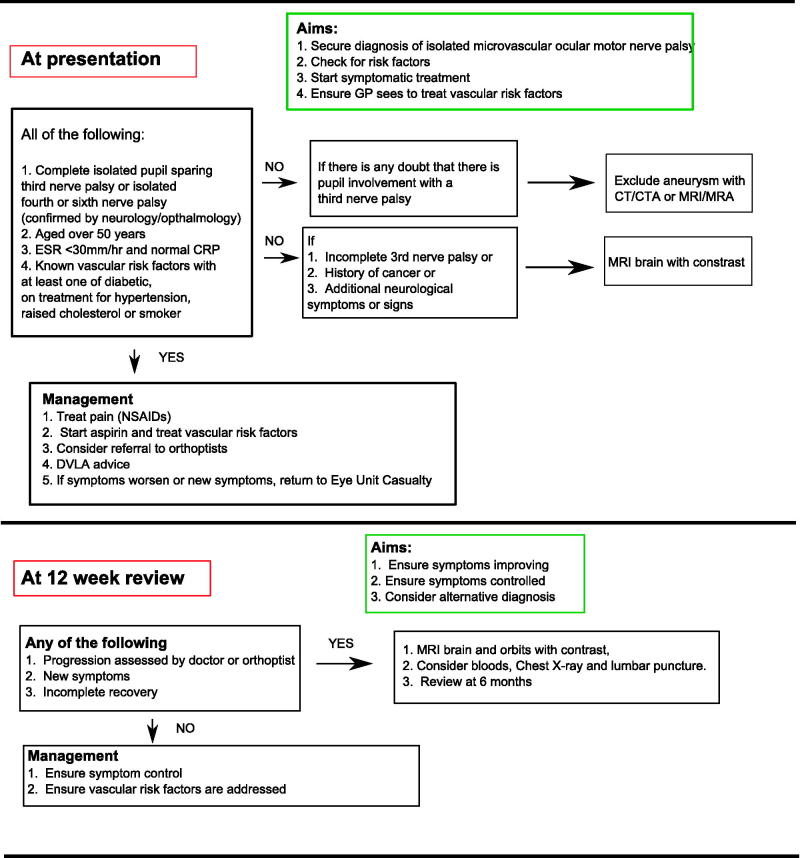
Suggesting a Practical Approach
At Presentation
We think this condition needs to be a positive diagnosis, made by a doctor (ophthalmologist or neurologist with adequate experience) from initial presentation, and suggest the following criteria:
An isolated complete pupil-sparing third or isolated fourth or sixth nerve palsy
Aged 50 years or over
ESR less than 30 and normal CRP
Known vascular risk factors: at least one of diabetes, hypertension, raised cholesterol or smoking.
If this is not possible, a senior clinician should decide if admission is indicated.
Patients diagnosed with microvascular palsies, as defined above, should not be sent home until GCA has been excluded. An unenhanced CT brain scan at presentation is not indicated, as this does not increase diagnostic sensitivity.18 All patients should have bloods sent for glucose and lipid profile and blood pressure taken to screen for diabetes, hypertension, and high cholesterol to assess cardiovascular disease risk, as cardiac risk is high in patients with microvascular nerve palsies.11,27
Patients with atypical features and those <50 years old need to be seen urgently, ideally within 24 hours, by an appropriate specialist who can decide on how to investigate further according to the presentation. Assessment by an orthoptist to confirm an isolated ocular motor nerve palsy (using a Hess chart) may be helpful at this juncture, especially if there is diagnostic doubt.
There are no trials on how to treat microvascular non-arteritic palsy, presumably because of the excellent rate of recovery. Common sense suggests that pain could be treated with non-steroidal anti-inflammatories and possibly a tricyclic antidepressant. In view of the high risk of stroke and cardiovascular disease, it is reasonable to start secondary prevention with aspirin where there are no contraindications and address any vascular risk factors. If they cannot tolerate aspirin, consider clopidogrel.
Practical management of double vision is with patching or Fresnel prisms (obtained via orthoptists’ input) as appropriate. The UK Driver and Vehicle Licensing Authority guidance states that patients should cease driving on developing double vision. If the diplopia is controlled by a patch or Fresnel prisms, they may drive.42
We propose the use of an information sheet (Supplementary Figure 1).
At 12-Week Review
Patients should be advised to present earlier to eye unit emergency department if their symptoms worsen.
All patients should be seen at about 12 weeks and if recovery is not considerable, contrast-enhanced MRI of brain and orbit should be requested. If the imaging is normal and the oculomotor nerve palsy is still isolated, it is still likely this is microvascular in origin, as 15–20% do not recover.2,13
However, the clinician should be aware this is atypical and repeat a detailed clinical history for other symptoms such as weight loss, fever, shortness of breath, and a full systemic and neurological examination. If there is any doubt about other symptoms, other investigations, including chest x-ray (for lymphadenopathy in sarcoidosis or lymphoma) and blood tests (e.g., antinuclear antibody [ANA] and anti-neutrophil cytoplasmic antibody [ANCA] for vasculitis, tumour markers for paraneoplastic syndromes), may be needed. If these are also normal, consider a lumbar puncture. Such patients should be reviewed again at 24 weeks.
Patients with isolated microvascular ocular motor nerve palsies are currently managed by a combination of ophthalmologists, neurologists, neuro-ophthalmologists, acute physicians, and stroke physicians. A single agreed clinical pathway after their initial presentation would be ideal. Neuro-ophthalmologists are best placed to diagnose, monitor, and manage these patients, with support from orthoptists. If not available, patients are best seen in a clinic led by any of the above specialists with a “special interest” and close liaison with others.
Conclusion
There is a great deal of evidence for the existence of a clinically uniform entity with a remarkably good prognosis of non-arteritic microvascular extraocular palsies. The controversies are around the clinical uncertainty at presentation. The risks of missing important alternative diagnoses at presentation are low as long as skilled clinical examination is sure of on isolated oculomotor palsy and the patients is over 50 years of age. Under 50 years, oculomotor palsies must always been investigated, as the incidence of other causes is high (63%).43 We suggest a pragmatic “user friendly” approach to diagnosis and management from initial presentation (Figure 4).
FIGURE 4.
Management of microvascular nerve palsies.
Acknowledgements
The authors thank Dr. Paul Riordan-Eva for detailed reading and helpful comments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figures 1, 2, 3, and 4 of this article are available in colour online at http://informahealthcare.com/oph.
Supplementary Material available online
Supplementary Figure 1: Supplementary content can be viewed and downloaded at http://informahealthcare.com/oph
References
- 1.Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology 2004;111:369–375 [DOI] [PubMed] [Google Scholar]
- 2.Akagi T, Miyamoto K, Kashii S, Yoshimura N. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol 2008;52:32–35 [DOI] [PubMed] [Google Scholar]
- 3.Park UC, Kim SJ, Hwang JM, Yu YS. Clinical features and natural history of acquired third, fourth, and sixth cranial nerve palsy. Eye (Lond) 2008;22:691–696 [DOI] [PubMed] [Google Scholar]
- 4.Chou KL, Galetta SL, Liu GT, Volpe NJ, Bennett JL, Asbury AK, Balcer LJ. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci 2004;219:35–39 [DOI] [PubMed] [Google Scholar]
- 5.Asbury AK, Algredge H, Hershberg R, Miller Fischer C. Oculomotor palsy in diabetes mellitus: a clinico-pathological study. Brain 1970;93:555–566 [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus PM, Hakim S, Adams RD. Diabetic ophthalmoplegia; report of case, with postmortem study and comments on vascular supply of human oculomotor nerve. AMA Arch Neurol Psychiatry 1957;77:337–349 [PubMed] [Google Scholar]
- 7.Weber RB, Daroff RB, Mackey EA. Pathology of oculomotor nerve palsy in diabetes. Neurology 1970;20:835–838 [DOI] [PubMed] [Google Scholar]
- 8.Kanda T. Biology of the blood–nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry 2013;84:208–212 [DOI] [PubMed] [Google Scholar]
- 9.Cahill M, Bannigan J, Eustace P. Anatomy of the extraneural blood supply to the intracranial oculomotor nerve. Br J Ophthalmol 1996;80:177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krisht A, Barnett DW, Barrow DL, Bonner G. The blood supply of the intracavernous cranial nerves: an anatomic study. Neurosurgery 1994;34:275–279 [DOI] [PubMed] [Google Scholar]
- 11.Sanders SK, Kawasaki A, Purvin VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol 2002;134:81–84 [DOI] [PubMed] [Google Scholar]
- 12.Capó H, Warren F, Kupersmith MJ. Evolution of oculomotor nerve palsies. J Clin Neuroophthalmol 1992;12:21–25 [PubMed] [Google Scholar]
- 13.Comer RM, Dawson E, Plant G, Acheson JF, Lee JP. Causes and outcomes for patients presenting with diplopia to an eye casualty department. Eye (Lond) 2007;21:413–418 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol 1994;112:961–966 [DOI] [PubMed] [Google Scholar]
- 15.Dhume KU, Paul KE. Incidence of pupillary involvement, course of anisocoria and ophthalmoplegia in diabetic oculomotor nerve palsy. Indian J Ophthalmol 2013;61:13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamhankar MA, Biousse V, Ying GS, Prasad S, Subramanian PS, Lee MS, Eggenberger E, Moss HE, Pineles S, Bennett J, Osborne B, Volpe NJ, Liu GT, Bruce BB, Newman NJ, Galetta SL, Balcer LJ. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology 2013;120:2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danchaivijitr C, Kennard C. Diplopia and eye movement disorders. J Neurol Neurosurg Psychiatry 2004;75:iv24–iv31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazerian P, Vanni S, Tarocchi C, Portaccio E, Vannucci N, Para O, Giannazzo G, Gigli C, Grifoni S. Causes of diplopia in the emergency department: diagnostic accuracy of clinical assessment and of head computed tomography. Eur J Emerg Med 2014;21:118–124 [DOI] [PubMed] [Google Scholar]
- 19.Moeller JJ, Kurniawan J, Gubitz GJ, Ross JA, Bhan V. Diagnostic accuracy of neurological problems in the emergency department. Can J Neurol Sci 2008;35:335–341 [DOI] [PubMed] [Google Scholar]
- 20.Flitcroft DI, Westcott M, Wormald R, Touquet R. Who should see eye casualties?: a comparison of eye care in an accident and emergency department with a dedicated eye casualty. J Accid Emerg Med 1995;12:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilker SC, Rucker JC, Newman NJ, Biousse V, Tomsak RL. Pain in ischaemic ocular motor cranial nerve palsies. Br J Ophthalmol 2009;93:1657–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders S, Kawasaki A, Purvin VA. Patterns of extraocular muscle weakness in vasculopathic pupil-sparing, incomplete third nerve palsy. J Neuroophthalmol 2001;21:256–259 [DOI] [PubMed] [Google Scholar]
- 23.Jacobson DM. Progressive ophthalmoplegia with acute ischemic abducens nerve palsies. Am J Ophthalmol 1996;122:278–279 [DOI] [PubMed] [Google Scholar]
- 24.Sunderland S, Hughes ES. The pupillo-constrictor pathway and the nerves to the ocular muscles in man. Brain 1946; 69:301–309 [DOI] [PubMed] [Google Scholar]
- 25.Jacobson DM. Pupil involvement in patients with diabetes-associated oculomotor nerve palsy. Arch Ophthalmol 1998;116:723–727 [DOI] [PubMed] [Google Scholar]
- 26.Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmol 2001;85:1225–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LN, Stetson SW, Krohel GB, Cipollo CL, Madsen RW. Aspirin use and the prevention of acute ischemic cranial nerve palsy. Am J Ophthalmol 2000;129:367–371 [DOI] [PubMed] [Google Scholar]
- 28.Murchison AP, Gilbert ME, Savino PJ. Neuroimaging and acute ocular motor mononeuropathies: a prospective study. Arch Ophthalmol 2011;129:301–305 [DOI] [PubMed] [Google Scholar]
- 29.Chi SL, Bhatti MT. The diagnostic dilemma of neuro-imaging in acute isolated sixth nerve palsy. Curr Opin Ophthalmol 2009;20:423–429 [DOI] [PubMed] [Google Scholar]
- 30.Kau HC, Tsai CC, Ortube MC, Demer JL. High-resolution magnetic resonance imaging of the extraocular muscles and nerves demonstrates various etiologies of third nerve palsy. Am J Ophthalmol 2007;143:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots—clinical applications. AJR Am J Roentgenol 2013;201:W326–W35 [DOI] [PubMed] [Google Scholar]
- 32.Pane A, Burdon M, Miller NR. Double vision. The neuro-ophthalmology survival guide. St. Louis, MO: Mosby, 2012:179–256 [Google Scholar]
- 33.Thurtell MJ, Longmuir RA. Third nerve palsy as the initialmanifestation of giant cell arteritis. J Neuroophthalmol 2014;34:243–245 [DOI] [PubMed] [Google Scholar]
- 34.Healey LA, Wilske KR. Presentation of occult giant cell arteritis. Arthritis Rheum 1980;23:641–643 [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta B; Giant Cell Arteritis Guideline Development Group Concise guidance: diagnosis and management of giant cell arteritis. Clin Med 2010;10:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014;371:50–55 [DOI] [PubMed] [Google Scholar]
- 37.Trobe JD. Third nerve palsy and the pupil. Footnotes to the rule. Arch Ophthalmol 1988;106:601–602 [DOI] [PubMed] [Google Scholar]
- 38.Motoyama Y, Nonaka J, Hironaka Y, Park YS, Nakase H. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo) 2012;52:202–205 [DOI] [PubMed] [Google Scholar]
- 39.Kupersmith MJ, Heller G, Cox TA. Magnetic resonance angiography and clinical evaluation of third nerve palsies and posterior communicating artery aneurysms. J Neurosurg 2006;105:228–234 [DOI] [PubMed] [Google Scholar]
- 40.Trobe JD. The rule of the pupil revisited: for pupil-involved third nerve palsy and aneurysm, is the diagnostic “gold standard” really a catheter angiogram in 2009? Presented at North American Neuroopthalmology Annual Meeting; Lake Tahoe, CA: 35th Annual NANOS Meeting, February 21–26, 2009. Available at: http://content.lib.utah.edu/cdm/ref/collection/ehsl-nam/id/127. Accessed August 2014
- 41.Elmalem VI, Hudgins PA, Bruce BB, Newman NJ, Biousse V. Underdiagnosis of posterior communicating artery aneurysm in noninvasive brain vascular studies. J Neuroophthalmol 2011;31:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Driver and Vehicle Licensing Agency. Current medical guidelines for professionals, 2013. Available at: https://www.gov.uk/current-medical-guidelines-dvla-guidance-for-professionals. Accessed August 2014
- 43.Bendszus M, Beck A, Koltzenburg M, Vince GH, Brechtelsbauer D, Littan T, Urbach H, Solymosi L. MRI in isolated sixth nerve palsies. Neuroradiology 2001;43:742–745 [DOI] [PubMed] [Google Scholar]
- 44.Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev 2012:CD006499. doi: 10.1002/14651858.CD006499.pub3 [DOI] [PubMed] [Google Scholar]