Abstract
Objective
The goal of the study was to investigate the role of histone deacetylases (HDACs) in adipocyte function associated with obesity and hypoxia.
Methods
Total proteins and RNA were prepared from human visceral adipose tissues (VAT) of human obese and normal weight subjects and from white adipose tissue (WAT) of C57Bl6-Rj mice fed a normal or high fat diet (HFD) for 16 weeks. HDAC activity was measured by colorimetric assay whereas the gene and protein expression were monitored by real-time PCR and by western blotting, respectively. RNA interference (RNAi) was used to silence the expression of genes in 3T3-L1 adipocytes.
Results
Total HDAC activity was decreased in VAT and WAT from obese individuals and from mice fed a HFD, respectively. The HDAC activity reduction was associated with decreased HDAC5/Hdac5 and HDAC6/Hdac6 expression in human and mice adipocyte fraction. Similarly, hypoxia hampered total Hdac activity and reduced the expression of Hdac5 and Hdac6 in 3T3-L1 adipocytes. The decrease of both Hdac5 and Hdac6 by hypoxia was associated with altered expression of adipokines and of the inducible cAMP early repressor (Icer), a key repressor that is defective in human and mice obesity. Silencing of Icer in adipocytes reproduced the changes in adipokine levels under hypoxia and obesity, suggesting a causative effect. Finally, modeling the defect of the two Hdacs in adipocytes by RNAi or selective inhibitors mimicked the effects of hypoxia on the expression of Icer, leading to impairment of insulin-induced glucose uptake.
Conclusion
Hdac5 and Hdac6 expression are required for the adequate expression of Icer and adipocyte function. Altered adipose expression of the two Hdacs in obesity by hypoxia may contribute to the development of metabolic abnormalities.
Keywords: Histone deacetylases, Adipocytes, Adipokines, Obesity, Insulin resistance
Highlights
-
•
Impaired adipose HDAC activity in human obese subjects and obese mice.
-
•
HDAC5 and HDAC6 expression is reduced in adipocytes of obese mice and human.
-
•
The expression of HDAC5, HDAC6 and ICER is altered by hypoxia in 3T3-L1 adipocytes.
-
•
ICER regulates hypoxia-sensitive adipokines expression.
-
•
Hdac5 and Hdac6 control the expression of ICER and glucose uptake in adipocytes.
1. Introduction
Insulin resistance is a key feature of obesity and is involved in the development of type 2 diabetes, fatty liver disease, cardiovascular disease, and cancer [1], [2], [3], [4]. Insulin resistance is associated with altered production of several adipokines (i.e. bioactive secreted products from adipocytes) that regulate insulin sensitivity and energy metabolism [5], [6], [7]. These adipokines include interleukin 6 (IL6), nicotinamide phosphoribosyltransferase (NAMPT, also called visfatin), leptin (LEP), angiotensinogen (AGT), Lipocalin 2 (LCN2), adiponectin (ADIPOQ), resistin (RETN), and SERPINE1 (also called plasminogen activator inhibitor type 1) [5], [6], [8]. These adipokines have been shown to induce insulin resistance in rodents [5], [9], [10].
In both human and mice obesity, hypoxia is thought to contribute to impaired adipokine production [11], [12], [13], [14]. Indeed, visceral adipose tissue (VAT) from obese subjects is characterized by impaired blood flow, defective capillary density, and impaired O2 partial pressure [12], [14]. Exposing mouse adipocytes to hypoxia leads to reduced expression of Adipoq, Agt, Lep, Nampt, and Retn, and, in contrast, to increased expression of Serpine1, Il6, and Lcn2 [8], [11]. However, the molecular mechanisms causing impaired adipokine production associated with hypoxia are still elusive. We postulate that both cAMP response element (CRE) binding protein activity in (CREB) and histone deacetylases are involved in these mechanisms. In support of this working hypothesis, hypoxia can stimulate CREB activity as observed in PC12 and lung cells [15], [16]. Adipocyte CREB activity is increased in obesity, leading to increased abundance of the activating transcription factor 3 (ATF3) [17], [18]. This increased ATF3 activity hampers the expression of Adipoq and glucose transporter GLUT4, ultimately leading to impairment in insulin-induced glucose uptake [17], [18]. The CREB-dependent activation mechanism is initiated by reduction in the content of inducible cAMP early repressor (ICER), a natural antagonist of CREB and other cAMP-dependent transcription factors [17]. Reduction of ICER was found in adipocytes of human obese individuals and mice fed a high fat diet (HFD) for 16 weeks [17]. In this study, therefore, we hypothesized that defective deacetylase activity may account for the collapse in the ICER level in obesity. Indeed, the expression of ICER is reported to be positively regulated by histone deacetylase activity (HDACs) in PC12 cells [19]. Overall, HDACs are pivotal in epigenetic mechanisms that permit gene expression adaptation to environmental changes [20]. There are 3 classes of HDACs [21], [22]: classes I, II and IV. Class I HDACs comprises HDAC1, HDAC2, HDAC3, and HDAC8. Class II HDACs is divided into subclass IIa (HDAC4, HDAC5, HDAC7, and HDAC9) and subclass IIb (HDAC6 and HDAC10). Class IV contains includes HDAC11 only. So far, selective inhibition of HDACs is a strategy for treating many cancers [22]. Additionally, there is emerging evidence implicating Hdac activity in the control of energy metabolism, thus opening an avenue for future targets in metabolic diseases [23]. In the hypothalamus of obese mice fed a HFD, the expression of Hdacs, including Hdac5, is modified when compared to that of mice fed a chow diet [24], [25]. Hdac5 is required for hypothalamic leptin signaling and food intake, as Hdac5 knockout mice display defective hypothalamic leptin signaling and are more prone to diet-induced obesity compared to wild-type mice [25]. Given the role of HDACs in obesity, we hypothesized the contribution of HDACs to the changes in adipokine expression elicited by hypoxia and obesity-associated adipocyte dysfunction.
2. Material and methods
2.1. Materials
Trichostatin (TSA), tubastatin and LMK293 were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Biopsies and RNA preparation
Total RNA was extracted from epididymal white adipose tissue (WAT) of mice fed a HFD (n = 10) or normal chow diet (n = 10). WAT isolation was performed in euthanized animals in accordance to the Swiss legislation for animal experimentation. Approximately 5 cm3 of VAT was obtained at the level of the omentum from five obese Caucasian women (BMI >35 kg/m2) who were referred for weight reduction surgery and five non-obese Caucasian women (24 < BMI <28 kg/m2) [17]. All patients provided informed consent, and the study was approved by the institutional review board [17]. The criteria for exclusion and phenotyping are those previously described [17]. Total RNA was isolated from adipose tissues and different cell fractions with the TriPure isolation reagent (Roche) as previously described [17]. Procedure for preparation of adipocytes and SVF fractions was done as described [17].
2.3. Cell culture and transfection
Culture and differentiation of 3T3-L1 cells were conducted as described [17]. Briefly, 3T3-L1 cells were grown and maintained in Dulbecco's modified Eagle's medium high glucose containing 50 units/ml penicillin, 50 μg/ml streptomycin, and 10% fetal calf serum (FCS) in a 10% CO2 environment. At postconfluency (2 days), the cells were differentiated by adding to the culture medium, isobutylmethylxanthine (500 μM), dexamethasone (25 μM), and insulin (4 μg/ml) for 3 days and then only with insulin for 3 more days. The medium was then changed every 3 days until the cells were fully differentiated, typically by 10 days. The 19-nt small interfering RNA (siRNA) duplex against Icer (siIcer) is described elsewhere [17]. The siRNA duplexes, targeting the GFP (5′-CGCTGACCCTGAAGTTCAT-3′), the mouse Hdac5 (5′-GCAAGCATTCTACAACGAT-3′), and Hdac6 (5′-CCAGGACGATCTCCAAGAT-3′) were purchased from Microsynth (Balgach, Switzerland). For silencing Icer, Hdac5, and Hdac6, on day 7 post-differentiation, 3T3-L1 adipocytes were electroporated with siRNAs using the GenePulser XCell (Bio-Rad) as previously described [17].
2.4. Western blotting and quantitative PCR
Protein extracts, western blotting, and real-time quantitative PCR were conducted as previously described [17]. PCR assays were carried out on a BioRad MyiQ Single-Color Real-Time PCR Detection System using the BioRad iQ SYBR Green Supermix, with 100 nM primers and 1 μl of template per 20 μl of PCR and an annealing temperature of 60 °C. The primer sequences are available in the supplementary materials.
2.5. 2-Deoxyglucose (2-DOG) uptake assay
2-deoxy-d-[1,2-3H]glucose (2-[3H]DOG, 26.2 Ci/mmol) uptake assays were conducted on fully differentiated 3T3-L1 adipocytes (days 7 and 8) as previously described [17]. Adipocytes were treated without (basal) or with insulin 10 nM for 10 min. 2-[3H]DOG (0.1 μCi; final concentration, 0.01 mmol/l) and 5 mM cold 2-DOG were then added for an additional 10 min at 37 °C. 2-DOG uptake was terminated by washing the cells three times with ice-cold PBS containing 10 mM glucose. Subsequently, cells were lysed in 1% (wt/vol) SDS and 0.2 M NaOH. Incorporated radioactivity was measured by liquid scintillation spectrometry.
2.6. Statistical analysis
The experiments including two groups were analyzed by t-test or with the non-parametric equivalent Wilcoxon.
3. Results
3.1. HDAC5/Hdac5 and HDAC6/Hdac6 mRNA levels are reduced in adipocytes from obese human subjects and from obese mice
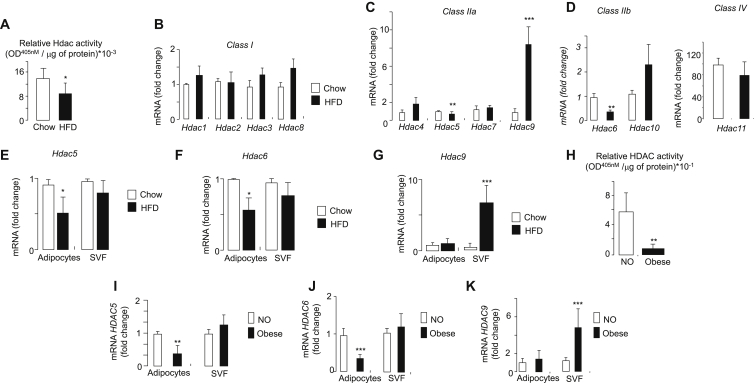
The total Hdac activity was monitored in WAT of mice that were fed a chow diet or a HFD for 16 weeks. We and others have shown previously that mice gain more weight, develop more adipose tissue, and develop systemic insulin resistance when fed a HFD [17]. The total Hdac activity was significantly decreased in the WAT of HFD mice (Figure 1A). Decreased Hdac activity was associated with a significant drop of classes IIa and IIb, Hdac5 and Hdac6 expression, respectively (Figure 1C,D). The level of all Hdac mRNA as well as the reduction of Hdac5 and Hdac6 expression was confirmed while the qRT-PCR analyses were normalized against the TATA box binding protein mRNA, for which we found that the level was also stable among the mice. In contrast, Hdac9 expression was significantly increased whereas the expression of class I Hdacs (Hdac1-3 and Hdac8) was unchanged in obese vs control mice (Figure 1B). The diminution of the two Hdacs had originated in the mice adipocyte fraction (Figure 1E,F), whereas the increased expression of Hdac9 was observed only in the adipose stromal-vascular fraction (SVF) of obese mice (Figure 1G). Similar results were found in human adipocytes and in SVF fractions of VAT from obese individuals (Figure 1H–K). The collapse of total HDAC activity (Figure 1H) was associated with a decrease in HDAC5 and HDAC6 expression in human adipocyte fraction (Figure 1I,J). As observed in mice, the HDAC9 expression was augmented in SVF (Figure 1K). These results indicate a decrease in adipocyte expression of HDAC5 and HDAC6 in human and mice obesity
Figure 1.
Measurement of Hdac/HDAC activity and expression from adipose tissues, adipocytes and svf in diet-induced obese mice, non-obese and obese individuals. A) Hdac activity in WAT of mice fed with regular or HFD. Total proteins were prepared from WAT of mice that were fed with regular (open bars, Chow, n = 10 mice) or HFD (filled bars, HFD, n = 10 mice). HDACs activity was measured by direct colorimetric assay kit (Epigentek). Data are the mean ± SEM of 3 independent experiments (*p < 0.05). Quantification of B) Class I Hdac1/2/3/8, C) Class IIa Hdac4/5/7/9D) Class IIb Hdac6/10 IIb and IV Hdac11 mRNA levels. The mRNA of E) Hdac5, F) Hdac6, and G) Hdac9 was quantified by PCR in adipocytes and stroma vascular fraction (SVF) that were collected from WAT from control (open bars, Chow) and obese mice (filled bars, HFD). The Hdac mRNA levels were determined by quantitative real-time PCR and were normalized against the housekeeping acidic ribosomal phosphoprotein P0 gene (Rplp0). Similar results were obtained while normalizing against TBP. The results were expressed as the fold changes over the controls. Data are the mean of ±SEM of 3 independent experiments (***P < 0.001; **P < 0.01; *P < 0.05). H) HDAC activity in VAT of non-obese and obese individuals. Total protein concentrations were prepared from VAT of non-obese (open bars, NO) or obese individuals (filled bars, Obese) and were subjected to colorimetric assay kit (Epigentek). Data are the mean ± SEM of 3 independent experiments (**P < 0.01). Quantification of I) HDAC5, J) HDAC6, and K) HDAC9 mRNA in adipocytes and SVF from VAT of non-obese (open bars, NO) or obese individuals (filled bars, Obese). The HDAC mRNA levels were determined by quantitative real-time PCR. The mRNA levels were normalized against the RPLP0 and were expressed as the fold changes over the controls. Data are the mean of ±SEM of 3 independent experiments (**P < 0.01; *P < 0.05).
3.2. Hypoxia mimics the reduction of Hdac5/HDAC5 and Hdac6/HDAC6 mRNA and the loss of Icer activity in obesity
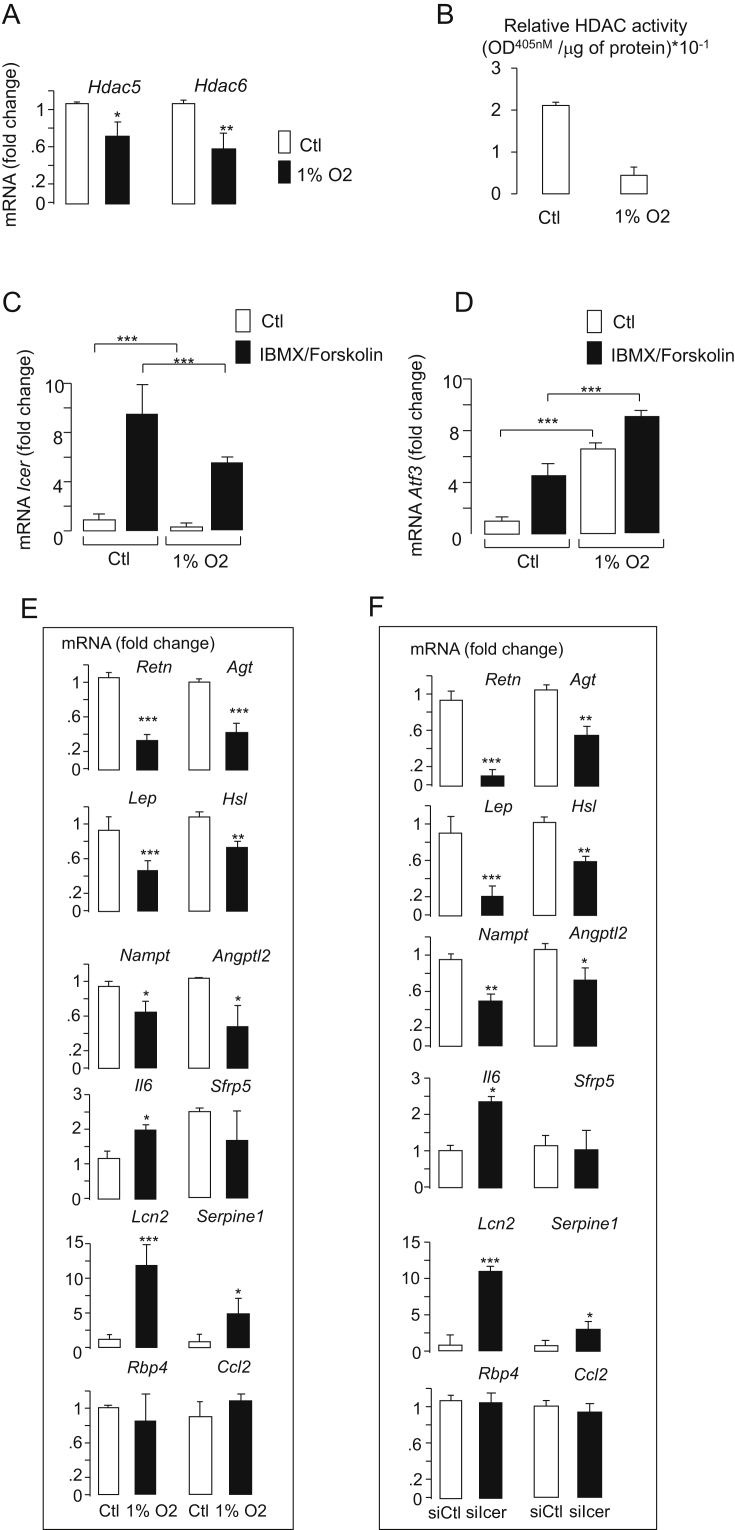
We hypothesized that the reduction of Hdac activity and mRNA level of Hdac5 and Hdac6 in adipocytes is produced by hypoxia. Hypoxia results in increased lactate release, which is associated with an increase of mRNAs of monocarboxylate transporters 1 and 4 (Mct1 and Mct4, respectively) [26]. Here, we confirmed that Mct1 and Mct4 levels were increased in 3T3-L1 adipocytes cultured under hypoxia for 24 h (Figure S1). Consistent with the induction of lactate metabolism by hypoxia, the expression of both lactate dehydrogenase gene (Ldha) and pyruvate dehydrogenase kinase 1 gene (Pdk1) were augmented (Figure S1). These hypoxia-induced changes were associated with decreased expression of both Hdac5 and Hdac6 (Figure 2A). Consistently, the reduction of both Hdac5 and Hdac6 in response to hypoxia was associated with a diminution of the total Hdac activity (Figure 2B). The expression of hypoxia-sensitive genes has been shown to rely on the increase of CREB activity [27], [28]. Alteration of Icer level expression may account for defective adipokine gene expression and increased CREB activity caused by hypoxia. The expression of Icer, therefore, was quantified in 3T3-L1 cells exposed to hypoxia. We found that hypoxia alleviated the expression of Icer mRNA under non-stimulatory conditions and in response to IBMX and Forskolin (Figure 2C). Parallel to the diminution of Icer expression, as expected, the CREB target Atf3 level was increased (Figure 2D), confirming an increase of CREB transcriptional activity by hypoxia. This result further suggested that reduced Icer expression modifies the expression of hypoxia-inducible adipokines. Indeed, hypoxia affects the expression of numerous genes encoding key adipokines involved in insulin resistance, including Il6, Nampt, Agt, Lep, Adipoq, Lcn2, Retn, and Serpine1 [8], [11], [12], [29], [30], [31], [32]. Culture of 3T3-L1 adipocytes under hypoxia significantly increased the level of Il6, Serpine1, and Lcn2, whereas it reduced the mRNA of Retn, Agt, Lep, Nampt, and Adipoq (Figure 2E). The expression of these adipokines was monitored in 3T3-L1 adipocytes in which the expression of Icer was silenced by small interfering RNAs (siIcer). The efficiency and specificity of siIcer for reducing Icer abundance in 3T3-L1 adipocytes has been reported in our previous study [17]. Here, we showed that Icer silencing significantly increased the expression of Il6, Lcn2, and Serpine1, whereas that of Retn, Agt, Lep, Nampt, and Adipoq was significantly reduced (Figure 2F). In humans, similar changes in adipokine levels were observed in the VAT of obese subjects (Figure S2), in whom we previously found a reduction in Icer activity [17]. Altogether, these data support a role for adipocyte Hdac5 and Hdac6 in the control of Icer level and thereby in hypoxia-sensitive adipokine expression in obesity.
Figure 2.
Effects of hypoxia on Hdac activity and gene expression. Measurement of A) Hdac5 and Hdac6, B) Hdac activity, C) Icer and D) Atf3 mRNA levels in hypoxic 3T3-L1 adipocyte cells. 3T3-L1 adipocyte cells were exposed for 12 h to normoxia or hypoxia (1% O2). For inducing the expression of Icer and Atf3 in C) and D), cells under normoxia or hypoxia were further incubated with DMSO (Ctl) or a mixture of cAMP-raising agents IBMX (100 μM)/Forskolin (10 μM) for 4 h. Quantification of adipokine mRNA by qRT-PCR in E) 3T3-L1 adipocyte cells that were exposed for 12 h to normoxia or hypoxia (1% O2) and F) upon silencing of Icer. 3T3-L1 adipocytes cells were electroporated with siGFP (siCtl, open bars) or siIcer (filled bars) for 72 h. The mRNA levels were normalized against the Rplp0 and were expressed as the fold changes over the controls. Data are the mean of ±SEM of 3 independent experiments (***P < 0.001; **P < 0.01; *P < 0.05).
3.3. Hdac5 and Hdac6 levels are required for the expression of Icer and glucose uptake
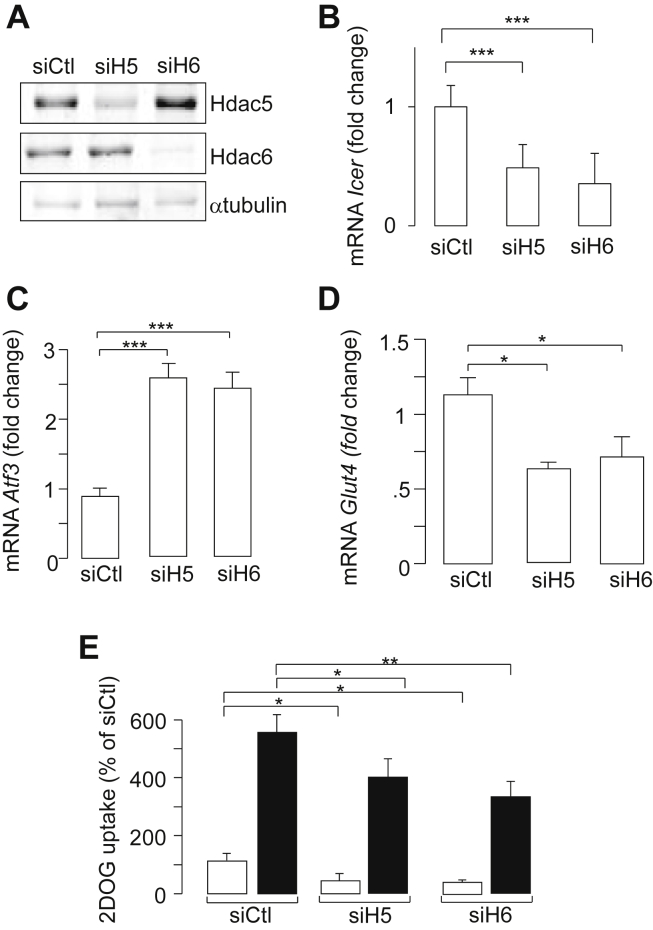
We next investigated the role of Hdac5 and Hdac6 levels on Icer expression. The efficient silencing of both Hdac5 and Hdac6 in 3T3-L1 adipocytes using small interfering RNAs duplexes (siH5 and siH6) was shown by Western Blotting analyses (Figure 3A). The reduction of Hdac5 or Hdac6 level diminished the expression of Icer in 3T3-L1 adipocytes (Figure 3B). The induction of Icer by cAMP raising agents IBMX and Forskolin was also attenuated upon silencing of both Hdacs (Figure S3a). This result was confirmed using Hdac inhibitors. The pan-HDAC inhibitor trichostatin (TSA) attenuated basal Icer expression (Figure S3b) and the induced expression of repressor in response to cAMP raising agents IBMX and Forskolin in 3T3-L1 adipocytes (Figure S3c). LMK 235 and tubastatin A are highly selective Hdac5 and Hdac6 inhibitors, respectively [33], [34]. Culture of 3T3-L1 adipocytes with either LMK 235 or tubastatin A reproduced the effects of TSA on the expression of Icer (Figure S3c). In agreement with the decrease of Icer level by siH5 or siH6, we observed that the expression of Atf3 and Glut4 was increased (Figure 3C,D). As anticipated, we found that the reduction of Icer by siH5 or siH6 was accompanied by an impairment of insulin-induced DOG uptake in 3T3-L1 adipocytes (Figure 3E), thus supporting a role for HDAC5 and HDAC6 in the glucose transport in a mechanism involving ICER (Figure 4).
Figure 3.
Effect of silencing of Hdac5 and Hdac6 levels in 3T3-L1 adipocytes. A) Efficiency of siRNAs on Hdac5 and Hdac6 content by Western Blotting. 3T3-L1 adipocytes were electroporated with 5 nmol of control small interfering RNA duplexes directed against GFP (siCtl) or with siRNAs against Hdac5 (siH5) or Hdac6 (siH6). Total proteins were subjected to Western blotting experiments for the quantification of Hdac5, Hdac6, and α-tubulin as a loading control. The figure shows the results of a representative experiment out of three. Effect of siH5 and siH6 on the B) expression of Icer, C) Atf3, and D) Glut4 mRNA. Gene expression was monitored by quantitative real-time PCR 72 h after transfection with either control small interfering RNA duplexes directed against GFP (siCtl), or siH5, or siH6. mRNA levels were normalized against the Rplp0 and were expressed as the fold changes over the controls. Data are the mean of ±SEM of at least 3 independent experiments (***P < 0.001). E) Efficiency of siH5 and siH6 on the 2-deoxy-glucose (DOG) uptake. Measurement of the labeled DOG in 3T3-L1 adipocytes cells was done 96 h after electroporation with siRNAs by measuring [1-3H]2-DOG (100 μm, 0.5 μCi/ml) uptake from unstimulated cells (open bar) or stimulated cell with 10 nM insulin (filled bar). Data are the mean of ±SEM of 4 independent experiments (**P < 0.01; *P < 0.05).
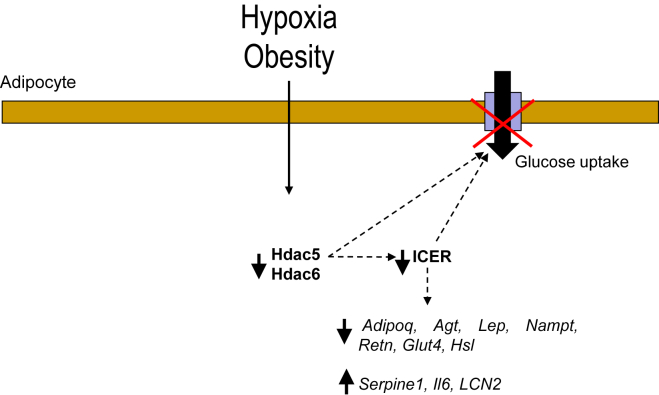
Figure 4.
Schematic representation for the role of Hdac5 and Hdac6 in coupling hypoxia and obesity to defective Icer expression and adipocyte dysfunction.
4. Discussion
Adipose reduction of Icer may be instrumental in the link between obesity and systemic insulin resistance. In the present study, we showed that the reduction of Icer in adipocytes of both obese mice and obese human subjects is the consequence of impaired HDAC5/Hdac5 and HDAC6/Hdac6 expression. Indeed, among the 11 Hdacs expressed in adipose tissue, only these two enzymes were down regulated, which was sufficient to lead to an overall reduction of Hdac activity and a reduction of Icer expression. This result was further supported by the silencing of Hdac5 or Hdac6 in 3T3-L1 adipocytes that also hampered Icer expression. Post-translational modifications can modulate nuclear activity and translocation of transcriptional regulators [35], [36]. We speculate that the defective activity of the two Hdacs in adipocytes leads to re-acetylation and thereby inhibition of some transcription factor(s) required for the Icer expression. In this case, increased Icer expression may result from silencing the transcriptional repressor. This mechanism has been suggested for the positive regulation of Icer by HDAC activity in PC12 cells [19]. We speculate that HDAC5 and HDAC6 activate the expression of Icer in adipocytes via a similar mechanism. Such regulation by the two HDACs may further rely on a concerted mechanism. The two HDACs may be co-localized in the nucleus within the gene coding for Icer. HDAC5 and HDAC6 can translocate from the nucleus to the cytosol depending on the cell type and stress condition [37], [38], [39]. Moreover interaction of class IIa and class IIb HDACs is possible. This was shown for HDCA6 and HDAC9 in GnRH neuronal cells for modulating cell movement and survival [40]. Therefore, it is possible that HDAC5 and HDAC6 act in concert for positively regulating the expression of Icer via an indirect mechanism involving similar nuclear or cytoplasmic targets in adipocyte. In contrast to adipocytes, in SVF of obese subjects and mice, ICER/Icer expression is increased [17]. We show in this study that increased ICER/Icer is associated with the elevation of Hdac9/HDAC9 expression. Future studies are needed to determine whether HDAC9 accounts for the increase of Icer and thereby impacts cell function in SVF.
Icer is a passive transcriptional repressor that prevents binding of the CREB transcriptional activator within CRE, leading to gene silencing. The antagonistic effect of ICER is a major mechanism that permits cells to return to their basal state upon cAMP-raising conditions such as fasting and glucagon and beta-adrenergic stimulation. In obesity, the decrease of ICER/Icer in adipocytes increases the binding of CREB within the CRE of its target genes [17], [18], increasing the transcriptional activity of CREB increases and the expression of its target ATF3 [17], [18]. Herein, we showed not only ATF3 but also IL6, Serpine1, and Lcn2 are increased, providing further evidence for a defective CREB pathway in obesity. In contrast, the expression of Retn, Agt, Lep, Nampt, and Adipoq were decreased upon Icer silencing. This suggests that they are indirect targets of CREB.
The dysregulation of adipokines and altered Hdac5 and Hdac6 levels in obesity are mimicked by hypoxia. We showed in 3T3-L1 adipocytes that the changes in adipokine levels caused by hypoxia are associated with impaired expression of the two Hdacs. We also found that adipokine dysregulation caused by hypoxia is the consequence of defective Icer activity. Silencing Icer in 3T3-L1 adipocytes mimicked the effect of hypoxia on the expression of adipokines. Finally, we propose that the reduction in Icer expression couples defective Hdac5 and Hdac6 activities to perturbed expression of adipokines and adipocyte dysfunction caused by hypoxia and/or obesity (Figure 4). Global methylation of histones and DNA is modified in adipose tissue from obese subjects [41], [42]. The main function of HDAC is to deacetylate histones and non-histone proteins. Moreover, each HDAC can have several targets. A reduction in Hdac5 and Hdac6 expression, therefore, may lead to re-acetylation of several cytosolic and/or nuclear proteins. In other words, the acetylome of adipocyte may be modified in obesity. Some nuclear re-acetylated proteins may contribute directly or indirectly to the deregulation of Icer and thereby to impaired adipokine production, adipocyte dysfunction, and, ultimately, systemic insulin resistance in the context of obesity.
Our study supports the requirement of maintaining Hdac5 and Hdac6 levels for optimal adipose function including glucose uptake and adipokine genes expression. Appropriate expression of Hdac5 has been shown to be required for energy metabolism in mice [25]. The expression of Hdac5 is indeed decreased in the hypothalami of obese mice [25]. Genetic and pharmacological inhibition of hypothalamic Hdac5 impairs leptin signaling and has deleterious effects on food intake and body-weight control [25]. Inhibition of some Hdacs, therefore, may be detrimental for energy metabolism in human. While inhibition of the class I HDAC Hdac3 has been seen as a promising therapeutic strategy for diabetes [23], [43], [44], [45], [46], inhibition of Hdac8, in contrast, may cause insulin resistance [47]. Moreover, there is a large spectra of class II HDAC inhibitors that are currently used for the treatment of cancers [22]. For example, belinostat, abexinostat, and trichostatin target several HDACs including HDAC5 and HDAC6 [22], [34]. Therefore, it is possible that inhibitors targeting Hdac5 and Hdac6 affect adipose function and ultimately lead to insulin resistance. For optimal therapeutic metabolic impact, our study suggests the need of refining highly specific HDAC inhibitors with low affinity for HDAC5 and HDAC6.
5. Conclusion
We have identified altered adipocyte Hdac5 and Hdac6 expression in obesity and associated hypoxia. Modeling the defect of the two Hdacs in adipocytes by genetic silencing and selective inhibitors mimicked the effect of hypoxia and obesity on the expression of key adipokines and of Icer, the key regulator of adipokine transcription. We believe that our findings provide a significant hint for better understanding the mechanism responsible of adipose dysfunction, obesity related insulin resistance, and metabolic complications.
Acknowledgements
This work was supported by INSERM, “Métropole Européenne de Lille (MEL)” and grants from “European Genomic Institute for Diabetes” (E.G.I.D., ANR-10-LABX-46) and European Commission, European Research Council (GEPIDIAB 294785 to P.F.). AA conceived and designed the project. JB, DF, SB, RB, RS, VP, MC, and AA performed the experiments and analyzed the data. GCG, BS, RC, FP provided the human samples, contributed to discussion and reviewed the manuscript. JB, PF, AB, and AA wrote and edited the paper.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.09.011.
Conflict of interest
The authors declare no conflict of interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Reaven G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Moller D.E., Flier J.S. Insulin resistance–mechanisms, syndromes, and implications. New England Journal of Medicine. 1991;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 3.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G.M. Pathophysiology of insulin resistance in human disease. Physiological Reviews. 1995;75(3):473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 5.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes & Metabolism. 2004;30(1):13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B., Wood I.S., Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455(3):479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law I.K.M., Xu A., Lam K.S.L., Berger T., Mak T.W., Vanhoutte P.M. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q., Li L., Li R., Yang M., Liu H., Nowicki M.J. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Annals of Medicine. 2009;41(4):311–320. doi: 10.1080/07853890902729760. [DOI] [PubMed] [Google Scholar]
- 11.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 12.Wood I.S., de Heredia F.P., Wang B., Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proceedings of the Nutrition Society. 2009;68(4):370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiological Reviews. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 14.Ye J., Gao Z., Yin J., He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(4):E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 15.n.d. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. http://www.ncbi.nlm.nih.gov.gate2.inist.fr/pmc/articles/PMC2643223/. [Accessed 11 May 2016]. [DOI] [PMC free article] [PubMed]
- 16.Chang J.H., Vuppalanchi D., van Niekerk E., Trepel J.B., Schanen N.C., Twiss J.L. PC12 cells regulate inducible cyclic AMP (cAMP) element repressor expression to differentially control cAMP response element-dependent transcription in response to nerve growth factor and cAMP. Journal of Neurochemistry. 2006;99(6):1517–1530. doi: 10.1111/j.1471-4159.2006.04196.x. [DOI] [PubMed] [Google Scholar]
- 17.Favre D., Le Gouill E., Fahmi D., Verdumo C., Chinetti-Gbaguidi G., Staels B. Impaired expression of the inducible cAMP early repressor accounts for sustained adipose CREB activity in obesity. Diabetes. 2011;60(12):3169–3174. doi: 10.2337/db10-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi L., Saberi M., Zmuda E., Wang Y., Altarejos J., Zhang X. Adipocyte CREB promotes insulin resistance in obesity. Cell Metabolism. 2009;9(3):277–286. doi: 10.1016/j.cmet.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fass D.M., Butler J.E., Goodman R.H. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. Journal of Biological Chemistry. 2003;278(44):43014–43019. doi: 10.1074/jbc.M305905200. [DOI] [PubMed] [Google Scholar]
- 20.Ling C., Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58(12):2718–2725. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra M. Class IIa HDACs – new insights into their functions in physiology and pathology. FEBS Journal. 2015;282(9):1736–1744. doi: 10.1111/febs.13061. [DOI] [PubMed] [Google Scholar]
- 22.West A.C., Johnstone R.W. New and emerging HDAC inhibitors for cancer treatment. The Journal of Clinical Investigation. 2014;124(1):30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen D.P., Dahllof M., Lundh M., Rasmussen D.N., Nielsen M.D., Billestrup N. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Molecular Medicine. 2011;17(5–6):378–390. doi: 10.2119/molmed.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funato H., Oda S., Yokofujita J., Igarashi H., Kuroda M. Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One. 2011;6(4):e18950. doi: 10.1371/journal.pone.0018950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabra D.G., Pfuhlmann K., García-Cáceres C., Schriever S.C., Casquero García V., Kebede A.F. Hypothalamic leptin action is mediated by histone deacetylase 5. Nature Communications. 2016;7:10782. doi: 10.1038/ncomms10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez de Heredia F., Wood I.S., Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch. 2010;459(3):509–518. doi: 10.1007/s00424-009-0750-3. [DOI] [PubMed] [Google Scholar]
- 27.Kvietikova I., Wenger R.H., Marti H.H., Gassmann M. The hypoxia-inducible factor-1 DNA recognition site is cAMP-responsive. Kidney International. 1997;51(2):564–566. doi: 10.1038/ki.1997.80. [DOI] [PubMed] [Google Scholar]
- 28.Kvietikova I., Wenger R.H., Marti H.H., Gassmann M. The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Research. 1995;23(22):4542–4550. doi: 10.1093/nar/23.22.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benita Y., Kikuchi H., Smith A.D., Zhang M.Q., Chung D.C., Xavier R.J. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Research. 2009;37(14):4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalán V., Gómez-Ambrosi J., Rodríguez A., Ramírez B., Silva C., Rotellar F. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. The Journal of Nutritional Biochemistry. 2011;22(7):634–641. doi: 10.1016/j.jnutbio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Liu S.-S., Wang H.-Y., Tang J.-M., Zhou X.-M. Hypoxia-induced collagen synthesis of human lung fibroblasts by activating the angiotensin system. International Journal of Molecular Sciences. 2013;14(12):24029–24045. doi: 10.3390/ijms141224029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trayhurn P., Wang B., Wood I.S. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? British Journal of Nutrition. 2008;100(2):227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 33.Butler K.V., Kalin J., Brochier C., Vistoli G., Langley B., Kozikowski A.P. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. Journal of the American Chemical Society. 2010;132(31):10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marek L., Hamacher A., Hansen F.K., Kuna K., Gohlke H., Kassack M.U. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. Journal of Medicinal Chemistry. 2013;56(2):427–436. doi: 10.1021/jm301254q. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Ma Z., Wang J., Li S., Zhang Y., Wang Y. Regulation of Hsf4b nuclear translocation and transcription activity by phosphorylation at threonine 472. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 2014;1843(3):580–589. doi: 10.1016/j.bbamcr.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Daitoku H., Sakamaki J., Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochimica et Biophysica Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Cho Y., Sloutsky R., Naegle K.M., Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hai Y., Christianson D.W. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nature Chemical Biology. 2016;12(9):741–747. doi: 10.1038/nchembio.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Shin D., Kwon S.H. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS Journal. 2013;280(3):775–793. doi: 10.1111/febs.12079. [DOI] [PubMed] [Google Scholar]
- 40.Salian-Mehta S., Xu M., McKinsey T.A., Tobet S., Wierman M.E. Novel interaction of class IIb histone deacetylase 6 (HDAC6) with class IIa HDAC9 controls gonadotropin releasing hormone (GnRH) neuronal cell survival and movement. Journal of Biological Chemistry. 2015;290(22):14045–14056. doi: 10.1074/jbc.M115.640482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benton M.C., Johnstone A., Eccles D., Harmon B., Hayes M.T., Lea R.A. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biology. 2015;16:8. doi: 10.1186/s13059-014-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamura M., Inagaki T., Tanaka T., Sakai J. Role of histone methylation and demethylation in adipogenesis and obesity. Organogenesis. 2010;6(1):24–32. doi: 10.4161/org.6.1.11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundh M., Christensen D.P., Damgaard Nielsen M., Richardson S.J., Dahllof M.S., Skovgaard T. Histone deacetylases 1 and 3 but not 2 mediate cytokine-induced beta cell apoptosis in INS-1 cells and dispersed primary islets from rats and are differentially regulated in the islets of type 1 diabetic children. Diabetologia. 2012;55(9):2421–2431. doi: 10.1007/s00125-012-2615-0. [DOI] [PubMed] [Google Scholar]
- 44.Plaisance V., Rolland L., Gmyr V., Annicotte J.-S., Kerr-Conte J., Pattou F. The class I histone deacetylase inhibitor MS-275 prevents pancreatic beta cell death induced by palmitate. Journal of Diabetes Research. 2014;2014:195739. doi: 10.1155/2014/195739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galmozzi A., Mitro N., Ferrari A., Gers E., Gilardi F., Godio C. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes. 2013;62(3):732–742. doi: 10.2337/db12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier B.C., Wagner B.K. Inhibition of HDAC3 as a strategy for developing novel diabetes therapeutics. Epigenomics. 2014;6(2):209–214. doi: 10.2217/epi.14.11. [DOI] [PubMed] [Google Scholar]
- 47.Tian Y., Wong V.W.S., Wong G.L.H., Yang W., Sun H., Shen J. Histone deacetylase HDAC8 promotes insulin resistance and β-catenin activation in NAFLD-associated hepatocellular carcinoma. Cancer Research. 2015;75(22):4803–4816. doi: 10.1158/0008-5472.CAN-14-3786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.