Abstract
The lipid transport protein, apolipoprotein E (apoE), is expressed in many peripheral tissues in vivo including the adrenal gland and testes. To investigate the role of apoE in adrenal cholesterol homeostasis, we have expressed a human apoE genomic clone in the Y1 mouse adrenocortical cell line. Y1 cells do not express endogenous apoE mRNA or protein. Expression of apoE in Y1 cells resulted in a dramatic decrease in basal steroidogenesis; secretion of fluorogenic steroid was reduced 7- to greater than 100-fold relative to Y1 parent cells. Addition of 5-cholesten-3 beta,25-diol failed to overcome the suppression of steroidogenesis in these cells. Cholesterol esterification under basal conditions, as measured by the production of cholesteryl [14C]oleate, was similar in the Y1 parent and the apoE-transfected cell lines. Upon incubation with adrenocorticotropin or dibutyryl cAMP, production of cholesteryl [14C]oleate decreased 5-fold in the Y1 parent cells but was unchanged in the apoE-transfected cell lines. These results suggest that apoE may be an important modulator of cholesterol utilization and steroidogenesis in adrenal cells.
Full text
PDF

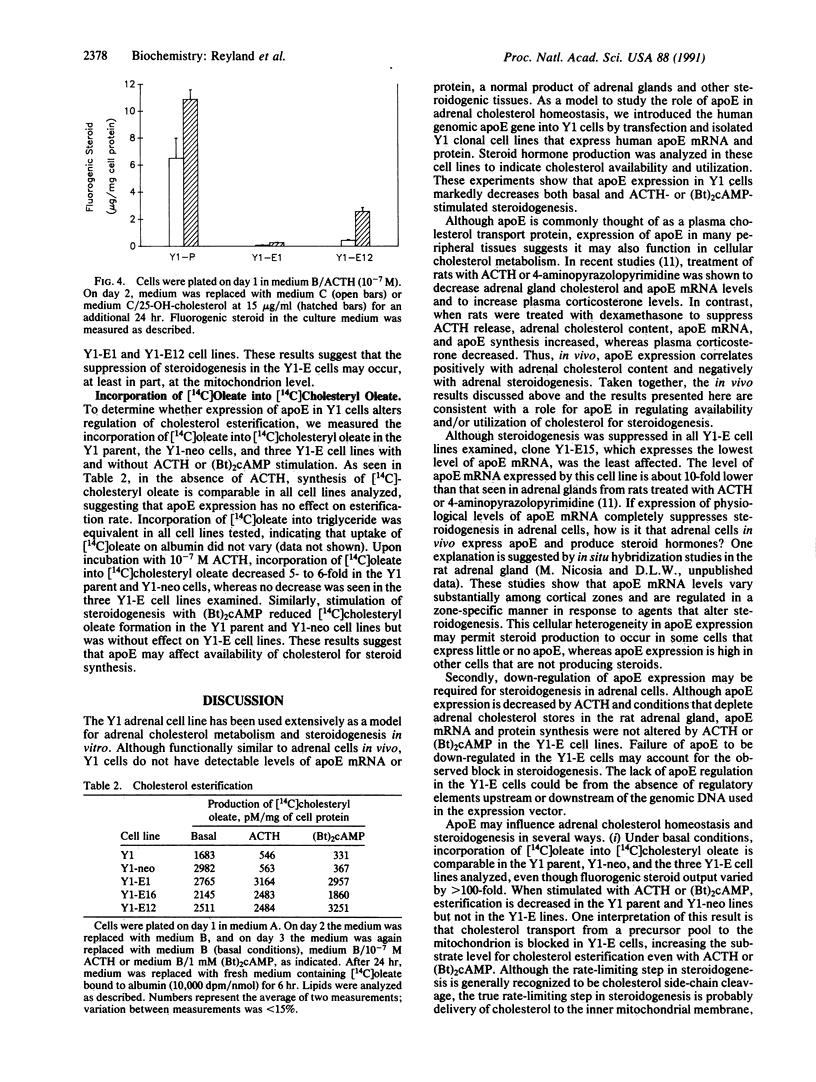

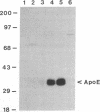
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basheeruddin K., Stein P., Strickland S., Williams D. L. Expression of the murine apolipoprotein E gene is coupled to the differentiated state of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):709–713. doi: 10.1073/pnas.84.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Williams D. L., Zucker S., Khan S. A., Blum C. B. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983 Jan;80(1):283–287. doi: 10.1073/pnas.80.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanderbhan R., Noland B. J., Scallen T. J., Vahouny G. V. Sterol carrier protein2. Delivery of cholesterol from adrenal lipid droplets to mitochondria for pregnenolone synthesis. J Biol Chem. 1982 Aug 10;257(15):8928–8934. [PubMed] [Google Scholar]
- Cheng B., Kowal J. A specific reversed-phase liquid chromatographic method for analysis of steroids in Y-1 adrenal cell cultures. J Chromatogr. 1988 Nov 18;432:302–307. doi: 10.1016/s0378-4347(00)80657-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Headon D. R., Olson C. D., Ungar F., Dempsey M. E. Intramitochondrial movement of adrenal sterol carrier protein with cholesterol in response to corticotropin. Proc Natl Acad Sci U S A. 1984 May;81(10):2970–2974. doi: 10.1073/pnas.81.10.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., Williams D. L., Alaupovic P. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res. 1989 Sep;30(9):1365–1373. [PubMed] [Google Scholar]
- Dawson P. A., Lukaszewski L. M., Ells P. F., Malbon C. C., Williams D. L. Quantification and regulation of apolipoprotein E expression in rat Kupffer cells. J Lipid Res. 1989 Mar;30(3):403–413. [PubMed] [Google Scholar]
- Dyer C. A., Curtiss L. K. Apoprotein E-rich high density lipoproteins inhibit ovarian androgen synthesis. J Biol Chem. 1988 Aug 5;263(22):10965–10973. [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977 Jul 25;252(14):4861–4871. [PubMed] [Google Scholar]
- Freeman D. A. Cyclic AMP mediated modification of cholesterol traffic in Leydig tumor cells. J Biol Chem. 1987 Sep 25;262(27):13061–13068. [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Hess B. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem. 1980 Nov 25;255(22):10875–10883. [PubMed] [Google Scholar]
- Kowal J. ACTH and the metabolism of adrenal cell cultures. Recent Prog Horm Res. 1970;26:623–687. doi: 10.1016/b978-0-12-571126-5.50020-0. [DOI] [PubMed] [Google Scholar]
- Kowal J., Fiedler R. Arenal cells in tissue culture. I. Assay of steroid products; steroidogenic responses to peptide hormones. Arch Biochem Biophys. 1968 Nov;128(2):406–421. doi: 10.1016/0003-9861(68)90047-7. [DOI] [PubMed] [Google Scholar]
- Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mazzone T., Gump H., Diller P., Getz G. S. Macrophage free cholesterol content regulates apolipoprotein E synthesis. J Biol Chem. 1987 Aug 25;262(24):11657–11662. [PubMed] [Google Scholar]
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2452–2457. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A. Immunochemical measurement of apolipoprotein synthesis in cell and organ culture. Methods Enzymol. 1986;129:254–271. doi: 10.1016/0076-6879(86)29074-6. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2444–2451. [PubMed] [Google Scholar]
- Wyne K. L., Schreiber J. R., Larsen A. L., Getz G. S. Rat granulosa cell apolipoprotein E secretion. Regulation by cell cholesterol. J Biol Chem. 1989 Oct 5;264(28):16530–16536. [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Kawamura M., Hall P. F. The regulation of intracellular transport of cholesterol in bovine adrenal cells: purification of a novel protein. Endocrinology. 1988 Oct;123(4):2075–2082. doi: 10.1210/endo-123-4-2075. [DOI] [PubMed] [Google Scholar]