Abstract
Ligands are essential for controlling the reactivity and selectivity of transition metal-catalyzed reactions. Access to large phosphine ligand libraries has become an essential tool for the application of metal-catalyzed reactions industrially, but these existing libraries are not well suited to new catalytic methods based on non-precious metals (i.e., Ni, Cu, Fe). The development of the requisite nitrogen- and oxygen-based ligand libraries lags far behind phosphines and the development of new libraries is anticipated to be time consuming. Here we show that this process can be dramatically accelerated by mining a typical pharmaceutical compound library that is rich in heterocycles for new ligands. Using this approach, we were able to screen a structurally diverse set of compounds with minimal synthetic effort and identify several new ligand classes for nickel-catalyzed cross-electrophile coupling. These new ligands gave improved yields for challenging cross-couplings of pharmaceutically relevant substrates compared to previously published ligands.
Graphical abstract
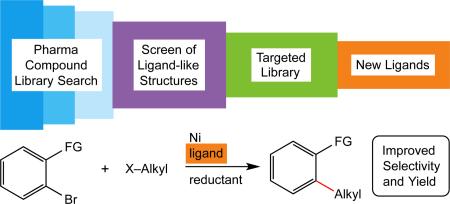
Pd-catalyzed cross-coupling reactions have become an essential tool in organic synthesis, both in academics and in industry.1, 2, 3 A large part of the utility of these reactions is due to the availability of ligand libraries to accelerate reaction discovery4, 5 and optimization6. The development of reactions catalyzed by metals of the first transition series (Fe, Co, Ni, Cu) can offer advantages of new reactivity, sustainability, and lower cost, but the application of existing, principally phosphine-based libraries to these metals can be difficult. Although there have been notable successes,7 phosphine ligands do not perform as well as those based upon nitrogen or oxygen in reactions such as nickel-catalyzed cross-electrophile coupling8,9 and copper-catalyzed C-N bond formation.10 There is an urgent need for N- and O-donor ligand libraries to ‘catch up’ to the diversity of phosphine ligands libraries. Existing, successful approaches to ligand discovery fall into two categories: variation of known ligands and design of new ligand families. Ligand variants can provide information on structure-activity relationships, but provide small increases in fundamental ligand diversity. Design of new ligands is riskier and time intensive, as a proposed ligand might not provide the expected reactivity or selectivity. As a result, only a few core structures are generally synthesized and evaluated at a time. Here we introduce an approach to speed ligand discovery: the mining of the large, structurally-diverse and nitrogen-heterocycle-rich compound libraries of pharmaceutical companies for new core structures. This approach differs from previous approaches because it minimizes bias towards one structural class and enables researchers to find unexpected ligands without requiring new synthetic effort. While truly high-throughput screening using libraries of multiple companies is a future goal, we first validated the approach screening Pfizer's compound library against nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides (Fig. 1).11 The resulting ligands were then applied to a variety of challenging substrates.
Fig. 1.
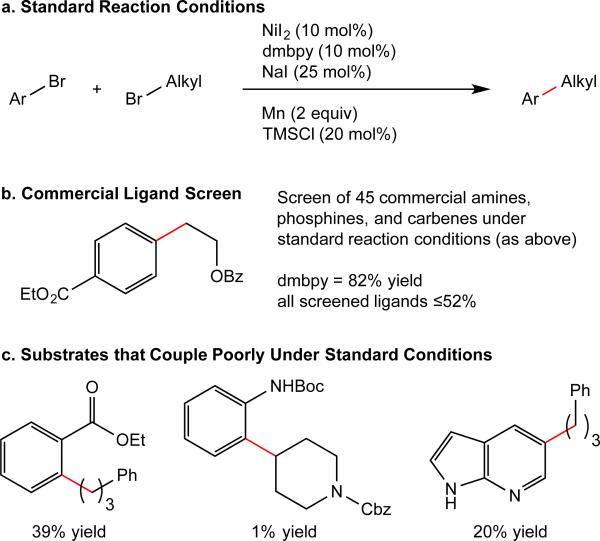
State of the art in cross-electrophile coupling. (a) Standard reaction conditions for nickel-catalyzed cross-electrophile coupling. dmbpy = 4,4’-dimethoxy-2,2’-bipyridine. (b) Results of a screen of commercially available ligands against a typical cross-electrophile coupling reaction demonstrate that existing ligand libraries fail to improve over known ligands. (c) Examples of coupling reactions that do not give high yields with dmbpy under standard conditions.
We began by screening commercially available ligands at Pfizer for activity in the nickel-catalyzed coupling of 2-benzyloxy-1-bromoethane with ethyl 4-bromobenzoate (Fig. 1b and Supplementary Fig. 1). Among the 45 commercially available phosphines, amines, and carbenes surveyed, none provided comparable yields to a standard bipyridine, 4,4’-dimethoxy-2,2’-bipyridine (dmbpy). That there is a lack of reactivity with phosphine ligands is limiting because there are many more commercially available phosphine ligands than nitrogen ligands (for example, Strem sells five times more P-ligands than N-ligands). To address this challenge, we turned to the Pfizer compound library as a potential source of diverse nitrogen-containing ligands. The Pfizer library is a collection of approximately 2.8 million distinct compounds that have been made by Pfizer scientists or contract research organizations. The collection is diverse in structure and size, containing both small building blocks and complex molecules available for biological testing.
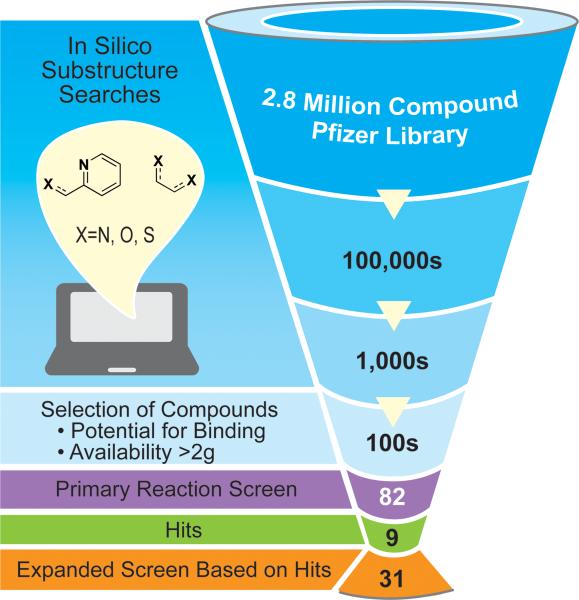
To begin, we identified the [(2-pyridyl)–C–X] motif as a conservative starting point and searched the Pfizer database for small molecules that contained this core (Fig. 2). While Strem sells only 25 ligands that fit this motif, the Pfizer library search returned greater than 1,500 compounds for X=N, even with the added constraint of requiring that >2 g of material be available for use! It quickly became evident that the challenge would not be finding new compounds, but in sorting through the vast quantity of compounds available. As a result, we conducted several more focused searches where X represents different heteroatom-containing functional groups. We chose a small subset (5-10 compounds) from each search, prioritizing low molecular weight, non-proprietary compounds with different steric and electronic properties for actual screening. The result was an 82 compound library that we screened against a typical coupling reaction (Fig. 2, for structures and a chart of results, see Supplementary Fig. 2).
Fig. 2.
Strategy employed for mining the Pfizer compound library for new ligands. Substructure searching of the 2.8 million compound library resulted in hundreds of thousands of hits. Refining these hits to motifs that looked likely to bind and were available in sufficient quantity narrowed this number to hundreds. Of this number, we screened 82 compounds against a typical cross-electrophile coupling reaction (Fig. 1a), resulting in nine hits with comparable yield and selectivity to dmbpy (C1). Based upon the nine hits, the compound library was mined again to generate a secondary library of 31 ligands used in further testing against more challenging reactions (Fig. 3).
We were pleased to find that even this narrow subset of compounds produced nine new hits with yields comparable to the standard ligand (4,4’-dimethoxy-2,2’-bipyridine) under the screening conditions. From these nine new ligands, two new ligand motifs emerged– primary pyridyl-2-carboxamidines and (2-pyridyl)-substituted aliphatic N-heterocycles. These results prompted us to search the Pfizer compound library a second time based upon these core metal-binding structures, resulting in a second generation screen of 31 compounds (Fig. 2, for structures and a chart of results, see Supplementary Fig. 3). Of all the compounds tested, seven ligands provided better results than the best available ligand, 4,4’-dimethoxy-2,2’-bipyridine (dmbpy), with many of these showing notable decreases in biaryl formation, the major side product with dmbpy (Fig. 1).
Nearly all of the best performing ligands from these screens contained the pyridyl-2-carboxamidine structure, and we decided to focus our efforts on this motif to explore this new ligand family. While cyclic amidines, such as pyridyl-2-imidazoline, have frequently been used as ligands in catalysis,12 unsubstituted pyridyl-2-carboxamidines have been neglected. For example, pyridyl-2-imidazolines were reported to be the optimal ligands for the nickel-catalyzed cross-electrophile coupling of aryl halides with allylic acetates13 and for a cross-Wurtz coupling,14 but we could find only a single example of a metal complex with an unsubstituted carboxamidine even though the two classes share a common starting material.15 The single literature example was a copper complex isolated as an unexpected side product and was not tested in catalysis. The closely related N-hydroxyamidines have a rich history of coordination chemistry,16 but while one of these ligands was found in the secondary screen (Supplementary Information) these ligands performed poorly in our focused screen (for example, Figure 3, A18). In contrast to their absence from coordination chemistry, unsubstituted pyridyl-2-carboxamidines show up in many pharmaceutical patents and medicinal chemistry articles (53 patents, 44 medicinal chemistry articles). This demonstrates the potential of looking at diverse compound collections for new reactivity.
Fig. 3.
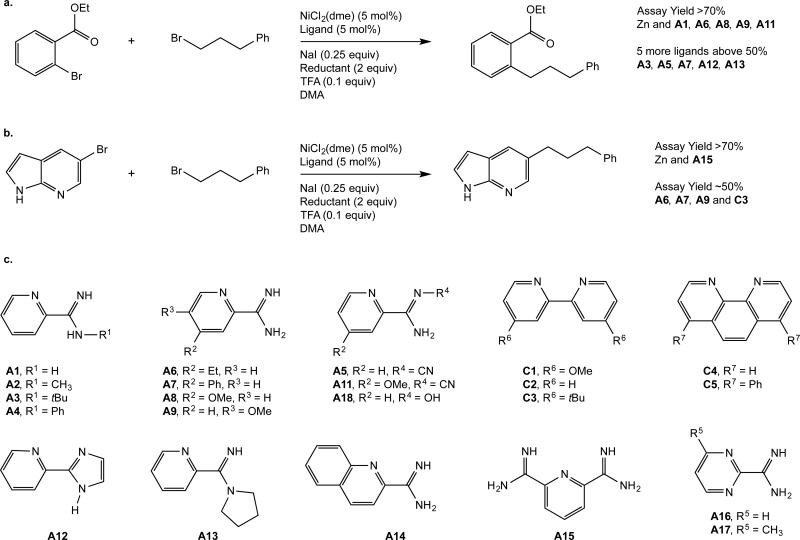
Results of screening a focused pyridyl carboxamidine ligand library (A1-A15) and a collection of known bipyridine and phenanthroline ligands (C1-C5) against two challenging reactions: (a) the coupling of a bromoarene with a coordinating group in the ortho position, (b) the coupling of a basic heteroaryl bromide. The structures of ligands tested (c) are also shown. Carboxamidine ligands provided superior results for both reactions tested. See Supplementary Information for complete tables. Note: Ligand A10 was discarded when it was found to be a different compound that what had been listed in the library.
Having exhausted the pyridyl-2-carboxamidine ligands available from our compound library, we decided to supplement a third generation ligand library with derivatives of the promising pyridyl-2-carboxamidine core in order to explore a wider range of substituents on the pyridyl and amidine functionalities. The amidines have the distinct advantage of being very easy to synthesize from 2-cyanopyridines,17 compared to the multistep syntheses of bipyridines18 and phenanthrolines.19 We decided on a library of 17 ligands for additional screening (Fig. 3).
In order to highlight utility of this compound-library-derived ligand collection, we first applied these ligands to a more challenging aryl bromide with coordinating functionality in the 2-position, ethyl 2-bromobenzoate (Fig. 3a). The aryl bromide has previously been coupled with bromododecane in 51% yield20, with 4-bromopiperidine in 44% yield,21 with tert-butyl bromide (4 equiv) in 65% yield,22 and with a variety of organometallic reagents in 13-91% yield.23,24 The control ligands (C1-C5) provided 20-40% yield and formed large amounts of biaryl. In contrast, most of the amidine ligands (A1-A18) performed better than the C-series ligands, and reactions run with five of these ligands (A1, A6, A8, A9, A11) provided >70% yield. The increased yield is due to improved selectivity for cross-product over dimerization and hydrodehalogenation of the aryl halide. Reactions with the control ligands contained 20-30% of these byproducts, but reactions with the best amidine ligands produced less than 10% (Supplementary Fig. 4). Several trends among the amidine ligands were observed: both alkyl and aryl substitution on the amidine resulted in lower yields (A2-A4, A13, A18), N-cyano substituted amidines were similar in reactivity to the unsubstituted amidines (A5 and A1 as well as A11 and A8 provided similar yields with Zn reductant), electron-donating groups at both the 4- and 5- positions on the pyridine ring gave improved yields (A8, A9), and pyrimidine-2-carboxamidines were less efficient than corresponding pyridine-2-carboxamidine ligands (A1 > A16).
We also applied the amidine ligand library to the coupling of 3-bromopyridine derivatives with alkyl halides (Fig. 3b). In this screen, a tridentate ligand, 2,6-pyridinedicarboxamidine (A15), was the only ligand to provide a high yield (>70% assay yield). The other amidine ligands provided results similar to the control ligands, with assay yields around 50% (Supplementary Fig. 4). While a few tridentate ligands have been used in cross-electrophile couplings, these have mostly been limited to pyridyl-bis(oxazolines)25 and terpyridine derivatives26 and neither ligand class has been reported to form a high-performing catalyst for the coupling of aryl halides with alkyl halides. The improved yield is associated with a decreased amount of homocoupling, but at this time it is not clear why catalysts derived from A15 diminish this side reaction.
In order to assess the generality of these results, we tested the best ligands from these two screens in reactions with related substrates (Fig. 4). The best ligands for reactions with 2-substituted bromoarenes (A1, A8, and A11) were found to be consistently better than the best control ligand (C1). Most notably, N-Boc-2-bromoaniline, 2-bromophenylboronic acid, and 2-bromobenzoic acid provided almost no detectible products with ligand C1 and 56-63% yields with ligands A8 and A1. These aryl halides are challenging to couple with alkyl substrates by any protocol, not just cross-electrophile coupling, and most were inspired by requests from medicinal chemists. For example, we were unable to find examples of the cross-coupling of 2-bromophenylboronic acid, 2-bromobenzamide, or N-Boc-2-bromoaniline with alkyl organometallic reagents at the C–Br bond. The reported yields are also unoptimized and certainly could be further improved through variation of the reaction conditions, but even without optimization it is clear that the carboxamidine ligands discovered in the Pfizer compound library are as good or better than the known bipyridine and phenanthroline ligands.
Fig. 4.
Examination of substrate scope for the Ni-catalysed cross-electrophile coupling. (a) Reaction conditions employed. (b) Results with new amidine ligands compared to dmbpy (C1) and 2,2’:6’,2”-terpyridine. These results demonstrate that the new primary amidine ligands A1, A8, and A15 as well as N-cyanoamidine A11 are generally superior to bipyridine C1. The advantage of A15 for heterocycle coupling reactions is not only due to it being tridentate because terpyridine gives a poor yield. iAssay yield from UPLC (UV-vis) or GC (FID). iiIsolated yield after chromatography or crystallization. ND = no product detected.
We also applied the amidine ligand library to the coupling of 3-bromopyridine derivatives with alkyl halides (Fig. 4). The tridentate 2,6-pyridinedicarboxamidine (A15) provided excellent results for coupling reactions with not only 3-bromopyridine, but also 5-bromoazaindole, and 5-bromonicotinonitrile. 5-Bromoazaindole had previously been coupled with 4-bromotetrahydropyran in 45% yield,27 4-bromoisoquinline coupled with N-Boc 4-bromopiperidine in 30% yield,27 and 3-bromoquinoline coupled to several secondary alkyl electrophiles in 30-80% yield.27, 28 Because A15 was the only tridentate ligand tested and because we had reported that terpyridines provided good results for the coupling of aryl halides with allylic acetates,26 we examined 2,2’:6’,2”-terpyridine in the coupling of 5-bromo-2-methoxypyridine. The reaction with the terpyridine ligand formed only 10% of the coupled product, even worse than C1 and A8. This demonstrates that the superior results of A15 are not merely due to it being the only tridentate ligand in the screen (Fig. 4), but also derive from the same carboxamidine effect seen for the bidentate ligands. Further examination of A15 derivatives for the coupling of halogenated heterocycles with alkyl halides is currently underway.
Given these results and the growing importance of nitrogen, oxygen, and sulfur-based ligands in catalysis, we think that pharmaceutical compound libraries should be considered invaluable sources of diversity not only for finding new drugs, but also for ligand discovery. We anticipate that similarly useful ligand motifs will be found for different reactions with various metals.
Supplementary Material
Acknowledgements
We gratefully acknowledge funding from Pfizer, Inc. and from the NIH (R01 GM097243). We thank D. Batesky (University of Rochester) for synthesis of several amidine ligands used in this study. We thank S. Monfette (Pfizer) for helpful discussions. This work has been influenced by the on-going efforts of the Non-Precious Metal Catalysis Alliance between Pfizer, Boehringer-Ingelheim and Abbvie.
Footnotes
Author Contributions N.J.G. conducted preliminary experiments with amidine ligands. E.C.H. and D.J.P. conducted the screens at Pfizer. E.C.H., D.J.P., and A.C.W. ran the reactions, isolated, and characterized the products in Fig. 3. Data analysis and study design were the product of meetings with all authors. All authors discussed the results and commented on the manuscript.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information The authors declare no competing financial interests.
References
- 1.Roughley SD, Jordan AM. The Medicinal Chemist's Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 2.Magano J, Dunetz JR. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 3.Busacca CA, Fandrick DR, Song JJ, Senanayake CH. The Growing Impact of Catalysis in the Pharmaceutical Industry. Adv. Synth. Catal. 2011;353:1825–1864. [Google Scholar]
- 4.Dreher SD, Dormer PG, Sandrock DL, Molander G. Efficient Cross-Coupling of Secondary Alkyltrifluoroborates with Aryl Chlorides–Reaction Discovery Using Parallel Microscale Experimentation. J. Am. Chem. Soc. 2008;130:9257–9259. doi: 10.1021/ja8031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins DW, Hartwig JF. A Simple, Multidimensional Approach to High-Throughput Discovery of Catalytic Reactions. Science. 2011;333:1423–1427. doi: 10.1126/science.1207922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buitrago Santanilla A, et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science. 2015;347:49–53. doi: 10.1126/science.1259203. [DOI] [PubMed] [Google Scholar]
- 7.Friedfeld MR, et al. Cobalt Precursors for High-Throughput Discovery of Base Metal Asymmetric Alkene Hydrogenation Catalysts. Science. 2013;342:1076–1080. doi: 10.1126/science.1243550. [DOI] [PubMed] [Google Scholar]
- 8.Knappke CEI, et al. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem.–Eur. J. 2014;20:6828–6842. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]
- 9.Biswas S, Weix DJ. Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones GO, Liu P, Houk KN, Buchwald SL. Computational Explorations of Mechanisms and Ligand-Directed Selectivities of Copper-Catalyzed Ullmann-Type Reactions. J. Am. Chem. Soc. 2010;132:6205–6213. doi: 10.1021/ja100739h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everson DA, Shrestha R, Weix DJ. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2010;132:920–921. doi: 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Du D-M. Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis. Adv. Synth. Catal. 2009;351:489–519. [Google Scholar]
- 13.Cui X, et al. Nickel-catalyzed reductive allylation of aryl bromides with allylic acetates. Org. Biomol. Chem. 2013;11:3094–3097. doi: 10.1039/c3ob40232k. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Zhao C, Qian Q, Deng W, Gong H. Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci. 2013;4:4022–4029. [Google Scholar]
- 15.Segl′a P, Koman M, Glowiak T. FORMATION OF 2-PYRIDINYL-2-OXAZOLINES AND PYRIDINE-2-CARBOXAMIDINE IN THE COORDINATION SPHERE OF COPPER(II). J. Coord. Chem. 2000;50:105–117. [Google Scholar]
- 16.Milios CJ, Stamatatos TC, Perlepes SP. The coordination chemistry of pyridyl oximes. Polyhedron. 2006;25:134–194. [Google Scholar]
- 17.Bacon ER, Singh B, Lesher GY. 6-heterocyclyl pyrazolo [3,4-d]pyrimidin-4-ones and compositions and method of use thereof USA. 1994 Patent US5294612 A.
- 18.Buonomo JA, Everson DA, Weix DJ. Substituted 2,2’-bipyridines by nickel-catalysis: 4,4’-di-tert-butyl-2,2’-bipyridine. Synthesis. 2013;45:3099–3102. doi: 10.1055/s-0033-1338520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luman CR, Castellano FN. In: Comprehensive Coordination Chemistry II. McCleverty JA, Meyer TJ, editors. Pergamon; 2003. pp. 25–39. [Google Scholar]
- 20.Czaplik WM, Mayer M, Jacobi von Wangelin A. Domino Iron Catalysis: Direct Aryl-Alkyl Cross-Coupling. Angew. Chem., Int. Ed. 2009;48:607–610. doi: 10.1002/anie.200804434. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Qian Q, Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate. Org. Lett. 2012;14:3352–3355. doi: 10.1021/ol3013342. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Wang S, Xue W, Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2015;137:11562–11565. doi: 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- 23.Blum J, et al. Palladium-Catalyzed Methylation of Aryl and Vinyl Halides by Stabilized Methylaluminum and Methylgallium Complexes. J. Org. Chem. 1997;62:8681–8686. [Google Scholar]
- 24.Han C, Buchwald SL. Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2009;131:7532–7533. doi: 10.1021/ja902046m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu J, Wang X, Xue W, Gong H. Nickel-catalyzed reductive coupling of alkyl halides with other electrophiles: concept and mechanistic considerations. Org. Chem. Front. 2015;2:1411–1421. [Google Scholar]
- 26.Anka-Lufford LL, Prinsell MR, Weix DJ. Selective Cross-Coupling of Organic Halides with Allylic Acetates. J. Org. Chem. 2012;77:9989–10000. doi: 10.1021/jo302086g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molander GA, Traister KM, O'Neill BT. Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides. J. Org. Chem. 2014;79:5771–5780. doi: 10.1021/jo500905m. [DOI] [PubMed] [Google Scholar]
- 28.Molander GA, Traister KM, O'Neill BT. Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem. 2015;80:2907–2911. doi: 10.1021/acs.joc.5b00135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.