Abstract
Acute kidney injury (AKI) is a major renal disease associated with a high mortality rate and increasing prevalence. Decades of research has suggested numerous chemical and biological agents with beneficial effects in AKI. In addition, cell therapy and molecular targeting have been explored for reducing kidney tissue damage and promoting kidney repair or recovery from AKI. Mechanistically, these approaches may mitigate oxidative stress, inflammation, cell death, and mitochondrial and other organellar damage, or activate cytoprotective mechanisms such as autophagy and pro-survival factors. However, none of these findings has been successfully translated into clinical treatment of AKI. In this review, we analyze these findings and propose experimental strategies for the identification of renoprotective agents or methods with clinical potential. Moreover, we propose the consideration of combination therapy by targeting multiple targets in AKI.
Keywords: Acute kidney injury, Kidney protection, Kidney repair, Renoprotection, Ischemia-reperfusion, Nephrotoxicity, Mitochondria, Apoptosis, Reactive oxygen species, Vascular dysfunction, Inflammation
Introduction
Acute kidney injury (AKI) is a syndrome characterized by the rapid loss of renal function resulting in the accumulation of end products of nitrogen metabolism (urea and creatinine) and/or decreased urine output (KDIGO, 2012). In clinic, AKI occurs mainly as the clinicopathological outcome of renal or extra-renal surgery, bacterial infection, and nephrotoxicity, Large epidemiological studies show a high incidence of AKI in hospitalized patients and in general population (Bellomo et al., 2012; Hsu et al., 2007; Lameire et al., 2013). AKI is considered to be an important independent risk factor for mortality (Uchino et al., 2006). Patients with uncomplicated AKI have a mortality rate of up to 10%. In contrast, patients presenting with AKI and multiorgan failure have been reported to have mortality rates of over 50%. If renal replacement therapy is required, the mortality rate rises further to as high as 80% (Shusterman et al., 1987; Liaño et al., 1998). In addition, AKI is an important factor in the development and progression of chronic kidney disease (CKD) (Chawla et al., 2014; Venkatachalam et al., 2015).
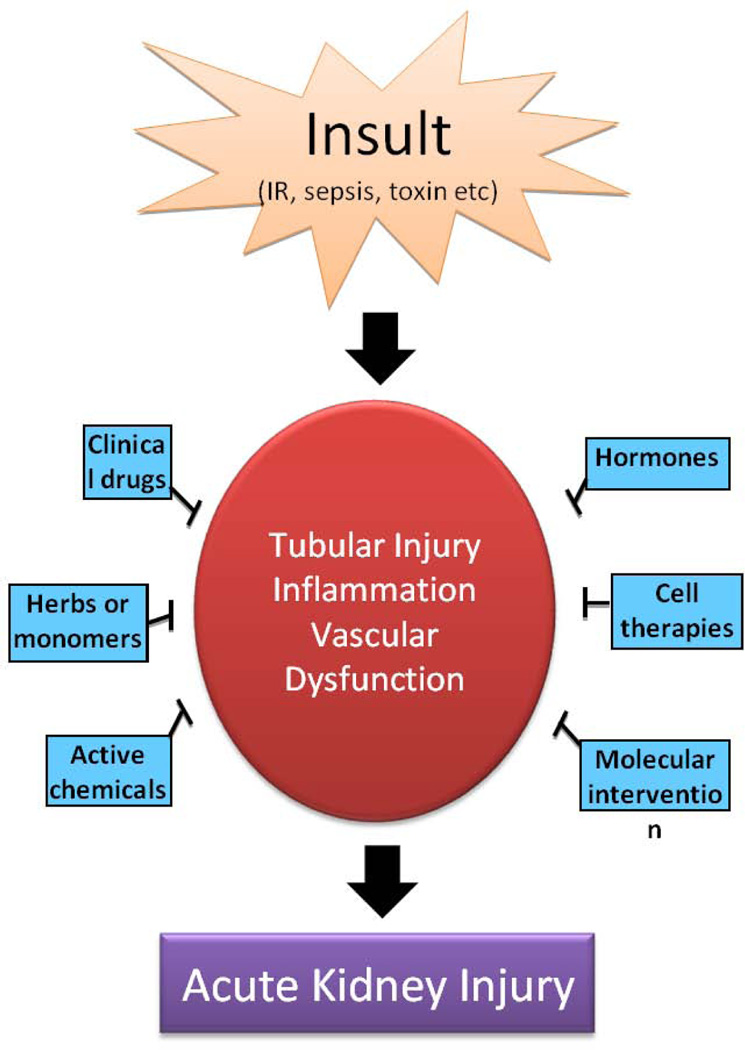
Pathogenetically, AKI is generally described as the injury of renal tubular epithelial cell and vasculature, accompanied by the activation of a robust inflammatory response (Bonventre & Yang, 2011; Molitoris, 2014; Linkermann et al., 2014). In addition, depending on its severity and duration, the damage may spread to glomerulus and interstitium resulting in a full blown, lasting disease. Along with the mechanistic research, a number of agents have been shown for their renoprotective effects in AKI models (Table 1–5), which include some clinical drugs, herbs, active chemicals, hormones, cytokines and growth factors. Moreover, molecular and cell therapies have been attempted with some promising results. In experimental models, these agents and approaches protected kidneys by suppressing inflammation, preserving vasculature, and/or directly preventing tubular cell injury and death (Figure 1). However, up-to-date none of them has been successfully translated to the bedside or the use in patients (Jo et al., 2007). In this review, we have summarized the main renoprotective agents and analyzed their effects in AKI models and relevant mechanisms. We have also discussed the experimental strategies for the discovery of efficacious therapies for AKI, including the use of comorbid models and the test of combination therapies.
Table 1.
Clinical drugs with renoprotective effects in AKI
| No | Name | Characteristics | Tested AKI model | Mechanism |
|---|---|---|---|---|
| 1 | Leflunomide | Pyrimidine synthesis inhibitor used in immunosuppressive diseases such as rheumatoid arthritis and psoriatic arthritis |
IRI in rat | reduce oxidative stress |
| 2 | Etanercept | TNF-α inhibitor used to treat autoimmune diseases |
IRI in rat | lower expression of TNF-α and MCP-1 |
| 3 | Statins drugs | Inhibitors of HMG-CoA reductase used to lower cholesterol |
drug-, septic- and ischemic -induced AKI in rat or mice |
antioxidant, anti-inflammatory and anti-apoptotic |
| 4 | Edaravone | Neuroprotective agent in acute brain ischemia and subsequent cerebral infarction |
IRI in rats | increase Bcl-2 expression |
| 5 | Paricalcitol | Analog of vitamin D2 active form, VDR agonist |
IRI in male C57BL/6 mice |
upregulate COX-2 and PGE2 |
| 6 | Tadalafil, Sildenafil |
Phosphodiesterase type 5 inhibitor | contrast-induced AKI in rabbits |
Inhibit protein kinase G |
| 7 | Milrinone | Phosphodiesterase type 3 inhibitor | IRI in mice | Inhibit NF-κB activation |
| 8 | Fidarestat | Aldose reductase inhibitor for diabetic complications |
LPS-induced endotoxic AKI |
suppress inflammation |
| 9 | Telmisartan | Angiotensin II receptor antagonist used in hypertension |
IRI in rats | decrease MDA, TNF-α, NO and homocysteine |
| 10 | Adrenomedullin | A potent endogenous vasodilatory peptide hormone |
contrast induced AKI in rats |
negative regulation of the RAAS |
| 11 | Rituximab | Monoclonal antibody against CD20 used in autoimmunity |
IRI in mice | Suppression of inflammation |
| 12 | Cyclosporin A | Immunosuppressant used in transplant medicine |
FA-induced AKI in mice |
block TWEAK expression and NF-κB activation |
| 13 | Mycophenolate mofetil |
Immunosuppressant used in transplant or autoimmune diseases |
IRI in rats | attenuate the increase of cytokines RANTES and AIF |
| 14 | Temsirolimus | Inhibitor of mammalian target of rapamycin (mTOR) |
septic-AKI in older adult mice |
induce autophagy |
| 15 | Doxycycline | Tetracycline antibiotics for treating infections or inflammation |
IRI in a rat model of ACS |
decrease IL-1β, TNF-α and MMP-2 |
| 16 | suramin | An antiparasitic drug used in treatment of trypanosomiasis |
IRI in mice | reduce tubular apoptosis and infiltrating leukocytes |
| 17 | Geranylgeranylac etone |
An antiulcer drug used in treatment of gastric ulcers |
IRI in rats | induction of Hsp70 |
Table 5.
Cytokines, growth factors and gene-interfered with renoprotective effects in AKI
| No | Name | Characteristics | Tested AKI model | Mechanism |
|---|---|---|---|---|
| 1 | IL-10 | cytokine synthesis inhibitory factor | Ischemic- and cisplatin- AKI in the mouse |
reduce levels of TNF-α, ICAM-1, and iNOS |
| 2 | CXCR4 antagonist |
Plerixafor, a small-molecule antagonist of CXCR4 |
IRI in rats | reduce chemokines CXCL1, CXCL5 and IL-6 |
| 3 | TNF-α inhibition |
Inhibitors of TNF-α production (GM6001, pentoxifylline), anti-TNF-α antibody, specific TNF-α knockout |
cisplatin- AKI in Swiss-Webster mice |
decrease levels of TNF-α, TGF-β, RANTES, MIP-2, MCP-1, and IL-1β |
| 4 | ICAM-1 inhibition |
Specific ICAM-1 knockout, Anti-1CAm-1 antibody |
IRI in mice IRI in rats |
attenuate neutrophil endothelial interactions |
| 5 | CT-1 | A member of IL-6 family and a potent pleiotropic cytokine |
contrast-induced AKI in rats |
prevent tubular desepithelization and obstruction |
| 6 | NGAL | A member of the lipocalin super family with diverse function |
IRI in rats | inhibit activation of caspase-3 and expression of Bax |
| 7 | L-FABP | A member of intracellular lipid-binding proteins involved in the transportation of fatty acids |
AA-induced AKI in mice |
suppress the production of HEL, HO-1, and receptor for AGEs |
| 8 | sTM | A glycoprotein present on the membrane surface of endothelial cells in many organs, including lung, liver, and kidney. |
IRI in rats | improve microvascular erythrocyte flow rates |
| 9 | COMP-Ang1 | A soluble and potent Ang1 variant, act as the ligand for Tie2 tyrosine kinase receptor that is expressed on EC. |
unilateral ureteral obstruction-induce d renal fibrosis |
improve peritubular capillary and enhance renal tissue (re)perfusion |
| 10 | IGF | A hormone similar in molecular structure to insulin |
IRI in rats cisplatin- RPTC cisplatin- or HgCl2- AKI in mice |
ameliorate acute tubular necrosis; produce pro-survival factor IGF-1 |
| 11 | MFG-E8 | A protein involved in marking apoptotic cells for phagocytosis |
IRI in C57BL/6 mice |
suppress renal inflammation |
| 12 | EGF | A potent growth promoter to renal tubule cells, produced in large amounts in the kidney |
HgCl2- AKI in mice |
attenuate tubular necrosis |
| 13 | EGFR inhibitor |
erlotinib, a selective tyrosine kinase inhibitor that can block EGFR activity |
cisplatin- AKI in rats |
decrease apoptosis and proliferation of tubular cells |
| 14 | HGF | A potent mitogen for parenchymal liver, epithelial and endothelial cells, as a ligand of MET oncoprotein |
glycerol-, gentamicin- AKI in rats |
attenuate tubulointerstitial injury, leukocyte infiltration and Th1 polarization |
| 15 | EPO | Glycoprotein produced by the kidney that regulats red blood cell production in the bone marrow |
IR-, cisplatin- and contrast- AKI in mice or rats or pigs |
inhibit apoptosis and promote cellular regeneration |
| 16 | G-CSF | Glycoprotein that stimulates bone marrow to produce granulocytes and stem cells |
glycerol- AKI In C57BL/6 mice |
induction of HO-1 |
| 17 | NF-κB Blockade |
NF-κB decoy oligodeoxynucleotides; NF-κB inhibitor milrinone, resveratrol |
IRI in rats or mice | decrease MCP-1 expression and monocyte infiltration |
| 18 | HIF-1 | Ubiquitously expressed hypoxia-inducible transcription factor |
IR- or cisplatin- in rats |
ameliorate tubulointerstitial and vascular damage |
| 19 | HIF-2 | Hypoxia-inducible transcription factor mainly expressed on endothelial cells |
IRI in mice | protect from vascular damage and fibrosis |
| 20 | PKCδ knockdown |
A member of PKC subfamily involved in cell apoptosis |
cisplatin- AKI in mice, cisplatin-RPTCs |
Activated MAPKs for apoptosis and tissue damage |
| 21 | OMA1 knockdown |
a zinc metalloprotease located at mitochondrial inner membrane that is involved in mitochondrial inner membrane disruption in cell stress |
ATP-depleted RPTC, IRI in C57BL/6 mice |
mediate OPA1 proteolysis and mitochondrial fragmentation |
| 22 | Dicer deletion |
A key ribonuclease for microRNA production, Dicer deletion leads to a global downregulation of microRNAs |
IRI in mice | depletion of the majority of microRNAs |
| 23 | miR-687, -24 blockade |
endogenous, noncoding, small RNAs that regulate expression and function of genes |
hypoxia-induced RPTC injury / apoptosis, IRI in mice |
attenuate cell cycle activation and apoptosis |
| 24 | miR-127, -34a, -155, -126 blockade |
ameliorate histologic tubular damage, apoptosis |
Fig. 1.
Overview of renoprotective approaches in acute kidney injury. Insults, such as ischemia/reperfusion, sepsis, and various nephrotoxins, induces injury and death of renal tubular cells, vascular dysfunction, and inflammation, resulting in acute kidney injury and renal failure. Renoprotective agents may protect tubular cells, suppress inflammatory response, and/or maintain renal vasculture in AKI.
I. Chemical Renoprotectants
1. Clinical drugs
Some clinical drugs have been shown to be protective in experimental models of AKI. These include disease-modifying antirheumatic drugs (DMARD), cholesterol-cutting statins, neuroprotective agents for cerebral infarction, selective vitamin D receptor agonist (VDRA), tetracycline antibiotics, phosphodiesterase-5 (PDE5) inhibitors, angiotensin II receptor antagonist, mammalian target of rapamycin (mTOR) inhibitor, immunosuppressant drug, and steroid hormones (Table 1). A notable advantage of clinical drugs is that they have been thoroughly tested for safety in human use and, if effective, they can be relatively rapidly applied for AKI treatment.
1.1 Antirheumatic and statin drugs
Leflunomide is known as an immunomodulating drug for the treatment of chronic inflammatory conditions, such as rheumatoid arthritis. In a rat model of renal ischemia-reperfusion injury (IRI), leflunomide markedly attenuated renal dysfunction and morphological alterations, and reduced oxidative stress (OS) (Karaman et al., 2006). Similarly, Etanercept (a soluble Tumor necrosis factor-alpha (TNF-α) receptor) showed anti-inflammatory and anti-apoptotic effects by lowering the expression of TNF-α and monocyte chemotactic protein-1 (MCP-1) in ischemic AKI rats (Choi et al., 2009). For statins, early postoperative statin use was associated with a lower incidence of AKI after cardiac surgery and decreased mortality risk as compared to preoperative statin use or acute statin withdrawal (Molnar et al., 2011; Billings et al., 2010). Several mechanisms have been suggested to contribute to the renoprotective effects of statins in AKI. Statins with their antioxidant, anti-inflammatory and anti-apoptotic effects may protect kidney against gentamicin-, cisplatin- and cyclosporine-induced nephrotoxicity, beyond their lipid-lowering capacity (Dashti-Khavidaki et al., 2013; Kostapanos et al., 2009). They may also block the activation of mitogen-activated protein kinase (MAPK) and the redox-sensitive NF-kB and activator protein-1 (AP-1) (Gueler et al., 2002). Also statins may ameliorate AKI by directly affecting renal vasculature, an observation that is particularly relevant to sepsis-associated AKI (Yasuda et al., 2006).
1.2 Neuroprotective drugs and Vitamin D receptor agonist
Edaravone is a neuroprotective drug used for treating cerebral infarction through its antioxidant property. In ischemic AKI, edaravone showed renoprotective effects as indicated by decreased serum creatinine (SCr) and blood urea nitrogen (BUN), and increased Bcl-2 expression (Watanabe et al., 2004; Li et al., 2010). Paricalcitol, an agonist of the vitamin D receptor, protected against ischemic AKI by upregulating cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) to attenuate inflammation (Hwang et al., 2013). In line with this observation, Vitamin D deficiency aggravated AKI induced by Tenofovir, a widely used component of antiretroviral regimens for HIV treatment (Canale et al., 2013).
1.3 Inhibitor of phosphodiesterase type 5
Tadalafil and Sildenafil are inhibitors of PDE5, the enzyme responsible for cyclic GMP degradation. Clinically, they are common drugs prescribed for the treatment of erectile dysfunction (ED) and pulmonary hypertension. In ischemic AKI, Tadalafil significantly improved renal function and preserved renal histology, which was associated with the attenuation of AKI biomarkers including kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) (Sohotnik et al., 2013). Similarly, Sildenafil reduced contrast medium-induced AKI in rabbits (Lauver et al., 2014).
1.4 Angiotensin II receptor antagonist and anti-ulcer drug
The angiotensin II receptor antagonist telmisartan was shown to attenuate the increases in BUN, SCr, malondialdehyde (MDA), TNF-α, NO and homocysteine levels in ischemic AKI (Fouad et al., 2010). Consistently, adrenomedullin (AM), a potent endogenous vasodilatory peptide hormone, also delayed the development of contrast-induced nephropathy (CIN) by negative regulation of the renin-angiotensin-aldosterone system (RAAS) (Charles et al., 2003; Inal et al., 2013). Interestingly, the combination of AM with AM binding protein-1 (AMBP-1) could markedly attenuate the inflammatory response in ischemic AKI, suggesting a mechanism of the renoprotective effect of AM (Shah et al., 2010).
1.5 Antibiotics and immunosuppressants
Tetracyclines exhibit significant anti-inflammatory and antiapoptotic properties in AKI induced by hypoxia, azide, cisplatin, or ischemia. For example, minocycline induced accumulation of Bcl-2 in mitochondria and suppression of death-promoting molecules including Bax, Bak, and Bid (Wang et al., 2004), and reduced leukocytes infiltration, leukocyte chemotaxis, and the expression of intercellular adhesion molecule-1 (ICAM-1) (Kelly et al., 2004). Minocycline also reduced renal microvascular leakage which may be related to diminished activity of matrix metallopeptidase 2 (MMP-2) and MMP-9 on the perivascular matrix (Sutton et al., 2005). However, in a clinical study minocycline did not show significant protective effects against AKI that developed post-cardiac bypass surgery (Golestaneh et al., 2015). Doxycycline as another tetracycline antibiotics exhibited renoprotective effects by decreasing levels of IL-1β, TNF-α and MMP-2 in renal tissue against IRI induced by abdominal compartment syndrome (ACS) (Ihtiyar et al., 2011). Both minocycline and doxycycline were effective in mitigating liver and kidney injury to improve survival in the mouse model of hemorrhagic shock/resuscitation (Kholmukhamedov et al., 2014).
Cyclosporin A, an immunosuppressant drug used in organ transplantation to prevent rejection, blocked the TNF-like weak inducer of apoptosis (TWEAK) expression and NF-κB activation in folic acid (FA)-induced AKI (Wen et al., 2012). Rituximab is a monoclonal antibody against the protein CD20 used in autoimmune diseases or anti-rejection treatment for organ transplants, which may suppress the inflammation in ischemic AKI (Hwang et al., 2013). In addition, treatment with mycophenolate mofetil together with polyphenolic bioflavonoids reduced tubular damage and attenuated the induction of inflammatory cytokines and renal inflammation (Jones et al., 2000). Thus, anti-inflammation appears to be a common mechanism underlying the renoprotective effects of antibiotics and immunosuppressants in AKI.
1.6 Inhibitors of mammalian target of rapamycin
Mammalian target of rapamycin (mTOR) is a serine/ threonine protein kinase with multiple functions. On one hand, mTOR is a key to cell growth and proliferation by promoting protein synthesis. On the other hand, recent work has demonstrated that mTOR is a crucial, negative regulator of autophagy in response to nutritional status, growth factor and stress signals (Jung et al., 2010; Datta et al., 2014). In AKI, the role of mTOR varies according to experimental models. In ischemic AKI, the inhibition of mTOR by rapamycin impaired or at least delayed kidney repair and recovery by suppressing tubular cell growth and proliferation (Lieberthal et al., 2012). However, in cisplatin nephrotoxic AKI, rapamycin showed protective effects (Jiang et al., 2012). Rapamycin also protected renal tubular cells from apoptosis during ER-stress (Dong et al., 2015). Similarly, rapamycin ameliorated renal injury in diabetic mice and the underlying mechanism may be related to autophagy induction in podocytes (Xiao et al., 2014). In endotoxic AKI induced by lipopolysaccharide (LPS), another mTOR inhibitor Temsirolimus induced autophagy and protected against kidney injury, even after established endotoxemia (Howell et al., 2013). Therefore depending on the models, inhibition of mTOR may protect against or exacerbate AKI. The exact cause of the different effects is unclear, but apparently it results from the multiple functions of mTOR. While mTOR may protect by promoting cell growth and proliferation, it may also enhance injury by inactivating autophagy. Also it is important to recognize that inhibitors of mTOR (e.g. rapamycin) are immune suppressants that may diminish the inflammatory response in AKI contributing to the observed protective effects of rapamycin in vivo.
1.7 Other clinical drugs
In addition to the drugs described above, several other clinically used drugs have shown renoprotective effects in AKI models. For example, suramin is an antiparasitic drug used for the treatment of trypanosomiasis. Zhuang and colleague demonstrated the beneficial effect of suramin in several kidney disease models, including ischemic AKI and renal fibrosis (Zhuang et al., 2009; Liu et al., 2011). Mechanistically, suramin may promote renal tubular cell proliferation and migration, processes important for kidney repair (Zhuang et al., 2005). Geranylgeranylacetone (GGA), a drug used in the treatment of gastric ulcers, ameliorated ischemic AKI via induction of heat shock protein 70 (Hsp70) (Suzuki et al., 2005). In addition, Fidarestat, an aldose reductase (AR) inhibitor used for treating diabetic complications, protected against LPS-induced endotoxic AKI probably by suppressing the inflammatory response (Kazunori et al., 2012).
2. Renoprotective Chemicals with Clinical Potential
Heme oxygenase-1 (HO-1) and its activators: HO-1 is an inducible enzyme that converts heme into biliverdin and bilirubin, releasing iron and carbon monoxide. The potent cytoprotective role of HO-1 has been recognized for over 20 years (Nath et al., 1992). In kidneys, HO-1 is induced in various AKI models including ischemia-reperfusion, sepsis, and nephrotoxicity (Nath, 2014; Shimizu et al., 2000; Maines et al., 1993). Mechanistically, HO-1 is known to promote the anti-oxidative capacity of the cell. Moreover, it may also dilate blood vessels, increase perfusion, and suppress inflammation in AKI as a result of tissue protection or indirectly by modulating immune cell trafficking (Hull et al., 2015). Several studies have tested the effects of HO-1 induction in AKI. For example, tin chloride (SnCl2) ameliorated ischemic AKI as shown by the decrease in serum creatinine and BUN and in tubular damage (Toda et al., 2002), while the tin protoporphyrin/ Tin mesoporphyrin/ stannous mesoporphyrin (SnMP, a competitive inhibitor of HO) exacerbated AKI induced by cisplatin (Agarwal et al., 1995; Salom et al., 2007).
Protein kinase C (PKC) inhibitors: PKC is a protein kinase family of multiple members, several of which are induced following renal IR injury in rats (Padanilam, 2001). In a rat model of kidney transplantation, the pan PKC inhibitor sotrastaurin attenuated tubular injury and accelerated renal recovery following transplantation (Fuller et al., 2012). In cisplatin nephrotoxicity, PKCδ was rapidly activated and the inhibition of PKCδ genetically or pharmacologically prevented kidney injury; notably PKCδ inhibitors also enhanced the chemotherapeutic effects of cisplatin in several tumor models, suggesting that blockade of PKCδ may be a “Kill two birds with one stone” strategy in cisplatin chemotherapy (Pabla et al., 2011).
Other renoprotective chemicals: Renoprotective effects have also been shown for the Rho kinase inhibitor Y27632-lysozyme in ischemic AKI (Prakash et al., 2008). Moreover, zafirlukast, the antagonist of cysteinyl leukotriene-1 receptor (CysLT1R, a member of G protein-coupled receptors superfamily), was shown to alleviate ischemic AKI by reducing neutrophil infiltration as well as P-selectin overexpression in renal tissues (Hanan et al., 2012). Necrostatin-1, a specific inhibitor of the receptor-interacting protein 1 (RIP1) kinase, prevented necrotic cell death and partially preserved renal function during AKI induced by ischemia-reperfusion, contrast media, and cisplatin nephrotoxicity (Linkermann et al., 2013; Linkermann et al., 2012; Xu et al., 2015). In addition, the inhibitor of Na+/ Ca2+ exchange KB-R7943 may attenuate renal tubular cell death by suppressing the increases of renal endothelin-1 (ET-1) and catalase during ischemic AKI and contrast medium-induced nephrotoxicity (Yamashita et al., 2001; Yang et al., 2013).
II. Herbs, Food and Dietary Nutrients
A variety of herbs, food and dietary nutrients that showed renoprotective effects in AKI models (Table 2).
Table 2.
Herbs, food and dietary nutrients with renoprotective effects in AKI
| No | Name | Characteristics | Tested AKI model | Mechanism |
|---|---|---|---|---|
| 1 | Korean Red Ginseng |
Perennial plants belonging to genus Panax of the Araliaceae |
AKI by cisplatin and gentamicin in rat |
reduce OS and inflammation |
| 2 | Radix Codonopsis (saponins) |
Perennial plants used frequently in traditional Chinese medicine |
IRI after kidney transplant in rat |
decrease lipid peroxidation and inhibit apoptosis |
| 3 | artemisia asiatica | Wormwood, traditional uses include treating liver problems, joint pain, gastric reflux |
IRI in male C57BL/6 mice |
increase the level of HO-1 and Bcl-2 |
| 4 | Ginkgo extract (ginaton) |
Herb extracts used for treating Alzheimer’s disease, memory loss, headache, et al. |
IRI in rats | suppress extrinsic apoptotic pathway induced by JNK |
| 5 | naringin (flavonoids) |
A flavonoid in grapefruit metabolized to flavanone naringenin |
IRI in rats | reduce TBARS, restore antioxidant enzymes |
| 6 | quercetin (flavonoids) |
A pigment with a molecular structure like or derived from flavone |
IRI in rats | increase GSH levels and activities of SOD and CAT |
| 7 | hesperidin (flavonoids) |
A flavanone glycoside found abundantly in citrus fruits |
cisplatin-induced AKI in rats |
attenuate OS, inflammation, apoptosis/necrosis |
| 8 | curcumin (Flavonoids) |
A diarylheptanoid which is a member of the ginger family |
IRI in rats | attenuate expression of RANTES, MCP-1 |
| 9 | Catechin (Flavanols) |
Derivatives of flavans that are abundant in teas |
IRI in rats | similar to naringin in rat kidney |
| 10 | Resveratrol (Polyphenols) |
A phenol found in red grapes, Japanese knotweed, etc |
septic-AKI in mice IRI in rats glycerol-ARF in rats cisplatin-AKI in mice |
Antooxidant, release NO, activate SIRT1 and inhibit p53 |
| 11 | Astragaloside IV | Marker compound in Astragali Radix |
IRI in rats | inhibit OS and p38 MAPK phosphorylation |
| 12 | Sulforaphane | A molecule within isothiocyanate from cruciferous vegetables |
H/R in HK2 RPTC IRI in mice |
induce Nrf2-dependent phase 2 enzymes |
| 13 | Sesame oil | Extraction from sesame seeds containing Vit E, Vit B6, etc |
AAs and contrast-induced AKI in rats |
inhibit renal OS |
| 14 | Polyenylphosphatid ycholine |
A lecithin soybean extract | IRI in rats | reduce levels of AST, BUN and NF-kB |
| 15 | Isoflavones | Phytoestrogens (plant estrogens) isolated from the soybean |
IRI in rats | induce heme oxygenase |
2.1 Herbs and derivatives
Korean red ginseng is a traditional herbal medicine in China, Korea, and Japan, which was shown to attenuated renal dysfunction, cell apoptosis and tubular damage in cisplatin- and gentamicin-induced AKI mainly by reducing ROS and inflammation (Kim et al., 2014; Lee et al., 2013). Similarly, Radix Codonopsis and the extract saponins increased superoxide dismutase (SOD) level and decreased apoptosis index in a model of kidney transplantation (He et al., 2011), artemisia asiatica extract increased the level of HO-1 and Bcl-2 in the setting of acute renal IRI damage (Jang et al., 2015), and Ginkgo extract (ginaton) was shown to possess anti-oxidation and anti-inflammation activities through suppressing extrinsic apoptotic signal pathway induced by c-Jun N-terminal kinase (JNK) signal pathway (Wang et al., 2008).
Interestingly, some bioactive extracts from herbs, such as flavonoids (naringin, quercetin, curcumin or hesperidin), flavanols (Catechin), Polyphenols (Resveratrol), and Saponin (Astragaloside IV), showed similar renoprotective effects with similar mechanisms. For example, quercetin, naringin, hesperidin, and catechin all reduced lipid perioxidation and restored the levels of antioxidant enzymes SOD and catalase in kidney tissues (Kahraman et al., 2003; Singh et al., 2004; Sahu et al., 2013; Singh et al., 2005). They also showed remarkable anti-inflammation effects (Shoskes, 1998).
Resveratrol is known for its effects on life extension, cancer prevention, and antidiabetic effects (Howitz et al., 2003; Baur et al., 2006; Su et al., 2006). In the animal models of AKI induced by sepsis, IR, glycerol, or cisplatin, Resveratrol improved kidney microcirculation and protected tubular epithelium. Mechanistically, Resveratrol may work by scavenging reactive oxygen/nitrogen species (ROS/RNS), releasing nitric oxide (NO), activating sirtuin 1 (SIRT1) and inhibiting p53 to block apoptosis (Holthoff et al., 2012; Sener et al., 2006; Chander & Chopra, 2006; Kim et al., 2011; Chander & Chopra, 2006). Saponin prevented renal damage through inhibiting ROS and p38 kinase-associated apoptosis pathways in AKI induced by renal IRI or contrast medium (Gui et al., 2013).
2.2 Food and dietary nutrients
Sulforaphane, an organosulfur compound enriched in cruciferous vegetables such as broccoli, protected against ischemic AKI probably by inducing the NF-E2-related factor-2 (Nrf2) antioxidative system (Yoon et al., 2008). Antioxidative activities were also shown for Sesame oil, which was renoprotective during aminoglycoside and iodinated contrast-induced AKI (Hsu et al., 2011; Hsu et al., 2010).
At least two extracts from soybean have been shown to be renoprotective in AKI models. First, polyenylphosphatidycholine was shown to reduce serum levels of aspartate aminotransferase, BUN and NF-kB expression (Demirbilek et al., 2006). Second, isoflavone extracted from soybeans protected against ischemic AKI probably by inducing heme oxygenase (Watanabe et al., 2007). In addition, isoflavones, such as daidzein, formononetin, and genistein, may activate the expression of SIRT1 and PGC-1α to induce mitochondrial biogenesis, leading to accelerated recovery of mitochondrial and cellular functions for renoprotection (Rasbach & Schnellmann, 2008).
III. Antioxidants and Mitochondrial Protectants
Other chemicals with renoprotective effects in AKI include antioxidants and mitochondrial Protectants (Table 3).
Table 3.
Other chemicals with renoprotective effects in AKI
| No | Name | Characteristics | Tested AKI model | Mechanism |
|---|---|---|---|---|
| 1 | N-acetylcysteine | A precursor of the antioxidant glutathione |
AKI by contrast in human, various AKI models in mouse and rat |
reduce oxidative stress |
| 2 | Glutamine | The abundant free amino acid in human blood while conditionally essential in states of illness or injury |
folic acid-induced AKI in CD-1 mice glycerol-induced AKI in rat |
inhibit JNK phosphorylation and enhancing Hsp70 |
| 3 | Glycine | The smallest amino acids found in proteins or natural products |
ATP-depleted MDCK cells, Menadione-induce d injury of RPTC |
target amino acid gated chloride channels |
| 4 | rMnSOD | MnSOD recombinant generated by DNA technique |
contrast-induced AKI in rat |
reduce renal oxidative stress |
| 5 | TDZD-8 | Pharmacological inhibitor of GSK3β |
ATP-depleted BUMPT cells, IRI in rats |
inhibit activation of GSK3β, Bax, and caspase 3 |
| 6 | Nutlin-3 | Small molecule antagonist of MDM2 |
Cisplatin-induced rat RPTC apoptosis |
suppress the activation of Bax/Bak |
| 7 | Minocycline | Semisynthetic derivative of tetracycline |
hypoxia, et al-RPTC apoptosis, IRI in rats |
induction of Bcl-2 |
| 8 | Mdivi-1 | Selective cell-permeable inhibitor of mitochondrial fission protein DRP1 |
Azide, cisplatin- RPTC apoptosis, IRI in C57BL/6 mice |
attenuate mitochondrial fragmentation and apoptosis |
| 9 | OMA1 | Mediator of mitochondrial inner membrane cleavage |
ATP-depleted RPTC, IRI in C57BL/6 mice |
mediate OPA1 proteolysis and mitochondrial fragmentation |
| 10 | SS-31 | Synthetic cell-permeable tetrapeptide that targets and concentrates in mitochondrial inner membrane |
IRI in rats | protect mitochondria by interacting with cardiolipin |
| 11 | SkQR1 | Cationic rhodamine derivative linked to a plastoquinone molecule |
glycerol-, IR-induced AKI in rats |
inhibit MPP and scavenge ROS |
| 12 | SRT1720 | Selective SIRT1 activator | IRI in rats | activate PGC-1α for mitochondrial biogenesis |
| 13 | Formoterol | Specific β2-adrenergic agonist | IRI in mice | promote mitochondrial biogenesis and recovery |
| 14 | sotrastaurin | Selective pan-PKC inhibitor | kidney transplantation in rat |
inhibit the induced PKC in transplantation |
| 15 | Y27632 | Coupled to lysozyme, selective Rho kinase inhibitor |
IRI in rats | reduce KIM-1, vimentin, MCP-1 |
| 16 | zafirlukast | Antagonist of CysLT1R | IRI in rats | reduce neutrophil infiltration, P-selectin overexpression |
| 17 | Necrostatin-1 | Specific inhibitor of RIP1 kinase | contrast-induced AKI in mice |
prevent dilation of peritubular capillaries |
| 18 | KB-R7943 | Inhibitor of Na+/ Ca2+ exchange | IRI in mice contrast-induced AKI in rat |
suppress the increased ET-1 and catalase |
3.1 Antioxidants
Oxidant stress is a well-recognized pathogenic factor in AKI. ROS are produced excessively during AKI by several mechanisms. a. disruption of mitochondrial homeostasis results in electronic leak from the respiratory chain; b. macrophage phagocytosis of cellular debris leads to the release of a large amount of ROS; c. hypoxia-reoxygenation in kidney tissues decreases the cellular antioxidant activity (glutathione-GSH, antioxidant enzymes) resulting in redox imbalance (Funk et al., 2012; Samarasinghe et al., 2000; Martins et al., 2003). Consequently, excess ROS in cells induces oxidative damage of proteins, lipid membranes and biological macromolecules, and promotes inflammation and tissue damage.
Glutathione (GSH) is a major cellular antioxidant that is synthesized by the precursors N-acetylcysteine (NAC), glutamine and glycine. NAC showed beneficial effects in various models of AKI and notably, in contrast-induced AKI patients (Kelly et al., 2008). In general, the renoprotective effect of NAC is attributed to improved levels of GSH and associated decrease of ROS in AKI (Duru et al., 2008; Briguori et al., 2011). However, in addition to antioxidation, glutamine may have other effects. For example, it may mitigate renal neutrophil infiltration and tubular cell apoptosis by inhibiting JNK and enhancing Hsp70 (Peng et al., 2013; Kim et al., 2009). Glycine is a classical cell plasma membrane protectant, which protects against kidney tubular cell death by a mechanism related to amino acid gated chloride channels rather than its anti-oxidant activity (Venkatachalam et al., 1996; Sogabe et al., 1996).
In addition, cellular antioxidant enzymes, such as recombinant manganese superoxide dismutase (rMnSOD), reduced OS following contrast medium-induced AKI (Pisani et al., 2014). Consistently, deletion of extracellular SOD3 led to a more pronounced functional deterioration in AKI, supporting the beneficial effect of SOD (Schneider et al., 2010).
3.2 Mitochondrial protectants
Pathologically, AKI is characterized by tubular cell injury and death. Under this condition, multiple forms of cell death are triggered and mediated by different pathways (Linkermann et al., 2014). Nonetheless, mitochondrial damage appears to be a common factor that induces tubular cell death in AKI. Mitochondrial permeability transition (MPT) at the inner membrane plays a critical role in tubular cell necrosis. As a result, the inhibition of MPT pharmacologically by cyclosporine A or genetically by cyclophilin D ablation led to an increased resistance of kidneys to ischemic AKI (Park et al., 2011; Feldkamp et al., 2009). At the outer membrane of mitochondria, Bax and Bak, two pro-apoptotic members of Bcl-2 family proteins, may co-operate to induce porous defects for the release of apoptotic factors, such as cytochrome c, leading to apoptosis. In ischemic AKI, GSK3β was suggested to activate Bax via phosphorylation and the pharmacological inhibitor of GSK3β, TDZD-8, could block Bax activation to afford significant renoprotective effects (Wang et al., 2010). Inerestingly, Nutlin-3, an murine double minute-2 (MDM2) inhibitor, was shown to directly antagonize Bax, resulting in the prevention of Bax/Bak oligomerization, inhibition of cytochrome c release, and suppression of apoptosis during cisplatin treatment of renal tubular cells (Jiang et al., 2007). Minocycline, a derivative of tetracycline, may up-regulate Bcl-2 in renal tubular cells to block Bax/Bak activation and apoptosis during hypoxia, ATP-depletion, and cisplatin injury (Wang et al., 2004). These studies support the therapeutic potential of the antagonists of Bax/Bak in AKI.
Mitochondria are highly dynamic organelles that undergo fission and fusion (Brooks & Dong, 2007). In AKI, mitochondrial dynamics is disrupted, resulting in mitochondrial fragmentation, which can be partially prevented by mdivi-1, a mitochondrial fission inhibitor. Importantly, mdivi-1 provided significant protection against AKI (Brooks et al., 2009). This study not only supports a role of mitochondrial dynamics disruption in the pathogenesis of AKI but has also identified a new therapeutic strategy. Mechanistically, it was shown that the fragmented mitochondria are more sensitive to Bax insertion (Brooks et al., 2011). More recent work by Xiao et al has further shown the regulation of mitochondrial fragmentation by inner membrane protease OMA1 cleaving (Optic atrophy 1) OPA1 in ischemic AKI (Xiao et al., 2014).
Several antioxidant agents have been reported to specifically target mitochondria and provide renoprotective effects in AKI. For example, Zorov and colleagues developed SKQR1, a positively charged mitochondrial-targeting compound carrying an antioxidative moiety, which showed renoprotective effects in rat models of ischemic and glycerol-induced AKI (Plotnikov et al., 2011). Mechanistically, SkQR1 may protect by inhibiting MPP and scavenging excessive ROS. Szeto and colleagues have synthesized SS-31, a mitochondria-targeted tetrapeptide with antioxidant property (Szeto et al., 2011). In ischemic AKI, SS-31 protected mitochondrial structure and function, reduced tubular cell death, and partially preserved renal function. Interestingly, the effects of SS-31 may be related to its interaction with cardiolipin (Birk et al., 2013), a specific type of liplid found in the inner membrane of mitochondria.
In addition to limiting mitochondrial damage, another strategy is to promote mitochondrial biogenesis during and following AKI. In this regard, Schnellmann and colleagues reported that the SIRT1 activator SRT1720 could activate PGC-1α for mitochondrial biogenesis, leading to the accelerated recovery from ischemic AKI (Funk & Schnellmann, 2013). Their more recent work further demonstrated that formoterol, a potent β2-adrenergic agonist, induced renal mitochondrial biogenesis and enhanced renal recovery from ischemic injury. Remarkably, formoterol was effective even when given 24 hours after injury (Jesinkey et al., 2014), expanding the time window of treatment of clinical significance.
IV. Hormones with Renoprotective Activities
Several kinds of hormones are known for their protective effects in AKI (Table 4).
Table 4.
Hormones with renoprotective effects in AKI
| No | Name | Characteristics | Tested AKI model | Mechanism |
|---|---|---|---|---|
| 1 | 17β-estradiol | The primary female hormone | Ischemic AKI in mouse, rat |
activate PI3K/Akt/eNOS pathway, suppress renal SNS |
| 2 | Relaxin | A hormone of insulin superfamily exists in ovary and breast of female or prostate and semen of male |
IRI in rats cisplatin-induced AKI in rat |
decrease plasma TNF-α levels and renal TNFR1 |
| 3 | Oxytocin | A neurohypophysial hormone stimulating uterine contraction during and after childbirth |
IRI in rats | decrease TNF-α and oxidative damage |
| 4 | AQGV | An oligopeptide related to the primary structure of beta-hCG |
IRI in mice | decrease TNF-α, INF-γ, IL-6 and IL-10 |
| 5 | Testosterone | A androgen hormone secreted primarily by testicles |
IRI in rats | attenuate the increase of urinary KIM-1and intrarenal TNF-α |
| 6 | α-MSH | Hormones causing increased pigmentation, named as Melanocortins |
ischemic AKI in mice and rats |
suppressneutrophil activation and infiltration |
| 7 | ACTH | septic AKI of cecal ligation puncture |
induce MC1R-mediated anti-apoptotic effect, |
|
| 8 | AP214 | an α-MSH analogue | septic AKI in mice, ischemic AKI in a porcine |
reduce NF-kB and splenocyte apoptosis |
| 9 | Melatonin | the physiological antagonist of α-MSH |
ischemic AKI in C57Bl/6N mice |
improve the migration and survival of eEPCs |
| 10 | Ghrelin | The hunger hormone produced in the gastrointestinal tract |
IRI in rats | decrease kidney IL-6 and MPO activity, increase Bcl-2/Bax ratio |
| 11 | STC-1 | A hormone regulating renal calcium/phosphate homeostasis |
IRI in mice | activate AMPK induce UCP-2 of mitochondria |
| 12 | PACAP | A hypophysiotropic hormone similar to vasoactive intestinal peptide |
IRI in rats | prevent Bcl-2 decrease and apoptotic effects |
| 13 | Dexamethasone | An artificial synthetic of Glucocorticoid hormone |
septic AKI in C57BL/6 mice |
reduce MD with preserved COI |
4.1 Sex hormones
Several female sex hormones are known to be renoprotective in AKI. 17β-estradiol (E2), the primary female hormone, is a good example. E2 was shown to protect renal endothelial barrier function in AKI following cardiac arrest and cardiopulmonary resuscitation (Hutchens et al., 2010; Hutchens et al., 2012). Mechanistically, E2 may attenuate renal injury through the activation of phosphatidylinositol-3 kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) pathway (Satake et al., 2008) and by suppressing the renal sympathetic nervous system (SNS) (Tanaka et al., 2012). The pregnancy hormone Relaxin was also protective in AKI and the underlying mechanism may be related to the suppression of TNF-α-related inflammation and apoptosis (Yoshida et al., 2013; Yoshida et al., 2014). Similarly, Oxytocin attenuated ischemic AKI by decreasing TNF-α and oxidative damage (Tuğtepe et al., 2007). Renoprotective effect has also been demonstrated for AQGV, an oligopeptide related to the primary structure of human chorionic gonadotropin (beta-hCG), another pregnancy hormone (Khan et al., 2009).
Currently, it is controversial whether the male hormone testosterone/ dihydrotestosterone is good or bad in AKI. Over a decade ago, Park and colleagues suggested a critical role for testosterone in the susceptibility of males to ischemic AKI (Park et al., 2004), and Attia, et al suggested that male gender increases sensitivity to renal injury due to lower renal NOS activity than female rats (Attia et al., 2003). Followup studies have further provided mechanistic insights into the effect of testosterone, referring to decreased expression of histone deacetylase HDAC11 that was accompanied by an increase in PAI-1 expression (Kim et al., 2013). However, a recent study showed a dramatic decrease of serum testosterone during ischemic AKI; further, infusion of testosterone during renal IR protected the kidneys (Soljancic et al., 2013). Interestingly, low dose of testosterone significantly decreased cisplatin-induced nephrotoxicity, while administration of high-dose testosterone enhanced it (Rostami et al., 2014), suggesting a dual role for testosterone at low- or high- doses, respectively.
4.2 Melanocortins
Melanocortins are a group of hormones causing increased pigmentation, which includes alpha-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH). Star and Colleagues (Chiao et al., 1997) demonstrated the renoprotective effect of α-MSH in ischemic AKI in mice and rats. Mechanistically, although the initial work suggested suppression of neutrophil activation and infiltration in kidneys as a mechanism, a followup study indicated the involvement of neutrophil-independent mechanism (Chiao et al., 1998). AP214, an analogue of α-MSH, was protective in septic and ischemic AKI by reducing NF-kB activation and splenocyte apoptosis (Doi et al., 2008; Simmons et al., 2010). Somewhat paradoxically, renoprotective effects were also shown for melatonin, the physiological antagonist of α-MSH. It was suggested that melatonin protected kidneys by improving the migration and survival of "early outgrowth" endothelial progenitor cells (eEPCs) (Patschan et al., 2012), a function that is unrelated to that of melanogenesis (Valverde et al., 1995). Moreover the protective effect of α-MSH appears to be AKI model dependent, because it did not ameliorate mercuric chloride (HgCl2)-induced AKI (Miyaji et al., 2002). Similar to α-MSH, ACTH also demonstrated renoprotective effects in specific AKI models. For example, Gong and colleagues recently showed that ACTH alleviated TNF-induced AKI. Moreover, ACTH appeared to be more efficacious than α-MSH in renoprotection in the septic AKI model of cecal ligation puncture (Si et al., 2013). The beneficial effects of ACTH may derive from both steroid-dependent mechanisms and melanocortin 1 receptor (MC1R)-mediated anti-apoptotic effect.
4.3 Other hormones
Beneficial effects of several other hormones have also been shown in some AKI models. For example, dexamethasone reduced mitochondrial damage, the release of proapoptotic proteins, and the production of pro-inflammatory cytokines in septic AKI following cecal ligation and puncture (Choi et al., 2013). In ischemic AKI, the stomach-derived peptide Ghrelin attenuated the vagus nerve-mediated systemic and kidney-specific inflammatory responses, resulting in significant preservation of renal histology and function (Rajan et al., 2012). Stanniocalcin-1 (STC1) is known as a regulator of calcium and phosphate transport and cellular calcium/phosphate homeostasis (Yeung & Wong, 2011). Sheikh-Hamad and colleagues demonstrated notable renoprotective effects of STC1 in ischemic AKI, which may be related to the induction of mitochondrial uncoupling protein 2 (UCP-2) and suppression of superoxide generation in ischemic AKI (Huang et al., 2012). Their latest work further suggested the activation of AMP-activated protein kinase (AMPK) as an upstream key to the effects of STC1 (Pan et al., 2015). Finally, the neuropeptide pituitary adenylate cyclase activating polypeptide (PACAP) prevented Bcl-2 decrease and apoptosis in ischemic AKI (Horvath et al., 2010), and consistently, PACAP deficiency was associated with an increased susceptibility to ischemic AKI (Szakaly et al., 2011).
V. Cytokines and Growth Factors
5.1 Cytokines
In general, increases of cytokines such as chemokines, TNF-α or ICAM-1 are implicated in the robust inflammatory response observed in AKI. Accordingly, blockade of these cytokines, their receptors or related signaling reduces inflammation and the associated kidney damage. In this regard, renoprotective effects in AKI have been demonstrated for ICAM-1 monoclonal antibodies, the CXCR4 (CXC chemokine receptor 4) inhibitor Plerixafor, and the TNF-α inhibitor pentoxifylline (Zuk et al., 2014; Ramesh et al., 2002; Kelly et al., 1996; Kelly et al., 1994).
On the other hand, some other cytokines have notable renoprotective effects. For example, IL-10 is known to inhibit the increases of TNF-α, ICAM-1, and iNOS and protect against ischemic and cisplatin-induced AKI (Deng et al., 2001). In recent work, cardiotrophin-1 (CT-1), a member of the interleukin 6 (IL-6) family, showed significant protective effects in AKI induced by contrast medium (Quiros et al., 2013). In addition, some cytokines may directly protect renal tubules. For example, the lipocalin NGAL inhibited the activation of caspase-3 and reduced Bax expression and renal tubular cell apoptosis in ischemic AKI in rats (An et al., 2013). L-FABP (Liver-type fatty acid-binding proteins) attenuated aristolochic acid-induced nephrotoxicity likely through its antioxidant activity in renal tubules (Matsui et al., 2011). Furthermore, there are cytokines that are beneficial to hemodynamics or angiogenesis in AKI and kidney recovery following AKI. This is well-exemplified by soluble thrombomodulin (sTM) and Cartilage oligomeric matrix protein-angiopoietin-1 (COMP-Ang1), which improved microvascular erythrocyte flow rates and reduced microvascular endothelial leukocyte rolling and attachment during ischemic AKI (Sharfuddin et al., 2009). By improving peritubular capillary and enhancing renal tissue (re)perfusion, these cytokines were shown to alleviate ischemic kidney injury (Kim et al., 2006; Jung et al., 2009).
5.2 Growth factors
Growth factors are signaling molecules between cells that promote cellular proliferation and differentiation by binding to specific receptors on the surface of their target cells. It is known that the activation of growth factor-mediated signaling pathways is important for the survival, migration and proliferation of renal tubular cell during AKI and subsequent renal recovery or repair (He et al., 2013; Tang et al., 2013; Zhou et al., 2013; Mason et al., 2014). In addition, various growth factors including insulin-like growth factor (IGF), epidermal growth factor (EGF), Milk fat globule-epidermal growth factor-factor VIII (MFG-E8), and hepatocyte growth factor (HGF), exerted beneficial effects in models of ischemic-, cisplatin-, HgCl2-, or glycerol- AKI (Miller et al., 1992; Yasuda et al., 2004; Friedlaender et al., 1995; Matsuda et al., 2011; Yen et al., 2015; Homsi et al., 2009; Chen et al., 2013). These growth factors, when added exogenously, protected against initial injury, enhanced kidney repair and accelerated recovery of renal function. In addition, HGF or IGF-1 expressing mesenchymal stem cells (MSCs) showed a high therapeutic efficacy in ischemic- or cisplatin- AKI models; notably, the efficacy appeared to rely on the growth factor expression on these cells, providing further support for the therapeutic potential of specific growth factors (Imberti et al., 2007; Chen et al., 2011).
Renoprotective effects of haematopoietic growth factors in AKI have also been reported. Especially, Erythropoietin (EPO), named for its function of stimulating red blood cell generation, has been shown to protect against AKI in several models. The tissue protective effect of EPO appears to be largely independent on red blood cell production; instead, EPO may inhibit cell death and promote cellular repair and regeneration (Sharples & Yaqoob, 2006; Moore & Bellomo, 2011). In addition, granulocyte colony-stimulating factor (G-CSF) has been shown to ameliorate rhabdomyolysis-associated AKI, and interestingly the protective effect may be mediated by the induction of HO-1 (Wei et al., 2011).
However, it is important to note that adverse effects of growth factors have also been reported. For example, IGF-1 enhanced the inflammatory response as indicated by increased neutrophil filtration in a rat model of ischemic AKI, which was associated with higher mortality rate (Fernández et al., 2001). More recently, it was shown that erlotinib (selective EGFR tyrosine kinase inhibitor) partially prevented cisplatin-induced AKI in rats, implying an injurious role for EGFR signaling (Wada et al., 2014). In addition, in post-AKI kidneys, growth factors may promote renal fibrosis. For example, EGFR mutant mice showed more severe AKI following renal ischemia (consistent with a protective role of EGFR signaling in acute injury), but these mice developed less interstitial fibrosis 28 days later, suggesting a role of EGFR signaling in renal fibrogenesis (Tang et al., 2013). Thus, in terms of AKI, the role played by a growth factor or its receptor-mediated signaling may depend on where and when the pathway is activated. This critical question requires detailed research using inducible, tissue-specific conditional gene knockout models (Chen et al., 2012).
VI. Agents targeting gene expression
6.1 Transcription factors
AKI is associated with a significant change in gene expression profile. Thus, it is not surprising that a number of transcription factors may participate in tissue injury as well as protection and repair. Here nuclear factor kappa B (NF-κB) and hypoxia inducible factors (HIF) are briefly discussed as examples.
NF-κB is well-known as an inflammation promoting transcription factor that contributes to immune cell infiltration and cytokine production in AKI. In 2004, Cao and colleagues reported that transfection of NF-κB decoy oligodeoxynucleotides abolished NF-κB activation in ischemic AKI, resulting in decreases in MCP-1 and ICAM-1 expression, suppression of monocyte/ macrophage infiltration, and significant attenuation of tissue damage (Cao et al., 2004). Consistently, NF-κB activation was inhibited by pharmacologic agents such as milrinone and resveratrol or overexpression of SIRT1, resulting in a better preservation of renal histology and function in ischemic-AKI and cisplatin nephrotoxic (Jung et al., 2014; Jung et al., 2012). Blockade of NF-κB was also implicated in the protective effect of Nrf2 signaling (Jiang et al., 2014).
In contrast to NF-κB, HIF are generally regarded as protective transcription factors in AKI. There are at least 3 members in the HIF family, i.e., HIF-1, -2, and -3. Functional HIF is a heterodimer protein consisting of α and β subunits. In response to hypoxia, HIF-α is stabilized and then associates with HIF-β to translocate into the nucleus to induce the transcription of target genes (Semenza, 2014). HIF-1 plays a pivotal role in the regulation of renal physiology and patho-physiology (Haase, 2013). Pharmacological as well as genetic up-regulation of HIF afforded renoprotective effects in ischemic and nephrotoxic AKI models (Matsumoto et al., 2003; Weidemann et al., 2008; Hill et al., 2008; Fähling et al., 2013; Conde et al., 2012), suggesting a therapeutic potential. The protective effect of HIF may involve the expression of genes for oxygen delivery, cell survival, and metabolic adaptation. It is noteworthy that HIF may function in different cell types in kidneys: while HIF-1 was generally believed to be the key HIF for renoprotection, recent work by Kapitsinou and colleague however suggests that HIF-2 of endothelial cells may be mainly responsible for the observed protective effects (Kapitsinou et al., 2014). From the point of therapeutics, it is important to note that HIF is also a critical factor for renal fibrosis following AKI (Kapitsinou et al., 2012), it is therefore critical to time the treatment to maximize the protective effect and minimize the fibrogenic effect.
6.2 microRNAs
MicroRNAs are endogenously produced, small RNA molecules that negatively regulate target gene expression mainly by blocking their translation. Recent work has demonstrated the important roles played by microRNAs in renal development, physiology, and pathogenesis of various kidney diseases (Trionfini et al., 2015; Chung & Lan, 2015; Badal & Danesh, 2015; Marrone & Ho, 2014). The role of microRNAs in AKI was first demonstrated by using a conditional knockout model in which Dicer, a key enzyme for microRNA biogenesis, was ablated specifically from renal proximal tubules in mice. In this model, microRNAs were largely depleted from kidney tissues and remarkably, the animals were resistant to ischemic AKI (Wei et al., 2010). By microarray analysis, 13 microRNAs were shown to be significantly up- or down-regulated during ischemic AKI and the latest work has begun to delineate the regulations of these microRNAs and determine their pathological roles. For example, microRNA-687 was shown to be induced dramatically via HIF-1 in ischemic AKI and, upon induction, this microRNA targets phosphatase and tensin homolog (PTEN) to mediate tubular cell death and renal tissue damage (Bhatt et al., 2015). Interestingly, the microRNA expression profiles of bilateral ischemic AKI (Wei et al., 2010) was quite different from that of unilateral ischemia (Godwin et al., 2010), suggesting the sensitivity of microRNA expression. In cisplatin nephrotoxicity, microRNA-34a was shown to be induced via p53 and contributed to cell survival because antagonism of miR-34a with specific antisense oligonucleotides increased cell death during cisplatin treatment (Bhatt et al., 2010). In addition to these earlier studies, more recent studies have further identified miR-24, miR-127, miR-687, and miR-126 as critical regulators of ischemic AKI. For example, Lorenzen and colleagues demonstrated that the silencing of miR-24 ameliorated apoptotic responses and histologic tubular damage in ischemic AKI, resulting in a significant improvement in survival and kidney function (Lorenzen et al., 2014). Also as alluded above, blockade of miR-687 also protected against ischemic AKI (Bhatt et al., 2015). While the induction of some microRNAs have also been reported as beneficial in AKI. For example, miR-127 was shown to protect against ischemic AKI by targeting kinesin family member 3B (KIF3B), which is involved in the regulation of cell-matrix and cell-cell adhesion maintenance (Aguado-Fraile et al., 2012). In cisplatin nephrotoxicity, miR-34a appeared to promote renal tubular cell survival. Consistently, miR-155-deficient mice demonstrated heightened kidney toxicity following cisplatin treatment, supporting a protective role of this microRNA (Pellegrini et al., 2014). The recent work by Bijkerk and colleagues further suggested that overexpression of miR-126 in the hematopoietic compartment can facilitate vascular regeneration and renal recovery from AKI likely by mobilizing and homing hematopoietic stem and progenitor cells (Bijkerk et al., 2014). Thus, some microRNAs are protective whereas others being injurious in AKI, and targeting of specific microRNAs may offer an effective strategy for the treatment of AKI.
6.3 Epigenetic regulators
A new development in AKI research is the recognition of the involvement of epigenetic regulation in kidney injury and subsequent recovery or repair (Tang, Dong, 2015; Tang, Zhuang, 2015). Epigenetics refers to heritable mechanisms that alter gene expression without changing DNA sequence. DNA methylation and post-translation histone modifications (e.g. acetylation) are major epigenetic mechanisms that may keep the chromatin in an ‘open’ or ‘closed’ configuration to facilitate or block gene expression. The earliest evidence for the contribution of epigenetic regulation in AKI came from the study of the effects of the inhibitors of histone deacetylase (HDAC). In 2008, we reported that two HDAC inhibtors, suberoylanilide hydroxamic acid and Trichostatin A, were toxic to renal tubular cells at relatively high concentrations (Dong et al., 2008), but at lower dosages they were protective against cisplatin-induced apoptosis in these cells (Dong et al., 2010). These studies suggested the involvement of epigenetic regulation in AKI and notably, the effect of HDAC inhibitors depended on their dosages. Consistently, MS-275 (another HDAC inhibitor) worsened AKI and prevented kidney repair in the mouse models of AKI induced by folic acid or rhabdomyolysis (Tang et al., 2014), whereas Trichostatin A and methyl-4-(phenylthio)butanoate were recently shown to be beneficial to ischemic AKI (Levine et al., 2015; Cianciolo et al., 2013). Thus, the effects of HDAC inhibitors depend on their specificity, dosages of use, and AKI models of test. Regardless, these studies support a role of epigenetic regulation in AKI and kidney repair following AKI. Recent studies have begun to delineate the specific epigenetic mechanisms in AKI. For example, Bomsztyk and colleagues have recently provided comprehensive information about the epigenetic modifications of histones in mouse models of AKI induced by renal ischemia/reperfusion and lipopolysaccharide (Mar et al., 2015). Further investigation in this area is expected to reveal specific epigenetic mechanisms that may provide effective therapeutic targets for AKI.
A partial list of cytokines, growth factors and proteins with renoprotective effects in AKI is provided in Table 5.
VII. Cell Therapy
7.1 Stem cells
Depending on their differentiation potentials, bone marrow derived stem cells (BMSC) are classified into hematopoietic stem cells (HSCs) and MSCs. BMSC showed renoprotective effects in different AKI models in numerous studies. Earlier studies suggested that BMSC may differentiate into renal tubules for kidney repair after AKI (Kale et al., 2003). But later studies indicated that differentiation of BMSC into renal tubular cells for repair, if any, is a very rare event (Li et al., 2007; Duffield et al., 2005). In these studies, the protective effects were mainly attributable to Mesenchymal stem cells (MSCs/BM-MSCs) (Tögel & Westenfelder, 2010; Morigi & Benigni, 2013; Fleig & Humphreys, 2014). As alluded above, rather than differentiation into renal tubular cells, MSCs home to the injury sites and mainly function by producing paracrine factors that limit injury in renal tubules in AKI and/or facilitate the kidney repair. For example, knockdown of IGF-1 in MSCs led to a marked reduction of the cells’ protective ability in cisplatin-induced AKI (Imberti et al., 2007). Similarly, knockdown of VEGF in MSCs significantly reduced their efficacy in protection against ischemic AKI in rats (Tögel et al., 2009). Interestingly, Hu and colleagues further reported that MSCs mainly accumulated in lung and spleen, and their renoprotective effect in AKI may be related to the induction of T regulatory cells (Hu et al., 2013), suggesting a renoprotective mechanism for MSCs from distant organs, especially the spleen.
In addition to bone marrow, MSCs derived from other tissues also showed the beneficial effects on AKI. For example, the Wharton's jelly-derived mesenchymal stromal cells (WJ-MSC) improved renal function following renal ischemia, which was associated with a stronger proliferative response, less apoptosis and less fibrotic lesions and HGF may be an important contributor to the effects of WJ-MSC (Du et al., 2012). Similarly, adipose tissue-derived MSCs ameliorated folic acid- and cisplatin-induced AKI by producing HGF, VEGF and other factors (Katsuno et al., 2013; Yasuda et al., 2012).
In addition to MSCs, recent work has demonstrated the beneficial effect of the exosomes derived from MSCs in AKI induced by ischemia and cisplatin (Gatti et al., 2011; Bruno et al., 2012). Exosomes, containing specific proteins, mRNAs and microRNAs, are released from various cells and can fuse with neighboring cells to deliver their contents as a means of communication or supplementation. Thus, the exosomes from MSCs may offer a more efficient way to getting access to injured renal tubules for protection and kidney repair.
Obviously, a focus of future investigation is to optimize the condition of MSCs or exosomes derived there from for therapeutic use. In this regard, several bioactive agents have been reported to enhance the renoprotective effects of MSCs. For example, Mias and colleagues reported that melatonin pretreatment could significantly increase the survival of MSCs, their paracrine activity of producing HGF and FGF, and the beneficial effect of MSCs in ischemic kidney (Mias et al., 2008). Genetic modification of MSCs is another option to improve the efficacy of renoprotection. For example, overexpression of CXCR4 (the alpha-chemokine receptor specific for SDF-1/CXCL12) improved the reparative ability of MSCs in AKI by enhancing their homing to injured kidneys and production of cytokines such as BMP-7, HGF, and IL-10 (Liu et al., 2013).
7.2 Endothelial progenitor cells
EPCs are bone marrow–derived, circulating progenitor cells of the endothelial lineage (Asahara et al., 1997). Interestingly, patients suffering from sepsis-induced AKI showed a significantly higher level of circulating EPCs (Patschan et al., 2011). In AKI, microvascular endothelial cell dysfunction results in a decline of perfusion in peritubular capillaries, leading to the suppression of kidney repair or recovery. In 2006, Patschan and colleagues (Patschan et al., 2006) demonstrated the mobilization and homing of EPCs to injured kidneys in ischemic AKI. Importantly, transplantation or systemic administration of EPCs afforded renoprotective effect (Patschan et al., 2010). Interestingly, Li and colleagues showed that prior induction of hematopoietic stem and progenitor cells (HSPC) before application may provide a better protection by producing renotrophic factors including VEGF, IGF-1, and HGF that promote epithelial proliferation and tubular repair (Li et al., 2012). Similarly to that of MSCs, microvesicles or exosomes derived from EPCs were shown to attenuate ischemic AKI, notably, by harboring endothelial-protective miRNAs such as miR-126 and microRNA-dependent reprogramming of resident renal cells (Bitzer et al., 2012).
7.3 T lymphocytes
In addition to their well-recognized injurious role, research in recent years has established a protective role for specific subsets of lymphocytes (Jang et al., 2015). Especially, the depletion of TXPβ(+)CD4(+)CD25(+)Foxp3(+) regulatory T cells (Tregs) after ischemic injury led to enhanced pro-inflammatory cytokines production, increased renal tubular damage, and reduced tubular proliferation, while infusion of Tregs enhanced kidney repair and recovery (Gandolfo et al., 2009; Kinsey et al., 2009). These and other follow-up studies indicate that the pathological role of T cells in AKI depends on the cell subtype and the stage of injury. How to specifically stimulate Tregs for renoprotection? Lai and colleagues identified the potential in N, N-dimethylsphingosine (DMS), a naturally occurring sphingosine derivative and sphingosine kinase inhibitor. DMS was shown to recruit Tregs and protect against ischemic AKI; notably, the protective effect of DMS was abolished when Tregs were depleted (Lai et al., 2012), suggesting that DMS protects kidneys by recruiting Tregs. Research in this direction may lead to the development of therapeutic agents for clinical application, whereas cell therapy using Tregs may be technically more challenging.
VIII. Final thoughts on the strategies for identifying renoprotective agents
As discussed, numerous agents and approaches have been reported to be effective in protecting against AKI in experimental models. However, most have yet to enter clinical trial (Faubel et al., 2012). For those tested in patients, none has been successfully translated into clinical use. The reason can be many, including the complexity of the pathogenesis of AKI, the heterogeneity of the patients, and the defects in the design of previous clinical trials, just to name a few. On the bench side, it is crucial to thoroughly verify the effects of potential protective agents before considering or proposing clinical tests. The verification needs to cross-checked against multiple AKI models and also considers comorbid factors.
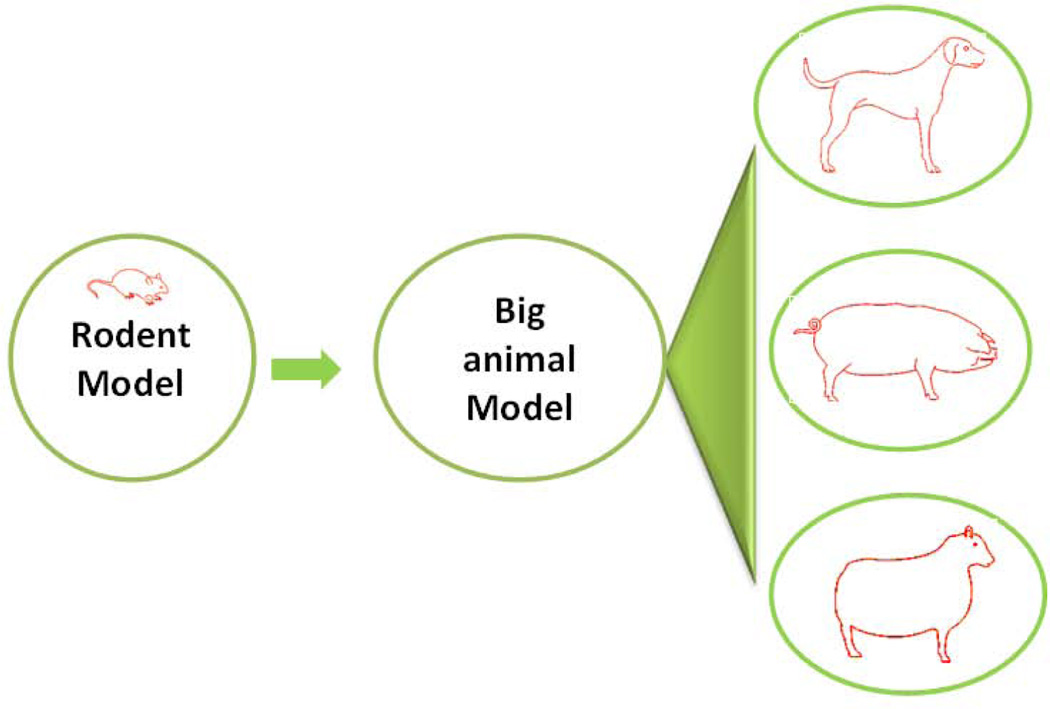
Currently, mouse and rat models are most commonly used for AKI research and for the test of potential renoprotective agents. Compared to mammals, the rodent models have notable merits, including the feasibility of transgenics. However, rodents are known to have major differences in the structural organization of kidneys. Especially, compared to mammals (e.g. dog), rodents have a relatively thicker renal medulla and a more complex vasculature that leads to the unique feature of “non-reflow” following ischemic injury. As such, many renoprotective agents shown in rodent ischemic models may fail in the models of higher animals since those agents mainly target the “non-reflow” phenomenon. Thus, it is important to verify the effect in rodent experiments by using higher animals, such as pig, dog, or sheep (Figure 2).
Fig. 2.
Experimental strategies for identifying renoprotective approaches for AKI: from rodent to mammalian models
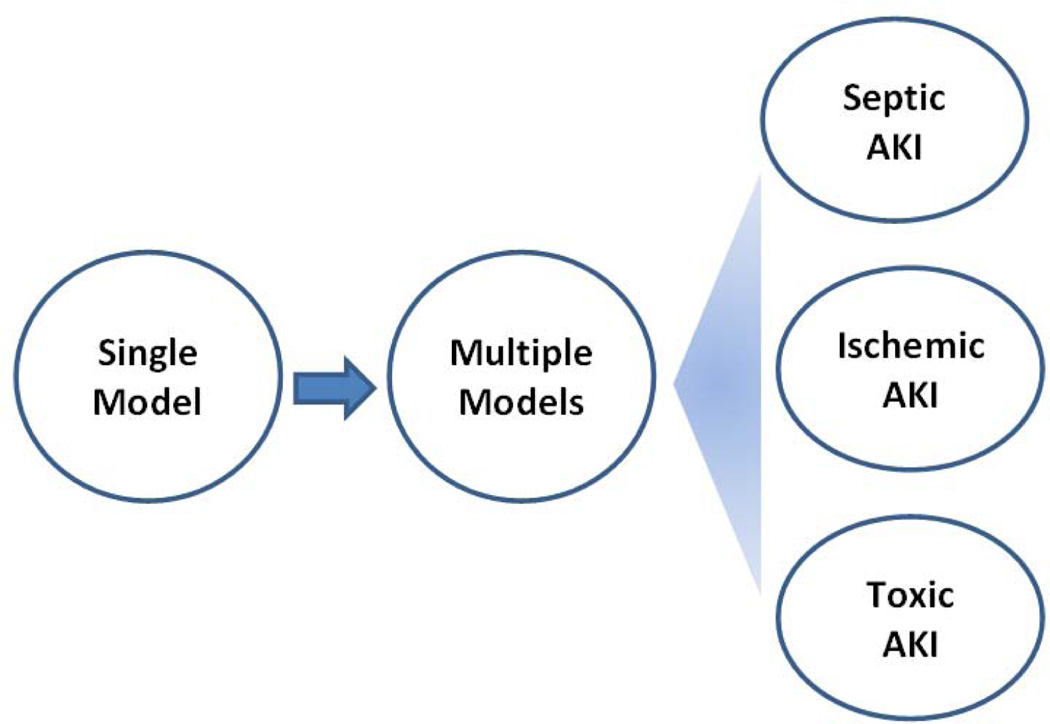
Clinically, there are various causes of AKI, which may be broadly divided into sepsis, nephrotoxicity, and renal ischemia-reperfusion. It is noteworthy that these causes are not mutually exclusive and in many cases, they co-exist. For example, ischemic injury may be an important component in nephrotoxic AKI due to toxic damage of vasculature and ensuing ischemia in kidney tissues. Importantly, while the cause of AKI is known for some patients (e.g. renal ischemia following cardiac surgery or nephrotoxicity after cisplatin chemotherapy), the cause of AKI for the majority of patients is unclear at admission. Under these conditions, it would be ideal to have a treatment that has a broad therapeutic spectrum. To discover such therapies, it is necessary to examine the effect in AKI models of different pathogenic origins (Figure 3). If the renoprotective effect of an agent is verified in two or more models, the chance of success in clinical trials is higher.
Fig. 3.
Experimental strategies for identifying renoprotective approaches for AKI: from single to multiple models
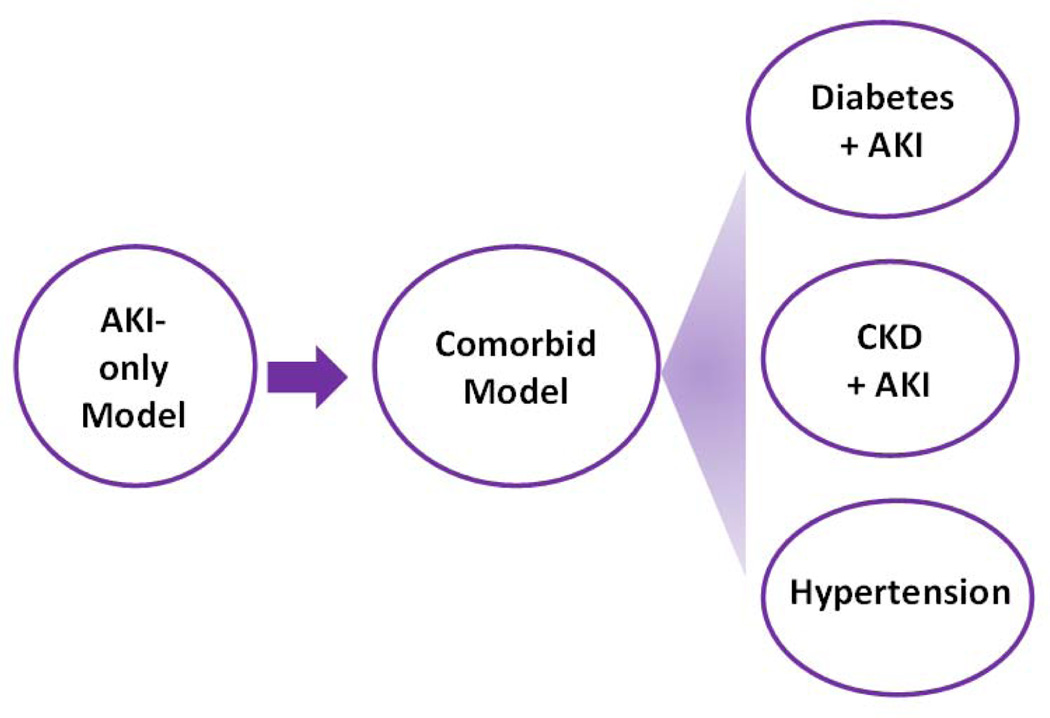
In addition, it is well recognized that AKI in young and otherwise healthy patients is mostly completely reversible. However, in clinic settings, a large portion of AKI patients also suffer from comorbid conditions, such as diabetes, hypertension, CKD, and/or aging. It is in this population of patients that AKI is severe, hard to recover, and likely to progress into end-stage renal disease or chronic kidney disease. Unfortunately, most previous studies investigated AKI in young and healthy adult animals without considering the comorbid factors that are known to have profound effects on the outcome in AKI patients. In this regard, AKI in aging has been studied for years (Rosner, 2013; Wang et al., 2014). Moreover, recent studies have begun to test comorbid models. For example, cisplatin nephrotoxicity has been investigated in tumor-bearing animal models (Pabla et al., 2011; Oh et al., 2014). and ischemic AKI examined in diabetic animals (Kelly et al., 2009; Peng et al., 2015; Gao et al., 2013). The comorbid models are obviously more complex; however, they are also more relevant to the patient condition and, as a result, renoprotective agents identified from these models are more likely to succeed at the bedside (Figure 4).
Fig. 4.
Experimental strategies for identifying renoprotective approaches for AKI: from AKI-only to comorbid models
Finally, depending on the etiology, AKI is mostly a combined result of the damage and dysfunction in kidney parenchymal and mesenchymal tissues, especially renal tubules, vasculatures, and immune response and inflammation. In view of such a complex pathogenesis, it is hard to envision a “silver bullet” for its optimal treatment. Rather, less specific, “dirty” drugs with multiple targets might be more effective. In this regard, cell therapy may be a good example. In addition, it is also important to consider the strategy of combination therapy, which takes advantage of the differential renoprotective effects of two or more agents. As presented in this review, various classes of renoprotective agents, including clinical drugs, herbs, natural or synthetic chemicals, bio-active proteins or peptides, and stem cells, have been described (Table 1–5). Notably, these agents have multiple and diverse mechanisms of protection, ranging from anti-oxidation, anti-inflammation, anti-apoptosis, and mitochondrial protection, to the activation of autophagy and other pro-survival pathways (Figure 1). Can the agents be used in combination to achieve better protective effects? Theoretically it is plausible. For example, it seems logical to combine a renal tubule protectant with an anti-inflammatory agent. However, the idea of combination therapy has rarely been tested, even in animal models (Liu et al., 2013).
In summary, decades of research has gained significant insights into the pathogenesis of AKI. Along the research, various renoprotective agents have been identified. Further investigation may cross-check their efficacy in multiple AKI models and also in comorbid models containing comorbid factors. Moreover, therapeutic efficacy may be improved or optimized by combination therapies.
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (81430017), the Hunan Province Natural Science Foundation, China (No.2009TP-1066-2), the National Basic Research Program of China 973, program No. 2012CB517601, the scientific research project of Hunan Province education department (14C0911), and the National Institutes of Health and Department of Veterans Administration of USA.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AIF
apoptosis inducing factor
- AKI
Acute kidney injury
- BMSC
bone marrow derived stem cells
- CIN
contrast-induced nephropathy
- COMP-Ang1
Cartilage oligomeric matrix protein-angiopoietin-1
- CysLT1R
cysteinyl leukotriene-1 receptor
- DMARD
disease-modifying antirheumatic drugs
- eNOS
endothelial nitric oxide synthase
- eEPCs
endothelial progenitor cells
- HDAC
histone deacetylase
- HSPC
hematopoietic stem and progenitor cells
- IRI
ischemia-reperfusion injury
- ICAM-1
intercellular adhesion molecule-1
- JNK
c-Jun N-terminal kinase
- KIF3B
kinesin family member 3B
- KIM-1
kidney injury molecule 1
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- α-MSH
alpha-melanocyte-stimulating hormone
- MFG-E8
Milk fat globule-epidermal growth factor-factor VIII
- MPT
Mitochondrial permeability transition
- MSCs
mesenchymal stem cells
- NGAL
neutrophil gelatinase-associated lipocalin
- MDM2
murine double minute-2
- MMP-2
matrix metallopeptidase 2
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol-3 kinase
- PACAP
pituitary adenylate cyclase activating polypeptide
- RAAS
renin-angiotensin-aldosterone system
- RANTES
regulated upon activation normal T-cell expressed and secreted
- RIP1
receptor-interacting protein 1
- TNF-α
Tumor necrosis factor-alpha
- TWEAK
TNF-like weak inducer of apoptosis
- VDRA
vitamin D receptor agonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
All authors declare that they have no commercial or other conflicting interests.
References
- Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48(4):1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- Aguado-Fraile E, Ramos E, Saenz-Morales D, Conde E, Blanco-Sanchez I, Stamatakis K, del Peso L, Cuppen E, Brune B, Garcia Bermejo ML. miR-127 Protects Proximal Tubule Cells against Ischemia/Reperfusion Identification of Kinesin Family Member 3B as miR-127 Target. PLOS ONE. 2012;7:e44305. doi: 10.1371/journal.pone.0044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Zang X, Yuan W, Zhuge Y, Yu Q. Neutrophil gelatinase-associated lipocalin (NGAL) may play a protective role against rats ischemia/reperfusion renal injury via inhibiting tubular epithelial cell apoptosis. Ren Fail. 2013;35(1):143–149. doi: 10.3109/0886022X.2012.741877. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Attia DM, Goldschmeding R, Attia MA, Boer P, Koomans HA, Joles JA. Male gender increases sensitivity to renal injury in response to cholesterol loading. Am J Physiol Renal Physiol. 2003;284(4):F718–F726. doi: 10.1152/ajprenal.00009.2002. [DOI] [PubMed] [Google Scholar]
- Badal SS, Danesh FR. MicroRNAs and their applications in kidney diseases. Pediatr Nephrol. 2015;30(5):727–740. doi: 10.1007/s00467-014-2867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 Induced by Hypoxia-Inducible Factor-1 Targets Phosphatase and Tensin Homolog in Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2015;26(7):1588–1596. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K, Zhou L, Mi QS, Huang S, She JX, Dong Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol Med. 2010;16(9–10):409–416. doi: 10.2119/molmed.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijkerk R, van Solingen C, de Boer HC, van der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM, Lievers E, et al. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J Am Soc Nephrol. 2014;25(8):1710–1722. doi: 10.1681/ASN.2013060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings FT, 4th, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24(6):913–920. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer M, Ben-Dov IZ, Thum T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 2012;82(4):375–377. doi: 10.1038/ki.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguori C, Quintavalle C, De Micco F, Condorelli G. Nephrotoxicity of contrast media and protective effects of acetylcysteine. Arch Toxicol. 2011;85(3):165–173. doi: 10.1007/s00204-010-0626-5. [DOI] [PubMed] [Google Scholar]
- Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300(3):C447–C455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins a key in Bak? Cell Cycle. 2007;6(24):3043–3047. doi: 10.4161/cc.6.24.5115. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale D, de Braganca AC, Goncalves J, Shimizu MHM, Volpini RA, Andrade L, Seguro AC. Vitamin D deficiency aggravates tenofovir induced metabolic syndrome and renal failure. Nephrol Dial Transplant. 2013;28:115. [Google Scholar]
- Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang L, Zhu CF. In vivo transfection of NF-kappaB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int. 2004;65(3):834–845. doi: 10.1111/j.1523-1755.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- Chander V, Chopra K. Protective effect of nitric oxide pathway in resveratrol renal ischemia-reperfusion injury in rats. Arch Med Res. 2006;37(1):19–26. doi: 10.1016/j.arcmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Chander V, Chopra K. Protective effect of resveratrol, a polyphenolic phytoalexin on glycerol-induced acute renal failure in rat kidney. Ren Fail. 2006;28(2):161–169. doi: 10.1080/08860220500531112. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Lainchbury JG, Nicholls MG, Rademaker MT, Richards AM, Troughton RW. Adrenomedullin and the renin-angiotensin-aldosterone system. Regul Pept. 2003;112(1–3):41–49. doi: 10.1016/s0167-0115(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82(1):45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen Z, Wang H, Xiong X, Liu X, Hu C, Han Y, Lu Y, Wu Z, Zhang Q. Plasmid pUDK-HGF encoding human hepatocyte growth factor gene attenuates gentamicin-induced kidney injury in rats. Exp Toxicol Pathol. 2013;65(5):541–547. doi: 10.1016/j.etp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, Peng X, Li W, Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20(1):103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone (α-MSH) protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99(6):1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. 1998;54(3):765–774. doi: 10.1046/j.1523-1755.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- Choi DE, Jeong JY, Lim BJ, Na KR, Shin YT, Lee KW. Pretreatment with the tumor nerosis factor-alpha blocker etanercept attenuated ischemia-reperfusion renal injury. Transplant Proc. 2009;41(9):3590–3596. doi: 10.1016/j.transproceed.2009.05.042. [DOI] [PubMed] [Google Scholar]
- Choi HM, Jo SK, Kim SH, Lee JW, Cho E, Hyun YY, Cha JJ, Kang YS, Cha DR, Cho WY, Kim HK. Glucocorticoids attenuate septic acute kidney injury. Biochem Biophys Res Commun. 2013;435(4):678–684. doi: 10.1016/j.bbrc.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Chung AC, Lan HY. MicroRNAs in renal fibrosis. Front Physiol. 2015;6:50. doi: 10.3389/fphys.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24(6):943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde E, Alegre L, Blanco-Sánchez I, Sáenz-Morales D, Aguado-Fraile E, Ponte B, et al. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS One. 2012;7(3):e33258. doi: 10.1371/journal.pone.0033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti-Khavidaki S, Moghaddas A, Heydari B, Khalili H, Lessan-Pezeshki M, Lessan-Pezeshki M. Statins against drug-induced nephrotoxicity. J Pharm Pharm Sci. 2013;16(4):588–608. doi: 10.18433/j3t30f. [DOI] [PubMed] [Google Scholar]
- Datta K, Suman S, Fornace AJ., Jr Radiation persistently promoted oxidative stress, activated mTOR via PI3K/Akt, and downregulated autophagy pathway in mouse intestine. Int J Biochem Cell Biol. 2014;57:167–176. doi: 10.1016/j.biocel.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirbilek S, Karaman A, Baykarabulut A, Akin M, Gürünlüoglu K, Edali MN, et al. Polyenylphosphatidylcholine pretreatment ameliorates ischemic acute renal injury in rats. Int J Urol. 2006;13(6):747–753. doi: 10.1111/j.1442-2042.2006.01397.x. [DOI] [PubMed] [Google Scholar]
- Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60(6):2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frøkiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73(11):1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Liu Y, Zhang L, Huang S, Ding HF, Dong Z. mTOR contributes to ER stress and associated apoptosis in renal tubular cells. Am J Physiol Renal Physiol. 2015;308(3):F267–F274. doi: 10.1152/ajprenal.00629.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Luo J, Kumar V, Dong Z. Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am J Physiol Renal Physiol. 2010;298(2):F293–F300. doi: 10.1152/ajprenal.00410.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Wang L, Wang CY, Yang T, Kumar MV, Dong Z. Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J Pharmacol Exp Ther. 2008;325(3):978–984. doi: 10.1124/jpet.108.137398. [DOI] [PubMed] [Google Scholar]
- Du T, Cheng J, Zhong L, Zhao XF, Zhu J, Zhu YJ, Liu GH. The alleviation of acute and chronic kidney injury by human Wharton's jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy. 2012;14(10):1215–1227. doi: 10.3109/14653249.2012.711471. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115(7):1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru M, Nacar A, Yönden Z, Kuvandik G, Helvaci MR, Söğüt S, et al. Protective effects of N-acetylcysteine on cyclosporine-A-induced nephrotoxicity. Ren Fail. 2008;30(4):453–459. doi: 10.1080/08860220801985942. [DOI] [PubMed] [Google Scholar]
- Fähling M, Mathia S, Paliege A, Koesters R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S, Rosenberger C. Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI. J Am Soc Nephrol. 2013;24(11):1806–1819. doi: 10.1681/ASN.2013030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD Acute Kidney Injury Advisory Group of the American Society of Nephrology. Ongoing clinical trials in AKI. Clin J Am Soc Nephrol. 2012;7(5):861–873. doi: 10.2215/CJN.12191111. [DOI] [PubMed] [Google Scholar]
- Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM. Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2009;297:F1632–F1646. doi: 10.1152/ajprenal.00422.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M, Medina A, Santos F, Carbajo E, Rodríguez J, Alvarez J, Cobo A. Exacerbated inflammatory response induced by insulin-like growth factor I treatment in rats with ischemic acute renal failure. J Am Soc Nephrol. 2001;12(9):1900–1907. doi: 10.1681/ASN.V1291900. [DOI] [PubMed] [Google Scholar]
- Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014;127(1–4):75–80. doi: 10.1159/000363680. [DOI] [PubMed] [Google Scholar]
- Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010;85(3):158–167. doi: 10.1159/000269779. [DOI] [PubMed] [Google Scholar]
- Friedlaender M, Popovtzer MM, Weiss O, Nefesh I, Kopolovic J, Raz I. Insulin-like growth factor-1 (IGF-1) enhances recovery from HgCl2-induced acute renal failure the effects on renal IGF-1, IGF-1 receptor, and IGF-binding protein-1 mRNA. J Am Soc Nephrol. 1995;5(10):1782–1791. doi: 10.1681/ASN.V5101782. [DOI] [PubMed] [Google Scholar]
- Fuller TF, Kusch A, Chaykovska L, Catar R, Pützer J, Hoff U, Dragun D, et al. Protein kinase C inhibition ameliorates posttransplantation preservation injury in rat renal transplants. Transplantation. 2012;94(7):679–686. doi: 10.1097/TP.0b013e318265c4d8. [DOI] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302(7):F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273(2):345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76(7):717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- Gao G, Zhang B, Ramesh G, Betterly D, Tadagavadi RK, Wang W, Reeves WB. TNF-α mediates increased susceptibility to ischemic AKI in diabetes. Am J Physiol Renal Physiol. 2013;304(5):F515–F521. doi: 10.1152/ajprenal.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26(5):1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107(32):14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh L, Lindsey K, Malhotra P, Kargoli F, Friedman A, El-Achkar TM, et al. Acute kidney injury after cardiac surgery is minocycline protective? J Nephrol. 2015;28(2):193–199. doi: 10.1007/s40620-014-0152-2. [DOI] [PubMed] [Google Scholar]
- Gueler F, Rong S, Park JK, Fiebeler A, Menne J, Kunter U, et al. Postischemic acute renal failure is reduced by short-term statin treatment in a rat model. J Am Soc Nephrol. 2002;13(9):2288–2298. doi: 10.1097/01.asn.0000026609.45827.3d. [DOI] [PubMed] [Google Scholar]
- Gui D, Huang J, Liu W, Guo Y, Xiao W, Wang N. Astragaloside IV prevents acute kidney injury in two rodent models by inhibiting oxidative stress and apoptosis pathways. Apoptosis. 2013;18(4):409–422. doi: 10.1007/s10495-013-0801-2. [DOI] [PubMed] [Google Scholar]
- Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol. 2013;24(4):537–541. doi: 10.1681/ASN.2012080855. [DOI] [PubMed] [Google Scholar]
- Hanan H Hagar, Raeesa Abd EI Tawab. Cysteinyl leukotriene receptor antagonism alleviates renal injury induced by ischemia-reperfusion in rats. J Surg Res. 2012;178(1):e25–e34. doi: 10.1016/j.jss.2012.02.022. [DOI] [PubMed] [Google Scholar]
- He B, Ying-Tian Z, Xin-Gang Y, Jing-Song S, Guang-Hui W, Tao L. Protective effects of Radix Codonopsis on ischemia–reperfusion injury in rats after kidney transplantation. Pediatr Surg Int. 2011;27(11):1203–1212. doi: 10.1007/s00383-011-2935-z. [DOI] [PubMed] [Google Scholar]
- He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304(4):F356–F366. doi: 10.1152/ajprenal.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19(1):39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81(4):370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am J Nephrol. 2009;29(4):283–291. doi: 10.1159/000159275. [DOI] [PubMed] [Google Scholar]
- Horvath G, Racz B, Reglodi D, Kovacs K, Kiss P, Gallyas F, Jr, Bognar Z, Szabo A, et al. Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia/reperfusion. J Mol Neurosci. 2010;42(3):411–418. doi: 10.1007/s12031-010-9342-0. [DOI] [PubMed] [Google Scholar]
- Howell GM, Gomez H, Collage RD, Loughran P, Zhang X, Matthew R Rosengart, et al. Augmenting Autophagy to Treat Acute Kidney Injury during Endotoxemia in Mice. PLoS ONE. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Sinclair DA, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DZ, Li YH, Chu PY, Periasamy S, Liu MY. Sesame oil prevents acute kidney injury induced by the synergistic action of aminoglycoside and iodinated contrast in rats. Antimicrob Agents Chemother. 2011;55(6):2532–2536. doi: 10.1128/AAC.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DZ, Liu CT, Li YH, Chu PY, Liu MY. Protective effect of daily sesame oil supplement on gentamicin-induced renal injury in rats. Shock. 2010;33(1):88–92. doi: 10.1097/SHK.0b013e3181a98de4. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q, Li Q, Shi S, Liu X, Chen X. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84(3):521–531. doi: 10.1038/ki.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82(8):867–877. doi: 10.1038/ki.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Agarwal A, et al. Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI. J Am Soc Nephrol. 2015;26(9):2139–2151. doi: 10.1681/ASN.2014080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol. 2012;303(3):F377–F385. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, Murphy SJ, Anderson S, Hurn PD. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology. 2010;112(2):395–405. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Choi YA, Park KC, Yang KJ, Choi HS, Kim SH, et al. Pretreatment with rituximab prevents subsequent ischemia-reperfusion injury in mice kidney. Nephrology Dialysis Transplantation. 2013;28:96. doi: 10.1093/ndt/gfs540. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Yang KJ, Park KC, Choi HS, Kim SH, Yang CW, et al. Pretreatment with paricalcitol attenuates inflammation in ischemia-reperfusion injury via the up-regulation of cyclooxygenase-2 and prostaglandin E2. Nephrol Dial Transplant. 2013;28(5):1156–1166. doi: 10.1093/ndt/gfs540. [DOI] [PubMed] [Google Scholar]
- Ihtiyar E, Yaşar NF, Erkasap N, Köken T, Tosun M, Oner S, Erkasap S. Effects of doxycycline on renal ischemia reperfusion injury induced by abdominal compartment syndrome. J Surg Res. 2011;167(1):113–120. doi: 10.1016/j.jss.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Inal S, Koc E, Okyay GU, Pasaoglu O, Gonul I, Oyar E, Pasaoglu H, Guz G. Protective effect of adrenomedullin on contrast induced nephropathy in rats. Nephrology Dialysis Transplantation. 2013;28(1 suppl):110. [Google Scholar]
- Jang HJ, Jeong EK, Kim SS, Lee JH, Oh MY, Kang KS, et al. Protective effect of artemisia asiatica extract against renal ischemia-reperfusion injury in mice. Exp Clin Transplant. 2015;13(1 Suppl):377–382. [PubMed] [Google Scholar]
- Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11(2):88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014;25(6):1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 Protects Kidney Cells during Cisplatin Therapy by Suppressing Bax/Bak Activation. J Biol Chem. 282(4):2636–2645. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82(12):1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Tian F, Zheng H, Whitman SA, Lin Y, Zhang Z, Zhang N, Zhang DD. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014;85(2):333–343. doi: 10.1038/ki.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2(2):356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- Jones EA, Shoskes DA. The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. J Urol. 2000;163(3):999–1004. [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Joo JD, Kim DW, In JH, Roh M, Jeong JT, Noh SJ, Choi JW. Effect of milrinone on the inflammatory response and NF-kB activeation in renal ischemia-reperfusion injury in mice. Korean J Anesthesiol. 2014;66(2):136–142. doi: 10.4097/kjae.2014.66.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, Jang KY, Sung MJ, Park SK, Kim W. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297(4):F952–F960. doi: 10.1152/ajprenal.00064.2009. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK, Lee SY, Han MK, Kim DH, Kim W. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun. 2012;419(2):206–210. doi: 10.1016/j.bbrc.2012.01.148. [DOI] [PubMed] [Google Scholar]
- Kahraman A, Erkasap N, Serteser M, Köken T. Protective effect of quercetin on renal ischemia/reperfusion injury in rats. J Nephrol. 2003;16(2):219–224. [PubMed] [Google Scholar]
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112(1):42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302(9):F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL, Sutton TA, Haase VH. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124(6):2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman A, Turkmen E, Gursul C, Tas E, Fadillioglu E. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol. 2006;13(11):1434–1441. doi: 10.1111/j.1442-2042.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, Yamamoto T, Sato W, Tsuboi N, Mizuno M, Ito Y, Imai E, Matsuo S, Maruyama S. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22(2):287–297. doi: 10.3727/096368912X655019. [DOI] [PubMed] [Google Scholar]
- Kazunori Takahashi, Hiroki Mizukami, Kosuke Kamata, Wataru Inaba, Noriaki Kato, Chihiro Hibi, Soroku Yagihashi. Amelioration of Acute Kidney Injury in Lipopolysaccharide-Induced Systemic Inflammatory Response Syndrome by an Aldose Reductase Inhibitor, Fidarestat. PLoS One. 2012;7:e30134. doi: 10.1371/journal.pone.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148(4):284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297(4):F923–F931. doi: 10.1152/ajprenal.00205.2009. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2004;287(4):F760–F766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A. 1994;91(2):812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Susa D, van Den Berg JW, Huisman M, Ameling MH, van den Engel S, Roest HP, Ijzermans JN, Dik WA, Benner R, de Bruin RW. Amelioration of renal ischaemia-reperfusion injury by synthetic oligopeptides related to human chorionic gonadotropin. Nephrol Dial Transplant. 2009;24(9):2701–2708. doi: 10.1093/ndt/gfp369. [DOI] [PubMed] [Google Scholar]
- Kholmukhamedov A, Czerny C, Hu J, Schwartz J, Zhong Z, Lemasters JJ. Minocycline and doxycycline, but not tetracycline, mitigate liver and kidney injury after hemorrhagic shock/resuscitation. Shock. 2014;42(3):256–263. doi: 10.1097/SHK.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease, Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int. 2012;(2 Suppl):1–138. [Google Scholar]
- Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Kim W, et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol. 2011;301(2):F427–F435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- Kim JI, Jung KJ, Jang HS, Park KM. Gender-specific role of HDAC11 in kidney ischemia- and reperfusion-induced PAI-1 expression and injury. Am J Physiol Renal Physiol. 2013;305(1):F61–F70. doi: 10.1152/ajprenal.00015.2013. [DOI] [PubMed] [Google Scholar]
- Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17(9):2474–2483. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee MY, Son HY, Park BK, Ryu SY, Jung JY. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014;80(8–9):645–654. doi: 10.1055/s-0034-1368571. [DOI] [PubMed] [Google Scholar]
- Kim YS, Jung MH, Choi MY, Kim YH, Sheverdin V, Kim JH, Ha HJ, Park DJ, et al. Glutamine attenuates tubular cell apoptosis in acute kidney injury via inhibition of the c-Jun N-terminal kinase phosphorylation of 14-3-3. Crit Care Med. 2009;37(6):2033–2044. doi: 10.1097/CCM.0b013e3181a005ba. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20(8):1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostapanos MS, Liberopoulos EN, Elisaf MS. Statin pleiotropy against renal injury. J Cardiometab Syndr. 2009;4(1):E4–E9. doi: 10.1111/j.1559-4572.2008.00052.x. [DOI] [PubMed] [Google Scholar]
- Lai LW, Yong KC, Lien YH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2012;81(10):983–992. doi: 10.1038/ki.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury an increasing global concern. Lancet. 2013;382(9887):170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- Lauver DA, Carey EG, Bergin IL, Lucchesi BR, Gurm HS. Sildenafil citrate for prophylaxis of nephropathy in an animal model of contrast-induced acute kidney injury. PLoS One. 2014;9(11):e113598. doi: 10.1371/journal.pone.0113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Chin YW, Choi YH. Effects of Korean red ginseng extract on acute renal failure induced by gentamicin and pharmacokinetic changes by metformin in rats. Food Chem Toxicol. 2013;59:153–159. doi: 10.1016/j.fct.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Levine MH, Wang Z, Bhatti TR, Wang Y, Aufhauser DD, McNeal S, Liu Y, Cheraghlou S, Han R, Wang L, Hancock WW. Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Am J Transplant. 2015;15(4):965–973. doi: 10.1111/ajt.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Black R, Ma Z, Yang Q, Wang A, Lin F. Use of mouse hematopoietic stem and progenitor cells to treat acute kidney injury. Am J Physiol Renal Physiol. 2012;302(1):F9–F19. doi: 10.1152/ajprenal.00377.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Truong P, Igarashi P, Lin F. Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol. 2007;18(12):3067–3077. doi: 10.1681/ASN.2007030284. [DOI] [PubMed] [Google Scholar]
- Li Y, Xia AZ, Xing SH. Protective effect of edaravone against renal ischemia/reperfusion injury and compared with ischemic postconditioning in rats. Yao Xue Xue Bao. 2010;45(7):840–848. [PubMed] [Google Scholar]
- Liaño F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int. 1998;(66 Suppl):S16–S24. [PubMed] [Google Scholar]
- Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. I. The signaling pathway. Am J Physiol Renal Physiol. 2012;303(1):F1–F10. doi: 10.1152/ajprenal.00014.2012. [DOI] [PubMed] [Google Scholar]
- Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81(8):751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25(12):2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Heller JO, Prókai A, Weinberg JM, De Zen F, Krautwald S, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24(10):1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Patzak A, Zhang J. CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol. 2013;305(7):F1064–F1073. doi: 10.1152/ajprenal.00178.2013. [DOI] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011;22(6):1064–1075. doi: 10.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Feng Y, Dong C, Liu D, Wu X, Wu H, Lv P, Zhou Y. Study on therapeutic action of bone marrow derived mesenchymal stem cell combined with vitamin E against acute kidney injury in rats. Life Sci. 2013;92:829–837. doi: 10.1016/j.lfs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, et al. MicroRNA-24 Antagonism Prevents Renal Ischemia Reperfusion Injury. J Am Soc Nephrol. 2014;25:2717–2729. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD, Mayer RD, Ewing JF, McCoubrey WK., Jr Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion possible role of heme as both promotor of tissue damage and regulator of HSP32. J Pharmacol Exp Ther. 1993;264(1):457–462. [PubMed] [Google Scholar]
- Mar D, Gharib SA, Zager RA, Johnson A, Denisenko O, Bomsztyk K. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int. 2015;88(4):734–744. doi: 10.1038/ki.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone AK, Ho J. MicroRNAs potential regulators of renal development genes that contribute to CAKUT. Pediatr Nephrol. 2014;29(4):565–574. doi: 10.1007/s00467-013-2599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PS, Kallas EG, Neto MC, Dalboni MA, Blecher S, Salomão R. Upregulation of reactive oxygen species generation and phagocytosis, and increased apoptosis in human neutrophils during severe sepsis and septic shock. Shock. 2003;20(3):208–212. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PubMed] [Google Scholar]
- Mason S, Hader C, Marlier A, Moeckel G, Cantley LG. Met activation is required for early cytoprotection after ischemic kidney injury. J Am Soc Nephrol. 2014;25(2):329–337. doi: 10.1681/ASN.2013050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A, Wu R, Jacob A, Komura H, Zhou M, Wang Z, Aziz MM, Wang P. Protective effect of milk fat globule-epidermal growth factor-factor VIII after renal ischemia-reperfusion injury in mice. Crit Care Med. 2011;39(9):2039–2047. doi: 10.1097/CCM.0b013e3182227a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Kamijo-Ikemorif A, Sugaya T, Yasuda T, Kimura K. Renal liver-type fatty acid binding protein (L-FABP) attenuates acute kidney injury in aristolochic acid nephrotoxicity. Am J Pathol. 2011;178(3):1021–1032. doi: 10.1016/j.ajpath.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14(7):1825–1832. doi: 10.1097/01.asn.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, Bourin P, Parini A, Cussac D. Ex vivo pretreatment with melatonin improves survival, proangiogenic / mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26(7):1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- Miller SB, Martin DR, Kissane J, Hammerman MR. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci U S A. 1992;89(24):11876–11880. doi: 10.1073/pnas.89.24.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Hu X, Star RA. alpha-Melanocyte-simulating hormone and interleukin-10 do not protect the kidney against mercuric chloride-induced injury. Am J Physiol Renal Physiol. 2002;282(5):F795–F801. doi: 10.1152/ajprenal.00203.2001. [DOI] [PubMed] [Google Scholar]
- Molitoris BA. Therapeutic translation in acute kidney injury the epithelial/endothelial axis. J Clin Invest. 2014;124(6):2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Coca S, Devereaux P, Jain A, Kitchlu A, Luo J, et al. Statin use is associated with a lower incidence of acute kidney injury after major elective surgery. J Am Soc Nephrol. 2011;22(5):939–946. doi: 10.1681/ASN.2010050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care. 2011;1(1):3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28(4):788–793. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Choi JH, Shen A, Choe SK, Karna A, Lee SH, Jo HJ, Yang SH, Kwak TH, Lee CH, Park R, So HS. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014;85(3):547–560. doi: 10.1038/ki.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121(7):2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121(7):2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001;59(5):1789–1797. doi: 10.1046/j.1523-1755.2001.0590051789.x. [DOI] [PubMed] [Google Scholar]
- Pan JS, Huang L, Belousova T, Lu L, Yang Y, Reddel R, Chang A, Ju H, DiMattia G, Tong Q, Sheikh-Hamad D. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol. 2015;26(2):364–378. doi: 10.1681/ASN.2013070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol. 2011;301(1):F134–F150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279(50):52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- Patschan D, Hildebrandt A, Rinneburger J, Wessels JT, Patschan S, Becker JU, Henze E, Krüger A, Müller GA. The hormone melatonin stimulates renoprotective effects of "early outgrowth" endothelial progenitor cells in acute ischemic kidney injury. Am J Physiol Renal Physiol. 2012;302(10):F1305–F1312. doi: 10.1152/ajprenal.00445.2011. [DOI] [PubMed] [Google Scholar]
- Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291(1):F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- Patschan D, Patschan S, Wessels JT, Becker JU, David S, Henze E, Goligorsky MS, Müller GA. Epac-1 activator 8-O-cAMP augments renoprotective effects of syngeneic [corrected] murine EPCs in acute ischemic kidney injury. Am J Physiol Renal Physiol. 2010;298(1):F78–F85. doi: 10.1152/ajprenal.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschan SA, Patschan D, Temme J, Korsten P, Wessels JT, Koziolek M, Henze E, Müller GA. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD) Crit Care. 2011;15(2):R94. doi: 10.1186/cc10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini KL, Han T, Bijol V, Saikumar J, Craciun FL, Chen WW, Fuscoe JC, Vaidya VS. MicroRNA-155 deficient mice experience heightened kidney toxicity when dosed with cisplatin. Toxicol Sci. 2014;141(2):484–492. doi: 10.1093/toxsci/kfu143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li X, Zhang D, Chen JK, Su Y, Smith SB, Dong Z. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015;87(1):137–150. doi: 10.1038/ki.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZY, Zhou F, Wang HZ, Wen XY, Nolin TD, Bishop JV, Kellum JA. The anti-oxidant effects are not the main mechanism for glutamine's protective effects on acute kidney injury in mice. Eur J Pharmacol. 2013;705(1–3):11–19. doi: 10.1016/j.ejphar.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Pisani A, Sabbatini M, Riccio E, Rossano R, Andreucci M, Capasso C, De Luca V, Carginale V, et al. Effect of a recombinant manganese superoxide dismutase on prevention of contrast-induced acute kidney injury. Clin Exp Nephrol. 18(3):424–431. doi: 10.1007/s10157-013-0828-2. [DOI] [PubMed] [Google Scholar]
- Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, Zorov DB. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta. 2011;1812(1):77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Prakash J, de Borst MH, Lacombe M, Opdam F, Poelstra K, Kok RJ, et al. Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. J Am Soc Nephrol. 2008;19(11):2086–2097. doi: 10.1681/ASN.2007070794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros Y, Sánchez-González PD, López-Hernández FJ, Morales AI, López-Novoa JM. Cardiotrophin-1 administration prevents the renal toxicity of iodinated contrast media in rats. Toxicol Sci. 2013;132(2):493–501. doi: 10.1093/toxsci/kft007. [DOI] [PubMed] [Google Scholar]
- Rajan D, Wu R, Shah KG, Jacob A, Coppa GF, Wang P. Human ghrelin protects animals from renal ischemia-reperfusion injury through the vagus nerve. Surgery. 2012;151(1):37–47. doi: 10.1016/j.surg.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110(6):835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. Isoflavones promote mitochondrial biogenesis. J Pharmacol Exp Ther. 2008;325(2):536–543. doi: 10.1124/jpet.107.134882. [DOI] [PubMed] [Google Scholar]
- Rosner MH. Acute kidney injury in the elderly. Clin Geriatr Med. 2013;29(3):565–578. doi: 10.1016/j.cger.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Rostami B, Nematbakhsh M, Pezeshki Z, Talebi A, Sharifi MR, Moslemi F, Eshraghi-Jazi F, Ashrafi F. Effect of testosterone on Cisplatin-induced nephrotoxicity in surgically castrated rats. Nephrourol Mon. 2014;6(5):e21546. doi: 10.5812/numonthly.21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu BD, Kuncha M, Sindhura GJ, Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine. 2013;20(5):453–460. doi: 10.1016/j.phymed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Salom MG, Cerón SN, Rodriguez F, Lopez B, Hernández I, Fenoy FJ, et al. Heme oxygenase-1 induction improves ischemic renal failure role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293(6):H3542–H3549. doi: 10.1152/ajpheart.00977.2007. (Salom et al., 2007) [DOI] [PubMed] [Google Scholar]
- Samarasinghe DA, Tapner M, Farrell GC. Role of oxidative stress in hypoxia-reoxygenation injury to cultured rat hepatic sinusoidal endothelial cells. Hepatology. 2000;31(1):160–165. doi: 10.1002/hep.510310124. [DOI] [PubMed] [Google Scholar]
- Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, Okumura K, Kitano K, Tsutsui H, Fujii K, Kobuchi S, Ohkita M, Matsumura Y. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008;73(3):308–317. doi: 10.1038/sj.ki.5002690. [DOI] [PubMed] [Google Scholar]
- Schneider MP, Sullivan JC, Wach PF, Boesen EI, Yamamoto T, Fukai T, Harrison DG, Pollock DM, Pollock JS. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int. 2010;78(4):374–381. doi: 10.1038/ki.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Sener G, Tuğtepe H, Yüksel M, Cetinel S, Gedik N, Yeğen BC. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res. 2006;37(7):822–829. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Shah KG, Rajan D, Jacob A, Wu R, Krishnasastry K, Wang P, et al. Attenuation of renal ischemia and reperfusion injury by human adrenomedullin and its binding protein. J Surg Res. 2010;163(1):110–117. doi: 10.1016/j.jss.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol. 2009;20(3):524–534. doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples EJ, Yaqoob MM. Erythropoietin and acute renal failure. Semin Nephrol. 2006;26(4):325–331. doi: 10.1016/j.semnephrol.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Takahashi T, Suzuki T, Yamasaki A, Fujiwara T, Akagi R, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28(3):809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury a new class of renoprotective agents [J] Transplantation. 1998;66(2):147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83(1):65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- Si J, Ge Y, Zhuang S, Wang LJ, Chen S, Gong R. Adrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and -independent mechanisms. Kidney Int. 2013;83(4):635–646. doi: 10.1038/ki.2012.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MN, Subramanian V, Crouzet S, Haber GP, Colombo JR, Jr, Ukimura O, Nielsen S, Gill IS. Alpha-melanocyte stimulating hormone analogue AP214 protects against ischemia induced acute kidney injury in a porcine surgical model. J Urol. 2010;183(4):1625–1629. doi: 10.1016/j.juro.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005;57(1):70–76. [PubMed] [Google Scholar]
- Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia-reperfusion induced renal injury in rats. Pharmacol Res. 2004;50(2):187–193. doi: 10.1016/j.phrs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM. Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int. 1996;50(3):845–854. doi: 10.1038/ki.1996.384. [DOI] [PubMed] [Google Scholar]
- Sohotnik R, Nativ O, Abbasi A, Awad H, Frajewicki V, Abassi Z, et al. Phosphodiesterase-5 inhibition attenuates early renal ischemia-reperfusion-induced acute kidney injury assessment by quantitative measurement of urinary NGAL and KIM-1. Am J Physiol Renal Physiol. 2013;304(8):F1099–F1104. doi: 10.1152/ajprenal.00649.2012. [DOI] [PubMed] [Google Scholar]
- Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, Juncos LA. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R951–R958. doi: 10.1152/ajpregu.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290(6):E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2005;288(1):F91–F97. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Maruyama S, Sato W, Morita Y, Sato F, Miki Y, Kato S, Katsuno M, Sobue G, Yuzawa Y, Matsuo S. Geranylgeranylacetone ameliorates ischemic acute renal failure via induction of Hsp70. Kidney Int. 2005;67(6):2210–2220. doi: 10.1111/j.1523-1755.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- Szakaly P, Laszlo E, Kovacs K, Racz B, Horvath G, Ferencz A, Lubics A, Kiss P, et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) show increased susceptibility to in vivo renal ischemia/reperfusion injury. Neuropeptides. 2011;45(2):113–121. doi: 10.1016/j.npep.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Tsutsui H, Kobuchi S, Sugiura T, Yamagata M, Ohkita M, Takaoka M, Yukimura T, Matsumura Y. Protective effect of 17β-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur J Pharmacol. 2012;683(1–3):270–275. doi: 10.1016/j.ejphar.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Tang C, Dong Z. Epigenetic regulation in acute kidney injury: new light in a dark area. Kidney Int. 2015;88(4):665–668. doi: 10.1038/ki.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183(1):160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yan Y, Zhao TC, Gong R, Bayliss G, Yan H, Zhuang S. Class I HDAC activity is required for renal protection and regeneration after acute kidney injury. Am J Physiol Renal Physiol. 2014;307(3):F303–F316. doi: 10.1152/ajprenal.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zhuang S. Epigenetics in acute kidney injury. Curr Opin Nephrol Hypertens. 2015;24(4):351–358. doi: 10.1097/MNH.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013;83(5):804–810. doi: 10.1038/ki.2012.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Takahashi T, Mizobuchi S, Fujii H, Nakahira K, Akagi R, et al. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit Care Med. 2002;30(7):1512–1522. doi: 10.1097/00003246-200207000-00020. [DOI] [PubMed] [Google Scholar]
- Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13(8B):2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tögel FE, Westenfelder C. Mesenchymal stem cells a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6(3):179–183. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol. 2015;11(1):23–33. doi: 10.1038/nrneph.2014.202. [DOI] [PubMed] [Google Scholar]
- Tuğtepe H, Sener G, Biyikli NK, Yüksel M, Cetinel S, Gedik N, Yeğen BC. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept. 140(3):101–108. doi: 10.1016/j.regpep.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- Valverde P, Benedito ESolano F, Oaknin S, Lozano JA, Garcia Borron JC. Melatonin Antagonizes α-Melanocyte-Stimulating Hormone Enhancement of Melanogenesis in Mouse Melanoma Cells by Blocking the Hormone-Induced Accumulation of the C Locus Tyrosinase. Eur J Biochem. 1995;232(1):257–263. doi: 10.1111/j.1432-1033.1995.tb20807.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26(8):1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam MA, Weinberg JM, Patel Y, Saikumar P, Dong Z. Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int. 1996;49(2):449–460. doi: 10.1038/ki.1996.64. [DOI] [PubMed] [Google Scholar]
- Wada Y, Iyoda M, Matsumoto K, Shindo-Hirai Y, Kuno Y, Yamamoto Y, Suzuki T, Saito T, Iseri K, Shibata T. Epidermal growth factor receptor inhibition with erlotinib partially prevents Cisplatin-induced nephrotoxicity in rats. PLoS One. 2014;9:e111728. doi: 10.1371/journal.pone.0111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- Wang X, Bonventre JV, Parrish AR. The aging kidney increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15(9):15358–15376. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pei DS, Ji HX, Xing SH. Protective effect of a standardized Ginkgo extract (ginaton) on renal ischemia/reperfusion injury via suppressing the activation of JNK signal pathway. Phytomedicine. 2008;15(11):923–931. doi: 10.1016/j.phymed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC. GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol. 2010;21(2):284–294. doi: 10.1681/ASN.2009080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, de Moura Neiva LB, da Costa Santos CX, Martins Laurindo FR, de Fátima Fernandes Vattimo M. Isoflavone and the heme oxygenase system in ischemic acute kidney injury in rats. Food Chem Toxicol. 45(12):2366–2371. doi: 10.1016/j.fct.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tanaka M, Watanabe K, Takamatsu Y, Tobe A. Research and development of the free radical scavenger edaravone as a neuroprotectant [Article in Japanese] Yakugaku Zasshi. 2004;124(3):99–111. doi: 10.1248/yakushi.124.99. [DOI] [PubMed] [Google Scholar]
- Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21(5):756–761. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Hill WD, Su Y, Huang S, Dong Z. Heme oxygenase-1 induction contributes to renoprotection by G-CSF during rhabdomyolysis-associated acute kidney injury. Am J Physiol Renal Physiol. 2011;301(1):F162–F170. doi: 10.1152/ajprenal.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Câmpean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C. HIF activation protects from acute kidney injury. J Am Soc Nephrol. 2008;19(3):486–494. doi: 10.1681/ASN.2007040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Peng Z, Li Y, Wang H, Bishop JV, Chedwick LR, Singbartl K, Kellum JA. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice. Nephrol Dial Transplant. 2012;27(8):3100–3109. doi: 10.1093/ndt/gfr766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Guan X, Nie L, Wang S, Sun L, Zhao J, et al. Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem. 2014;394(1–2):145–154. doi: 10.1007/s11010-014-2090-7. [DOI] [PubMed] [Google Scholar]
- Xiao X, Hu Y, Quirós PM, Wei Q, López-Otín C, Dong Z. OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol. 306(11):F1318–F1326. doi: 10.1152/ajprenal.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ma H, Shao J, Wu J, Zhou L, Han J, et al. A Role for Tubular Necroptosis in Cisplatin-Induced AKI. J Am Soc Nephrol. 2015;26(11):2647–2658. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, Ogata M, Takaoka M, Matsumura Y. KB-R7943, a selective Na+/Ca2+ exchange inhibitor, protects against ischemic acute renal failure in mice by inhibiting renal endothelin-1 overproduction. J Cardiovasc Pharmacol. 2001;37(3):271–279. doi: 10.1097/00005344-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Yang D, Yang D, Jia R, Tan J. Na+/Ca2+ exchange inhibitor, KB-R7943, attenuates contrast-induced acute kidney injury. J Nephrol. 2013;26(5):877–885. doi: 10.5301/jn.5000259. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Kato A, Miyaji T, Zhou H, Togawa A, Hishida A. Insulin-like growth factor-I increases p21 expression and attenuates cisplatin-induced acute renal injury in rats. Clin Exp Nephrol. 2004;8(1):27–35. doi: 10.1007/s10157-003-0263-x. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69(9):1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Ozaki T, Saka Y, Yamamoto T, Gotoh M, Ito Y, Yuzawa Y, Matsuo S, Maruyama S. Autologous cell therapy for cisplatin-induced acute kidney injury by using non-expanded adipose tissue-derived cells. Cytotherapy. 2012;14(9):1089–1100. doi: 10.3109/14653249.2012.693157. [DOI] [PubMed] [Google Scholar]
- Yen TH, Alison MR, Goodlad RA, Otto WR, Jeffery R, Cook HT, Wright NA, Poulsom R. Epidermal growth factor attenuates tubular necrosis following mercuric chloride damage by regeneration of indigenous, not bone marrow-derived cells. J Cell Mol Med. 2015;19(2):463–473. doi: 10.1111/jcmm.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung BHY, Wong CKC. Stanniocalcin-1 Regulates Re-Epithelialization in Human Keratinocytes. PLoS ONE. 2011;6:e27094. doi: 10.1371/journal.pone.0027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75(11):2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kumagai H, Kohsaka T, Ikegaya N. Relaxin prorects against renal ischemia reperfusion injury. Am J Physiol Renal Physiol. 2013;305(8):F1169–F1176. doi: 10.1152/ajprenal.00654.2012. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kumagai H, Kohsaka T, Ikegaya N. Protective effects of relaxin against cisplatin-induced nephrotoxicity in rats. Nephron Exp Nephrol. 2014;128(1–2):9–20. doi: 10.1159/000365852. [DOI] [PubMed] [Google Scholar]
- Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84(3):509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009;75(3):304–311. doi: 10.1038/ki.2008.506. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. Suramin promotes proliferation and scattering of renal epithelial cells. J Pharmacol Exp Ther. 2005;314(1):383–390. doi: 10.1124/jpet.104.080648. [DOI] [PubMed] [Google Scholar]
- Zuk A, Gershenovich M, Ivanova Y, MacFarland RT, Fricker SP, Ledbetter S. CXCR4 antagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Renal Physiol. 2014;307(7):F783–F797. doi: 10.1152/ajprenal.00685.2013. [DOI] [PubMed] [Google Scholar]