Abstract
Immunoglobulin (Ig) E is the least abundant Ig isotype, yet plays a critical role in allergic reactions and host protection from helminth infection. While IgE was discovered 50 years ago, the ultimate evidence for its role in human allergic diseases was obtained by the efficacy of anti-IgE therapy in many clinical trials on asthma and other allergic diseases. Beginning from the discovery of IgE 50 years ago followed by studies of IgE receptors and activation mechanisms, this review provides a historic perspective of allergy research that has led to the development of anti-IgE therapy and other strategies targeting IgE and its receptors. Current IgE studies towards future precision medicine will also be reviewed.
Text
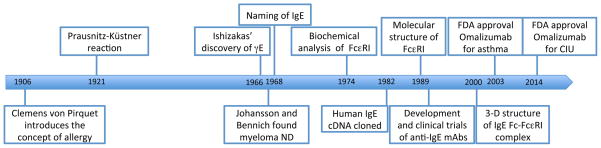
The year 2016 is special in the history of immunology (Figure 1). It marks the 50th anniversary of the discovery of IgE and the 100th anniversary of the inauguration of The Journal of Immunology. The discovery of IgE has been celebrated by special sessions and symposia organized by several research societies around the theme of IgE and allergy. Indeed, the realization that IgE was the “long-sought” reaginic antibody that causes allergic reactions sparked an exciting time of research that also led to the identification of its culprit target cells, mast cells and basophils, which express high-affinity receptors for IgE (FcεRI). A low-affinity IgE receptor (FcεRII or CD23) was found to be expressed on mature B cells and other immune cells. Along with the development of molecular biological and hybridoma techniques in subsequent years, recombinant anti-IgE antibodies were generated, and tested in human asthmatics and other allergy patients. Numerous clinical trials of the humanized monoclonal anti-IgE omalizumab clearly demonstrated its effectiveness in treating these diseases. Currently, omalizumab is a US Food and Drug Administration (FDA)-approved medicine to treat moderate-to-severe asthma and chronic idiopathic urticaria. This review will place the successful translational research into historic and future perspectives, eventually leading towards precision medicine.
Figure 1. Timeline of IgE-related research.
The major discoveries related to IgE research that led to the development of IgE-targeting therapeutics are highlighted.
Discovery of IgE
Readers who are interested in details recalling the story of the discovery of IgE are referred to two recent papers by Kimishige Ishizaka and Teruko Ishizaka (1), as well as by S.G.O. Johansson (2), the latter describing an atypical myeloma protein that turned out to be physicochemically identical to the antibody the Ishizakas discovered. At the time when the Ishizakas and other scientists were trying to identify the agent that causes allergic reactions, the only assay available for this purpose was the so-called Prausnitz-Küstner (PK) reaction. Prausnitz and Küstner described this reaction in 1921 showing that an intracutaneous injection of the serum of Küstner who was allergic to fish allergen to Prausnitz (who was not allergic to fish), followed by injection of the allergen into the same skin site the next day resulted in the induction of a wheal and erythema reaction. The antibody-like substance in the patient serum responsible for erythema-wheal reactions was called ‘reagin’. In the beginning of the 1960s reagin was believed to belong to a newly discovered Ig isotype IgA (3), as a serum fraction, largely composed of IgA, isolated from hay fever patients was shown to have such skin-sensitizing activity. However, further studies by the Ishizakas showed that their IgA antibodies lacked the ability to sensitize human skin in PK reactions (4). Because he believed that biological activities of an antibody are decided by the structure of the Fc portion of the antibody, Kimishige Ishizaka surmised that the reaginic activity in the IgA fraction in hay fever patients might be due to an impurity in the IgA preparation, an idea supported experimentally. This observation created a big hurdle, as it suggested that the concentration of reaginic activity in the original serum was less than 1 μg/ml. Such low levels of a substance made it extremely difficult, if not impossible, to purify it in amounts substantial enough for further physicochemical analyses, which at that time were the accepted approach to identify a novel protein. He then changed strategy focusing on the preparation of rabbit antibodies specific for reagin for identification. They immunized rabbits with a reagin-rich fraction of the serum of hay fever patients and adsorbed the obtained serum with human IgG, IgA, and IgD (5). Using this antiserum in PK tests, they demonstrated that, even after extensive adsorption, the rabbit serum contained antibodies specific for human reagin. Using this antiserum, reagin-rich fractions were identified after ion-exchange column chromatography of the serum from ragweed-sensitive patients and the reaginic activity, as quantified by PK reactions, was well correlated with their reactivity to the antiserum (5, 6). Radio-immunoelectrophoresis and sucrose density gradient ultracentrifugation showed that the reagin belongs to the fast γ globulin fraction with a molecular weight of 190,000, which differed from the properties of the other Ig isotypes. Perfect correlation between antigen-binding activity of this antibody, initially termed γE (‘E’ meaning erythema) (5), and its reaginic activity convinced them to believe that γE is associated with reaginic activity. They subsequently purified γE from ragweed-sensitive patients. Their rabbit anti-γE antibodies were also used to confirm that the atypical myeloma protein IgND isolated by Johansson and Bennich was identical to γE (7). IgND was shown to block the PK reaction (8). An immunoassay for IgND was developed to show increased levels of IgND in allergic asthma (9), followed by the development of radioallergosorbent test (RAST), to detect and quantify allergen-specific IgE antibodies (10). IgE myeloma protein from the second patient (PS) was used for purification of anti-IgE antibody and labeling IgE-bound cells, leading to the description of basophils and mast cells as major cell types that bind IgE via the Fc portion of the molecule (11, 12) among others.
IgE receptors, FcεRI and CD23
Using rat basophilic leukemia cells the binding properties of IgE and its high-affinity IgE receptor, FcεRI, was characterized by Henry Metzger and his associates. They showed that IgE binds as a monomer to a single univalent receptor with very high affinity (≈ 1010 M−1) and slow off-rate explaining the long persistence of reaginic activity in the skin (13, 14). Furthermore, using crosslinking agents and conditions of solubilization in mild detergents, they found that the receptor is a multisubunit structure containing besides one IgE-binding α subunit, one β subunit (15) and a dimer of disulfide-linked γ subunits (16). The cDNA cloning of all components of the rodent receptor (17–19), by Henry Metzger, Jean-Pierre Kinet and colleagues confirmed that the receptor contained the three subunits (20, 21). Successful expression of a functional receptor (in terms of IgE binding) on the surface of COS-7 cells was, however, only achieved when the cDNAs for all three subunits were co-transfected in the case of rodent receptors, while human receptors could also be expressed as trimers in the absence of β chains (21). Later, Kinet and his associates showed that the β subunit plays a critical role in receptor stabilization/maturation and signal amplification (22, 23). The cloning and expression of the receptor subunits also contributed to advancing the study of signal transduction of the receptor. Important steps in the understanding constituted the discovery by M. Reth that the signal-transducing β and γ subunits share a signaling motif, called immunoreceptor tyrosine-based activation motif (ITAM), with T and B cell receptors signaling chains (24). Early studies also showed that that crosslinking of IgE receptors induced tyrosine phosphorylation events (25) including phosphorylation of β and γ subunits (26). Several tyrosine kinases were shown to be involved in the early steps of FcεRI signaling, including Src (27), Syk (28), and Tec (29) family kinases. The availability of cDNAs of the α chain also allowed large scale production for studies of the 3-dimensional structure with the first complex between an immunoglobulin and its receptor being published in year 2000 (30).
A low-affinity receptor for IgE, FcεRII, was found to be expressed on lymphocytes and monocytes (31, 32). Tadamitsu Kishimoto’s laboratory demonstrated that this receptor is identical to the B cell-specific differentiation antigen CD23 (33). They showed that immature B cells do not express CD23/FcεRII; but IL-4 induces CD23/FcεRII expression; B cells lose CD23/FcεRII expression after they undergo isotype switching; increased soluble CD23/FcεRII as a complex with IgE was observed in the serum of atopic patients. Several groups including those of T. Kishimoto, J. Yodoi and G. Delespesse independently cloned the cDNA of the CD23/FcεRII molecule (34–36). CD23/FcεRII expressed on B cells binds IgE, and upon allergen binding gets internalized and digested in the lysosomes allowing allergen presentation to T cells along with MHC II molecules. This antigen-presentation pathway is efficient in taking up low-concentration allergens. CD23/FcεRII is also involved in feedback regulation of IgE production after crosslinking of membrane IgE receptors and CD23-bound IgE with specific allergen (37, 38). Anti-CD23 mAbs were shown to reduce blood IgE levels by about 50% in human clinical studies (39, 40). However, it is not clear whether CD23 contributes to human diseases (41).
Properties and clinical application of anti-IgE antibodies
Allergic diseases such as allergic asthma, allergic rhinitis, atopic dermatitis, and food allergy are caused by IgE-mediated type I hypersensitivity reactions. However, this dogma, i.e., that IgE and their target cells are essential for these diseases, has not been rigorously proven in some of these diseases even nowadays. This was particularly troubling when observations were made that IgE (42) and mast cells (43, 44) are dispensable in multiple asthma models. Later, it was shown that sensitization of animals with allergen in the presence of alum or large doses of allergen obliterates the necessity of mast cells and IgE in airway inflammation induced by allergen challenge of sensitized mice (45, 46). Regardless of this controversy, anti-IgE mAbs were developed to target both membrane-bound and soluble IgE as potential therapeutics (47–49). Omalizumab is a humanized monoclonal anti-IgE antibody with a dissociation constant (KD) of 6–8 nM for IgE. It inhibits allergic responses by binding to serum IgE molecules, thereby preventing their interactions with IgE receptors. It can also be used in allergen-specific immunotherapy to reduce signs of anaphylaxis associated with allergy shots and to accelerate immunization schedule and dosing. Numerous excellent reviews on omalizumab have been published (50–57).
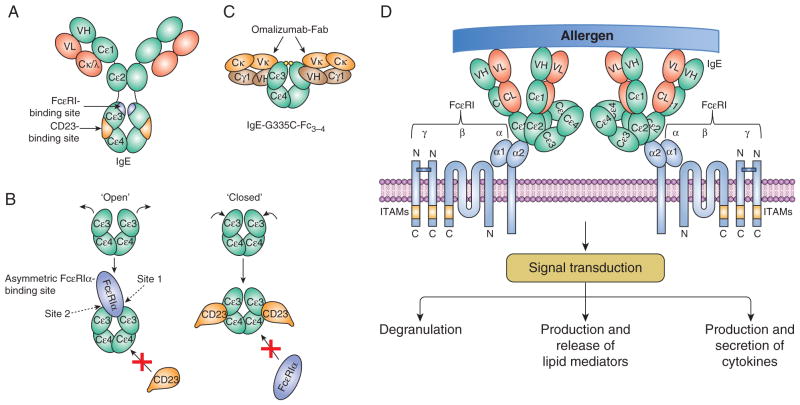
Unlike many anti-IgE antibodies that can crosslink FcεRI-bound IgE, thus causing activation of mast cell and basophils, omalizumab is not anaphylactogenic. It binds the Cε3 domain of free IgE preventing it from binding to FcεRI (58, 59), but it does not bind IgE already bound by FcεRI or CD23 on the cell surface (48). By depleting IgE, omalizumab down-regulates the expression of FcεRI on mast cells and basophils as well as antigen-presenting cells. The positive correlation between the basophil FcεRI surface expression levels and serum IgE concentrations was known for long (60). Later it was shown that IgE-free FcεRI is unstable and internalized for degradation (61, 62), thus limiting activation of mast cells and basophils. The structural basis for the ability of omalizumab to block IgE from binding to both CD23 and FcεRI was recently revealed by X-ray crystallography of the IgE-omalizumab complex (63). Omalizumab inhibition of FcεRI binding involves substantial steric conflict with the receptor at site 2, while its inhibition of CD23 binding is due to a greater steric overlap of omalizumab with CD23 than that for FcεRI α and a more extensive overlap between IgE residues that engage omalizumab and CD23 as compared with FcεRI α (Figure 2). In addition to the depletion of free IgE and downregulation of FcεRI, anti-IgE effects might be related to accumulated soluble immune complexes of anti-IgE:IgE 5–10 times the basal levels of IgE (64–67), inhibition of IgE-committed B cells (68, 69), and neutralization of cytokinergic activity of IgE (70).
Figure 2. IgE, its interaction with omalizumab, and FcεRI-mediated mast cell activation.
(A) IgE and the relative locations of the FcεRIα- and CD23-binding sites. (B) Open and closed conformations of the IgE-Fcε3–4 domains interacting with FcεRIα and CD23, respectively. (C) Symmetric interaction of two omalizumab Fabs with IgE-Fcε3–4. Modified from Pennington et al. (63). (D) Crosslinking of IgE-bound FcεRI with a multivalent allergen leads to activation of mast cells. The activated cells degranulate and produce/release lipid mediators and cytokines. Signal transduction via the FcεRI was recently reviewed (124–127). Domains of IgE and omalizumab are represented by ovals. ITAMs, immunoreceptor tyrosine-based activation motifs.
Omalizumab is indicated for moderate to severe asthma with serum IgE levels of 30–700 IU/ml. It is ineffective to asthmatics who have very high IgE levels. Omalizumab’s effects on allergic asthma are shown by improvements in the quality of life, as well as marked reduction of asthma exacerbations, emergency room visits, and use of systemic corticosteroids and rescue bronchodilators (71–73). However, it has less effects on lung function (51, 56, 57). As noted above, the primary outcome of omalizumab effects is the prevention of asthma exacerbations, which has been noted also with other monoclonal antibodies that affect the type 2 inflammation (74–76). This suggests the existence of a feed forward pathway(s) that involves Th2 cytokines, IgE and mast cells as the main mechanism for allergic asthma. The major cause of asthma exacerbations is viral respiratory infections (77, 78). The Inner City Asthma Study demonstrated that omalizumab treatment reduces the frequency of asthma flares associated with seasonal virus exposure (79). Long-term effects of omalizumab in some studies include a reduced airway wall area, reduced sputum eosinophilia, increased baseline lung function and reduced epithelial reticular basement membrane thickening (80, 81). Moreover, potential benefits of omalizumab were also shown in non-allergic phenotypes of severe asthma (82, 83).
Although not approved by FDA, efficacy of omalizumab or another anti-IgE TNX-901 was shown by decreased nasal and ocular symptoms, reduced antihistamine use, and improved quality of life scores in allergic rhinitis (84–88) and by increased threshold dose of allergenic food required to trigger hypersensitivity reactions in food allergy (89–92). However, clinical improvements by omalizumab were not seen in two double-blind, placebo-controlled trials on atopic dermatitis (93, 94), despite ample evidence for the involvement of IgE in murine models of atopic dermatitis (95, 96). Use of omalizumab was approved for the treatment of chronic idiopathic urticaria, although little is understood regarding the pathogenesis of this syndrome with multiple phenotypes (97–99). In case IgG autoantibodies against IgE or FcεRIα or IgE autoantibodies against thyroperoxidase (100) are involved, the effect of omalizumab might be understood by reduced levels of IgE. Interestingly, many omalizumab-responding patients had low or undetectable levels of IgE, therefore how anti-IgE works in these patients is unclear.
As with other humanized antibodies, omalizumab occasionally causes anaphylactic reactions (101). Related to this adverse effect, the recently demonstrated ability of omalizumab to promote the dissociation of IgE/FcεRIα complexes (102) implies that omalizumab might have some ability to bind to IgE that has bound to FcεRI. Fortunately, a concern of increased risk of malignancy was refuted (103).
Other therapies targeting IgE and IgE receptors
New strategies targeting IgE have been explored (51), including anti-IgE mAb QGE031 with higher affinity (KD of 139 pM) to the Cε3 domain of IgE than omalizumab (104, 105) and Designed Ankyrin Repeat Protein (DARPin) E2_79 that can accelerate the dissociation of IgE:FcεRI complexes (102, 106). An interesting strategy is to disrupt IgE production by targeting a segment (the M1′ domain or CεmX (107)) of membrane IgE on human IgE-switched B cells that is not present in serum IgE (108). FcεRI could also be targeted to desensitize allergy patients. Khodoun et al. showed that rapid desensitization with anti-FcεRI mAb suppresses both active and passive IgE-mediated anaphylaxis without inducing disease (109): the procedure starts with a mAb too small to induce disease, which is doubled hourly until a saturating dose is reached. Desensitization was accomplished by decreasing mast cell signaling through FcεRI and slow, but eventual removal of nearly all IgE from the mast cell surface. However, we have to point out that the clinical relevance of the potential therapeutics discussed in this section has not been established.
IgE heterogeneity and personalized precision medicine
Asthma and allergic diseases are syndromes presenting with a set of signs and symptoms (110). Efforts are currently directed toward defining endotypes of these diseases (71). The measurement of an increasing number of clinical parameters and biomarkers will allow to stratify patients along specific pathophysiologic pathways. IgE has not been a good biomarker whereas eosinophils or exhaled nitric oxide levels correlate to greater responsiveness to omalizumab (111).
Like IgG, IgE consists of variable and constant regions in both heavy and light chains. Given that biologic activities reside in the constant Fc portion of the molecule, all IgE molecules are believed to behave in the same way except for their unique ability to recognize specific antigens. However, the antigen-recognition ability of the variable Fab portion might not be simply one Fab-to-one antigen. A well-characterized dinitrophenyl-specific IgE molecule SPE-7 can recognize several haptens as well as a protein thioredoxin (112). Thus, this observation raises the possibility that an IgE molecule on mast cells or basophils might fortuitously recognize multiple antigens and activate these cells. Interestingly, SPE-7 IgE was recognized as the most potent cytokinergic IgE molecule. Originally, two groups found that monomeric IgE molecules promote mast cell survival in the absence of growth factors, but two studies differed in the cytokine-producing abilities of IgE molecules (113, 114). Subsequently, this difference was shown to be due to the ability of IgE molecules used, resulting in the proposal of the existence of two types of IgE, i.e., highly cytokinergic vs. poorly cytokinergic IgEs (115). Highly cytokinergic IgEs can induce large aggregates of FcεRI, whereas poorly cytokinergic IgEs induce much smaller FcεRI aggregates (115). Therefore, highly cytokinergic IgEs can activate mast cells almost as strongly as IgE plus antigen can. A recent study reported the Fv-Fv interacting ability of SPE-7 IgE as a mechanism for high cytokinergic activity (116). By contrast, poorly cytokinergic IgEs can only promote cell survival weakly.
Another known heterogeneity among IgE molecules is its reactivity to histamine-releasing factor (HRF). HRF can activate mast cells and basophils primed with a certain type (so-called IgE+) of IgE molecules, leading to the release of histamine, IL-4 and IL-13 (117). IgE+ can be derived from some, but not all, atopic patients. HRF is found in body fluids of allergic reactions thus implicated in the pathogenesis of allergic diseases (118, 119). Interestingly, ~25% of the tested IgE molecules directly bind HRF via IgE Fab interactions of two IgE-binding sites within the HRF molecule (120). Because HRF can be present as a disulfide-linked dimer, dimeric HRF-bound IgE can crosslink FcεRI molecules to activate mast cells and basophils. HRF inhibitors that block interactions between IgE and HRF inhibit IgE+HRF-induced activation of mast cells in vitro and airway inflammation in IgE-dependent in vivo models of asthma (120). Since HRF, also known as translationally controlled tumor protein or fortilin (121, 122), is one of the most abundantly expressed proteins in all tested cell types, secreted HRF works as a kind of autoantigen to induce allergic reactions (123).
Concluding Remarks
It was a long journey of 36 years from the discovery of IgE before omalizumab was first approved for the treatment of asthma. The research from the identification of IgE as the reaginic antibody to successful translation to omalizumab is a triumphant example of how immunologic research can bring a benefit to patients. As richly illustrated in this case, one cannot overemphasize the importance of basic science to further enrich therapeutic options to treat allergic diseases.
Acknowledgments
T.K. is supported by the NIH grants (HL124283, AR064418, and AI115534) and the Nipponham Foundation for the Future of Food.
References
- 1.Ishizaka K, Ishizaka T. Identification of IgE. The Journal of allergy and clinical immunology. 2016;137:1646–1650. doi: 10.1016/j.jaci.2015.12.1343. [DOI] [PubMed] [Google Scholar]
- 2.Johansson SG. The discovery of IgE. The Journal of allergy and clinical immunology. 2016;137:1671–1673. doi: 10.1016/j.jaci.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Heremans JF, Vaerman JP. Beta-2A-Globulin as a possible carrier of allergic reaginic activity. Nature. 1962;193:1091–1092. doi: 10.1038/1931091b0. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaka K, Ishizaka T, Lee EH, Fudenberg H. Immunochemical properties of human gamma-A isohemagglutinin. I. Comparisons with gamma-G and gamma-M-globulin antibodies. Journal of immunology. 1965;95:197–208. [PubMed] [Google Scholar]
- 5.Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. Journal of immunology. 1966;97:75–85. [PubMed] [Google Scholar]
- 6.Ishizaka K, Ishizaka T, Hornbrook MM. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity wth gamma-E-globulin antibody. Journal of immunology. 1966;97:840–853. [PubMed] [Google Scholar]
- 7.Bennich H, Ishizaka K, Ishizaka T, Johansson SG. A comparative antigenic study of gamma E-globulin and myeloma-IgND. Journal of immunology. 1969;102:826–831. [PubMed] [Google Scholar]
- 8.Stanworth DR, Humphrey JH, Bennich H, Johansson SG. Inhibition of Prausnitz-Kustner reaction by proteolytic-cleavage fragments of a human myeloma protein of immunoglobulin class E. Lancet. 1968;2:17–18. doi: 10.1016/s0140-6736(68)92889-4. [DOI] [PubMed] [Google Scholar]
- 9.Johansson SG. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967;2:951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 10.Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2:1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaka K, Tomioka H, Ishizaka T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. Journal of immunology. 1970;105:1459–1467. [PubMed] [Google Scholar]
- 12.Tomioka H, Ishizaka K. Mechanisms of passive sensitization. II. Presence of receptors for IgE on monkey mast cells. Journal of immunology. 1971;107:971–978. [PubMed] [Google Scholar]
- 13.Kulczycki A, Jr, Isersky C, Metzger H. The interaction of IgE with rat basophilic leukemia cells. I. Evidence for specific binding of IgE. The Journal of experimental medicine. 1974;139:600–616. doi: 10.1084/jem.139.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulczycki A, Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. The Journal of experimental medicine. 1974;140:1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holowka D, Hartmann H, Kanellopoulos J, Metzger H. Association of the receptor for immunoglobulin E with an endogenous polypeptide on rat basophilic leukemia cells. Journal of receptor research. 1980;1:41–68. doi: 10.3109/10799898009039254. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Montfort R, Kinet JP, Metzger H. A previously unrecognized subunit of the receptor for immunoglobulin E. Biochemistry. 1983;22:5722–5728. doi: 10.1021/bi00294a007. [DOI] [PubMed] [Google Scholar]
- 17.Kinet JP, Blank U, Ra C, White K, Metzger H, Kochan J. Isolation and characterization of cDNAs coding for the beta subunit of the high-affinity receptor for immunoglobulin E. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6483–6487. doi: 10.1073/pnas.85.17.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinet JP, Metzger H, Hakimi J, Kochan J. A cDNA presumptively coding for the alpha subunit of the receptor with high affinity for immunoglobulin E. Biochemistry. 1987;26:4605–4610. doi: 10.1021/bi00389a002. [DOI] [PubMed] [Google Scholar]
- 19.Ra C, Jouvin MH, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. The Journal of biological chemistry. 1989;264:15323–15327. [PubMed] [Google Scholar]
- 20.Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 21.Miller L, Blank U, Metzger H, Kinet JP. Expression of high-affinity binding of human immunoglobulin E by transfected cells. Science. 1989;244:334–337. doi: 10.1126/science.2523561. [DOI] [PubMed] [Google Scholar]
- 22.Donnadieu E, Jouvin MH, Kinet JP. A second amplifier function for the allergy-associated Fc(epsilon)RI-beta subunit. Immunity. 2000;12:515–523. doi: 10.1016/s1074-7613(00)80203-4. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Cicala C, Scharenberg AM, Kinet JP. The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 24.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 25.Benhamou M, Gutkind JS, Robbins KC, Siraganian RP. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolini R, Jouvin MH, Kinet JP. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991;353:855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 27.Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 28.Hutchcroft JE, Geahlen RL, Deanin GG, Oliver JM. Fc epsilon RI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami Y, Yao L, Miura T, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Molecular and cellular biology. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature. 2000;406:259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 31.Capron A, Dessaint JP, Capron M, Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975;253:474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence DA, Weigle WO, Spiegelberg HL. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. The Journal of clinical investigation. 1975;55:368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikutani H, Suemura M, Owaki H, Nakamura H, Sato R, Yamasaki K, Barsumian EL, Hardy RR, Kishimoto T. Fc epsilon receptor, a specific differentiation marker transiently expressed on mature B cells before isotype switching. The Journal of experimental medicine. 1986;164:1455–1469. doi: 10.1084/jem.164.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikuta K, Takami M, Kim CW, Honjo T, Miyoshi T, Tagaya Y, Kawabe T, Yodoi J. Human lymphocyte Fc receptor for IgE: sequence homology of its cloned cDNA with animal lectins. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:819–823. doi: 10.1073/pnas.84.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikutani H, Inui S, Sato R, Barsumian EL, Owaki H, Yamasaki K, Kaisho T, Uchibayashi N, Hardy RR, Hirano T, et al. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986;47:657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- 36.Ludin C, Hofstetter H, Sarfati M, Levy CA, Suter U, Alaimo D, Kilchherr E, Frost H, Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. The EMBO journal. 1987;6:109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo HY, Hofstetter H, Banchereau J, Delespesse G. Cross-linking of CD23 antigen by its natural ligand (IgE) or by anti-CD23 antibody prevents B lymphocyte proliferation and differentiation. Journal of immunology. 1991;146:2122–2129. [PubMed] [Google Scholar]
- 38.Sherr E, Macy E, Kimata H, Gilly M, Saxon A. Binding the low affinity Fc epsilon R on B cells suppresses ongoing human IgE synthesis. Journal of immunology. 1989;142:481–489. [PubMed] [Google Scholar]
- 39.Nakamura T, Kloetzer WS, Brams P, Hariharan K, Chamat S, Cao X, LaBarre MJ, Chinn PC, Morena RA, Shestowsky WS, Li YP, Chen A, Reff ME. In vitro IgE inhibition in B cells by anti-CD23 monoclonal antibodies is functionally dependent on the immunoglobulin Fc domain. International journal of immunopharmacology. 2000;22:131–141. doi: 10.1016/s0192-0561(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 40.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. The Journal of allergy and clinical immunology. 2003;112:563–570. doi: 10.1016/s0091-6749(03)01861-x. [DOI] [PubMed] [Google Scholar]
- 41.Poole JA, Meng J, Reff M, Spellman MC, Rosenwasser LJ. Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects. The Journal of allergy and clinical immunology. 2005;116:780–788. doi: 10.1016/j.jaci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kung TT, Stelts D, Zurcher JA, Jones H, Umland SP, Kreutner W, Egan RW, Chapman RW. Mast cells modulate allergic pulmonary eosinophilia in mice. Am J Respir Cell Mol Biol. 1995;12:404–409. doi: 10.1165/ajrcmb.12.4.7695919. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. The Journal of experimental medicine. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, Ishizaka K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. Journal of immunology. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 46.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. The Journal of experimental medicine. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang TW. The pharmacological basis of anti-IgE therapy. Nature biotechnology. 2000;18:157–162. doi: 10.1038/72601. [DOI] [PubMed] [Google Scholar]
- 48.Chang TW, Davis FM, Sun NC, Sun CR, MacGlashan DW, Jr, Hamilton RG. Monoclonal antibodies specific for human IgE-producing B cells: a potential therapeutic for IgE-mediated allergic diseases. Bio/technology. 1990;8:122–126. doi: 10.1038/nbt0290-122. [DOI] [PubMed] [Google Scholar]
- 49.Chang TW, Wu PC, Hsu CL, Hung AF. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Advances in immunology. 2007;93:63–119. doi: 10.1016/S0065-2776(06)93002-8. [DOI] [PubMed] [Google Scholar]
- 50.Holgate S, Buhl R, Bousquet J, Smith N, Panahloo Z, Jimenez P. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respiratory medicine. 2009;103:1098–1113. doi: 10.1016/j.rmed.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Holgate ST. New strategies with anti-IgE in allergic diseases. The World Allergy Organization journal. 2014;7:17. doi: 10.1186/1939-4551-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humbert M, Busse W, Hanania NA, Lowe PJ, Canvin J, Erpenbeck VJ, Holgate S. Omalizumab in asthma: an update on recent developments. The journal of allergy and clinical immunology. In practice. 2014;2:525–536. e521. doi: 10.1016/j.jaip.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Stokes JR, Casale TB. The use of anti-IgE therapy beyond allergic asthma. The journal of allergy and clinical immunology. In practice. 2015;3:162–166. doi: 10.1016/j.jaip.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 54.D’Amato G, Stanziola A, Sanduzzi A, Liccardi G, Salzillo A, Vitale C, Molino A, Vatrella A, D’Amato M. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): a review. Multidisciplinary respiratory medicine. 2014;9:23. doi: 10.1186/2049-6958-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respiratory medicine. 2010;104:188–196. doi: 10.1016/j.rmed.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Logsdon SL, Oettgen HC. Anti-IgE therapy: clinical utility and mechanistic insights. Current topics in microbiology and immunology. 2015;388:39–61. doi: 10.1007/978-3-319-13725-4_3. [DOI] [PubMed] [Google Scholar]
- 57.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. The Cochrane database of systematic reviews. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Presta L, Shields R, O’Connell L, Lahr S, Porter J, Gorman C, Jardieu P. The binding site on human immunoglobulin E for its high affinity receptor. The Journal of biological chemistry. 1994;269:26368–26373. [PubMed] [Google Scholar]
- 59.Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, Jardieu PM. Humanization of an antibody directed against IgE. Journal of immunology. 1993;151:2623–2632. [PubMed] [Google Scholar]
- 60.Malveaux FJ, Conroy MC, Adkinson NF, Jr, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. The Journal of clinical investigation. 1978;62:176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubo S, Matsuoka K, Taya C, Kitamura F, Takai T, Yonekawa H, Karasuyama H. Drastic up-regulation of Fcepsilonri on mast cells is induced by IgE binding through stabilization and accumulation of Fcepsilonri on the cell surface. Journal of immunology. 2001;167:3427–3434. doi: 10.4049/jimmunol.167.6.3427. [DOI] [PubMed] [Google Scholar]
- 62.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. Journal of immunology. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 63.Pennington LF, Tarchevskaya S, Brigger D, Sathiyamoorthy K, Graham MT, Nadeau KC, Eggel A, Jardetzky TS. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nature communications. 2016;7:11610. doi: 10.1038/ncomms11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corne J, Djukanovic R, Thomas L, Warner J, Botta L, Grandordy B, Gygax D, Heusser C, Patalano F, Richardson W, Kilchherr E, Staehelin T, Davis F, Gordon W, Sun L, Liou R, Wang G, Chang TW, Holgate S. The effect of intravenous administration of a chimeric anti-IgE antibody on serum IgE levels in atopic subjects: efficacy, safety, and pharmacokinetics. The Journal of clinical investigation. 1997;99:879–887. doi: 10.1172/JCI119252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox JA, Hotaling TE, Struble C, Ruppel J, Bates DJ, Schoenhoff MB. Tissue distribution and complex formation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. The Journal of pharmacology and experimental therapeutics. 1996;279:1000–1008. [PubMed] [Google Scholar]
- 66.Liu J, Lester P, Builder S, Shire SJ. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry. 1995;34:10474–10482. doi: 10.1021/bi00033a020. [DOI] [PubMed] [Google Scholar]
- 67.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 68.Haba S, Nisonoff A. Effects of syngeneic anti-IgE antibodies on the development of IgE memory and on the secondary IgE response. Journal of immunology. 1994;152:51–57. [PubMed] [Google Scholar]
- 69.Sun LK, Liou RS, Sun NC, Gossett LA, Sun C, Davis FM, MacGlashan DW, Jr, Chang TW. Transfectomas expressing both secreted and membrane-bound forms of chimeric IgE with anti-viral specificity. Journal of immunology. 1991;146:199–205. [PubMed] [Google Scholar]
- 70.Kawakami T, Kitaura J. Mast Cell Survival and Activation by IgE in the Absence of Antigen: A Consideration of the Biologic Mechanisms and Relevance. Journal of immunology. 2005;175:4167–4173. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. The Journal of allergy and clinical immunology. 2015;135:299–310. doi: 10.1016/j.jaci.2014.12.1871. quiz 311. [DOI] [PubMed] [Google Scholar]
- 72.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. The Journal of allergy and clinical immunology. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 73.Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respiratory medicine. 2013;107:1141–1151. doi: 10.1016/j.rmed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Gallelli L, Busceti MT, Vatrella A, Maselli R, Pelaia G. Update on anticytokine treatment for asthma. BioMed research international. 2013;2013:104315. doi: 10.1155/2013/104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kau AL, Korenblat PE. Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Current opinion in allergy and clinical immunology. 2014;14:570–575. doi: 10.1097/ACI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walsh GM. Anti-IL-4/-13 based therapy in asthma. Expert opinion on emerging drugs. 2015;20:349–352. doi: 10.1517/14728214.2015.1050377. [DOI] [PubMed] [Google Scholar]
- 77.Gern JE. Virus/Allergen Interaction in Asthma Exacerbation. Annals of the American Thoracic Society. 2015;12(Suppl 2):S137–143. doi: 10.1513/AnnalsATS.201503-153AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kloepfer KM, Gern JE. Virus/allergen interactions and exacerbations of asthma. Immunology and allergy clinics of North America. 2010;30:553–563. vii. doi: 10.1016/j.iac.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration; international review of thoracic diseases. 2012;83:520–528. doi: 10.1159/000334701. [DOI] [PubMed] [Google Scholar]
- 81.Riccio AM, Dal Negro RW, Micheletto C, De Ferrari L, Folli C, Chiappori A, Canonica GW. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. International journal of immunopathology and pharmacology. 2012;25:475–484. doi: 10.1177/039463201202500217. [DOI] [PubMed] [Google Scholar]
- 82.de Llano LP, Vennera del CM, Alvarez FJ, Medina JF, Borderias L, Pellicer C, Gonzalez H, Gullon JA, Martinez-Moragon E, Sabadell C, Zamarro S, Picado C, Spanish R. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. The Journal of asthma: official journal of the Association for the Care of Asthma. 2013;50:296–301. doi: 10.3109/02770903.2012.757780. [DOI] [PubMed] [Google Scholar]
- 83.Domingo C, Pomares X, Angril N, Rudi N, Amengual MJ, Mirapeix RM. Effectiveness of omalizumab in non-allergic severe asthma. Journal of biological regulators and homeostatic agents. 2013;27:45–53. [PubMed] [Google Scholar]
- 84.Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. The journal of allergy and clinical immunology. In practice. 2014;2:332–340. e331. doi: 10.1016/j.jaip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Adelroth E, Rak S, Haahtela T, Aasand G, Rosenhall L, Zetterstrom O, Byrne A, Champain K, Thirlwell J, Cioppa GD, Sandstrom T. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. The Journal of allergy and clinical immunology. 2000;106:253–259. doi: 10.1067/mai.2000.108310. [DOI] [PubMed] [Google Scholar]
- 86.Casale TB, I, Bernstein L, Busse WW, LaForce CF, Tinkelman DG, Stoltz RR, Dockhorn RJ, Reimann J, Su JQ, Fick RB, Jr, Adelman DC. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. The Journal of allergy and clinical immunology. 1997;100:110–121. doi: 10.1016/s0091-6749(97)70202-1. [DOI] [PubMed] [Google Scholar]
- 87.Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, McAlary M, Fowler-Taylor A, Racine A, Gupta N, Fick R, Della Cioppa G G. Omalizumab Seasonal Allergic Rhinitis Trail. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. Jama. 2001;286:2956–2967. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- 88.Chervinsky P, Casale T, Townley R, Tripathy I, Hedgecock S, Fowler-Taylor A, Shen H, Fox H. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2003;91:160–167. doi: 10.1016/S1081-1206(10)62171-0. [DOI] [PubMed] [Google Scholar]
- 89.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, Wong DA. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. The Journal of allergy and clinical immunology. 2011;127:1309–1310. e1301. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 90.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. The Journal of allergy and clinical immunology. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunology and allergy clinics of North America. 2012;32:111–133. doi: 10.1016/j.iac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Lieberman JA, Chehade M. Use of omalizumab in the treatment of food allergy and anaphylaxis. Current allergy and asthma reports. 2013;13:78–84. doi: 10.1007/s11882-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 93.Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG. 2010;8:990–998. doi: 10.1111/j.1610-0387.2010.07497.x. [DOI] [PubMed] [Google Scholar]
- 94.Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Chiang D, Umetsu DT, Nadeau KC. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. International archives of allergy and immunology. 2013;162:89–93. doi: 10.1159/000350486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. The Journal of investigative dermatology. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Current opinion in immunology. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS, Canvin J, Rahmaoui A, Georgiou P, Alpan O, Spector S, Rosen K. Efficacy and Safety of Omalizumab in Patients with Chronic Idiopathic/Spontaneous Urticaria who Remain Symptomatic on H1 Antihistamines: A Randomized, Placebo-Controlled Study. The Journal of investigative dermatology. 2015;135:925. doi: 10.1038/jid.2014.512. [DOI] [PubMed] [Google Scholar]
- 98.Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, Agarwal S, Doyle R, Canvin J, Kaplan A, Casale T. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. The New England journal of medicine. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 99.Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. The Journal of allergy and clinical immunology. 2015;135:337–342. doi: 10.1016/j.jaci.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 100.Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, Brehler R, Grabbe J, Hunzelmann N, Jakob T, Jung A, Kleine-Tebbe J, Mempel M, Meurer M, Reich K, Rueff F, Schakel K, Sengupta K, Sieder C, Simon JC, Wedi B, Zuberbier T, Mahler V, Staubach P. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. The Journal of allergy and clinical immunology. 2011;128:202–209. e205. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 101.Kim HL, Leigh R, Becker A. Omalizumab: Practical considerations regarding the risk of anaphylaxis. Allergy, asthma, and clinical immunology: official journal of the Canadian Society of Allergy and Clinical Immunology. 2010;6:32. doi: 10.1186/1710-1492-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, Shin JS, Vogel M, Stadler BM, Dahinden CA, Jardetzky TS. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. The Journal of allergy and clinical immunology. 2014;133:1709–1719. e1708. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, Iribarren C, Chen H, Carrigan G, Rosen K, Szefler SJ. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. The Journal of allergy and clinical immunology. 2014;134:560–567. e564. doi: 10.1016/j.jaci.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. British journal of clinical pharmacology. 2009;68:61–76. doi: 10.1111/j.1365-2125.2009.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, Owen CE, Jones I, Lowe PJ. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014;44:1371–1385. doi: 10.1111/cea.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim B, Eggel A, Tarchevskaya SS, Vogel M, Prinz H, Jardetzky TS. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature. 2012;491:613–617. doi: 10.1038/nature11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng C, Davis FM, Sun LK, Liou RS, Kim YW, Chang TW. A new isoform of human membrane-bound IgE. Journal of immunology. 1992;148:129–136. [PubMed] [Google Scholar]
- 108.Brightbill HD, Jeet S, Lin Z, Yan D, Zhou M, Tan M, Nguyen A, Yeh S, Delarosa D, Leong SR, Wong T, Chen Y, Ultsch M, Luis E, Ramani SR, Jackman J, Gonzalez L, Dennis MS, Chuntharapai A, DeForge L, Meng YG, Xu M, Eigenbrot C, Lee WP, Refino CJ, Balazs M, Wu LC. Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice. The Journal of clinical investigation. 2010;120:2218–2229. doi: 10.1172/JCI40141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Lewkowich I, Morris SC, Finkelman FD. Rapid polyclonal desensitization with antibodies to IgE and FcepsilonRIalpha. The Journal of allergy and clinical immunology. 2013;131:1555–1564. doi: 10.1016/j.jaci.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nature immunology. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA, Jr, Schleimer RP, Minnicozzi M. Asthma outcomes: biomarkers. The Journal of allergy and clinical immunology. 2012;129:S9–23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 113.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 114.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 115.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, Kawakami T. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bax HJ, Bowen H, Dodev TS, Sutton BJ, Gould HJ. Mechanism of the antigen-independent cytokinergic SPE-7 IgE activation of human mast cells in vitro. Scientific reports. 2015;5:9538. doi: 10.1038/srep09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 118.MacDonald SM, Lichtenstein LM, Proud D, Plaut M, Naclerio RM, MacGlashan DW, Kagey-Sobotka A. Studies of IgE-dependent histamine releasing factors: heterogeneity of IgE. Journal of immunology. 1987;139:506–512. [PubMed] [Google Scholar]
- 119.Warner JA, Pienkowski MM, Plaut M, Norman PS, Lichtenstein LM. Identification of histamine releasing factor(s) in the late phase of cutaneous IgE-mediated reactions. Journal of immunology. 1986;136:2583–2587. [PubMed] [Google Scholar]
- 120.Kashiwakura J, Ando T, Matsumoto K, Kimura M, Kitaura J, Matho MH, Zajonc DM, Ozeki T, Ra C, Macdonald SM, Siraganian RP, Broide DH, Kawakami Y, Kawakami T. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. The Journal of clinical investigation. 2012;122:218–228. doi: 10.1172/JCI59072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bommer UA, Thiele BJ. The translationally controlled tumour protein (TCTP) Int J Biochem Cell Biol. 2004;36:379–385. doi: 10.1016/s1357-2725(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 122.Bommer UA. Cellular function and regulation of the translationally controlled tumor protein TCTP. The Open Allergy Journal. 2012;5:19–32. [Google Scholar]
- 123.Kawakami T, Kashiwakura J, Kawakami Y. Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy, Asthma & Immunology Research. 2013:6. doi: 10.4168/aair.2014.6.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu LC. Immunoglobulin E receptor signaling and asthma. The Journal of biological chemistry. 2011;286:32891–32897. doi: 10.1074/jbc.R110.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunological reviews. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Advances in immunology. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Draber P, Halova I, Polakovicova I, Kawakami T. Signal transduction and chemotaxis in mast cells. European journal of pharmacology. 2016;778:11–23. doi: 10.1016/j.ejphar.2015.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]