Abstract
Objective
To provide evidence-based recommendations for the treatment of patients with ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA).
Methods
A core group led the development of the recommendations, starting with the treatment questions. A literature review group conducted systematic literature reviews of studies that addressed 57 specific treatment questions, based on searches conducted in OVID Medline (1946–2014), PubMed (1966–2014), and the Cochrane Library. We assessed the quality of evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method. A separate voting group reviewed the evidence and voted on recommendations for each question using the GRADE framework.
Results
In patients with active AS, the strong recommendations included use of nonsteroidal antiinflammatory drugs (NSAIDs), use of tumor necrosis factor inhibitors (TNFi) when activity persists despite NSAID treatment, not to use systemic glucocorticoids, use of physical therapy, and use of hip arthroplasty for patients with advanced hip arthritis. Among the conditional recommendations was that no particular TNFi was preferred except in patients with concomitant inflammatory bowel disease or recurrent iritis, in whom TNFi monoclonal antibodies should be used. In patients with active nonradiographic axial SpA despite treatment with NSAIDs, we conditionally recommend treatment with TNFi. Other recommendations for patients with nonradiographic axial SpA were based on indirect evidence and were the same as for patients with AS.
Conclusion
These recommendations provide guidance for the management of common clinical questions in AS and nonradiographic axial SpA. Additional research on optimal medication management over time, disease monitoring, and preventive care is needed to help establish best practices in these areas.
Ankylosing spondylitis (AS) is a form of chronic inflammatory arthritis characterized by sacroiliitis, enthesitis, and a marked propensity for sacroiliac joint and spinal fusion (1). AS is a condition in the spondyloarthritis (SpA) family of diseases, which share several clinical, genetic, and immunologic features (2). AS is distinguished in this family by universal involvement with sacroiliac joint inflammation or fusion, and more prevalent spinal ankylosis (3); these more advanced sacroiliac changes form the core of the modified New York criteria for the classification of AS (4). Radiographic features may take years to develop, which limits these classification criteria by potentially excluding patients early in the disease course.
Recently, the Assessment of SpondyloArthritis international Society (ASAS) proposed classification criteria that apply to both patients in the early stage of the disease and those in the later stages, included under the umbrella term axial SpA (5). These criteria follow the rubric of prior criteria for the SpA family of diseases (6,7). In this classification, the designation “nonradiographic axial SpA” encompasses patients who have chronic back pain and features suggestive of SpA but who do not meet the classification criteria for AS.
The goals of treatment of AS and nonradiographic axial SpA are to reduce symptoms, maintain spinal flexibility and normal posture, reduce functional limitations, maintain work ability, and decrease disease complications. The mainstays of treatment have been nonsteroidal antiinflammatory drugs (NSAIDs) and exercise, with the additional use of slow-acting antirheumatic drugs (SAARDs) in patients with peripheral arthritis. Over the past 15 years, the availability of tumor necrosis factor inhibitors (TNFi) has greatly altered the approach to the treatment of AS. More recently, additional biologic agents have been developed. With more treatment options, recommendations are needed to help optimize care of these patients. Although there are clinical similarities between AS and nonradiographic axial SpA, we considered these conditions separately because studies typically have included either patients with AS or those with nonradiographic axial SpA.
Several international organizations, including ASAS and the European League Against Rheumatism, have published recommendations for the treatment of AS, and rheumatology professional organizations in many countries have published guidelines on the use of TNFi in AS (8–11). The focus of these prior recommendations was on the use of particular medications or interventions, rather than on the treatment of patients in specific clinical circumstances. Although these recommendations were evidence based, the processes used to guide the translation of the evidence into recommendations were often not specified. Our effort differs in that we used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method to develop the recommendations (12–14). Important aspects of this method include identification of the most important clinical questions for which treatment recommendations are needed, specification of the important outcomes, and use of a tested approach for deriving recommendations from the evidence.
This project was developed by members of the Spondyloarthritis Research and Treatment Network (SPARTAN), a group of North American rheumatologists with special interest in SpA, in response to a request for proposals from the American College of Rheumatology (ACR) and with support from the Spondylitis Association of America (SAA), a patient advocacy organization. The project began in late 2012 after the ACR and SAA boards approved the proposal, and was modified based on community and stakeholder feedback received during a public comment period in spring 2013.
The primary objective of these recommendations was to provide optimal advice on the treatment of patients with AS and nonradiographic axial SpA, based on the evidence from the literature on benefits and harms associated with specific treatment decisions, the quality of that evidence, and patients’ values and preferences. Expert opinion was used in weighing these factors when developing the recommendations. The target populations are adults with AS or nonradiographic axial SpA (regardless of age at onset). The target users of these recommendations are primary care clinicians, rheumatology providers, physiatrists, and physical therapists.
METHODS
Overview of the project
Three groups were assembled to perform different functions (Figure 1): a core leadership group, a literature review group, and a voting panel. The core group comprised 4 rheumatologists, a GRADE methods expert, and ACR staff. The core group was responsible for defining the scope of the project, formulating the clinical questions to be addressed by the recommendations, supervising members of the literature review and voting groups, conducting the voting panel meeting, and drafting the manuscript. The literature review group comprised 12 rheumatologists, a physical therapist, and an orthopedic surgeon. They screened articles, abstracted data, and rated the quality of evidence. The voting group was independent of the literature review group and comprised 8 rheumatologists, a patient, a physical therapist, and an orthopedic surgeon. They were responsible for collectively reviewing the evidence and providing final recommendations. The GRADE methods expert provided guidance in methodology, but was not directly involved in the systematic review or grading the evidence. Adhering to ACR policy, at least 51% of each group was required to have no relevant conflicts of interest. The principal investigator and literature review committee leader were also required to have no relevant conflicts of interest.
Figure 1.
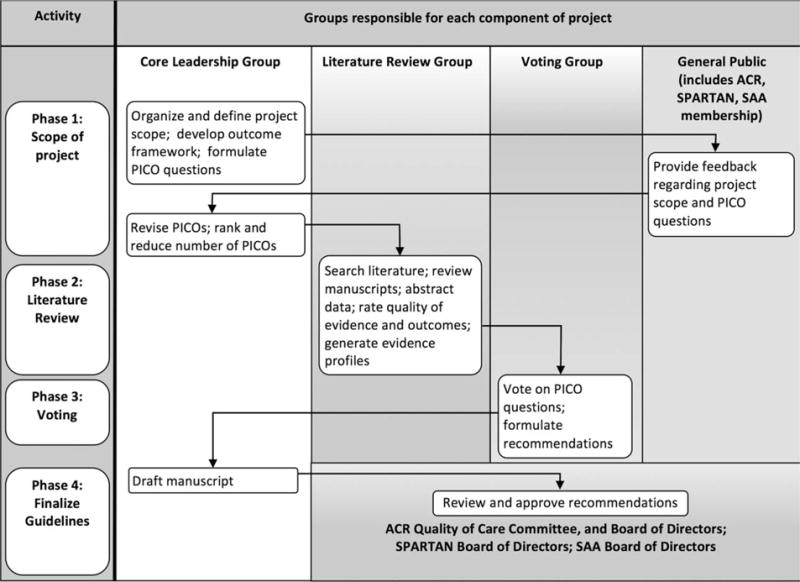
Flow chart showing the recommendation development process. PICO questions = clinical questions including the elements Patient (or Population) to whom the recommendation will apply, the Intervention being considered, the Comparison (which may be “no action” or an alternative intervention), and the Outcomes affected by the intervention; ACR = American College of Rheumatology; SPARTAN = Spondyloarthritis Research and Treatment Network; SAA = Spondylitis Association of America.
Developing the PICO questions
Guidelines are most useful when they provide specific actionable advice on choosing between alternative approaches in particular clinical situations (14). Therefore, the GRADE method advocates specifying 4 elements for each clinical question to be addressed by the recommendations: the Patient (or Population) to whom the recommendation will apply; the Intervention being considered; the Comparison (which may be “no action” or an alternative intervention); and the Outcomes affected by the intervention (PICO) (14). These PICO elements are arranged into the questions to be addressed in the literature searches. Each PICO question then forms the basis for a recommendation.
The core group initially generated 90 PICO questions in the following 6 topic areas: pharmacologic therapy, rehabilitation, surgery, specific comorbidities, disease monitoring, and preventive care. After public comment, we reduced the scope to 57 PICO questions (37 for AS and 20 for nonradiographic axial SpA) thought to address the central aspects of treatment (see Supplements A and B, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract).
Because therapy goals of most treatments are similar, we developed a common outcomes framework to apply across PICO questions. The framework included 5 major outcomes: mortality, health status, functional status, serious adverse events, and comorbidities (Table 1). For health status and functional status, we used patient-reported outcomes as the primary outcome measures, because these more directly reflect the impact of the condition on the individual. We considered data on surrogate outcomes (e.g., spinal range of motion) only when data on patient-reported outcomes were not available. For rehabilitation interventions, the outcomes were health status, functional status, and adverse events.
Table 1.
Outcomes framework*
The framework included the following 5 major outcomes:
|
AS = ankylosing spondylitis.
Literature searches
Systematic literature reviews were conducted for the following domains: pharmacologic therapy, physical therapy and rehabilitation, surgery, disease activity assessment, education and preventive care, iritis, and inflammatory bowel disease. The search strategies were developed by 2 experienced medical librarians in consultation with the core group and were reciprocally reviewed (see Supplement C, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). We searched OVID Medline since 1946, PubMed since its inception in the mid-1960s, and the Cochrane Library. Search filters were applied to retrieve study designs of interest, as well as articles on safety. Searches for medications were limited to those approved by the US Food and Drug Administration for any indication. The initial searches were performed in September 2013 and updated in July 2014.
For each PICO question, one literature reviewer screened titles and abstracts of retrieved articles and, when indicated, the full manuscript to identify relevant articles. A second reviewer independently screened the titles and abstracts excluded in the first review to ensure that all relevant articles were included. We also checked prior systematic reviews. We excluded articles focused on children (ages <18 years), those in languages other than English, narrative reviews, meeting abstracts, and case reports. The literature search results appear in Supplement D, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract.
Data abstraction and rating the quality of evidence
A major principle of the GRADE method is to base recommendations on the best available evidence identified through a systematic literature review and summarized in quantitative estimates of treatment effects, such as pooled mean differences or relative risks (15,16). Studies not reporting results in sufficient detail to calculate these values (e.g., those that did not include standard deviations) were not included in the evidence report. The review group synthesized these data to produce an effect estimate for each outcome, and assessed the quality of the evidence based on the risk of bias, imprecision in the estimates of effect, inconsistency among studies, indirectness (e.g., the study examined a similar but distinct patient group or intervention), and publication bias (16).
In GRADE, randomized controlled trials are generally assumed to provide higher-quality evidence than observational studies (16). Therefore, if a PICO question was addressed by one or more trials, we only summarized data from these trials in the Evidence Report (see Supplement E, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). If a PICO question was addressed only in observational studies (and not in any clinical trial), we summarized the data from the observational studies in the Evidence Report.
GRADE uses 4 categories to rate the quality of evidence: high, moderate, low, and very low (16). High-quality evidence, which usually comes from controlled trials, indicates that we have high confidence in the effect estimate and future studies are thought unlikely to alter that effect. Very low-quality evidence implies very little certainty about the estimate and that the true effect may be quite different.
Deriving recommendations
According to the GRADE method, in addition to the quality of evidence, the voting group weighed the overall balance of desirable and undesirable consequences, and the variation in how patients might value an intervention’s potential benefits and risks relative to the comparison (13,17). Although the GRADE method allows for considerations of costs in making recommendations, the ACR does not routinely do so, and we did not explicitly consider costs in these recommendations.
The strength of recommendation refers to the extent to which the panel was confident that the desirable effects of an intervention outweigh the undesirable effects. The GRADE method specifies 4 possible strengths of recommendations, each with particular implications (17) (Table 2). Strong recommendations usually require high-quality evidence and reflect a high degree of confidence that future research will not change the results. Strong recommendations usually involve interventions sufficiently clear in their benefits and risks that almost all informed patients would accept the recommendation. A conditional recommendation is more appropriate when the quality of evidence is low or very low, or the balance between desirable and undesirable consequences of an intervention is close, or if patients vary widely in their preferences. It is important to recognize that strong recommendations in GRADE do not necessarily imply large effects or benefits, nor a priority in which interventions should be used. Another principle of GRADE is that judgments at each step are documented so that the process of recommendation development can be traced and verified (15).
Table 2.
Strength of recommendations in GRADE*
| Strength | Interpretation | Implications for clinicians | Implications for policymakers |
|---|---|---|---|
| Strongly in favor | Almost all informed patients would choose to receive the intervention | Should be accepted by most patients to whom it is offered | Should be adopted as policy |
| Conditionally in favor | Most informed patients would choose the intervention, but a sizable minority would not | Large role for education and shared decision-making | Requires stakeholder engagement and discussion |
| Conditionally against | Most informed patients would not choose the intervention, but a small minority would | Large role for education and shared decision-making | Requires stakeholder engagement and discussion |
| Strongly against | Most patients should not receive the intervention | Should not be offered to patients | Should be adopted as policy |
GRADE = Grading of Recommendations, Assessment, Development and Evaluation.
Voting process
Voting group members were sent the Evidence Report and asked to submit a nonbinding vote on their recommendation for each PICO question 2 weeks before the voting group meeting. At the meeting, held July 19–20, 2014 in Arlington, VA, the Evidence Report was reviewed, questions were discussed and in some cases clarified, and members voted anonymously by electronic ballots on the recommendation for each PICO question. Iterative voting and discussion continued until at least 80% of the members agreed on one of the 4 possible recommendation options.
Definitions and key assumptions
Patients with AS were those meeting the modified New York criteria (4). For purposes of these recommendations, we defined active disease as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to SpA. We defined stable disease as that which was asymptomatic or causing symptoms that were bothersome but at an acceptable level as reported by the patient. A status of stable disease required a minimum of 6 months of clinical stability. The specific medications included in each drug category are in listed Supplement F, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract.
These guidelines are meant to apply to typical patients, rather than exceptional cases. Treatment decisions should always involve education of the patient as to anticipated benefits and potential harms. In instances when a particular medication is not recommended, it does not imply that it is contraindicated.
Endorsements
Prior to publication, these recommendations were reviewed and endorsed by the ACR Committee on Quality of Care, as well as the Boards of Directors of the ACR, SAA, and SPARTAN.
RESULTS
Figure 2 provides a summary of the main recommendations.
Figure 2.

Key recommendations for the treatment of patients with ankylosing spondylitis (AS) or nonradiographic axial spondyloarthritis (SpA). NSAIDs = nonsteroidal antiinflammatory drugs; TNFi = tumor necrosis factor inhibitor. Correction added after online publication 24 September 2015: The fourth bullet point in Figure 2 has been revised.
A. Recommendations for the treatment of patients with active AS
A1. Pharmacologic treatment
In adults with active AS:
We strongly recommend treatment with NSAIDs over no treatment with NSAIDs (PICO 2; low-quality evidence; vote 100% agreement).
We conditionally recommend continuous treatment with NSAIDs over on-demand treatment with NSAIDs (PICO 1; very low-quality evidence; vote 90% agreement).
We do not recommend any particular NSAID as the preferred choice (PICO 3; moderate- to low-quality evidence; conditional recommendation; vote 100% agreement).
Evidence and rationale
Evidence for the efficacy of NSAIDs in active AS comes from several placebo-controlled trials (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). The overall quality of evidence was low based on serious risk of bias and imprecision for several critical outcomes. Despite this, the panel believed most patients benefit from treatment with NSAIDs and that the desirable consequences far outweighed undesirable consequences for the large majority of patients, justifying a strong recommendation. Although some patients have contraindications to treatment, the panel thought there was likely little variation among patients in preferences for treatment with NSAIDs.
Continuous NSAID treatment was compared to on-demand treatment in one controlled trial, which showed no significant differences between groups in any clinical end point, wide confidence intervals, and high risk of bias (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art39298/abstract). Hypertension and depression were more common in the continuous treatment group. Despite this, the panel favored daily NSAID treatment for the period of active AS for most patients. The decision to use NSAIDs continuously may vary depending on the severity and intermittency of symptoms, comorbidities, and patient preferences. The dose may also be changed depending on symptom level.
We did not identify any formal comparative effectiveness analyses of different NSAIDs in AS. Evidence of the relative efficacy of NSAIDs was provided by several head-to-head controlled trials that used either indomethacin (n = 12), celecoxib (n = 2), or naproxen (n = 1) as the comparator drug (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). There was no evidence to suggest that indomethacin had different effects on pain or stiffness compared to other NSAIDs, nor differences in efficacy between celecoxib and either diclofenac or ketoprofen. Many studies had small samples and imprecise estimates of effects, and the overall quality of evidence was rated as moderate to low, depending on the drug. Only one study had a noninferiority design. Based on this evidence, the panel recommended against designating any particular NSAIDs as the preferred treatment option. Choice of NSAID should be based on consideration of the patient’s past history of NSAID use, risk factors for adverse effects, and comorbidities.
In adults with active AS despite treatment with NSAIDs, we conditionally recommend against treatment with SAARDs (PICO 7; very low- to moderate-quality evidence, depending on the drug; vote 90% agreement).
Evidence and rationale
Evidence on the efficacy of SAARDs was based on controlled trials of sulfasalazine (n = 8), methotrexate (n = 3), leflunomide (n = 1), pamidronate (n = 1), thalidomide (n = 1), and apremilast (n = 1) (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). The quality of evidence for critical outcomes was moderate to very low. Sulfasalazine had a small beneficial effect on spinal pain but not on other outcomes, and had a higher risk of side effects than placebo. Although treatment with sulfasalazine did not improve peripheral joint counts, small benefit was seen in a composite measure of peripheral arthritis symptoms. The other medications were tested in small numbers of patients. Trials of methotrexate were limited by use of weekly doses of 10 milligrams or less. Treatment with high-dose pamidronate was associated with improved patient-reported outcomes compared to low-dose pamidronate and deserves further study. Based on a very low to moderate level of evidence of small or no clinical effects, the panel recommended against the use of SAARDs in most patients whose AS remained active despite NSAID use; treatment with TNFi would be recommended instead (see PICO 6 below). Treatment with sulfasalazine or pamidronate could be considered for patients with contraindications to TNFi or those who decline treatment with TNFi. Sulfasalazine could be considered for those with prominent peripheral arthritis.
In adults with active AS despite treatment with NSAIDs:
We strongly recommend treatment with TNFi over no treatment with TNFi (PICO 6; moderate-quality evidence; vote 80% agreement).
We do not recommend any particular TNFi as the preferred choice, except for patients with concomitant inflammatory bowel disease or recurrent iritis (PICO 5; moderate-quality evidence; conditional recommendation; vote 100% agreement).
Evidence and rationale
Evidence supporting the use of TNFi in this setting comes from 13 randomized controlled trials, which showed large and consistent improvements in clinical outcomes (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10. 1002/art.39298/abstract). The trials were short term, with most limited to 24 weeks. Although the panel believed a strong recommendation was justified based on the overall balance between desirable and undesirable consequences of treatment, the panel emphasized that the decision to begin treatment with a TNFi should be made only after education of the patient on the potential risks and benefits of the medication, and with consideration that some patients may value the relief of symptoms and risk of side effects differently. The panel judged that lack of response (or intolerance) to at least 2 different NSAIDs over 1 month, or incomplete responses to at least 2 different NSAIDs over 2 months, would be adequate trials with which to judge NSAID responses.
Evidence to guide the choice of TNFi, based on either superior efficacy or lower toxicity of one drug versus another, is limited. In a small 2-year open-label trial, there were no differences in clinical outcomes between patients randomized to receive infliximab or etanercept (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary. wiley.com/doi/10.1002/art.39298/abstract). Two meta-analyses that tested indirect comparisons among TNFi using data from controlled trials showed no differences in ASAS criteria for 20% improvement in disease activity responses, while other clinical outcomes were not examined. The quality of evidence was judged to be moderate and insufficient to support recommendation of the use of one TNFi over another. However, the panel thought that in patients with inflammatory bowel disease or frequently recurrent iritis, treatment with infliximab or adalimumab should be preferred over etanercept (see PICO 29 and 32 below).
In adults with active AS despite treatment with NSAIDs and who have contraindications to TNFi, we conditionally recommend treatment with a SAARD over treatment with a non-TNFi biologic agent (PICO 8; very low to low-quality evidence, depending on the drug; vote 100% agreement).
Evidence and rationale
No studies have directly compared treatment responses to non-TNFi biologic agents and SAARDs in patients with active AS who have contraindications to TNFi. Similarly, these treatments have not been compared in patients who are nonresponders to TNFi, which might provide indirect evidence. Therefore, qualitative comparisons of responses to SAARDs (PICO 7) and non-TNFi biologic agents were used to address this question. Data from small single-arm studies comparing patients before and after treatment with abatacept and tocilizumab failed to demonstrate benefit for important patient outcomes, while a study of ustekinumab showed some evidence of effectiveness but had serious risk of bias (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). A trial of rituximab showed little benefit in patients who had not responded to prior treatment with TNFi. The overall level of evidence supporting use of non-TNFi biologic agents was very low. The panel thought there were insufficient data currently to support the use of presently available non-TNFi biologic agents in active AS, and favored treatment with sulfasalazine or pamidronate before the use of these medications, despite the limited benefit of sulfasalazine and limited evidence for pamidronate.
In adults with active AS despite treatment with the first TNFi used:
We conditionally recommend treatment with a different TNFi over adding a SAARD (PICO 9; very low-quality evidence; vote 100% agreement).
We conditionally recommend treatment with a different TNFi over treatment with a non-TNFi biologic agent (PICO 10; very low-quality evidence; vote 90% agreement).
Evidence and rationale
This recommendation addresses TNFi failures due to ineffectiveness rather than toxicity. No studies directly compared treatment with SAARDs to treatment with TNFi for patients with active AS whose treatment with the first TNFi was unsuccessful. The recommendation was therefore based on indirect comparison of outcomes of patients who switched to a different TNFi after failure of the first TNFi, and outcomes of patients treated with either SAARDs or non-TNFi biologic agents (addressed in PICO 7 and PICO 8, respectively). Data from 4 observational studies suggested that patients who switched TNFi improved on average in important clinical outcomes, although the improvement was often less than in primary responses (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/ art.39298/abstract). These improvements were larger than those reported with either SAARDs or non-TNFi biologic agents. The overall quality of evidence was low given the risk of bias associated with open-label treatment, and the lack of studies that directly addressed these questions. The panel recommended that after failure of the first TNFi, switching to a different TNFi be considered for most patients. The panel did not consider the question of treatment options after failure of the second TNFi.
In adults with active AS, we strongly recommend against treatment with systemic glucocorticoids (PICO 4; very low-quality evidence; vote 100% agreement).
Evidence and rationale
Treatment with systemic glucocorticoids was examined in 3 case series and 1 short-term (2-week) randomized placebo-controlled trial of high-dose glucocorticoids (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10. 1002/art.39298/abstract). In the randomized trial, 5 of 10 outcomes favored prednisolone 50 milligrams daily over placebo. In the case series, there were modest improvements with treatment over 4–6 months, but the studies had a serious risk of bias and imprecise estimates of effect. The overall quality of evidence was therefore rated as very low. The panel concluded that there was little evidence to support long-term treatment with systemic glucocorticoids. However, it was recognized that short-term glucocorticoid treatment with rapid tapering could be considered in a very limited number of circumstances, including a polyarticular flare of peripheral arthritis, flares during pregnancy, or concomitantly with flares of inflammatory bowel disease.
In adults with AS and isolated active sacroiliitis despite treatment with NSAIDs, we conditionally recommend treatment with locally administered parenteral glucocorticoids over no treatment with local glucocorticoids (PICO 13; very low-quality evidence; vote 100% agreement).
Evidence and rationale
Sacroiliac joint glucocorticoid injections were tested in 2 small controlled trials, only one of which was blinded. These results, along with those of 2 observational studies, showed improvement in pain for up to 9 months (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). However, the studies had serious risk of bias and some included patients with undifferentiated SpA. The panel recognized that this treatment may be a useful option when weighed against the alternative of escalating systemic treatment.
In adults with AS with stable axial disease and active enthesitis despite treatment with NSAIDs, we conditionally recommend using treatment with locally administered parenteral glucocorticoids over no treatment with local glucocorticoids. Peri-tendon injections of Achilles, patellar, and quadriceps tendons should be avoided. (PICO 14; very low-quality evidence; vote 100% agreement).
In adults with AS with stable axial disease and active peripheral arthritis despite treatment with NSAIDs, we conditionally recommend using treatment with locally administered parenteral glucocorticoids over no treatment with local glucocorticoids (PICO 15; very low-quality evidence; vote 100% agreement).
Evidence and rationale
Our search identified no studies of local glucocorticoid injections for the treatment of enthesitis in patients with AS. The recommendation was based on extrapolation from experiences in other diseases, which showed modest short-term benefit from peri-tendon injections, depending on the site (18). The panel specifically recommended against local injections around the Achilles, patellar, and quadriceps tendons, given the risk of tendon rupture (19). Injections at other sites, such as the greater trochanter, pelvic rim, and plantar fascia attachment, could be considered based on symptom severity and patient preferences for local versus systemic treatment with a SAARD or TNFi.
Similarly, no studies reported on the use of intraarticular glucocorticoid injections in the treatment of active peripheral arthritis in AS. The panel recommended this treatment as an option, based on evidence from other rheumatic diseases (20). This option may be considered for patients who prefer local treatment over systemic treatment, and when only 1 or 2 joints are inflamed.
A2. Rehabilitation
In adults with active AS:
We strongly recommend treatment with physical therapy over no treatment with physical therapy (PICO 16; moderate-quality evidence; vote 100% agreement).
We conditionally recommend active physical therapy interventions (supervised exercise) over passive physical therapy interventions (massage, ultrasound, heat) (PICO 17; very low-quality evidence; vote 82% agreement).
We conditionally recommend land-based physical therapy interventions over aquatic therapy interventions (PICO 18; moderate-quality evidence; vote 100% agreement).
Evidence and rationale
There was moderate-level evidence from 2 controlled trials in support of the efficacy of physical therapy in patients with active AS (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary. wiley.com/doi/10.1002/art.39298/abstract). Given the evidence of benefit and the very low likelihood of harms, the panel judged that a strong recommendation was justified.
No studies were identified that compared active with passive interventions. Because one of the goals of physical therapy is to educate patients in self-management in using an independent exercise program, the panel judged that active interventions should be stressed over passive interventions. Passive interventions could supplement, but not substitute for, active physical therapy interventions.
Four controlled trials compared aquatic and land-based interventions in active AS, with no significant short-term differences in changes in disease activity, pain, or stiffness between treatment groups, but some slightly better outcomes with aquatic interventions (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley. com/doi/10.1002/art.39298/abstract). Given the absence of strong evidence favoring aquatic interventions, the panel judged that aquatic therapy should not take precedence over land-based therapy. While aquatic therapy can be used by those with access to a swimming pool or hydrotherapy tub, land-based therapy was conditionally preferred because access to land-based therapy is often greater.
B. Recommendations for the treatment of patients with stable AS
B1. Pharmacologic treatment
In adults with stable AS, we conditionally recommend on-demand treatment with NSAIDs over continuous treatment with NSAIDs (PICO 1; very low-quality evidence; vote 100% agreement).
Evidence and rationale
This recommendation differs from that for patients with active AS (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/ doi/10.1002/art.39298/abstract). The panel considered that the undesirable consequences of continuous treatment with NSAIDs outweighed the desirable consequences in patients with stable AS. Continuous NSAID treatment in clinically stable patients might be considered for those with early AS, no comorbidities, and a higher propensity to develop progressive spinal fusion (for example, men, smokers, those with persistently high serum C-reactive protein [CRP] concentrations, and those with existing syndesmophytes) (21), based on the patient’s preferences for taking daily medication for possible future benefits, even if those benefits are unclear.
In adults with stable AS receiving treatment with TNFi and NSAIDs, we conditionally recommend continuing treatment with TNFi alone compared to continuing both treatments (PICO 11; very low-quality evidence; vote 100% agreement).
In adults with stable AS receiving treatment with TNFi and SAARDs, we conditionally recommend continuing treatment with TNFi alone over continuing both treatments (PICO 12; very low-quality evidence; vote 100% agreement).
Evidence and rationale
The literature search did not identify any studies that addressed the effects of withdrawal of either NSAIDs or SAARDs on the outcomes in patients with stable AS who were also receiving TNFi. We did not consider the option of withdrawing TNFi rather than NSAIDs or SAARDs, based on evidence from an observational study that suggested a high likelihood of relapse after withdrawal of TNFi (22). In stable patients, a trial of withdrawing either NSAIDS or SAARDs could be considered, based on the likelihood that undesirable consequences of combined treatment may outweigh the desirable consequences. Continuing treatment with either NSAIDs or SAARDs in this setting has uncertain but likely little benefit, but entails risk of gastrointestinal, renal, cardiac, and hematologic toxicity (23,24).
It is important to note that the recommendation regarding SAARDs does not apply to the question of using low-dose methotrexate with TNFi treatment to decrease the potential development of antidrug antibodies. The panel did not address this question.
B2. Rehabilitation
In adults with stable AS, we strongly recommend treatment with physical therapy over no treatment with physical therapy (PICO 19; low-quality evidence; vote 82% agreement).
Evidence and rationale
The use of physical therapy in patients with stable AS was examined in 8 controlled trials, with improvement in disease activity and physical functioning but no significant improvement in pain or stiffness (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/ abstract). The overall level of evidence was rated as low based on risk of bias and heterogeneous results across studies. Given the evidence of benefit and low likelihood of harms, the panel judged that a strong recommendation was justified. Physical therapy in stable patients was most important for periodic reassessment and appropriate modifications of home exercises.
C. Recommendations for the treatment of patients with either active or stable AS
In adults with active or stable AS:
We conditionally recommend the regular-interval use and monitoring of a validated AS disease activity measure (PICO 54; very low-quality evidence; vote 100% agreement).
We conditionally recommend regular-interval use and monitoring of the CRP concentrations or erythrocyte sedimentation rate (ESR) over usual care without regular CRP or ESR monitoring (PICO 55; very low-quality evidence; vote 100% agreement).
Evidence and rationale
No studies addressed the effect of routine monitoring of a disease activity measure, such as the Bath AS Disease Activity Index or the AS Disease Activity Score, or acute-phase reactants on outcomes in patients with AS. The panel thought that monitoring would be most helpful in patients with active symptoms as a guide to treatment. Monitoring was not thought necessary at every clinic visit, and could be omitted in patients who were clinically stable for some time.
In adults with active or stable AS, we conditionally recommend advising unsupervised back exercises (PICO 20; moderate-quality evidence; vote 91% agreement).
Evidence and rationale
Unsupervised back exercise refers to exercises done by patients at home without prior training by a therapist. This could include recommendations by physicians or in educational pamphlets or videos. This intervention was examined in one controlled trial, which showed no benefit in health status or functional status favoring the intervention, although with serious risk of bias (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). The panel recommended unsupervised back exercise as part of a general endorsement of physical activity, given that desirable consequences were likely to outweigh undesirable consequences. The recommendation is conditional in that unsupervised back exercises should not substitute for initial instruction in back exercises by a physical therapist.
In adults with active or stable AS and spinal fusion or advanced spinal osteoporosis, we strongly recommend against treatment with spinal manipulation (PICO 21; very low-quality evidence; vote 100% agreement).
Evidence and rationale
We did not identify any studies of the effects of spinal manipulation with high-velocity thrusts on outcomes in patients with AS. Several case reports of spine fractures, spinal cord injury, and paraplegia following chiropractic spinal manipulation, particularly of the cervical spine in patients with spinal fusion or advanced osteoporosis of the spine, have been reported (25). Based on the absence of evidence of benefit and evidence of potential severe harms, the panel recommended strongly against spinal manipulation with high-velocity thrusts in patients with AS who have spinal fusion or advanced spinal osteoporosis.
D. Recommendations for the treatment of patients with AS and specific impairments or comorbidities
In adults with AS and advanced hip arthritis, we strongly recommend treatment with total hip arthroplasty over no surgery (PICO 25; very low-quality evidence; vote 100% agreement).
Evidence and rationale
Evidence for the effectiveness of total hip arthroplasty in patients with AS included observational studies and case series which demonstrated postoperative improvements in pain, functioning, and hip range of motion (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). Weak study designs resulted in the overall quality of evidence being rated very low, although this question can practicably be addressed only in observational studies. Arthroplasty is the best treatment option for patients with advanced hip arthritis and severe hip pain, who often would otherwise experience progressive limitations in mobility and reliance on opiates. The panel judged that a strong recommendation for surgery was justified for all patients with hip arthritis that was substantially impacting their mobility or quality of life. An exception is among patients in whom surgery is contraindicated by comorbid conditions. The surgery should be performed by orthopedic surgeons and at hospitals that are highly experienced in joint replacement in patients with AS.
In adults with AS and severe kyphosis, we conditionally recommend against elective spinal osteotomy (PICO 26; very low-quality evidence; vote 100% agreement).
Evidence and rationale
Evidence for the effectiveness of spinal osteotomy in patients with AS and severe kyphosis is from case series, which most commonly only reported restoration of horizontal gaze or degrees of correction as outcomes (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/ abstract). Perioperative mortality of 4% and permanent neurologic sequelae of 5% were noted. Given the high procedure-associated risks and the potential for large benefits with respect to physical functioning, cardiopulmonary functioning, and psychological well-being, decisions about surgery clearly require extensive discussion and consideration of the risks, benefits, and preferences of individual patients. The panel thought that in most patients the risks would outweigh the potential benefits. However, elective spinal osteotomy could be considered in those patients with severe kyphosis who lack horizontal vision and for whom this causes major physical and psychological impairments. In this highly selected subgroup, surgery could be recommended if the procedure is performed at specialized centers by surgeons with extensive experience in the technique. This recommendation does not apply to nonelective surgery, including stabilization of spinal fractures or pseudoarthroses, and relief of spinal cord or nerve root compression.
In adults with AS and acute iritis, we strongly recommend treatment by an ophthalmologist to decrease the severity, duration, or complications of episodes (PICO 27; very low-quality evidence; vote 100% agreement).
Evidence and rationale
No studies were identified that examined the relative effectiveness of treatment of iritis by specialists or nonspecialists. However, given the expertise of ophthalmologists in diagnosing iritis, evaluating the severity of episodes, and selecting the best local treatments, the panel strongly recommended treatment by an ophthalmologist.
In adults with AS and recurrent iritis, we conditionally recommend prescription over no prescription of topical glucocorticoids for prompt at-home use in the event of eye symptoms to decrease the severity or duration of iritis episodes (PICO 28; very low-quality evidence; vote 91% agreement).
Evidence and rationale
No studies examined the effectiveness of patient-initiated at-home treatment with topical glucocorticoids at the onset of symptoms of iritis compared to physician-directed treatment. Prompt treatment may lessen the severity of episodes and decrease the likelihood of ocular complications (26). Therefore, the committee favored provision of prescriptions of topical glucocorticoids so that patients could initiate treatment at the onset of typical symptoms of iritis. However, this should be restricted to patients with recurrent episodes who are knowledgeable about the symptoms of iritis. Prescription of topical glucocorticoids for at-home use should be done in the context of a care plan that includes a prompt ophthalmologic examination.
In adults with AS and recurrent iritis, we conditionally recommend treatment with infliximab or adalimumab over treatment with etanercept to decrease recurrences of iritis (PICO 29 and 30; very low-quality evidence; vote 82% agreement).
Evidence and rationale
In 4 observational studies or pooled analyses of clinical trial results, treatment with infliximab or adalimumab was associated with lower rates of iritis than was treatment with etanercept (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/ doi/10.1002/art.39298/abstract). Data on adalimumab were less extensive than data on infliximab. With some evidence of differential effectiveness through indirect comparisons and no evidence of increased harms, the panel recommended infliximab or adalimumab over etanercept for patients with frequently recurrent iritis episodes.
The panel decided to delete the question (PICO 30) that addressed switching TNFi in patients who develop iritis while receiving treatment, since the important decision is the specific TNFi to be used, which is addressed in PICO 29.
In adults with AS and inflammatory bowel disease:
We do not recommend any particular NSAID as the preferred choice to decrease the risk of worsening of inflammatory bowel disease symptoms (PICO 31; very low-quality evidence, conditional recommendation; vote 100% agreement).
We strongly recommend using treatment with TNFi monoclonal antibodies over treatment with etanercept (PICO 32; very low-quality evidence; vote 100% agreement).
Evidence and rationale
We did not identify any studies of the relative harms of different NSAIDs in patients with AS and inflammatory bowel disease. Evidence of whether treatment with conventional NSAIDs is associated with intestinal relapses in patients with inflammatory bowel disease (without AS) is limited and controversial. While case reports suggest that conventional NSAID treatment can lead to relapses (or onset of inflammatory bowel disease), epidemiologic studies do not support an association (27). Rates of exacerbations of inflammatory bowel disease among patients treated with celecoxib were not significantly different from rates among those treated with placebo in a 2-week trial (28). The panel considered that there was only limited evidence to support use of celecoxib over other NSAIDs, but that short courses of treatment with celecoxib may have less potential for harm.
Evidence of the choice of TNFi in patients with AS and inflammatory bowel disease comes from a pooled analysis, which indicated lower risks of either flare or new onset of inflammatory bowel disease with infliximab than with etanercept (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). Adalimumab was also associated with lower risks, although these associations were not as strong as those of infliximab. The overall level of evidence was rated as very low because of risk of bias, inconsistency, and imprecision. In making the recommendation, the panel extrapolated from evidence from trials demonstrating the efficacy of TNFi monoclonal antibodies in the treatment of inflammatory bowel disease, but inefficacy of etanercept (29).
E. Education and preventive care
In adults with AS, we conditionally recommend participation in formal group or individual self-management education (PICO 48; moderate-quality evidence; vote 91% agreement).
Evidence and rationale
Self-management education interventions in AS were tested in 5 controlled trials, with generally small but significant improvements in measures of disease activity and health status (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). Attrition in some studies introduced a risk of bias, and the overall level of evidence was rated as moderate. The durability of short-term improvements was unclear. The panel considered that there were few potential undesirable consequences of formal patient education interventions, but participation represents a time commitment for a potentially small and transient benefit. Stratifying group sessions into patients with similar durations or severity of AS may lessen anxiety for some patients. Only programs that have been demonstrated to be effective should be used. Apart from formal self-management education, instruction of patients in the nature, treatments, and prognosis of AS is an important aspect of good clinical care.
In adults with AS, we conditionally recommend fall evaluation and counseling (PICO 51; very low-quality evidence; vote 100% agreement).
Evidence and rationale
No studies were found that examined the effectiveness of fall evaluations or fall counseling in patients with AS. Because falls can lead to spinal fractures and devastating neurologic consequences in some patients, the panel recommended fall evaluation and counseling for patients with osteoporosis, extensive spinal fusion, postural instability, or concomitant neurologic or musculoskeletal diseases that affect balance.
In adults with AS, we conditionally recommend screening for osteopenia/osteoporosis with dual x-ray absorptiometry (DXA) scanning over no screening (PICO 49; very low-quality evidence; vote 100% agreement).
In adults with AS and syndesmophytes or spinal fusion, we conditionally recommend screening for osteoporosis/ osteopenia with DXA scanning of the spine as well as the hips, compared to DXA scanning solely of the hip or other non-spine sites (PICO 50; very low-quality evidence; vote 100% agreement).
Evidence and rationale
We did not identify any studies that compared different strategies of osteoporosis screening in patients with AS. Because osteoporosis is a treatable complication of AS, the panel thought that screening was indicated. The age and sex of the patient, the patient’s level of physical activity, the duration and severity of AS, and presence or absence of other risk factors for osteoporosis should be considered in deciding when to begin screening and at what interval scans should be done.
Evidence on the validity of the spine DXA readings in the presence of syndesmophytes is limited and conflicting (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http:// onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). The panel thought that restricting DXA scans to non-spine sites in patients with syndesmophytes or spinal fusion could potentially miss spinal osteoporosis in some patients, and therefore recommended that spine and hip DXA scans be included in the initial screening of most patients. The sites to be scanned subsequently should be those that are most informative on the initial screening.
In adults with AS:
We strongly recommend against screening for cardiac conduction defects with electrocardiograms (PICO 52; very low-quality evidence; vote 82% agreement).
We strongly recommend against screening for valvular heart disease with echocardiograms (PICO 53; very low-quality evidence; vote 90% agreement).
Evidence and rationale
We did not identify studies that examined the effectiveness of screening for cardiac conduction defects or valvular heart disease in asymptomatic patients with AS. Conduction defects affect fewer than 10% of patients with AS (30). Because treatment would not be indicated in the absence of symptoms, the panel ascribed little value to screening asymptomatic patients. Detection of minor conduction disturbances may cause patients unnecessary anxiety and generate repeated medical tests. Syncope, dizziness, palpitations, fatigue, angina, and heart failure are indications for investigation.
Screening of asymptomatic patients with echocardiography for aortic valve disease would not likely detect occult abnormalities that could be treated to prevent progression to a symptomatic stage. The highly sensitive nature of echocardiography may lead to detection of minor abnormalities and cause anxiety. The panel judged that the undesirable consequences of screening, including its costs, outweighed the potential benefits.
F. Recommendations for the treatment of patients with nonradiographic axial SpA
The panel considered 20 PICO questions on pharmacologic treatment, use of rehabilitation, and monitoring of nonradiographic axial SpA that were analogous to the questions for AS (see Supplement B, available on the Arthritis & Rheumatology web site at http://onlinelibrary. wiley.com/doi/10.1002/art.39298/abstract). Because non-radiographic axial SpA has only recently been defined, the literature on treatment of this condition is limited (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39298/abstract). Therefore, the panel relied on the AS literature as the basis for most recommendations, which are also provided in Supplement B. These recommendations were the same as for AS, with the exception of the PICO question on use of TNFi. This question also had the highest level of evidence among those for nonradiographic axial SpA.
In adults with active nonradiographic axial SpA despite treatment with NSAIDs, we conditionally recommend treatment with TNFi over no treatment with TNFi (PICO 38; moderate-quality evidence; vote 90% agreement).
Evidence and rationale
This recommendation was based on evidence from 5 controlled trials of adalimumab, certolizumab, etanercept, and infliximab (see Supplement E: Evidence Report, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley. com/doi/10.1002/art.39298/abstract). Results consistently favored TNFi over placebo, but with imprecise estimates of effect. One trial included an unknown number of patients with AS. Overall, the quality of evidence for the critical outcomes was judged to be moderate, and the panel judged that treatment be considered in patients with active nonradiographic axial SpA not responsive to NSAIDs, particularly those with evidence of sacroiliitis on magnetic resonance imaging and/or an elevated CRP level. Patients should be thoroughly educated about the therapy and actively engaged in the treatment decision.
DISCUSSION
In the scope of these recommendations, we tried to identify the most common, consequential, and unsettled questions in the care of patients with AS and nonradiographic axial SpA, so that the recommendations would be useful in guiding clinical decision making. We prioritized symptoms, health status, functional status, quality of life, mortality, and toxicities to guide comparisons of treatment options. A quick reference guide that summarizes the main recommendations in AS is provided in Figure 3.
Figure 3.
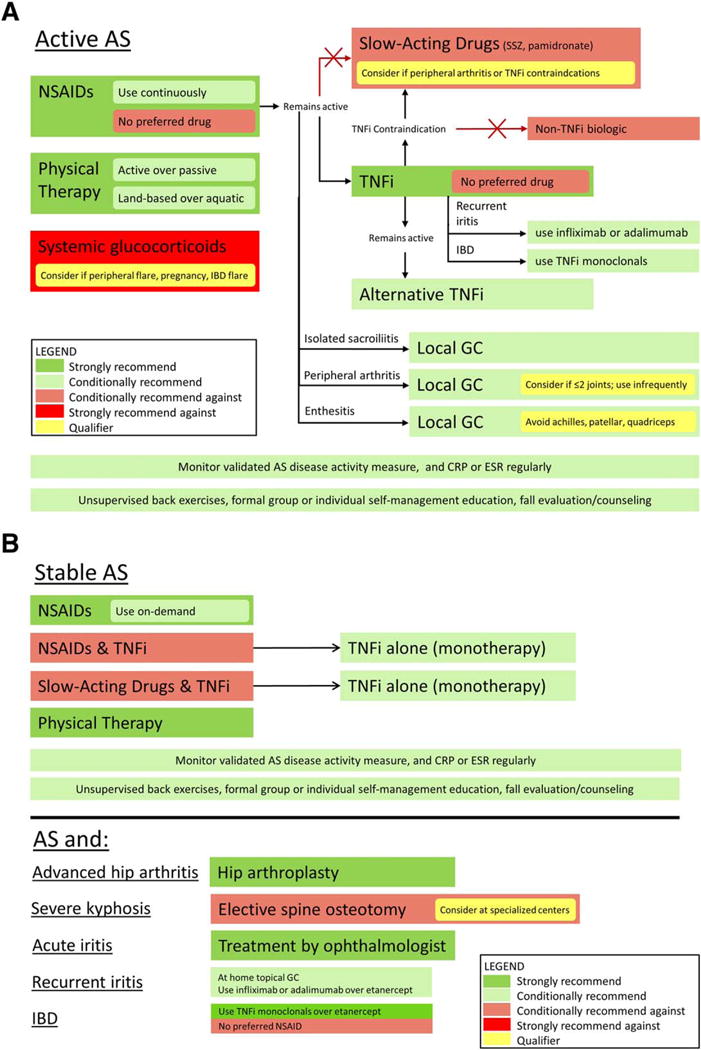
Summary of the main recommendations for the treatment of patients with active ankylosing spondylitis (AS) (A) or stable AS (B). NSAIDs = nonsteroidal antiinflammatory drugs; SSZ = sulfasalazine; TNFi = tumor necrosis factor inhibitors; IBD = inflammatory bowel disease; GC = glucocorticoid; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
We used the GRADE method to develop these recommendations. Important features of this method are 1) specification of the patient groups, interventions, competing alternatives, and outcomes so each recommendation is clearly focused on a particular clinical situation; 2) grading of the quality of evidence; and 3) basing the strength of recommendations on the quality of evidence, balance of benefits and harms, and patients’ preferences for different treatment options. The systematic, transparent, and explicit process of developing recommendations through GRADE is a major feature accelerating its adoption by professional groups internationally (12).
Detailing the interventions and treatment alternatives to specific patient subgroups in particular clinical circumstances is a factor that distinguishes these recommendations from previous AS guidelines. We did not include recommendations on diagnostic evaluations, good clinical practice, or overall principles of care, since these were considered foundational. Our recommendations are limited in that we did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring. These topics should be addressed in future updates, which should also include searches of additional literature databases and studies in languages other than English, if resources allow. While we did not develop recommendations for the treatment of juvenile SpA, these recommendations can be extrapolated to patients with AS who are younger than 18 years of age. We also did not examine the cost effectiveness of treatment options.
For some questions, including most of those for patients with nonradiographic axial SpA, we did not identify any directly relevant data from the literature. In these cases, recommendations were based on the experience and knowledge of voting panel members, and using indirect evidence from other conditions. While there was substantial evidence for some interventions, particularly for pharmacologic treatments, there were few studies of treatment strategies or the sequencing of medications in the event of contraindications or nonresponse, or of the efficacy of different strategies of monitoring disease activity and response. More studies are needed on the role of systemic and local glucocorticoids, on the use of NSAIDs in patients with coexisting inflammatory bowel disease, and comparison of active versus passive physical therapy treatments. Studies of appropriate methods to screen for osteoporosis and cardiovascular disease are also needed. This review highlights the many knowledge gaps in the treatment of AS and nonradiographic axial SpA.
While these recommendations can address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences. Treatment recommendations also cannot address all permutations that might affect treatment decisions. Application of these recommendations therefore requires careful assessment and sound clinical judgment.
In addition to this publication, these recommendations will be disseminated through posting on the ACR web site (www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/Axial-Spondyloarthritis), and through the membership of the Spondylitis Association of America and the Spondyloarthritis Research and Treatment Network. The main potential barrier to implementation of these guidelines is limited access to care. Financial barriers to TNFi are substantial, even for patients with medical insurance. Philanthropic organizations and pharmaceutical patient-assistance programs can in some cases help supply or defray the costs of TNFi. Many community-based health centers and public hospitals either support or can refer patients to physical therapists at reduced costs. Public hospitals also provide orthopedic surgery services to patients unable to pay for care.
As new evidence is generated and new treatments become available, these recommendations will require updating. We anticipate an update will be done within 5 years of this publication. However, the recommendations may be modified before then if warranted by major changes in treatments or recognition of new harms with the current interventions.
Supplementary Material
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to these guidelines and recommendations to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed or endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice.
The American College of Rheumatology is an independent, professional, medical and scientific society which does not guarantee, warrant, or endorse any commercial product or service.
Acknowledgments
This work was supported by the American College of Rheumatology and the Spondylitis Association of America. We thank our patient representative for adding valuable perspectives. We thank Laurie Savage and Charlotte Howard of the Spondylitis Association of America for their partnership on this project. We thank Regina Parker for administrative assistance and Tamara Rader, who with Janet Joyce, developed and reviewed the literature search strategies. We appreciate the contributions of Drs. Robert Hart, Siba Raychaudhuri, and James Witter to the literature review.
Drs. Ward and Colbert’s work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Dr. Deodhar has received consulting fees, speaking fees, and/ or honoraria from Abbott, Amgen, Pfizer, and Novartis (less than $10,000 each) and from AbbVie and UCB (more than $10,000 each), and research grants from Novartis, UCB, Johnson & Johnson, and Amgen. Dr. Ermann has received honoraria from AbbVie (less than $10,000) for Advisory Board service. Dr. Gensler has received consulting fees, speaking fees, and/or honoraria from AbbVie, UCB, and Amgen (less than $10,000 each) and research grants from Celgene and AbbVie. Dr. Borenstein has received consulting fees, speaking fees, and/or honoraria from Iroko and Abbott (less than $10,000 each) and honoraria from Clinical Care Options (more than $10,000). Dr. Inman has received consulting fees, speaking fees, and/or honoraria from AbbVie, Abbott, Janssen, Amgen/Pfizer, UCB, Novartis, and Celgene (less than $10,000 each). Dr. Majithia has received consulting fees, speaking fees, and/or honoraria from GlaxoSmithKline (less than $10,000). Dr. Haroon has received consulting fees, speaking fees, and/ or honoraria from AbbVie, Abbott, Amgen, Janssen/Johnson & Johnson/Centocor/Ortho Biotech Products, Janssen Biotech, Celgene, UCB, and Pfizer (less than $10,000 each). Dr. Maksymowych has received consulting fees, speaking fees, and/or honoraria from AbbVie, UCB, Pfizer, Amgen, Janssen, and Augurex (less than $10,000 each) and receives licensing fees and royalties from Augurex for the 14-3-3 biomarker. Mr. Clark has received consulting fees, speaking fees, and/or honoraria from AbbVie, Abbott, Amgen, Janssen, and Bristol-Myers Squibb (less than $10,000 each). Dr. Figgie has received consulting fees, speaking fees, and/or honoraria from Medtronic and Ethicon (less than $10,000 each). Dr. Hallegua has received consulting fees, speaking fees, and/or honoraria from AbbVie, Q-Med AB, UCB, Bristol-Myers Squibb, Centocor, Amgen, IDEC, Xoma, Novartis, Roche, Isis, Pharmacia, La Jolla Pharma, Genentech, Proctor & Gamble, Genelabs, MedImmune, Human Genome Sciences, Array Biopharma, and Cipher (less than $10,000 each). Dr. Prete has received consulting fees, speaking fees, and/or honoraria from Abbott (less than $10,000). Dr. Rosenbaum has received consulting fees, speaking fees, and/or honoraria from UCB, Regeneron, Xoma, Lux Biosciences, Elan, Allergan, Santen, Teva, Novartis, Sanofi, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, and Genentech (less than $10,000 each) and is a contributor to UpToDate. Dr. van den Bosch has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Janssen/Johnson & Johnson, Pfizer, and UCB (less than $10,000 each). Dr. Reveille has received consulting fees, speaking fees, and/or honoraria from Abbott and UCB (less than $10,000 each).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ward had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Ward, Deodhar, Akl, Inman, Maksymowych, Joyce, Colbert, Yu, Miller, Reveille, Caplan.
Acquisition of data. Deodhar, Lui, Ermann, Gensler, Smith, Borenstein, Hiratzka, Weiss, Inman, Majithia, Haroon, Maksymowych, Joyce, van den Bosch, Yu, Reveille, Caplan.
Analysis and interpretation of data. Ward, Deodhar, Akl, Lui, Ermann, Gensler, Smith, Hiratzka, Weiss, Inman, Haroon, Maksymowych, Clark, Colbert, Figgie, Hallegua, Prete, Rosenbaum, Stebulis, van den Bosch, Caplan.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Zochling J, Smith EU. Seronegative spondyloarthritis. Best Pract Res Clin Rheumatol. 2010;24:747–56. doi: 10.1016/j.berh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Perez Alamino R, Maldonado Cocco JA, Citera G, Arturi P, Vazquez-Mellado J, Sampaio-Arros PD, et al. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol. 2011;38:1656–60. doi: 10.3899/jrheum.101049. [DOI] [PubMed] [Google Scholar]
- 4.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 5.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 6.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–9. In French. [PubMed] [Google Scholar]
- 7.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. the European Spondylarthropathy Study Group The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 8.Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. doi: 10.1136/ard.2011.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidiropoulos PI, Hatemi G, Song IH, Avouac J, Collantes E, Hamuryudan V, et al. Evidence-based recommendations for the management of ankylosing spondylitis: systematic literature search of the 3E Initiative in Rheumatology involving a broad panel of experts and practising rheumatologists. Rheumatology (Oxford) 2008;47:355–61. doi: 10.1093/rheumatology/kem348. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Braun J, Dougados M, Emery P, FitzGerald O, Helliwell P, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73:6–16. doi: 10.1136/annrheumdis-2013-203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Berg R, Stanislawska-Biernat E, van der Heijde DM. Comparison of recommendations for the use of anti-tumour necrosis factor therapy in ankylosing spondylitis in 23 countries worldwide. Rheumatology (Oxford) 2011;50:2270–7. doi: 10.1093/rheumatology/ker270. [DOI] [PubMed] [Google Scholar]
- 12.Grade Working Group web site. URL: www.gradeworkinggroup.org.
- 13.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. for the GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–7. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Coombes BK, Bisset L, Vincenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–67. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe D, Achten J, Costa ML. Glucocorticoid injections in lesions of the Achilles tendon. Foot Ankle Int. 2009;30:661–5. doi: 10.3113/FAI.2009.0661. [DOI] [PubMed] [Google Scholar]
- 20.Wallen M, Gillies D. Intra-articular steroids and splints/rest for children with juvenile idiopathic arthritis and adults with rheumatoid arthritis. Cochrane Database Syst Rev. 2006:CD002824. doi: 10.1002/14651858.CD002824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poddubnyy D, Sieper J. Radiographic progression in ankylosing spondylitis/axial spondyloarthritis: how fast and how clinically meaningful? Curr Opin Rheumatol. 2012;24:363–9. doi: 10.1097/BOR.0b013e328352b7bd. [DOI] [PubMed] [Google Scholar]
- 22.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Alten R, Burmester G, et al. Safety and efficacy of readministration of infliximab after longterm continuous therapy and withdrawal in patients with ankylosing spondylitis. J Rheumatol. 2007;34:510–5. [PubMed] [Google Scholar]
- 23.Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindran V, Scott DL, Choy EH. A systematic review and meta-analysis of efficacy and toxicity of disease-modifying antirheumatic drugs and biological agents for psoriatic arthritis. Ann Rheum Dis. 2008;67:855–9. doi: 10.1136/ard.2007.072652. [DOI] [PubMed] [Google Scholar]
- 25.Liao CC, Chen LR. Anterior and posterior fixation of a cervical fracture induced by chiropractic spinal manipulation in ankylosing spondylitis: a case report. J Trauma. 2007;63:E90–4. doi: 10.1097/01.ta.0000246957.22573.63. [DOI] [PubMed] [Google Scholar]
- 26.Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150:534–42. doi: 10.1016/j.ajo.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrest K, Symmons D, Foster P. Systematic review: is ingestion of paracetamol or non-steroidal anti-inflammatory drugs associated with exacerbations of inflammatory bowel disease? Aliment Pharmacol Ther. 2004;20:1035–43. doi: 10.1111/j.1365-2036.2004.02270.x. [DOI] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Stenson WF, Brynskov J, Lorenz RG, Steidle GM, Robbins JL, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006;4:203–11. doi: 10.1016/j.cgh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Cohen LB, Nanau RM, Delzor F, Neuman MG. Biologic therapies in inflammatory bowel disease. Transl Res. 2014;163:533–56. doi: 10.1016/j.trsl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Lautermann D, Braun J. Ankylosing spondylitis: cardiac manifestations. Clin Exp Rheumatol. 2002;20(Suppl 28):S11–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


