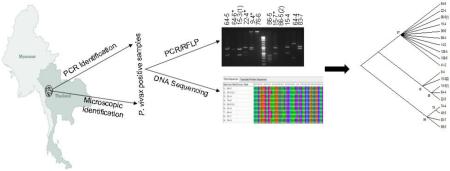
Abstract
Malaria transmission along international borders of the Greater Mekong Subregion is a big challenge for regional malaria elimination. At the Thai-Myanmar border, Plasmodium falciparum cases have dropped dramatically; however, increasing P. vivax prevalence and the emerging reports on hidden malaria burden due to asymptomatic infections demand attention. We conducted cross-sectional surveys to detect asymptomatic malaria infections in a small village located at Thai-Myanmar border and genotyped P. vivax infections in order to understand the level of genetic diversity on such a microgeographic scale. PCR/RFLP and DNA sequencing identified high levels of genetic polymorphisms at both Pvmsp3α and Pvmsp3β loci among P. vivax infections. Combining the PCR/RFLP patterns of Pvmsp3α and Pvmsp3β, a total of 10 genotypes were observed among 17 samples, while concatenated DNA sequences of Pvmsp3α and 3β generated 14 haplotypes with haplotype diversity of 0.97. These markedly diverse parasites on a microgeographic scale suggest the circulation of a considerably large parasite population at the international border.
Keywords: Plasmodium vivax, Thai-Myanmar, Microgeographic, Genetic diversity, Pvmsp3α, Pvmsp3β
Graphical Abstract
1. Introduction
Malaria remains a global public health problem, with an estimated 214 million cases and 0.4 million deaths in 2015 (WHO, 2015a). In recent years, malaria has been declining globally; 54% (57/106) malarious countries have reduced the incidence of malaria by >75% between 2000 and 2015 (WHO, 2015a). Countries within the Greater Mekong Subregion (Cambodia, China, Laos, Myanmar, Thailand and Vietnam) are aiming to achieve regional malaria elimination by 2030. Thailand has seen 50-75% reduction in malaria incidence in the last five years (WHO, 2015a). As in other countries of this region, the geographical distribution of malaria is highly heterogeneous (Cui et al., 2012). Central Thailand has been malaria-free for several decades; malaria predominantly occurs along the international borders shared with Myanmar, Cambodia and Malaysia. Provinces bordering Myanmar account for the highest burden of malaria in Thailand, and Tak Province among them, contributes ~70% of the total P. falciparum cases in the country (Wongsrichanalai et al., 2001). Among various possible reasons for this epidemiology in Thailand, importation of malaria cases from Myanmar poses a major challenge for controlling malaria in the border areas. Adding to the concerns of malaria control programs, huge burden of asymptomatic cases has been observed recently in Tak Province (Baum et al., 2015; Parker et al., 2015a; b). The situation is further worsened since the populations living there appear reluctant to seek proper health care (Sonkong et al., 2015).
To obtain detailed knowledge of malaria epidemiology in the Thai-Myanmar border, we recently conducted cross-sectional surveys in 2011-2012 in the Tha Song Yang District of Tak Province (Parker et al., 2015b). This study revealed a high level of undiagnosed malaria cases at a microgeographic scale (in a village of ~500 residents and spanning ~321 m north to south and ~500 m east to west). Whereas an expert microscopist identified 34 positive cases (18 P. vivax and 16 P. falciparum), PCR revealed 81 parasite-infected blood samples (55 P. vivax and 20 P. falciparum, 5 mixed, and 1 P. malariae). The infections missed by microscopy have been considered as submicroscopic asymptomatic cases. Noticeably, this study revealed a threefold increase in P. vivax cases identified by PCR, while most of the P. falciparum cases were symptomatic. Similar trends were observed by Baum and coworkers (2015) from another sentinel village in Tak Province. Such microgeographic studies of malaria prevalence can potentially reveal the accurate burden of malaria, which can help present a big picture of the whole region where malaria cases are being under-diagnosed by routine microscopic examinations. Conversely, without an accurate estimation of malaria epidemiology, it would be difficult for the national malaria program to realize the goal of malaria elimination.
For the last few years, Thailand's annual malaria incidence has continuously recorded P. vivax as the predominant species in the provinces along the Thai-Myanmar border (Parker et al., 2015b). Analyses in these regions have shown natural P. vivax populations to be highly genetically diverse with frequent multiple-strain infections. Yet, these studies have been conducted in different and distant geographical locations and on relatively large geographical scales (Cui et al., 2003; Rungsihirunrat et al., 2011; Putaporntip et al., 2014). The genetic diversity and transmission dynamics of malaria parasites at a microgeographic scale are much less understood. Thus, we determined the genetic diversity of P. vivax isolates from a small village in Tak Province using Pvmsp3α- and Pvmsp3β-based PCR/RFLP and sequencing techniques. Continuous surveillance of the genetic complexity in regions pursuing malaria elimination could reveal hidden reservoirs of malaria and also provide useful baseline molecular epidemiology data to monitor the effectiveness and progress in malaria elimination efforts.
2. Materials and methods
2.1. Study site and sample collection
The study site is a small village (SO) in the Tha Song Yang District of Tak Province on the Thai side of Thai-Myanmar border (Fig. 1). The village spans over ~321 m from east to west and 500 m from north to south on a side of hill. This village has been inhabited by ~550 people with ~80% of residents being of the Karen ethnicity (Parker et al., 2015). Three mass blood surveys were conducted each year in 2012 and 2013. The study population included all the residents of the village present at the time of the surveys, the average population examined during six mass blood surveys was approximately 527. Some villagers could not be included in the surveys as they frequently moved out of the village and even crossed the international border in search of the work. The detailed demographic information of the study site has been published in Parker et al., 2015b. The identification codes for each family and the residents used by Parker and coworkers (Parker et al., 2015b) were maintained in the present study.
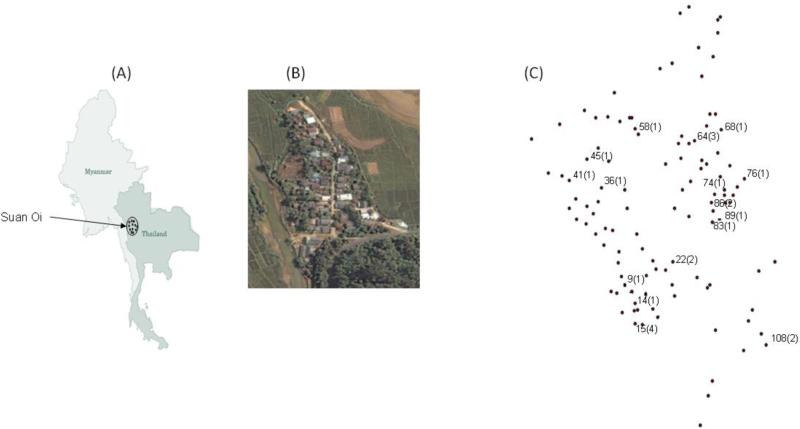
Fig. 1.
Maps showing location of the study area. (A) Map of Thailand showing area of sample collection in Tak Province. (B) Satellite image of the SO village in Tak (wikimapia.org). (C) Village map created using spatial coordinates. Dots represent each house and the numbers written are the house numbers having P. vivax-positive patients and the figures in the parenthesis are the number of P. vivax infected samples detected from each house during these surveys.
Finger-pricked blood samples (100 μl each) on Whatman filter paper were obtained from all patients on enrolment and blood smears were prepared and stained with Giemsa. Written informed consent was obtained from the participants or guardians. This study was approved by the Institutional Review Boards of Pennsylvania State University and the Thai Ministry of Health.
2.2. Malaria diagnosis
The presence of malaria parasites in all blood samples from participants were confirmed by microscopic examination of Giemsa-stained blood films and by PCR. Thick films were screened for 200 oil-immersion fields. For molecular identification, the DNA was extracted from dried blood spots using a QiaAmp DNA Mini Kit (Qiagen, Germany). Plasmodium species identification was carried out using genus- and species-specific nested PCR as described (Snounou et al., 1993) and samples with mixed infections were omitted from the analysis. The persons with P. vivax infections were visited by public health workers. The patients were advised and treated with antimalarials following the Thai national antimalarial drug policy.
2.3. PCR-RFLP genotyping of P. vivax isolates at the Pvmsp3α and Pvmsp3β loci
Our earlier studies using two highly polymorphic genetic markers Pvmsp3α and Pvmsp3β have demonstrated high levels of genetic diversity of the P. vivax populations in western Thailand (Cui et al., 2003; Yang et al., 2006). Allelic diversity of the Pvmsp3α and Pvmsp3β genes was assessed using established PCR-RFLP techniques (Bruce et al., 1999; Yang et al., 2006). Pvmsp3α was amplified by nested PCR using primers P1 (5′-CAGCAGACACCATTTAAGG-3′) and P2 (5′-CCGTTTGTTGATTAGTTGC-3′) for the primary PCR and primers N1 (5′-GACCAGTGTGATACCATTAACC-3′) and N2 (5′- ATACTGGTTCTTCGTCTTCAGG-3′) for the nested PCR. Primary PCR of Pvmsp3β was performed with primers F1 (5′-GTATTCTTCGCAACACTC- 3′) and R1 (5′-CTTCTGATGTTATTTCCAG-3′), while nested reactions were done with primers F2 (5′-CGAGGGGCGAAATTGTAAACC-3′) and R2 (5′-GCTGCTTCTTTTGCAAAGG-3′). PCR was performed for 35 cycles using the following conditions: 94 °C for 20 s, 54 °C for 30 s, and 68 °C for 2.5 min. For RFLP analysis, the PCR products of Pvmsp3α and Pvmsp3β were digested with HhaI and PstI, respectively, and DNA was separated in a 1.5% agarose gel. Alleles were identified based on unique restriction banding patterns and were classified as described by earlier studies (Cui et al., 2003; Yang et al., 2006). If the molecular size of the bands was measured to be within 25 bp, they were considered the same.
2.4. Sequence analysis and phylogenetics
Amplified fragments of Pvmsp3α and Pvmsp3β from the P. vivax samples were purified using the High Pure PCR cleanup microkit (Roche) and sequenced in both directions using BigDye Terminator v3.1. DNA sequences obtained were assembled using Lasergene software (DNASTAR) with manual editing, and Pvmsp3α and Pvmsp3β sequences were aligned with the Salvador I (Sal-I) reference gene sequences PVX_097720 and PVX_097680, respectively, using ClustalW. All sequences obtained in this study have been deposited in GenBank with accession numbers KX656749-KX656784.
Phylogenetic analysis was conducted using MEGA v6.0 (Tamura et al., 2013).Unrooted trees were generated using the neighbor-joining method with the Jukes-Cantor distance option after applying partial deletion. Support for a node was assessed by 500 bootstrap resampling of the original dataset. The N- and C-termini of the Pvmsp3β sequence were determined as in Rayner et al. (2004) on the basis of the Sal-I strain sequence (PVX_097680).
3. Results
3.1. Detection of Plasmodium vivax using microscope and nested PCR method
To determine the extent of P. vivax genetic diversity and transmission dynamics at a microgeographic scale along the Thai-Myanmar border, we analyzed parasite samples from mass blood surveys conducted during 2012 and 2013 in a small border village in western Thailand (Fig. 1). Our earlier molecular analysis identified a large number of asymptomatic infections of P. vivax infections in the study village residential population (Parker et al., 2015a). In the three cross sectional surveys of 2012, 10 single-species P. vivax infections were identified by PCR analysis using the 18S rRNA gene (4 in April, 2 in July-August, and 4 in October-December), while in the two surveys of 2013, 14 single-species P. vivax infections were identified (5 in March, and 9 in May - June) (Table 1). However, only six isolates were identified as P. vivax infections by microscopy.
Table 1.
Details of P. vivax infected persons from Suan Oi village in Tak province, their date of sampling, age, sex, occupation and nationality.
| S.No. | Date of sampling (Month/Year) | Identification code (Family-Member) | Age (Years) | Sex | Occupation | Ethnicity/Nationality |
|---|---|---|---|---|---|---|
| 1 | 03/2013 | 64-5 | 4 | F | Child | Foreigner |
| 2 | 03/2013 | 64-6 | 1 | F | Child | |
| 3 | 08/2012 | 64-4 | 20 | M | Farmer | |
| 4 | 03/2013 | 15-3(1) | 16 | M | Student | Foreigner |
| 5 | 04/2012 | 15-7 | 4 | M | Child | |
| 6 | 04/2012 | 15-4 | 14 | M | Student | |
| 7 | 06/2013 | 15-3(2) | 16 | M | Student | |
| 8 | 03/2013 | 22-4 | 8 | M | Student | Foreigner |
| 9 | 05/2013 | 22-5 | 2 | F | Child | |
| 10 | 06/2013 | 86-5(1) | 1 | F | Child | Foreigner |
| 11 | 11/2012 | 86-5(2) | 1 | F | Child | |
| 12 | 04/2012 | 108-4 | 10 | M | Student | Foreigner |
| 13 | 04/2012 | 108-6 | 6 | F | Student | |
| 14 | 03/2013 | 74-4 | 30 | F | Farmer | Foreigner |
| 15 | 10/2012 | 76-6 | 7 | M | Student | Foreigner |
| 16 | 07/2012 | 83-7 | 3 | F | Child | Foreigner |
| 17 | 05/2013 | 89-2 | 36 | F | Farmer | Foreigner |
| 18 | 12/2012 | 36-6 | 4 | M | Child | Foreigner |
| 19 | 06/2013 | 45-5 | 10 | M | Student | Foreigner |
| 20 | 05/2013 | 41-2 | 49 | F | Farmer | Foreigner |
| 21 | 06/2013 | 58-3 | 23 | M | Farmer | Thai Citizen |
| 22 | 06/2013 | 68-4 | 5 | M | Child | Foreigner |
| 23 | 12/2012 | 9-4 | 7 | F | Student | Foreigner |
| 24 | 06/2013 | 14-2 | 19 | M | Farmer | Foreigner |
• The cases of recurrent infection are shown in bold.
• F- Female, M- Male
Of the total 24 P. vivax infections, two cases were probably recurrent as they occurred after three and five months of the first infections, respectively (Table 1). Twelve of the infected residents were males and ten were females, and they were grouped into three age groups: 0-5 years (children), 6-17 years (students) and 18 and above (adults) (Table1). Eight of the infections were detected each in the children and students groups, and six infections were observed among adults. All the adults were recorded as farmers who moved out of the village and crossed the international border frequently. Whereas age and sex were not correlated with the infections, 21 of the 22 infected persons were migrants or without Thai citizenship, indicating significant association of malaria infections cross-border migration. Infected individuals were from 16 families. Multiple members of the four families were found infected either at the same time or at different time points during the surveys (Table 1), leading to statistically significant clustering of the cases (Parker et al., 2015b).
3.2. Genetic diversity of the Pvmsp3α gene
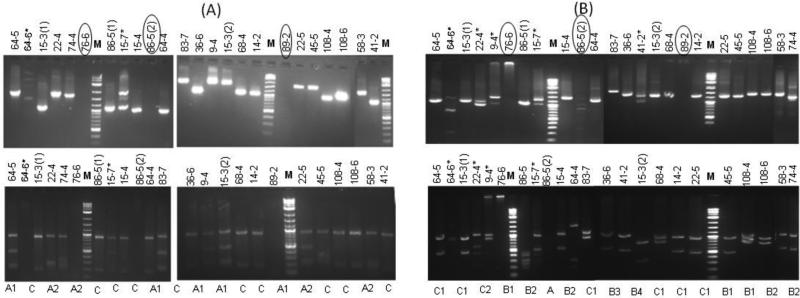
Pvmsp3α gene was successfully amplified in 21 of the 24 P. vivax isolates (Fig. 2A). Two PCR length variant types A (~1.9 Kb) and C (~1.1 Kb) were observed, whereas type B (1.5 Kb) was not observed in the tested samples (Cui et al., 2003) (Fig. 2A). Type A was observed in nine isolates, while type C was found in ten isolates. Two samples produced two bands, indicating mixed-strain infections (Fig. 2A). Pvmsp3α fragments from the 19 isolates were subjected to restriction digestion with HhaI. Totally three RFLP patterns (two in type A and one in C) were observed, and one ~950 bp fragment was present in all 20 isolates (Fig. 2A). In silico mapping of HhaI sites in Pvmsp3α of the Sal-I strain using RestrictionMapper (restrictionmapper.org) could not identify restriction site in the 1123 bp fragment from position 1912 to 3034, suggesting that this region may correspond to the ~950 bp fragment observed in all HhaI digested PCR products.
Fig. 2.
PCR-RFLP typing of the P vivax isolates using (A) Pvmsp3α and (B) Pvmsp3β gene. Upper panel, undigested PCR products Lower panel, Pvmsp3α PCR products digested with Hha I and Pvmsp3β with PstI. Lane M is 2 log DNA marker. Sample names on each lane showing house number and the member of the family and the numbers in the parenthesis are showing the P. vivax infections that occurred twice in the same individual during the study period. Mixed infections are shown with asterisk on the sample names and samples with no amplification are circled. RFLP-based genotype of each sample is also shown in lower panels.
Sequencing of single PCR bands from the 19 isolates generated fragments of 1072 - 1856 bp. The isolates with type C contained a ~750 bp deletion when aligned with type A isolates and the ~810 bp region from position 2009 to 2818 (corresponding to the Sal-I PVX_097720) was found conserved in all 19 isolates. A total of 35 single nucleotide polymorphisms (SNPs) were found among the 19 sequences, resulting in nine haplotypes with haplotype diversity of 0.77.
3.3. Genetic diversity of the Pvmsp3β gene
PCR amplification of Pvmsp3β was successful in 22 samples, of which five samples showed mixed-strain infections (Fig. 2B). The remaining 17 samples generated three genotypic variants based on the size of amplified fragments (Fig. 2B). As previously described in the isolates from Tak Province by Yang et al. (2006), type A (1.7-2.2 Kb) and type B (1.4-1.5 Kb) were observed in the present study, however, type C (0.65 Kb) was not found (Fig. 2B). We observed some fragments in the range of 1.0 to 1.3 Kb that we have named as type C in the present study (Fig. 2B). Type A was observed in one sample, type B in nine samples and type C was observed in seven samples (Fig. 2B). Restriction digestion of PCR amplified products with PstI generated seven banding patterns including one in A, and four in B and two in C types (Fig. 2B)
Seventeen isolates could be successfully sequenced ranging from 906 bp to 1866 bp. A large number of indels and polymorphic sites were observed in the Pvmsp3β sequences. However, the 752 bp in the N-terminal (143 to 894 bp) and 501 bp C-terminal portions (1890 to 2390 bp) were relatively less polymorphic. N-terminal region was obtained from 17 isolates, while C-terminal portion could be obtained from only 13 isolates. Thus, only N-terminal region was used for further analysis. Excluding gaps due to indels, a total of 175 SNPs were observed in the N-terminal that produced 13 haplotypes with haplotype diversity of 0.968.
3.4. Spread of P. vivax infections based on Pvmsp3α and Pvmsp3β genetic and phylogenetic relationships
A high level of diversity was observed among P. vivax samples. Among two cases of possible recurrent infections, distinct genotypes were observed in one case, while in the other case no amplification was observed at both Pvmsp3α and Pvmsp3β loci. Combining both Pvmsp3α and Pvmsp3β RFLP patterns, a total of 10 genotypes were observed among 17 samples. However, when the Pvmsp3α and 3β sequences were concatenated in the 17 isolates (excluding four samples with mixed-strain infections), 14 haplotypes were obtained, giving haplotype diversity of 0.97.
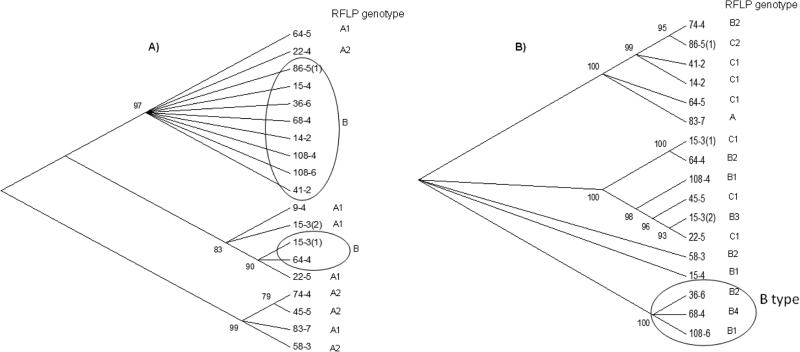
Phylogenetic analysis of the 19 Pvmsp3α sequences revealed three major clusters (Fig. 3A). Most of the infections detected in the same or the different members of the family were found in different clusters. Noticeably, the majority of the A and B RFLP-based subtypes formed separate groups (Fig. 2A). In contrast, the phylogenetic tree of Pvmsp3β showed even higher genetic diversity with multiple branches, and only three samples of the B subtypes were found clustered together in a group (Fig. 2B).
Fig. 3.
Phylogenetic relationships among the SO P. vivax isolates using (A) 19 sequences of Pvmsp3α and (B) 17 sequences of Pvmsp3β gene. Unrooted phylogenetic trees were constructed using neighbor-joining method in MEGA 6.0. Bootstrap support (>75%) is shown as the percentage from 500 pseudo replications. Corresponding PCR/RFLP genotype of each sample has been shown on both trees. The identical haplotypes sharing the same RFLP genotypes within a cluster are encircled.
We also constructed sequence relationship tree of 19 Pvmsp3α sequences with 158 published Pvmsp3α sequences from different geographical locations in Asia and America (18 from Thailand, 24 from Myanmar, 22 from South Korea, 17 from Sri Lanka, 4 from India, 33 from Peruvian Amazon, 21 from Venezuela, 6 from Brazil, 5 from Greece, Sal-I strain, Chesson strain, and one each from Pakistan, North Korea, Papua New Guinea, Bangladesh, Ong olé and Ecuador). All the sequences clustered into four distinct groups (Fig. S1). One group contained ten parasite samples from the present study along with the Chesson strain and one isolate from Thailand. Two other groups contained isolates from this study (SO) along with mixed strains from all the Asian and American countries, while the fourth group contained only South Korean isolates (Fig. S1). Nine SO isolates found in the first group were 100% identical to the Chesson strain. In the third group, one SO isolate showed 100% similarity with sequences from Myanmar and Thailand, while another SO isolate was identical to a Myanmar isolate.
Similarly, a Pvmsp3β phylogenetic tree was constructed using 17 sequences from the present study and 58 published sequences including 32 from Thailand, 4 from China, 2 India, 2 Pakistan, 8 Brazil, 2 Ecuador, Sal-I, Chesson, one each from Papua New Guinea, Bangladesh, North Korea, South Korea, Ong olé and Sri Lanka (Fig. S2). No clear clustering pattern was observed as the tree showed many branches. The majority of the SO samples were found clustered with Thailand, Brazil, South Korea isolates and the Chesson strain (Fig. 2B), while only one SO isolate was identical to a previously published isolate from Thailand.
4. Discussion
P. vivax is gaining increased attention due to its unique biology and changing epidemiology, which poses a big challenge to malaria control and elimination. In areas where both P. vivax and P. falciparum coexist, while P. falciparum has been found responsive to the control measures (especially artemisinin-based combination therapies), P. vivax incidences have significantly increased over the years (WHO, 2015b). A similar trend of increasing P. vivax/P. falciparum ratio has been observed in the Thai side of the Thai-Myanmar border in the past few years (Parker et al., 2015b). The high prevalence of P. vivax in this area might be the consequence of i) appearance of P. vivax resistance to chloroquine (Muhamad et al., 2011; Bhumiratana et al., 2013), ii) importation of infections from Myanmar, iii) the large number of asymptomatic cases that serve as the reservoir of transmission (Baum et al., 2015; Parker et al., 2015b), and iv) occurrence of relapsing cases. To develop effective P. vivax control measures that meet the goal of malaria elimination in this region, an in-depth understanding of genetic structure and transmission dynamics of these parasite species is highly needed. In the present study, we determined the genetic diversity of P. vivax isolates among asymptomatic infections in a malaria endemic village in Tak Province by genotyping two highly polymorphic merozoite surface protein genes Pvmsp3α and Pvmsp3β.
While earlier studies of P. vivax clinical isolates from acute cases in this region have identified highly diverse P. vivax populations (Cui et al., 2003), the genetic diversity of asymptomatic infections, especially at a microgeographic scale, has not been characterized. In a small border village of western Thailand, we found that P. vivax isolates were highly diverse, showing 14 distinct genotypes from 17 isolates with haplotype diversity of 0.97 (combining both Pvmsp3α and Pvmsp3β). This is comparable to the high level of diversity (haplotype diversity of 1.0) recently reported from a neighboring district of the same province, where samples were collected from acute malaria cases from a larger geographical area (Putaporntip et al., 2014). As found earlier, there is significant clustering of malaria cases in the village (Parker et al., 2015b), and there were families with members being infected at the same time or in different times. Despite this, the infections recorded from the same or different members of the family were distinct genotypes, further adding to the genetic diversity pool of the parasites. Since malaria infections were significantly associated with people with no citizenship suggesting of migrant status, it is likely that this high level of genetic diversity in the P. vivax isolates might be the consequence of frequent migration of the villagers who acquired infections outside of the village (Parker et al., 2015b). Whereas different parasite genotypes occurring in the same individuals indicated the result of new infections, that also could be due to relapses from heterologous hypnozoites of different genotypes (Chen et al., 2007; Imwong et al., 2007). In this study, at least 20% of the asymptomatic infections showed multiple-strain infections, similar to the previous observations from the Tak Province (Cui et al., 2003; Putaporntip et al., 2014). If these were infectious reservoirs sustaining local malaria transmission, they could generate recombinant genotypes as earlier reported in P. falciparum populations from Tanzania (Tanabe et al., 2007), thus further increasing genetic diversity of the parasite populations. Altogether, the high prevalence of asymptomatic infections and highly diverse parasite populations suggest a relative large effective population size of the parasites, which needs to be considered for the design and deployment of more effective control measures.
Both Pvmsp3α and Pvmsp3β used in this study are the most polymorphic genetic markers and PCR-RFLP technique based on these two genes have been widely used to identify multiple strains of natural P. vivax infections. However, according to a recent analysis, PCR-RFLP patterns of Pvmsp3α revealed less diversity as compared to that shown by sequencing (Rice et al., 2013). Similar observations were later observed in Pvmsp3β by different studies (Putaporntip et al., 2014; Li et al., 2015). This pattern has been further confirmed in this study, as we observed much higher diversity from the sequencing data as compared to RFLP patterns in both Pvmsp3α and Pvmsp3β genes. However, some of the identical haplotypes in both Pvmsp3α and Pvmsp3β had RFLP patterns belonging to the same genotypes (Figs. S1 & S2). Moreover, the majority of the isolates clustered together in the phylogenetic tree of Pvmsp3α shared common RFLP patterns, suggesting some levels of congruence between the two techniques. In corroboration with the earlier observations, Pvmsp3β was found more diverse with a large number of inserts and mutations (Rayner et al., 2004; Rungsihirunrat et al., 2011) as compared to Pvmsp3α. Moreover, a band size different from the ones previously reported for Pvmsp3b PCR amplification by Yang and coworkers (2006) was observed in this study, which may be related to the high transmission intensity in this region, or may have been generated from recombination events between asymptomatic, multiple clone infections. In addition to the higher level of genetic diversity in Pvmsp3β, this gene detected a higher number of mixed-strain infections than Pvmsp3α. The reasons for the higher diversity in Pvmsp3β are not known, which might be due to different selection pressures acting on these two genes (Gupta et al., 2015). However, these distinct observations between two genes suggested that using multiple loci for genetic diversity analysis may increase the resolution of genotypic characterization and eventually separate the common haplotype into two distinct haplotypes.
Comparison of the Pvmsp3α and Pvmsp3β sequences with the published sequences from different countries, the SO isolates were clustered with the isolates from different countries. This might be the consequence of global distribution of the genotypes due to a high level of gene flow between different geographical regions. Interestingly, some of the SO isolates showed 100% similarity with the previously published sequences especially from Thailand and Myanmar. This implies the impact of frequent human migrations across the border between Thailand and Myanmar on malaria epidemiology.
In conclusion, genotyping of asymptomatic P. vivax infections collected from a small village located at the Thai-Myanmar border showed markedly diverse parasite populations. This finding is highly encouraging, and such micro-geographically focused studies are needed to get an accurate estimate of the potential burden and the extent of genetic diversity of malaria parasites. The genetic diversity information obtained may also provide a valuable baseline to compare the extent and patterns of P. vivax diversity in later years, which could help assess the impact of ongoing malaria interventions on the P. vivax burden in that region. Therefore, continuous malaria genetic monitoring in the regions that are under malaria elimination phase should be an essential component of malaria control efforts.
Supplementary Material
Highlights.
Cross-sectional survey was conducted in a small village at Thai-Myanmar border
Asymptomatic P. vivax infections were genotyped using PvMSP3α and 3β genes
Data revealed highly diverse parasite populations at microgeographic scale
Genetic diversity among P. vivax isolates was evaluated at a microgeographic scale
Cross sectional surveys identified large number of asymptomatic P. vivax infections
PCR/RFLP and DNA sequence polymorphisms in Pvmsp3α and Pvmsp3β gene were evaluated
High level of genetic polymorphism was observed at both loci
Acknowledgements
This research was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19AI089672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Davies D, Jain A, Lo E, Lee M-C, Randall AZ, Molina DM, Liang X, Cui L, Felgner PL, Yan G. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand - molecular and serological evidence. Malar. J. 2015;14:95. doi: 10.1186/s12936-015-0611-9. doi:10.1186/s12936-015-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumiratana A, Intarapuk A, Sorosjinda-nunthawarasilp P, Maneekan P, Koyadun S. Border malaria associated with multidrug resistance on Thailand-Myanmar and Thailand-Cambodia borders : transmission dynamic , vulnerability , and surveillance. Biomed Res. Int. 2013;2013:13. doi: 10.1155/2013/363417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MC, Galinski MR, Barnwell JW, Snounou G, Day KP. Polymorphism at the merozoite surface protein-3alpha locus of Plasmodium vivax: global and local diversity. Am. J. Trop. Med. Hyg. 1999;61:518–525. doi: 10.4269/ajtmh.1999.61.518. [DOI] [PubMed] [Google Scholar]
- Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J. Infect. Dis. 2007;195:934–41. doi: 10.1086/512242. doi:10.1086/512242. [DOI] [PubMed] [Google Scholar]
- Cui L, Mascorri CN, Fan Q, Rzomp KA, Khuntirat B, Zhou G, Chen H, Yan G, Sattabongkot J. Genetic diversity and multiple infections of Plasmodium vivax malaria in Western Thailand. Am. J. Trop. Med. Hyg. 2003;68:613–619. doi: 10.4269/ajtmh.2003.68.613. [DOI] [PubMed] [Google Scholar]
- Cui L, Yan G, Sattabongkot J, Cao Y, Chen B. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. doi:10.1016/j.actatropica.2011.02.016. Malaria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Reddy BPN, Fan Q, Yan G, Sirichaisinthop J, Sattabongkot J, Escalante A. a, Cui L. Molecular evolution of PvMSP3α Block II in Plasmodium vivax from diverse geographic origins. PLoS One. 2015;10:e0135396. doi: 10.1371/journal.pone.0135396. doi:10.1371/journal.pone.0135396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann J, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NPJ, Anderson TJC, White NJ. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 2007;195:927–933. doi: 10.1086/512241. doi:10.1086/512241. [DOI] [PubMed] [Google Scholar]
- Li Y-C, Wang G, Meng F, Zeng W, He C, Hu X, Wang S. Genetic diversity of Plasmodium vivax population before elimination of malaria in Hainan province. Malar. J. 2015;14:78. doi: 10.1186/s12936-015-0545-2. doi:10.1186/s12936-015-0545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhamad P, Ruengweerayut R, Chacharoenkul W, Rungsihirunrat K, Na-Bangchang K. Monitoring of clinical efficacy and in vitro sensitivity of Plasmodium vivax to chloroquine in area along Thai Myanmar border during 2009-2010. Malar. J. 2011;10:44. doi: 10.1186/1475-2875-10-44. doi:10.1186/1475-2875-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DM, Carrara VI, Pukrittayakamee S, McGready R, Nosten FH. Malaria ecology along the Thailand–Myanmar border. Malar. J. 2015a;14:388. doi: 10.1186/s12936-015-0921-y. doi:10.1186/s12936-015-0921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DM, Matthews SA, Yan G, Zhou G, Lee M-C, Sirichaisinthop J, Kiattibutr K, Fan Q, Li P, Sattabongkot J, Cui L. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar. J. 2015b;14:198. doi: 10.1186/s12936-015-0712-5. doi:10.1186/s12936-015-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaporntip C, Miao J, Kuamsab N, Sattabongkot J, Sirichaisinthop J, Jongwutiwes S, Cui L. The Plasmodium vivax merozoite surface protein 3β sequence reveals contrasting parasite populations in Southern and Northwestern Thailand. PLoS Negl. Trop. Dis. 2014;8:e3336. doi: 10.1371/journal.pntd.0003336. doi:10.1371/journal.pntd.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Huber CS, Feldman D, Ingravallo P, Galinski MR, Barnwell JW. Plasmodium vivax merozoite surface protein PvMSP-3β is radically polymorphic through mutation and large insertions and deletions. Infect. Genet. Evol. 2004;4:309–319. doi: 10.1016/j.meegid.2004.03.003. doi:10.1016/j.meegid.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Rice BL, Acosta MM, Pacheco MA, Escalante A. a. Merozoite surface protein-3 alpha as a genetic marker for epidemiologic studies in Plasmodium vivax: a cautionary note. Malar. J. 2013;12:288. doi: 10.1186/1475-2875-12-288. doi:10.1186/1475-2875-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungsihirunrat K, Chaijaroenkul W, Siripoon N, Seugorn A, Na-Bangchang K. Genotyping of polymorphic marker (MSP3α and MSP3β) genes of Plasmodium vivax field isolates from malaria endemic of Thailand. Trop. Med. Int. Heal. 2011;16:794–801. doi: 10.1111/j.1365-3156.2011.02771.x. doi:10.1111/j.1365-3156.2011.02771.x. [DOI] [PubMed] [Google Scholar]
- Snounou G, Viriyakosol S, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. doi:10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- Sonkong K, Chaiklieng S, Neave P, Suggaravetsiri P. Factors affecting delay in seeking treatment among malaria patients along Thailand-Myanmar border in Tak Province, Thailand. Malar. J. 2015;14:1–8. doi: 10.1186/1475-2875-14-3. doi:10.1186/1475-2875-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski a., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. doi:10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Sakihama N, Rooth I, Björkman A, Färnert A. High frequency of recombination-driven allelic diversity and temporal variation of Plasmodium falciparum msp1 in Tanzania. Am. J. Trop. Med. Hyg. 2007;76:1037–45. [PubMed] [Google Scholar]
- Wongsrichanalai C, Sirichaisinthop J, Karwacki JJ, Congpuong K, Miller RS, Pang L, Thimasarn K. Drug resistant malaria on the Thai-Myanmar and Thai-Cambodian borders. Southeast Asian J. Trop. Med. Public Health. 2001;32:41–49. [PubMed] [Google Scholar]
- World Health Organization World Malaria Report. 2015a [Google Scholar]
- World Health Organization . Control and elimination of Plasmodium vivax malaria: a technical brief. World Health Organization; 2015b. [Google Scholar]
- Yang Z, Miao J, Huang Y, Li X, Putaporntip C, Jongwutiwes S, Gao Q, Udomsangpetch R, Sattabongkot J, Cui L. Genetic structures of geographically distinct Plasmodium vivax populations assessed by PCR/RFLP analysis of the merozoite surface protein 3α gene. Acta Trop. 2006;100:205–212. doi: 10.1016/j.actatropica.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.