Abstract
Food allergy is a pathological, potentially deadly, immune reaction triggered by normally innocuous food protein antigens. The prevalence of food allergies is rising and the standard of care is not optimal, consisting of food-allergen avoidance and treatment of allergen-induced systemic reactions with adrenaline. Thus, accurate diagnosis, prevention and treatment are pressing needs, research into which has been catalysed by technological advances that are enabling a mechanistic understanding of food allergy at the cellular and molecular levels. We discuss the diagnosis and treatment of IgE-mediated food allergy in the context of the immune mechanisms associated with healthy tolerance to common foods, the inflammatory response underlying most food allergies, and immunotherapy-induced desensitization. We highlight promising research advances, therapeutic innovations and the challenges that remain.
The atopic diseases tend to occur in a progression termed the atopic march1–3, in which the initial manifestation of atopic disease in infancy or early childhood is often atopic dermatitis, followed by the staggered development of food allergy, allergic rhinitis and allergic asthma. Food allergy is a pathological reaction of the immune system triggered by the ingestion of a food protein antigen. Exposure to very small amounts of allergenic foods can trigger clinical symptoms such as gastrointestinal disorders, urticaria and airway inflammation, ranging in severity from mild to life-threatening. Food allergy is distinct from food intolerance in that intolerance does not arise from immune system dysregulation; for example, lactose intolerance arises from non-immune factors, such as lactose malabsorption and lactase deficiency.
Food allergy is both common and costly. In a recently published epidemiological study defining a retrospective cross-sectional cohort of 333,200 children in the United States, food allergy prevalence was found to be 6.7%4. A global survey of food allergy in children found that only 9 of the 89 countries surveyed had accurate prevalence data determined by oral food challenge (OFC), 7 of which reported a prevalence ranging from 0.45% to 10% among children less than 5 years old; the remaining 28 countries that had food allergy prevalence data relied on methods such as self-reporting, which is known to overestimate food allergy prevalence5. Annually, food allergy results in costs to the US healthcare system of approximately $24.8 billion6. In addition, the global prevalence of all food allergy seems to be increasing7. Between 1997 and 2007, the self-reported prevalence of food allergy in children under 18 years old in the United States increased by 18% to an estimated prevalence of 3.9%8, and prevalence had reached ~5% by 2011 (REF. 9). Another study of 38,480 children under 18 years of age in the United States yielded an estimated food allergy prevalence of 8%, as well as the findings that approximately 40% of patients with food allergy have experienced a life-threatening allergic reaction, and 30% of children with food allergy have multiple food allergies10. Increases in the numbers of food allergy anaphylaxis-related hospital admissions and fatalities may reflect this apparent increase in food allergy prevalence, although other factors (such as increased recognition of anaphylaxis, increased severity of food allergy or increased exposure of allergic individuals to food allergens) may also contribute. Between 1998 and 2012, the number of food allergy anaphylaxis-related hospital admissions increased by 137% in children 14 years and younger in England and Wales without an increase in the number of fatalities11. Between 1997 and 2013, the number of food allergy anaphylaxis-related deaths increased by an average of 9.7% per year in Australia12. The apparent increase in food allergy prevalence over a short time period suggests that environmental factors have a role in its aetiology. Although the mechanisms by which environmental factors could promote food allergy are not well understood, recent findings indicate that environmental exposures may interfere with the normal ability of the immune system to promote tolerance to food allergens13–15.
In light of the prevalence and costs of food allergy, effective methods of prevention and treatment would be of substantial clinical value. The current standard of care is food-allergen avoidance and the treatment of food allergen-induced systemic reactions with adrenaline16. Recent advances in food allergy research have led to the development of new therapies, many of which are currently in clinical trials. This Review presents a summary of food allergy classification and mechanisms of disease, diagnostic methods, strategies for prevention and treatment, and future directions in food allergy research.
Classification of food allergies
Food allergies are atopic disorders, which can be broadly classified into those that are IgE mediated, those that are mediated by both IgE-dependent and IgE-independent pathways (mixed), and those that are not IgE mediated (TABLE 1). Food allergies are mechanistically distinct from non-atopic disorders, such as coeliac disease.
Table 1.
Classification of food allergies
| Subtype | Prevalence | Age group affected |
Common allergens | Usual symptoms | Diagnosis | Treatment |
|---|---|---|---|---|---|---|
| IgE-mediated food allergy | ||||||
| No subtype | 0.4–10% | Children > adults | Milk, egg, wheat, soy, peanut, tree nuts, shellfish and fish | Pruritus, urticaria, angioedema, abdominal pain, vomiting, wheezing and hypotension | sIgE levels, SPT and OFC | • Standard: food-allergen avoidance and emergency medication • Research: OIT, SLIT, EPIT and omalizumab |
| Mixed IgE- and cell-mediated food allergy | ||||||
| Food-allergy-associated atopic dermatitis | 27–37% of patients with atopic dermatitis183; 14–27% of patients with self-reported atopic dermatitis184 |
Children > adults | Milk, egg, wheat, soy, peanut, tree nuts, shellfish and fish | Exacerbation of dermatitis with allergen ingestion (as an addition to typical IgE-mediated food allergy symptoms) | sIgE levels, SPT and OFC | • Standard: food-allergen avoidance • Research: OIT, SLIT, EPIT and omalizumab |
| EoE | Up to ~50 patients per 100,000 (REF. 185) | Children and adults (3:1 male to female ratio)185 | Milk, wheat, egg, beef, soy and chicken186 | Vomiting, failure to thrive, dysphagia, food impaction and heartburn | Oesophageal biopsies showing eosinophil infiltrates after a 2–3 month course of protein pump inhibitors to exclude gastro-oesophageal reflux disease as a cause | Standard: topical steroids (in the oesophagus) or food-allergen avoidance |
| Other eosinophilic gastrointestinal disorders (EC, EG or EGE) | Rare187 | • EC: infants • EG: adults > children • EGE: adults |
• EC: milk and soy • EG: possibly milk, wheat, soy, egg, nuts, seafood and red meats • EGE: may not have food allergy aetiology |
Manifestations vary with affected gastrointestinal tract region and layer (mucosal, muscular or serosal) | Increased numbers of eosinophils seen in gastrointestinal biopsy; eosinophils in ascites if serosal layer affected; other, more common, causes of eosinophilia must be ruled out | Standard: steroids for EC and EG; avoidance of specific foods |
| Non-IgE-mediated food allergy32 | ||||||
| FPIES | Few data: one study reports 0.34% of infants with FPIES to cow's milk188 | Infants and children | Milk, soy, rice, oat and egg | • Intermittent allergen exposure: severe vomiting • Chronic allergen exposure: diarrhoea and failure to thrive |
Food-allergen avoidance and food challenge | Standard: food-allergen avoidance |
| FPIP | Few data: one study reports 0.16% of infants with potential FPIP to cow's milk189 | Infants | Milk, soy, wheat and egg | Rectal bleeding | Food-allergen avoidance and food challenge | Standard: food-allergen avoidance |
| FPE | Few data | Infants and toddlers | Milk, soy, wheat and egg | Steatorrhoea from malabsorption, diarrhoea and failure to thrive | Food-allergen avoidance and food challenge together with jejunal biopsy showing villous atrophy and crypt hyperplasia | Standard: food-allergen avoidance |
EC, eosinophilic colitis; EG, eosinophilic gastritis; EGE, eosinophilic gastroenteritis; EoE, eosinophilic oesophagitis; EPIT, epicutaneous immunotherapy; FPE, food protein enteropathy; FPIES, food protein-induced enterocolitis syndrome; FPIP, food protein-induced proctocolitis; OFC, oral food challenge; OIT, oral immunotherapy; sIgE, allergen-specific IgE; SLIT, sublingual immunotherapy; SPT, skin prick test.
IgE-mediated food allergies
This class of food allergy is associated with the risk of severe or fatal reactions; accordingly, it is the most fully characterized type of food allergy and is the main focus of this Review. Whereas IgE-mediated allergies to cow's milk, egg, wheat and soy tend to be outgrown, atopic responses to peanuts, tree nuts and shellfish usually persist into adulthood17. The most common IgE-mediated food allergies vary somewhat by region and with dietary habits; for example, children in Ghana between 5 and 16 years of age are commonly allergic to pineapple, pawpaw, orange and mango, as well as to peanut18, whereas children in North America are commonly allergic to peanut, milk, egg, shellfish and soy4. The most common food allergen in Asia is shellfish; in most Asian countries wheat allergy is uncommon, but in Japan and Korea it is the leading cause of anaphylaxis19. In Singapore, allergy to bird's nest was the leading cause of anaphylaxis in children more than 15 years ago, whereas peanut allergy is currently the most common type of food allergy19.
In food allergen-sensitized individuals — those who have been exposed to the allergen and had an initial immune response — subsequent exposure to the food allergen triggers IgE-mediated degranulation of immune effector cells, such as mast cells and basophils, resulting in the rapid manifestation of symptoms. Food allergen-derived epitopes attach to IgE molecules bound to FcεRI receptors on the surface of these effector cells; epitope-specific crosslinking of IgE-bound receptors then occurs, leading to the release of preformed histamine and other inflammatory mediators of the immediate allergic reaction20. After this immediate phase of the response, the de novo production of leukotrienes, platelet activating factor and cytokines such as interleukin-4 (IL-4), IL-5 and IL-13 maintains allergic inflammation20. Gastrointestinal manifestations can include oral tingling, pruritus and/or swelling, as well as nausea, abdominal pain and/or vomiting. Respiratory effects include wheezing and/or airway inflammation. Skin manifestations include flushing, urticaria, angioedema and/or pruritus. Systemic responses may also occur, such as hypotension due to fluid leakage from the vasculature and/or hypothermia. Anaphylaxis is a serious allergic reaction that involves multiple organ systems and can rapidly become life-threatening21.
Variants of IgE-mediated food allergy include oral allergy syndrome (OAS), in which individuals with allergic rhinitis produce IgE molecules specific for pollen-derived epitopes that are crossreactive with fruit- or vegetable-protein epitopes22. Signs and symptoms of OAS include immediate oral pruritus, mucosal angioedema and/or abdominal pain. As the cross reactive IgE antibodies may be specific for a heat-labile food epitope, uncooked fruits or vegetables should be used in diagnostic testing for OAS. In the context of peanut allergy, testing for IgE antibodies specific for particular components of peanut protein (IgE component testing) can be used to distinguish OAS from standard IgE-mediated allergy: in individuals with birch-pollen allergy, IgE antibodies specific for the pollen antigen Bet v 1 may crossreact with the peanut component Ara h 8 to cause OAS23, but reactivity against Ara h 8 is also associated with a decreased risk of clinically relevant IgE-mediated peanut allergy; by contrast, reactivity against the peanut components Ara h 1 or Ara h 2 is more likely to be associated with clinically relevant IgE-mediated peanut allergy24. IgE- and T cell-mediated crossreactivities have also been observed between Bet v 1 and the hazelnut allergen Cor a 1 (REF. 25).
Another variant of IgE-mediated food allergy occurs in individuals who produce IgE antibodies that are specific for the red meat carbohydrate galactose-α-1,3-galactose, rather than specific for a protein epitope26. As patients with this type of food allergy have usually consumed red meat uneventfully for many years, it has been proposed that sensitivity is initiated through tick bites, via exposure to antigens transmitted in the saliva of ticks that have fed on mammals26. Although these reactions can be severe, they are unlike other IgE-mediated allergic reactions to food in that symptom onset is delayed by 4–6 hours or longer26,27.
Mixed food allergies
This class of food allergy is characterized by both IgE-dependent and IgE-independent pathways. Atopic manifestations arising from IgE-independent factors include delayed food-allergy-associated atopic dermatitis (6–48 hours post exposure28) caused by the action of T helper 2 (TH2) cells29, and eosinophilic gastrointestinal disorders, such as eosinophilic oesophagitis (EoE), which are often triggered by milk allergens and caused by the eosinophilic infiltration of tissues30,31. Further research is underway to discern the possible role of food allergies in these conditions and to assess the relative contributions of IgE-dependent and -independent pathways in individuals with these disorders.
Non-IgE-mediated food allergies
Most of the known non-IgE-mediated food allergies primarily affect the gastrointestinal tract, rather than the skin and respiratory tracts. For example, allergen-specific T cells are thought to have roles in the largely unknown aetiologies of food protein-induced enterocolitis syndrome (FPIES), food protein-induced proctocolitis (FPIP) and food protein enteropathy (FPE)32. FPIES, FPIP and FPE primarily involve infants and toddlers who are allergic to cow's milk, and they commonly resolve after 1 to 5 years32. Their prevalence is uncertain owing to a lack of diagnostic tests, and treatment consists of food-allergen avoidance.
Immune mechanisms
For overall health, it is essential that the immune system distinguishes pathogenic antigens from innocuous environmental antigens. Accordingly, a state of unresponsiveness to common food antigens occurs in non-food-allergic (in other words, healthy or immune-tolerant) individuals. In individuals with food allergy, sensitization to food allergens results in inappropriate inflammatory immune responses to common foods. Many clinical trials in food allergy aim to establish functional immune tolerance in place of this sensitization. This is defined by clinical endpoints in a clinical trial after a period of withdrawal from therapy, otherwise known as sustained unresponsiveness. These studies involve food allergen immunotherapy, in which an individual with food allergy is exposed to initially small but gradually increasing amounts of allergenic food proteins over many months, so that the immune system slowly adjusts to the exposure, and the probability of an allergic reaction decreases. Immunotherapy often results in desensitization — a temporary increase in the threshold for allergen reactivity — instead of durable sustained unresponsiveness. The mechanisms of desensitization are not well understood and may differ from those of the healthy state of immune tolerance. Because it is unknown whether a patient with food allergy who has been desensitized through immunotherapy will remain permanently unresponsive to that food allergen, we refer to sustained unresponsiveness as distinct from the healthy, pre-allergic state of immune tolerance.
The deduction of disease mechanisms lies at the fore-front of food allergy research. Technological advances, such as high-throughput instruments for CyTOF (cytometry by time of flight mass spectrometry) and single-cell quantitative PCR, that are capable of quantifying multiple parameters simultaneously have converged with rapid advances in big data analytics to enable unprecedented discoveries in the signalling pathways underlying immune tolerance, sensitization and desensitization to food allergens. This section examines our current understanding of the mechanisms involved in the progression of food allergy from health (tolerance), to disease (sensitization), to treatment (desensitization), focusing mainly on research in humans but drawing from research in mouse models where relevant.
Tolerance
The establishment of immune tolerance to orally administered antigens is a subject of intensive study33 (FIG. 1). The mechanisms have been covered in detail elsewhere34, and only the main points are highlighted here. The healthy immune system is tolerant to foods, characterized by a sustained (that is, memory) unresponsiveness towards potential food allergens even in the absence of regular allergen exposure. Immune tolerance is an active process that begins with the uptake of potential food antigens in the small intestine, which contains much of the gut-associated lymphoid tissue (GALT)35. Diverse cell types collaborate in the series of events that maintains immune tolerance, involving transmission of antigen from the gut lumen to the lamina propria, transfer of antigen to the lymphoid tissue, antigen presentation and induction of a T cell response in the lymphoid tissue, and subsequent return of immune effector cells to the gut.
Figure 1. Immune tolerance to oral antigens in the gut.
CX3CR1+ cells (most likely to be macrophages) extend dendrites between the intestinal epithelial cells, sample antigens in the gut lumen59,162 and transfer captured antigens via gap junctions to CD103+ dendritic cells (DCs). A subset of these DCs migrates from the lamina propria to the draining lymph nodes where the DCs express transforming growth factor-β (TGFβ) and retinoic acid, thereby inducing naive T cells to differentiate into regulatory T (Treg) cells44,46. Macrophages also seem to secrete interleukin-10 (IL-10), leading to Treg cell proliferation43,59,162; however, this is debated163. Several types of regulatory T cell (resting, effector, and memory164) have been reported to be associated with mucosal tolerance, including induced forkhead box P3 (FOXP3)+ Treg cells, IL-10-secreting Tr1 cells and TGFβ-secreting T helper 3 (TH3) cells165. Retinoic acid also induces Treg cell expression of integrin α4β7, which results in homing to the gut where Treg cells may dampen the immune response49,50,56. CD103+ DCs also sample antigens that pass through the epithelial barrier via M cell-mediated transcytosis or through translocation by mucin-secreting goblet cells; under some circumstances, CD103+ DCs may capture antigens from the lumen directly, via periscoping behaviour (extending a process through a tight junction) or by extending a process through a transcellular pore in an M cell38. B cell clones expressing antibody specific for food allergen may undergo isotype switching in the secondary lymphoid organs with the aid of follicular T helper (TFH) cells. Food tolerance and allergen desensitization are associated with IgA (FIG. 1) and IgG4 (FIG. 3), respectively86,166. By contrast, food allergen-specific IgE (FIG. 2) will be bound by FcεRI on mast cells (which are normally found in tissues forming environmental barriers167) and basophils, thus leading to immediate hypersensitivity reactions to food. High-dose exposure to oral antigens has been reported to lead to the anergy or deletion of antigen-specific T cells, possibly after DC interaction168. TFH cells secreting different cytokine combinations favour B cell switch recombination to produce particular antibody isotypes, whereas follicular Treg cells suppress the germinal centre reaction169,170. The roles of tissue-resident T cells, CD8+ T cells and γδ T cells remain to be determined. The relationship between TFH cells and the conventional TH cell subsets is not clear, and conversion between the two has been reported171,172. CTLA4, cytotoxic T lymphocyte antigen 4.
Potential food antigens can be captured from the gut lumen by passing between (paracytosis) or through (transcytosis) epithelial cells35–37. Specialized cells, such as the M (microfold) cells that overlie the GALT, can endocytose antigen38, and myeloid cells such as dendritic cells (DCs) and/or macrophages may capture potential food antigens from the gut lumen39,40. For example, CD103+ DCs sample antigens that pass through the epithelial barrier via M cell-mediated transcytosis or through translocation by mucin-secreting goblet cells; under some circumstances, CD103+ DCs may capture antigens directly from the lumen via periscoping behaviour (extending a process through a tight junction) or by extending a process through a transcellular pore in an M cell38. An estimated 2% of proteins pass through the gut epithelium intact and could be transported directly or indirectly to the liver or to secondary lymphoid tissues for antigen presentation by other cell types41,42.
CX3C-chemokine receptor 1 (CX3CR1)+ cells (probably macrophages) extend dendrites between the epithelial cells, sample antigens in the gut lumen and may promote tolerance through IL-10 secretion43. These CX3CR1+ cells also seem to transfer captured antigens via gap junctions to CD103+ DCs, a subset of which migrates from the lamina propria to the draining lymph nodes44. Here, the CD103+ DCs produce transforming growth factor-β (TGFβ) and retinoic acid, thereby inducing naive T cells to differentiate into antigen-specific forkhead box P3 (FOXP3)+ regulatory T (Treg) cells35,44–48. CD103+ DCs may also induce the differentiation of naive CD4+ T cells into FOXP3−, IL-10-secreting T cells, possibly type 1 regulatory T cells (Tr1 cells)35,49,50.
The cell-mediated trafficking of antigen to the secondary lymphoid tissue promotes the establishment of tolerance: mice deficient in CCR7, which is required for DC trafficking to secondary lymphoid tissue, have impaired oral tolerance51. The relative importance of the mesenteric lymph nodes, Peyer's patches and other types of secondary lymphoid tissue remains unclear52–54, although many reports have suggested that the mesenteric lymph nodes are a major destination of migrating DCs52,53. This hypothesis is supported by a mouse model showing that the absence of mesenteric lymph nodes impairs oral tolerance53. CD103+ DCs are thought to be one of the major DC subsets responsible for the development of mucosal tolerance35. In addition, CCR9+ DCs have been identified in lymphoid tissues and have been shown to induce Treg cell function, thereby suppressing antigen-specific immune responses55.
Retinoic acid, produced by CD103+ DCs, also induces Treg cell expression of integrin α4β7, which results in the homing of Treg cells to the gut where they may dampen the immune response49,50,56. In mouse models, the retinoic acid-induced expression of integrin α4β7 by T cells is required for the development of T cell-mediated oral tolerance57. Peripherally induced regulatory T cells return to the lamina propria, where they maintain their regulatory phenotype in response to IL-10 secretion by CX3CR1+ cells58,59. The presence of a large number of regulatory T cells in the gut (including FOXP3+ Treg cells and Tr1 cells) depends upon the availability of food antigens60.
Further evidence supporting the role of Treg cells in oral tolerance is provided by human clinical studies reporting hypomethylation (and thus increased accessibility for transcription) of the FOXP3 locus of Treg cells in those patients who develop and maintain functional tolerance in response to oral immunotherapy (OIT)61. Also, the IL-4 receptor (IL-4R) has been implicated in food allergy pathogenesis, and IL-4R inhibition may augment Treg cell induction and the enforcement of tolerance, as shown in mouse models62.
Normal immune tolerance is not well understood. A study comparing the responses of CD4+ T cells to the birch pollen allergen Bet v 1 in healthy and allergic individuals found that allergen-specific CD4+ T cells from healthy individuals secrete IFNγ and IL-10, in contrast to those from patients with allergy: tolerance to the allergen was associated with the expansion of these protective T cells63. Although food antigen-specific lymphocytes are very rare in the peripheral blood, a flow cytometry study compared peanut-specific cells among children who are allergic to peanuts, children with no peanut allergy, and those who have naturally outgrown a peanut allergy. Cells from peanut-allergic individuals have a TH2-polarized cytokine production, whereas those from the non-allergic and no-longer allergic groups have a TH1-type cytokine response to peanut antigens64.
Sensitization
DC-mediated tolerance to food antigens can be reprogrammed by inflammatory stimuli to give rise to food-allergic immune responses mediated by TH2 cells, as well as by additional cell types (BOX 1). Because several excellent reviews65–67 address the immune mechanisms of sensitization in detail, we touch only upon research concerning a pivotal step in the postulated atopic march from cutaneous sensitization to food allergy. A current model of the induction of a TH2 cell-mediated inflammatory immune response in the gut is given in FIG. 2.
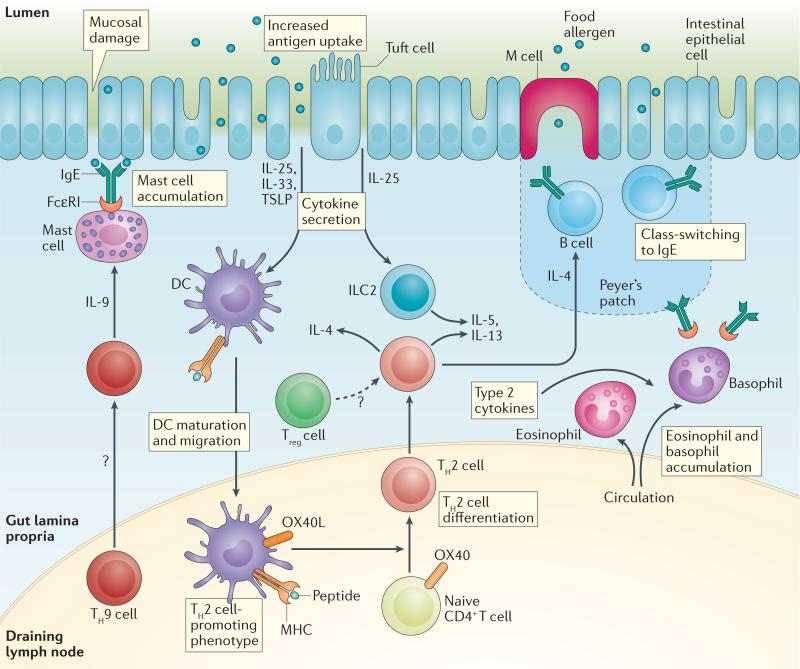
Figure 2. TH2 cell-mediated inflammatory response to oral antigen in the gut.
Epithelial damage or inflammation (for example, due to toxin exposure or trauma) in the gut, skin (not shown) or airways (not shown) allows increased antigen entry and promotes the secretion of the epithelium-derived cytokines interleukin-25 (IL-25), IL-33 and thymic stromal lymphopoietin (TSLP)157. These mediators ‘set’ the immune system towards a T helper 2 (TH2) cell response, and it is thought that the initial sensitization to food allergens often takes place at the skin70,120. In particular, TSLP may promote dendritic cell (DC) differentiation into a TH2 cell-promoting phenotype157,173. For example, OX40L may be upregulated in DCs that promote TH2 cell differentiation of naive CD4+ T cells173. IL-25 secretion by epithelial tuft cells also may aid the expansion of type 2 innate lymphoid cell (ILC2) populations174, which together with TH2 cells secrete cytokines that promote the TH2 cell-mediated immune response175, which includes tissue eosinophil accumulation and IgE class-switching by B cells20. TH9 cells also contribute to the allergic immune response by increasing tissue mast cell accumulation176, and IL-4-mediated signalling may convert regulatory T cells into TH2 cells62. The roles of follicular T cells, tissue-resident T cells, CD8+ T cells and γδ T cells remain to be determined. Treg cell, regulatory T cell.
Cutaneous exposure to food allergens may promote sensitization. Inflammatory cytokines released by the skin epithelium, including IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), act on DCs and other cells to deviate the immune response towards TH2 cell-related allergic responses, rather than tolerogenic responses68. For example, the cutaneous sensitization of mice with ovalbumin (OVA) and TSLP, followed by repeated oral challenge with OVA, results in IL-25-dependent diarrhoea and anaphylaxis69. Also, repeated application of peanut protein extract to undamaged skin in mice leads to a TH2 type immune response through IL-33 induction70, and mechanical injury to the skin results in cutaneous TSLP expression and the elicitation of an inflammatory TH2 cell-mediated response71. The mechanisms by which a TH2 cell-mediated response of cutaneous origin can reach the gut are unclear. One possibility is that DCs in the skin may secrete retinoic acid, which induces allergen-specific T cells to express gut-homing markers as they differentiate down the TH2 cell pathway72.
Desensitization
The immune changes that accompany food allergen immunotherapy are thought to occur as a progression of several elements73–75 (FIG. 3). The mechanisms by which mast cell and basophil responsiveness to degranulation are decreased early during immunotherapy are under investigation76. Other changes to the immune system observed after immunotherapy include increased numbers of anergic antigen-specific T cells77, apoptotic antigen-specific T cells78–81 and FOXP3+ Treg cells80,82, as well as increased levels of TGFβ1 (REFS 77,83). For example, over the course of OIT, antigen-specific CD4+ T cells were found to transition from allergic and regulatory phenotypes to anergic and non-allergic phenotypes, in association with decreased allergic symptoms and the development of sustained unresponsiveness77. Allergen-specific immunotherapy has also been associated with the preferential deletion of allergen-specific TH2 cells, without significant concomitant changes in the frequencies of TH1 and Tr1 cells78.
Figure 3. Desensitization to oral antigens in the gut.
Differences between the immune responses associated with desensitization and tolerance are under active investigation. Initially during desensitization, mast cell and basophil activation are decreased through an unclear mechanism, and a shift occurs from the predominance of T helper 2 (TH2) cells177 to that of allergen-specific Treg cells77, which in turn may lead to the observed shift in allergen-specific antibodies from the IgE to the IgG4 isotype86,178. As has been proposed in immunotherapy for venom allergies, IgG4 antibodies may compete with IgE antibodies for food allergens, further dampening the TH2 cell-mediated immune response179,180. The source of these IgG4 antibodies may be regulatory B cells, which also promote immune tolerance through the secretion of IL-10 (REF. 90). The proposed mechanism of dendritic cell (DC) inhibition by regulatory T (Treg) cells is possibly mediated via cytotoxic T lymphocyte antigen 4 (CTLA4) and lymphocyte activation gene 3 (LAG3)181. Treg cells inhibit mast cells via contact involving OX40–OX40L interaction182. The roles of follicular T cells, tissue-resident T cells, CD8+ T cells and γδ T cells remain to be determined.
In summary, Treg cells are likely to be a part of, but not unique to, the mechanisms involved in immune tolerance. These observations are consistent with a model involving a shift from allergen-specific TH2 cells to another cell subset as the key event in sustained unresponsiveness. This shift reduces the inflammatory response of tissues that encounter food allergen, thereby mitigating the recruitment and/or activation of innate immune cells such as mast cells and basophils84. Recently published data in mouse models show some similarities and differences among the mechanisms of desensitization induced by OIT, sublingual immunotherapy (SLIT) and epicutaneous immunotherapy (EPIT), which seem to induce different subsets of regulatory T cells85.
Other changes observed over the course of OIT include those in allergen-specific IgE levels, which decrease, whereas allergen-specific IgG4 levels increase86. Although levels of all IgG subclasses rise during OIT and may contribute differentially to the suppression of IgE-mediated responses through binding FcγRIIb, the increase in the ratio of IgG4 to IgE is particularly notable, and IgG4 has become an important research focus87. In tests of basophil activation, sera from peanut-sensitive patients drawn at week 52 of OIT were found to suppress the responsiveness to peanut antigens of basophils from normal, non-peanut-allergic donors that had been activated with allergic sera; when IgG was removed from these sera, their suppressive effect was mitigated, and this suppressive effect was restored when the IgG fractions were restored87. In tests of mast cell activation, plasma from peanut-sensitive patients with detectable peanut-specific IgG4 was found to inhibit mast cell activation, which was restored when the plasma was depleted of IgG4 (REF. 88). Other findings suggest that food allergen OIT can stimulate the somatic mutation of allergen-specific IgG4 (REF. 89).
Furthermore, an increase in the number of regulatory B cells (Breg cells) that secrete IL-10 and IgG4 specific for bee venom phospholipase A2 has been observed in individuals undergoing treatment for bee-sting allergy, which indicates that Breg cells may have an important role in immunotherapy90. In addition, immunotherapy-related increases in IgA2 levels have been observed in a study of children with egg allergy, who had lower IgA2 levels than did control individuals at baseline91. Successful OIT has resulted in desensitization over a period of months to a year, and in some cases has succeeded in establishing sustained unresponsiveness to food allergens77.
Diagnostic tests for food allergies
Clinical history and physical examination are the first-line approach to diagnosing a food allergy, which should address specific risk factors such as atopic dermatitis and a family history of atopic disease16. When IgE-mediated food allergy is suspected, measurements of total and allergen-specific serum IgE (sIgE) levels and skin prick tests (SPTs) are recommended to identify the causative food, although they are not in themselves diagnostic16. Increased sIgE levels may suggest food allergy, and sIgE thresholds with a 95% positive predictive value have been determined for some food allergens; for example, in peanut-allergic patients, the threshold sIgE level for peanut antigen is 14 kUA/L (UA = allergen-specific units; 1 unit ≈ 2.4 ng IgE)17,92,93. SPTs provoke allergen-mediated mast cell degranulation in the skin, leading to wheal-and-flare reactions. A lancet coated with allergen extract is used to puncture the epithelial barrier to elicit a skin reaction; if a wheal forms, it is measured after 15 minutes. Wheal size thresholds indicating a high probability of food allergy have been defined for some common foods; for example, a peanut SPT resulting in a wheal of ≥8 mm diameter has a 95% positive predictive value94.
However, sIgE levels and SPTs indicate allergen sensitization only (which is necessary, but not sufficient, for food allergy), rather than clinically significant food allergy, and both tests are associated with a high rate of false positives, due in part to crossreactivity between homologous proteins95. False-positive SPTs may also arise from differences in the manner in which the skin and intestinal epithelia encounter food proteins: the skin epithelium encounters intact protein molecules in a SPT, whereas the intestinal epithelium also encounters digested proteins, which may have altered capacities to elicit immune responses.
The only definitive diagnostic method for food allergy is the OFC, in which increasing doses of a potential food allergen are ingested over fixed time intervals, until either an allergic reaction is observed or a maximum dose is reached17. However, this diagnostic method is infrequently used outside properly equipped and staffed specialist centres because it is resource-intensive and carries the risk of inducing a severe anaphylactic reaction. Ideally, a clinical setting in which food allergy SPT and sIgE measurements are carried out should have a referral system in place for obtaining definitive diagnoses using OFC.
Of substantial clinical value would be simple and reliable in vitro assays for the accurate diagnosis of food allergy, as well as tests capable of predicting the severity of an allergic reaction upon OFC. These assays could draw from our mechanistic understanding of the submolecular specificity of the inflammatory TH2 cell-mediated response, as well as our understanding of the roles and behaviours of the immune cells that are involved.
Towards this end, diagnostic tests that are in development include component-resolved diagnosis (CRD)96 and the basophil activation test (BAT)97. CRD uses purified or recombinant allergens (such as rAra h 2)98 to identify allergen-specific IgE and IgG4 antibodies99, which may distinguish various allergenic, non-allergenic and crossreactive molecules in standardized amounts. BATs are carried out using allergenic proteins as stimulants. Upon stimulation, basophils induce surface expression or upregulate the surface level of proteins such as CD63 and CD203c97. Although the BAT provides highly accurate diagnoses of peanut97 and other food allergies, it is currently used primarily in research settings. With further standardization, these in vitro tests for food allergy may become more widely accepted in clinical settings.
Prevention
Increasing attention is being devoted to research on risk factors and preventive measures to mitigate the rising prevalence of food allergies. Whereas the avoidance of common food allergens during pregnancy and breastfeeding has been previously advocated100, some evidence now suggests that early exposure to potential food allergens may decrease the risk of developing the respective allergy. For example, maternal consumption of common food allergens, such as peanuts, tree nuts, milk and wheat, during pregnancy may reduce the risk of food allergy in infants101. Similarly, the introduction of an increased diversity of foods during infancy may be associated with a reduced risk of allergic disease102. A landmark study and its follow-up indicate that the early initiation of peanut consumption (at 4–11 months) may prevent peanut allergy; increased levels of peanut-specific IgG4 characterized the peanut-consuming group, whereas the avoidance group had increased levels of peanut-specific IgE103,104. On the basis of this Learning Early About Peanut Allergy (LEAP) trial, global consensus guidance has been published recommending the early introduction of peanuts to high-risk infants because of the potential association between delaying peanut introduction and an increased risk of peanut allergy105. Another large, prospective, randomized trial from the Enquiring About Tolerance (EAT) study group, which enrolled more than 1,300 infants, found that the introduction of six commonly allergenic foods (peanut, cow's milk, egg, sesame, whitefish and wheat) to breastfeeding infants at 3 months of age may be feasible without interfering with breastfeeding, although difficulty with compliance precluded further definitive findings regarding the prevention of food allergy in this feasibility study106.
These studies indicate that the early introduction of allergenic foods may be a promising prevention strategy for food allergy. However, many questions regarding the effects of allergen type, dose and frequency require further consideration before recommendations can be made105, and a systematic review has concluded that there is insufficient evidence to support a role for maternal allergen consumption in the risk of food allergy107. Although a definitive, protective value of early exposure to potential food allergens has not been established, current guidelines no longer recommend allergen avoidance; instead, it is recommended that mothers follow a normal diet during pregnancy and breastfeeding, and they avoid delaying the introduction of solid foods — including potentially allergenic foods — beyond 4–6 months of age, even in infants at high risk of developing allergic disease.
The role of the microbiota in the induction of immune tolerance is a very active area of research. Microorganisms that normally colonize the skin and the gut influence immune system development and can promote or restrain the development of immune tolerance to foods108,109. Although causality has not been established, one epidemiological study found an association between the use of antibiotics and an increased risk of cow's milk allergy110. Variations in the composition of the human skin and gut microbiota have been correlated with food allergy, but the observed changes in bacterial groups are inconsistent across several studies111–113. Many studies have investigated whether caesarean birth (which is thought to alter the microbiota of the infant compared with the maternal vaginal microbiota acquired during vaginal delivery) is associated with an increased risk of food allergy; a systematic review of 13 studies reports that 6 observed significant associations, although only 2 of those relied on clinical food allergy diagnosis114. Similarly, many studies have examined the ingestion of probiotics during pregnancy or breastfeeding, or by individuals at risk of or suffering from food allergy; however, uncertainty persists as to whether the incidence of food allergy is altered115.
Recent studies suggest that higher serum levels of vitamin D are inversely associated with risk of food allergy. Vitamin D is an immunomodulator, the deficiency of which not only interferes with its general tolerogenic effects but also may increase an individual's susceptibility to gastrointestinal infection, thereby compromising the integrity of the gastrointestinal epithelial barrier and undermining tolerogenic encounters of immune cells with food protein antigens116. The incidence of peanut or egg allergy was 3 times higher in vitamin D-deficient infants, and it was 6 times higher among food-sensitized vitamin D-deficient infants; those infants with lower levels of vitamin D-binding protein, and thus higher levels of biologically available serum vitamin D, experienced some compensation for the unfavourable effects of low serum levels of vitamin D117. The immunological mechanisms underlying these observations are ripe for further investigation.
Other factors associated with increased risk of food allergy include atopic dermatitis and family history of atopic disease118. Predisposition to the development of TH2 cell-mediated diseases, including food allergy, has been associated with mutations in filaggrin, which is a protein important for skin barrier integrity119,120. Additional study of the mechanisms that mediate this association, which may involve an improper or alarming introduction of food proteins to the immune system through a weakened skin barrier, are warranted. Studies of the application of skin emollients to maintain skin barrier integrity have shown beneficial effects in terms of atopic dermatitis, but they have not yet focused on the development of food allergies121,122. Genetic studies have identified seven susceptibility loci that are associated with the atopic march from atopic dermatitis to asthma, but their role in susceptibility to food allergy was not studied123. Further investigation of the extent to which the prevention and treatment of atopic dermatitis may also function in food allergy prevention and treatment may be very valuable.
Therapy
Currently, there is no definitive treatment for food allergy: the avoidance of food allergens and treatment of food allergen-induced systemic reactions with adrenaline remain the standard of care16. Adrenaline can reverse oedema, urticaria, bronchospasm, hypotension and gastrointestinal symptoms within minutes. Early treatment with adrenaline after allergen exposure (such as within the first 6 minutes after exposure) is more effective than later treatment (such as more than 20 minutes after the onset of reaction), and early response is a crucial factor in preventing death from anaphylaxis124. Between 1% and 20% of people who experience an allergic food reaction may have a biphasic reaction that involves a recurrence of their symptoms within hours after initial adrenaline treatment; a late or insufficient first dose of adrenaline has been postulated to increase the risk of a biphasic reaction125.
Other pharmaceuticals, such as antihistamines, including diphenhydramine, or the more specific H1 receptor blockers such as cetirizine, are used to treat localized food allergy symptoms. Gastrointestinal symptoms can be treated with H2 receptor blockers such as famotidine. However, currently available pharmaceuticals only control the symptoms of food allergy, and they do not address the underlying immune disorder.
Immunotherapy to desensitize individuals to potential food allergens represents tremendous progress in treating food allergy. In this section, we discuss the different types of immunotherapy, ongoing clinical trials (TABLE 2) and other innovative treatments in clinical development.
Table 2.
Immunotherapy for food allergy
| Treatment | Type of drug | Mechanism | Trial examples | (Preliminary) results |
|---|---|---|---|---|
| ANB020 | IL-33-specific antibody | Blocks IL-33-induced signalling, which normally favours TH2 cell-mediated allergic responses | Phase I | NA |
| Reslizumab | Humanized mouse monoclonal antibody | Binds free IL-5, preventing IL-5 receptor activation | Off label137 | No clinical effect in EoE, though decrease in eosinophilic infiltration of oesophagus |
| Mepolizumab | Humanized mouse monoclonal antibody | Binds free IL-5, preventing IL-5 receptor activation | Off label136 | Decrease in eosinophilic infiltration of oesophagus in EoE |
| Omalizumab | Humanized mouse monoclonal antibody | Binds Fc region of IgE, blocking FceRI binding | Phase I and II (see main text and REFS 131–134) | Reduces allergen-mediated mast cell degranulation; FceRI signal blockade blunts TH2 cell-mediated response; mitigates allergen side effects and allows rapid escalation of OIT dose |
| OIT for treatment | Allergen taken orally | Potentially functions via expansion of allergen-specific CD4+FOXP3+ Treg cell population | Egg (CoFAR study)128 | 75% desensitization and 28% sustained unresponsiveness |
| OIT for prevention | Allergen taken orally | Potentially functions via expansion of allergen-specific CD4+FOXP3+ Treg cell population | Peanut (LEAP study)104 | 13.7% of SPT-negative infants developed peanut allergy in the avoidance group versus 1.9% in the consumption group |
| SLIT | Allergen administered sublingually | Potentially functions via expansion of allergen-specific CD4+FOXP3+ Treg cell population | Hazelnut126 and peanut127 | 45% of the treatment group reached the highest dose of hazelnut (20 g), versus 9% of the placebo group; modest desensitization to peanut in most test subjects versus placebo |
| EPIT | Allergen absorbed through skin | Potentially functions via expansion of allergen-specific CD4+FOXP3+ Treg cell population | Phase I (for milk)190 | Abstracts report promising increases in dose threshold for food allergic symptoms |
| EMP-123 | Recombinant Ara h 1, Ara h 2 and Ara h 3 | Rectal administration to induce mucosal tolerance | Phase I (REF. 191) | Increased food allergic reactions with drug |
| Peanut vaccine | Ara h 1, Ara h 2 and Ara h 3 | Presentation of peanut protein without inflammation induces tolerance | Phase I and preclinical | NA |
CoFAR, Consortium for Food Allergy Research; EoE, eosinophilic oesophagitis; EPIT, epicutaneous immunotherapy; IL, interleukin; LEAP, Learning Early About Peanut Allergy; NA, not applicable; OIT, oral immunotherapy; SLIT, sublingual immunotherapy; SPT, skin prick test; TH2, T helper 2; Treg cell, regulatory T cell.
Antigen desensitization
New immunotherapies for food allergy are being designed based on our increasing mechanistic understanding of how desensitization to food protein antigens occurs. Desensitizing immunotherapy is generally delivered sublingually, orally or through the skin. The first double-blind, placebo-controlled trial of SLIT for food allergy was published in 2005 (REF. 126). SLIT involves administering a liquid extract of the allergen under the tongue, where it is held for several minutes. Daily allergen doses begin in the submilligram range and increase gradually over a period of days or weeks. A large, multicentre, randomized, placebo-controlled, double-blind, crossover study evaluated SLIT for peanut allergy in 40 subjects, with a response defined as tolerating a dose of 5 g peanut powder or a ≥10-fold increase in the tolerated dose: after 44 weeks, 70% of the 20 subjects in the treatment group had responded, compared with 15% in the control group; the median tolerated dose of peanut powder in the treatment group rose from an initial 3.5 mg to 496 mg (the equivalent of about two peanuts)127.
OIT is a promising approach that enables the majority of children with a food allergy to become desensitized to substantial amounts of the allergenic food. In OIT, a low dose of allergen (in the milligram range) is ingested daily and the dose is gradually increased (for example, every two weeks) over a period of several months. Because of the larger allergen doses that are used in OIT compared with other forms of immunotherapy that patients may choose, patients can often be desensitized not only to amounts of the allergen sufficient to avoid a life-threatening reaction due to accidental exposure, but also to the extent that they are able to consume gram amounts of allergenic foods.
The question of sustained unresponsiveness to higher doses of allergen, distinct from desensitization requiring continued, regular allergen exposure, was addressed in a double-blind, randomized, placebo-controlled trial for egg OIT involving 55 children128. After an initial build-up and maintenance phase, 55% and 75% of the 40 children in the treatment arm passed an OFC at 10 and 22 months, respectively, whereas none in the placebo group did, showing again that OIT can effectively induce allergen desensitization. Those children who passed the OFC at 22 months avoided egg for 2 months and were retested by OFC at 24 months. Only 11 children passed the OFC at 24 months (28% of the 40 participants in the treatment arm), but all who passed were able to pass a subsequent OFC one year later (after 36 months on an ad lib diet). In conclusion, although desensitization does not necessarily lead to tolerance, sustained unresponsiveness is achievable in a subset of patients.
EPIT uses an adhesive containing microgram amounts of allergen to deliver antigen to the skin surface. This route of delivery seems to have fewer and less intense side effects than OIT, and some subjects may prefer wearing a skin patch to orally consuming the same food allergen each day129. A phase II study evaluating a milk patch and a phase III study evaluating a peanut patch are currently underway.
Monoclonal antibodies
Our mechanistic understanding of the molecular processes involved in sensitization has inspired the development of monoclonal antibodies as therapeutic agents to help block these processes. For example, because allergic reactions are common during OIT and can be severe, the use of monoclonal anti bodies specific for IgE in conjunction with OIT has been explored to increase safety. The monoclonal antibody omalizumab binds to the Fc region of IgE antibodies, blocking IgE binding to FcεRI and thus preventing the Fc receptor-mediated activation and degranulation of mast cells and basophils130. Omalizumab was originally approved for the treatment of allergic asthma, but has now been tested in combination with OIT for the treatment of food allergies in a series of smaller studies131–134. Promising results indicate that OIT plus omalizumab may allow the immune system to be desensitized to food allergens more quickly and safely than OIT alone. Patients initially receive injections of omalizumab on a biweekly to monthly basis for at least 8 weeks to deplete free IgE and downregulate surface expression of FcεRI on mast cells135. Then, OIT begins: after an initial overlap period of several weeks in which both omalizumab and food allergen are given, the mono clonal antibody is discontinued134. Additional clinical trials are being carried out to confirm the safety and efficacy of omalizumab, to optimize maintenance doses and to evaluate the sustainability of desensitization or the establishment of tolerance.
Additional examples include monoclonal antibodies that target upstream mediators of food allergy. For example, pilot studies have been carried out using IL-5-specific monoclonal antibodies (such as mepolizumab and reslizumab) to treat EoE. In a study of mepolizumab, 59 patients received three, monthly antibody infusions, which resulted in a decrease in oesophageal eosinophil counts (peak count decreased from 122.5 to 40.2 eosinophils per high-powered field; mean decreased from 39.1 to 9.3)136. In a study of reslizumab, 226 patients received four, monthly antibody infusions137, which resulted in an approximately 60% reduction in the median oesophageal eosinophil count, compared with a 24% reduction for the placebo group. However, physician assessment of EoE severity improved in both placebo and treatment groups, with no statistically significant difference between the groups.
Future treatments
Our increasing understanding of the biology of allergic responses gives rise to new targets for the experimental treatment of food allergy138 (TABLE 2). The IL-33-mediated signalling pathway is one such target: knocking out the IL-33 receptor, ST2, in a mouse model of house dust mite and peanut allergy showed that this pathway is necessary for driving the TH2 cell-mediated allergic response139.
Mutations in the α-subunit of the IL-4 receptor (IL-4Rα)140 and in IL-13 (REF. 141) have been found to be associated with an increased risk of food allergy. Accordingly, another strategy for treating food allergy is directly to block signalling by the canonical TH2 cell cytokines, IL-4 and IL-13. Although this approach has yet to be tested in food allergy, there is reason to believe that it may provide an effective treatment. Dupilumab is a fully human monoclonal antibody directed against IL-4Rα, which is a component of type I and type II IL-4 receptors as well as of the IL-13 receptor (which is a heterodimer composed of IL-4Rα and IL-13RA1). This monoclonal antibody has been found to be effective for the treatment of asthma and atopic dermatitis142,143. These findings support the potential clinical benefit in allergic diseases of blockade of the IL-4- and IL-13-induced signalling pathways, and dupilumab is currently being tested in patients with EoE.
In a mouse model of peanut allergy featuring a mutation in IL-4Rα that increases ligand-activated signalling, TH2 cell responses and IgE production are enhanced; the additional IgE then binds FcεRI on mast cells, promoting atopic TH2 cell-mediated responses to food allergens and leading to Treg cell suppression140. To focus specifically on the IgE-mediated activation of mast cells in this model, a mast-cell-specific deletion of spleen tyrosine kinase (Syk), which is essential for FcεRI signalling, was engineered; this deletion restored the induction of tolerance-promoting Treg cells140. Furthermore, the transfer of Treg cells from mice treated with both peanut and Syk inhibitor blocked allergic sensitization to peanut in recipient mice, which is consistent with the hypothesis that Treg cells have an important role in tolerizing the immune system to allergens and could be useful as a means of monitoring patients or as a target of OIT (BOX 2).
As FIG. 3 suggests, the balance between atopy and tolerance can be understood in mechanistic terms as a balance between the predominance of TH2 cells (atopy) and that of allergen-specific Treg cells (desensitization and tolerance). Adjuvants that can deviate the immune response from a TH2 cell-mediated state when administered with food allergen thus may offer a potential treatment for food allergy. Chitin plus chitosan, and outer membrane protein 16 (OMP16), have been found in mouse models to reduce atopic responses to peanut and cow's milk, respectively144,145.
Toll-like receptor (TLR) agonists also have been used to treat atopic conditions other than food allergy by immune deviation, and are therefore of interest as potential therapies for food allergy. R848, a TLR7 agonist, was found to decrease airway inflammation in a mouse model of asthma through its action on natural killer T (NKT) cells146; also in mice, CpG-oligodeoxynucleotides (TLR9 agonists), when combined with protamine to form nanoparticles and then complexed with the peanut protein Ara h 2, were found to blunt the peanut-allergic TH2 cell-mediated response147. In humans, a TLR9 agonist has been shown to be effective in the treatment of allergic rhinitis148, and a modified allergoid preparation including a TLR4 agonist has been effective in treating ragweed pollen allergy149. Taken together, these studies suggest that TLR modulation in humans may be a fruitful approach to food allergy treatment.
CRTH2, a G protein-coupled receptor expressed by TH2 cells, eosinophils and basophils, provides another therapeutic target. Activated mast cells release the CRTH2 ligand, prostaglandin D2, which is thought to attract and activate CRTH2+ cells and thereby to augment the TH2 cell-mediated immune response. CRTH2 antagonists have been tested in humans for the treatment of asthma and allergic rhinitis, with promising results150,151. It would be informative to test the effectiveness of CRTH2 antagonists in food allergy, which also involves many nonrespiratory symptoms.
Another potential approach to tolerizing the immune system uses a DNA vaccine that expresses peanut protein. In 1999, this approach was demonstrated in mice using oral delivery of a DNA plasmid encoding the Ara h 2 protein on a nanoparticle carrier152. Subsequent Ara h 2 expression in the gut epithelium resulted in partial protection from anaphylaxis. A clinical trial is currently underway to test a DNA vaccine for peanut allergy.
As our understanding of the biology of food allergy develops through the immune monitoring of individuals enrolled in both preventive and therapeutic clinical studies, we anticipate that cellular and molecular markers will identify subgroups of patients who differ in their disease behaviour and in their response to targeted therapies. This classification of endotypes and phenotypes of food allergy will enable the design of safer and more effective treatments. In addition, markers may be developed to aid in monitoring the course of food allergy treatment. For example, an in vitro diagnostic test on a blood sample could be used to identify when a patient has been tolerized to an allergen, rather than simply desensitized, so that the need for maintenance ingestion of allergen in immunotherapy can be determined.
Conclusions
Food allergy is a common and potentially fatal condition that affects the daily lives of a growing number of individuals and their families. Although the traditional standard of care has been food-allergen avoidance, there is recent progress in using allergen exposure both in the prevention of food allergies and as a method of desensitization. Although desensitization therapies for food allergy have been shown to be effective, many practical barriers restrict their general use. Diagnostic in vitro tests that can be carried out reliably and inexpensively without the risk of an anaphylactic reaction are required to enable immunotherapy to become the standard of care and prevention for food allergy. The frequent, extended clinical visits that are required for immunotherapy also pose a barrier to its general adoption. Furthermore, desensitization resulting from immunotherapy is often temporary, and recurrence of the food allergy is often observed after regular intake of the maintenance dose of the food allergen is stopped. The goal and the challenge are to achieve sustained unresponsiveness, a possibly permanent state of nonreactivity to a food allergen, without requiring regular, continued exposure to the food allergen. Gaps persist in our understanding of the immune mechanisms of food allergy and of how desensitization and conversion to sustained unresponsiveness take place. New technologies for monitoring and analysing the human immune system in individuals undergoing immunotherapy may help to elucidate these mechanisms and to reveal new cell types that are important in this process. In particular, understanding the markers and mechanisms that differentiate tolerance, desensitization and sustained unresponsiveness will be useful for monitoring immunotherapy and providing the next generation of treatment targets.
Box 1 | Additional cell types involved in food allergy pathogenesis.
Tuft cells
These are component cells of the small intestinal epithelium that secrete interleukin-25 (IL-25) to maintain the homeostasis of type 2 innate lymphoid cells (ILC2s)153. Under certain conditions, tuft cells can induce ILC2s to secrete IL-13, thereby initiating a T helper 2 (TH2) cell-mediated response153.
γδ T cells
Found in the intestinal epithelium and the lamina propria, these cells can modulate and contribute to the breakdown of oral tolerance in mice, and may have a similar role in humans154.
CD8+ T cells
These cells may influence gastrointestinal manifestations of food allergy: increased numbers of CD8+ T cells have been observed in a mouse model of eosinophilic oesophagitis155, and CD8+ T cells are reported to suppress food allergen-triggered diarrhoea in another mouse model156.
Epithelial cells
Cells of the gut and respiratory mucosae and skin that secrete thymic stromal lymphopoietin (TSLP), IL-25 and IL-33; these cytokines tend to elicit a TH2 cell-mediated response through their actions on dendritic cells (DCs) and ILC2s157. Mutations in filaggrin impair epithelial barrier function and can be associated with an increased risk of atopic dermatitis and food allergy119,120,158,159.
Type 2 innate lymphoid cells
ILC2s can be influenced by TSLP, IL-25 or IL-33 to secrete IL-5 and IL-13, which promote TH2 cell-mediated immune responses157,160.
Mast cells
Mast cells provide both immediate and delayed release of inflammatory mediators, and their activation is a hallmark of atopic disease20,66.
CD14+ monocytes
These cells occur at an increased ratio to CD4+ T cells in the cord blood of infants who develop food allergies161. CD14+ monocytes are hyperresponsive to lipopolysaccharide (LPS), secreting increased levels of the inflammatory cytokines IL-1β, IL-6 and tumour necrosis factor in response to LPS stimulation161.
Box 2 | Tolerance versus desensitization versus sustained unresponsiveness.
Tolerance is the state of healthy immunological nonresponsiveness to common food antigens. Desensitization is a temporary increase in the threshold for allergen reactivity, the mechanisms of which are not well understood and may differ from those of the permanently allergen-unresponsive state of immune tolerance.
An individual with food allergy who has been desensitized to a food allergen must continue to consume that allergen regularly, or risk losing his or her desensitization. Immunotherapy often results in desensitization rather than a durable state of sustained unresponsiveness. Because it is unknown whether a food-allergic patient who has been desensitized through immunotherapy will remain permanently unresponsive to that food allergen, we refer to sustained unresponsiveness as distinct from the healthy, pre-allergic state of immune tolerance.
The immune changes that are observed in oral desensitization to food allergens include a decrease in the number of allergen-specific T helper 2 (TH2) cells and in IgE levels, as well as an increase in the number of allergen-specific regulatory T cells and in IgG4 levels. Other cells are probably eliminated through depletion, exhaustion or anergic mechanisms. One study found preferential depletion of allergen-specific TH2 cells after repeated stimulation with high-dose antigen78. Another recent study demonstrated that peanut-specific CD4+ T cells from subjects who developed sustained unresponsiveness tended to transition more strongly towards the gene-expression clusters defined as ‘non allergic’ or ‘anergic memory’ (REF. 77).
Although preliminary research has identified certain immunological changes that occur with immunotherapy, further research is necessary to identify appropriate biomarkers of successful immunotherapy. There are no reliable markers to distinguish desensitization from durable, sustained unresponsiveness.
Atopic diseases
Disorders characterized by pathological immune responses to normally innocuous antigens.
Oral food challenge
(OFC). Used for food allergy diagnosis. Under supervision, the patient is asked to consume small but increasing amounts of the suspected food allergen until a predetermined maximum dose is reached or a food allergic reaction is observed.
IgE component testing
Purified allergen extracts or recombinant allergens are used to diagnose food allergies in a more accurate manner than is possible with crude allergen extracts.
Sustained unresponsiveness
The state in which an individual with food allergy no longer requires regular ingestion of an allergen to maintain desensitization to it; phenotypically, it resembles the normal immune tolerance of a healthy individual but may be mechanistically distinct.
Gut-associated lymphoid tissue
(GALT). The immune system of the gastrointestinal tract is collectively called the GALT; examples include the tonsils, Peyer's patches and lymphoid cells in the lamina propria.
Type 1 regulatory T cells
(Tr1 cells). A subset of regulatory T cells that are FOXP3−. Tr1 cells mediate suppressive effects through IL-10 secretion.
Type 2 innate lymphoid cells
(ILC2s). Cells that produce T helper 2 cell cytokines, such as IL-4, IL-5, IL-9 and IL-13, and are implicated in the development of allergic inflammation.
Sublingual immunotherapy
(SLIT). An immunotherapeutic method in which the food allergen (in the form of a tablet or drops) is administered under the tongue.
Regulatory B cells
(Breg cells). These cells support immune tolerance through secretion of the anti-inflammatory cytokines IL-10, IL-35 and TGFβ.
H1 receptor blockers
H1 receptor antagonists that block histamine action, dampening allergic reactions.
Acknowledgements
The authors would like to acknowledge the assistance of T. A. Chatila, S. J. Galli, M. T. Graham, V. Sampath and J. M. Spergel.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Thomsen SF. Epidemiology and natural history of atopic diseases. Eur. Clin. Respir. J. 2015;2:24642. doi: 10.3402/ecrj.v2.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anto JM, et al. Understanding the complexity of IgE-related phenotypes from childhood to young adulthood: a Mechanisms of the Development of Allergy (MeDALL) seminar. J. Allergy Clin. Immunol. 2012;129:943–954. doi: 10.1016/j.jaci.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 3.Alduraywish SA, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 4.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott SL, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026–1031. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 7.Koplin JJ, Mills EN, Allen KJ. Epidemiology of food allergy and food-induced anaphylaxis: is there really a Western world epidemic? Curr. Opin. Allergy Clin. Immunol. 2015;15:409–416. doi: 10.1097/ACI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 8.Branum AM, Lukacs SL. NCHS data brief, no 10. National Center for Health Statistics; Hyattsville, MD: 2008. Food allergy among U. S. children: trends in prevalence and hospitalizations. [PubMed] [Google Scholar]
- 9.Jackson KD, Howie LD, Akinbami LJ. CDC NCHS data brief, no 121. National Center for Health Statistics; Hyattsville, MD: 2013. Trends in allergic conditions among children: United States, 1997–2011. [PubMed] [Google Scholar]
- 10.Gupta RS, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 11.Turner PJ, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J. Allergy Clin. Immunol. 2015;135:956–963. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia 1997 to 2013. Clin. Exp. Allergy. 2016;46:1099–1110. doi: 10.1111/cea.12748. [DOI] [PubMed] [Google Scholar]
- 13.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J. Allergy Clin. Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Brough HA, et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. J. Allergy Clin. Immunol. 2013;132:630–638. doi: 10.1016/j.jaci.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Benedé S, Blázquez AB, Chiang D, Tordesillas L, Berin MC. The rise of food allergy: environmental factors and emerging treatments. EBioMedicine. 2016;7:27–34. doi: 10.1016/j.ebiom.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyce JA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinthrajah RS, et al. Diagnosis of food allergy. Pediatr. Clin. North Am. 2015;62:1393–1408. doi: 10.1016/j.pcl.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obeng BB, et al. Food allergy in Ghanaian schoolchildren: data on sensitization and reported food allergy. Int. Arch. Allergy Immunol. 2011;155:63–73. doi: 10.1159/000318704. [DOI] [PubMed] [Google Scholar]
- 19.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac. Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson HA, et al. Second symposium on the definition and management of anaphylaxis: summary report — Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 22.Ivković-Jureković I. Oral allergy syndrome in children. Int. Dent. J. 2015;65:164–168. doi: 10.1111/idj.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurlburt BK, et al. Structure and function of the peanut panallergen Ara h 8. J. Biol. Chem. 2013;288:36890–36901. doi: 10.1074/jbc.M113.517797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asarnoj A, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J. Allergy Clin. Immunol. 2012;130:468–472. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann C, et al. Cor a 1-reactive T cells and IgE are predominantly cross-reactive to Bet v 1 in patients with birch pollen-associated food allergy to hazelnut. J. Allergy Clin. Immunol. 2013;131:1384–1392. doi: 10.1016/j.jaci.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to α-gal, an oligosaccharide in mammalian meat. Allergol. Int. 2016;65:16–20. doi: 10.1016/j.alit.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015;135:589–596. doi: 10.1016/j.jaci.2014.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidbury R, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J. Am. Acad. Dermatol. 2014;71:1218–1233. doi: 10.1016/j.jaad.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spergel JM. Nonimmunoglobulin e-mediated immune reactions to foods. AAllergy Asthma Clin. Immunol. 2006;2:78–85. doi: 10.1186/1710-1492-2-2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo L, Rothenberg ME. Gastrointestinal eosinophilia. Immunol. Allergy Clin. North Am. 2007;27:443–455. doi: 10.1016/j.iac.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon D, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71:611–620. doi: 10.1111/all.12846. [DOI] [PubMed] [Google Scholar]
- 32.Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015;135:1114–1124. doi: 10.1016/j.jaci.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr. Biol. 2013;23:R389–R400. doi: 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.du Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J. Allergy Clin. Immunol. 2016;137:998–1010. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247–259. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 37.McDole JR, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 40.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 41.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016;137:984–997. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warshaw AL, Walker WA, Isselbacher KJ. Protein uptake by the intestine: evidence for absorption of intact macromolecules. Gastroenterology. 1974;66:987–992. [PubMed] [Google Scholar]
- 43.Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm. Bowel Dis. 2014;20:166–175. doi: 10.1097/MIB.0b013e3182a69dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [CX3CR1+ macrophages capture food antigens and transfer them to CD103+ DCs in a connexin 43-dependent manner, cooperating in the process of antigen capture and presentation that contributes to Treg cell differentiation involved in tolerance.] [DOI] [PubMed] [Google Scholar]
- 45.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur. J. Immunol. 2013;43:3098–3107. doi: 10.1002/eji.201343740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8:265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 50.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 51.Worbs T, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraus TA, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J. Clin. Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spahn TW, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur. J. Immunol. 2002;32:1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 54.Fujihashi K, et al. Peyer's patches are required for oral tolerance to proteins. Proc. Natl Acad. Sci. USA. 2001;98:3310–3315. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hadeiba H, et al. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans TI, Reeves RK. All-trans-retinoic acid imprints expression of the gut-homing marker α4β7 while suppressing lymph node homing of dendritic cells. Clin. Vaccine Immunol. 2013;20:1642–1646. doi: 10.1128/CVI.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cassani B, et al. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 2011;141:2109–2118. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadis U, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Kim KS, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 61.Syed A, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [Hypomethylation of the FOXP3 locus of antigen-induced Treg cells may predict the achievement of operationally defined clinical tolerance during peanut OIT.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noval Rivas M, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [IL-4R signalling drives Treg cell reprogramming into TH2-like cells and interferes with Treg cell-mediated suppression of mast cell activation and expansion; IL-4R inhibition has a role in Treg cell induction and the enforcement of tolerance, as shown in mouse models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Overtvelt L, et al. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J. Immunol. 2008;180:4514–4522. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 64.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J. Clin. Invest. 2003;111:1065–1072. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat. Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J. Allergy Clin. Immunol. 2014;133:309–317. doi: 10.1016/j.jaci.2013.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J. Clin. Invest. 2014;124:5442–5452. doi: 10.1172/JCI77798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tordesillas L, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J. Clin. Invest. 2014;124:4965–4975. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010;126:976–984. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammerschmidt SI, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burks AW, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma and Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J. Allergy Clin. Immunol. 2013;131:1288–1296. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 74.Jones SM, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J. Allergy Clin. Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 76.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015;8:17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan JF, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc. Natl Acad. Sci. USA. 2016;113:E1286–E1295. doi: 10.1073/pnas.1520180113. [Antigen-specific CD4+ T cells transition from allergic and regulatory phenotypes to an anergic TH2-cell phenotype that is largely absent in both pretreatment participants and healthy controls, as patients develop sustained unresponsiveness via OIT.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wambre E, et al. Specific immunotherapy modifies allergen-specific CD4+ T-cell responses in an epitope-dependent manner. J. Allergy Clin. Immunol. 2014;133:872–879. doi: 10.1016/j.jaci.2013.10.054. [In allergen-specific immunotherapy, allergen-specific TH2 cells are preferentially deleted whereas the frequencies of TH1 and Tr1 cells are not significantly changed.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wambre E. Effect of allergen-specific immunotherapy on CD4+ T cells. Curr. Opin. Allergy Clin. Immunol. 2015;15:581–587. doi: 10.1097/ACI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwok WW. Modulation of Peanut-specific humoral and cellular responses pre- and post-oral immunotherapy. Clin. Exp. Allergy. 2015;45:1146–1149. doi: 10.1111/cea.12552. [DOI] [PubMed] [Google Scholar]
- 81.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–673. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 82.Wisniewski JA, et al. Analysis of cytokine production by peanut-reactive T cells identifies residual Th2 effectors in highly allergic children who received peanut oral immunotherapy. Clin. Exp. Allergy. 2015;45:1201–1213. doi: 10.1111/cea.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol. Int. 2010;59:43–51. doi: 10.2332/allergolint.09-OA-0107. [DOI] [PubMed] [Google Scholar]
- 84.Berin MC, Shreffler WG. Mechanisms underlying induction of tolerance to foods. Immunol. Allergy Clin. North Am. 2016;36:87–102. doi: 10.1016/j.iac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Dioszeghy V, et al. Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut. Cell. Mol. Immunol. 2016;13:1–13. doi: 10.1038/cmi.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugimoto M, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr. Allergy Immunol. 2016;27:276–282. doi: 10.1111/pai.12535. [DOI] [PubMed] [Google Scholar]
- 87.Burton OT, et al. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J. Allergy Clin. Immunol. 2014;134:1310–1317. doi: 10.1016/j.jaci.2014.05.042. [IgG in sera from patients with peanut allergies undergoing OIT suppresses peanut-induced basophil activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santos AF, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J. Allergy Clin. Immunol. 2015;135:1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoh RA, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J. Allergy Clin. Immunol. 2016;137:157–167. doi: 10.1016/j.jaci.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van de Veen W, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 91.Konstantinou GN, et al. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr. Allergy Immunol. 2014;25:64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamilton R,G, Adkinson NF., Jr. In vitro assays for the diagnosis of IgE-mediated disorders. J. Allergy Clin. Immunol. 2004;114:213–225. doi: 10.1016/j.jaci.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 93.Spergel JM, et al. Food allergy in infants with atopic dermatitis: limitations of food-specific IgE measurements. Pediatrics. 2015;136:e1530–e1538. doi: 10.1542/peds.2015-1444. [DOI] [PubMed] [Google Scholar]
- 94.Roberts G, Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J. Allergy Clin. Immunol. 2005;115:1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 95.Kattan JD, Wang J. Allergen component testing for food allergy: ready for prime time? Curr. Allergy Asthma Rep. 2013;13:58–63. doi: 10.1007/s11882-012-0311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tuano KS, Davis CM. Utility of component-resolved diagnostics in food allergy. Curr. Allergy Asthma Rep. 2015;15:32. doi: 10.1007/s11882-015-0534-0. [DOI] [PubMed] [Google Scholar]
- 97.Santos AF, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014;134:645–652. doi: 10.1016/j.jaci.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suratannon N, Ngamphaiboon J, Wongpiyabovorn J, Puripokai P, Chatchatee P. Component-resolved diagnostics for the evaluation of peanut allergy in a low-prevalence area. Pediatr. Allergy Immunol. 2013;24:665–670. doi: 10.1111/pai.12125. [DOI] [PubMed] [Google Scholar]
- 99.Wright BL, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71:1552–1560. doi: 10.1111/all.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lack G. Update on risk factors for food allergy. J. Allergy Clin. Immunol. 2012;129:1187–1197. doi: 10.1016/j.jaci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 101.Bunyavanich S, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 2014;133:1373–1382. doi: 10.1016/j.jaci.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roduit C, et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014;133:1056–1064. doi: 10.1016/j.jaci.2013.12.1044. [DOI] [PubMed] [Google Scholar]
- 103.Du Toit G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N. Engl. J. Med. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PubMed] [Google Scholar]
- 104.Du Toit G, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fleischer DM, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. J. Allergy Clin. Immunol. 2015;136:258–261. doi: 10.1016/j.jaci.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Perkin MR, et al. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016;137:1477–1486. doi: 10.1016/j.jaci.2015.12.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst. Rev. 2012;9:CD000133. doi: 10.1002/14651858.CD000133.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen N, Clemente JC. Engineering the microbiome: a novel approach to immunotherapy for allergic and immune diseases. Curr. Allergy Asthma Rep. 2015;15:39. doi: 10.1007/s11882-015-0538-9. [DOI] [PubMed] [Google Scholar]
- 110.Metsala J, et al. Mother's and offspring's use of antibiotics and infant allergy to cow's milk. Epidemiology. 2013;24:303–309. doi: 10.1097/EDE.0b013e31827f520f. [DOI] [PubMed] [Google Scholar]
- 111.Berni Canani R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016;10:742–750. doi: 10.1038/ismej.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling Z, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014;80:2546–2554. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Azad MB, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin. Exp. Allergy. 2015;45:632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 114.Marrs T, et al. Is there an association between microbial exposure and food allergy? A systematic review. Pediatr. Allergy Immunol. 2013;24:311–320. doi: 10.1111/pai.12064. [DOI] [PubMed] [Google Scholar]
- 115.Fiocchi A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): probiotics. World Allergy Organ. J. 2015;8:4. doi: 10.1186/s40413-015-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vassallo MF, Camargo CA., Jr. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J. Allergy Clin. Immunol. 2010;126:217–222. doi: 10.1016/j.jaci.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 117.Allen KJ, Koplin JJ. Prospects for prevention of food allergy. J. Allergy Clin. Immunol. Pract. 2016;4:215–220. doi: 10.1016/j.jaip.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Koplin JJ, et al. The impact of family history of allergy on risk of food allergy: a population-based study of infants. Int. J. Environ. Res. Public Health. 2013;10:5364–5377. doi: 10.3390/ijerph10115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marenholz I, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J. Allergy Clin. Immunol. 2006;118:866–871. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 120.Venkataraman D, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J. Allergy Clin. Immunol. 2014;134:876–882. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Horimukai K, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:824–830. doi: 10.1016/j.jaci.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 122.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014;134:769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marenholz I, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat. Commun. 2015;6:8804. doi: 10.1038/ncomms9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ho MH, Wong WH, Chang C. Clinical spectrum of food allergies: a comprehensive review. Clin. Rev. Allergy Immunol. 2014;46:225–240. doi: 10.1007/s12016-012-8339-6. [DOI] [PubMed] [Google Scholar]
- 125.Lieberman P. Biphasic anaphylactic reactions. Ann. Allergy Asthma Immunol. 2005;95:217–226. doi: 10.1016/S1081-1206(10)61217-3. [DOI] [PubMed] [Google Scholar]
- 126.Enrique E, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J. Allergy Clin. Immunol. 2005;116:1073–1079. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 127.Fleischer DM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J. Allergy Clin. Immunol. 2013;131:119–127. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burks AW, et al. Oral immunotherapy for treatment of egg allergy in children. N. Engl. J. Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sindher S, Fleischer DM, Spergel JM. Advances in the treatment of food allergy: sublingual and epicutaneous immunotherapy. Immunol. Allergy Clin. North Am. 2016;36:39–54. doi: 10.1016/j.iac.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Pennington LF, et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat. Commun. 2016;7:11610. doi: 10.1038/ncomms11610. [The crystal structure of the complex of IgE with omalizumab is presented and used to generate a mutant omalizumab-resistant IgE–Fc fragment which, in combination with omalizumab, more efficiently blocks basophil activation than does either agent alone.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitisation in combination with omalizumab therapy in patients with cow's milk allergy. J. Allergy Clin. Immunol. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schneider LC, et al. A pilot study of omalizumab to facilitate rapid oral desensitisation in high-risk peanut-allergic patients. J. Allergy Clin. Immunol. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wood RA, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J. Allergy Clin. Immunol. 2016;137:1103–1110. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bégin P, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin. Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [The first study to use omalizumab with OIT to multiple allergens simultaneously demonstrates the safe and rapid desensitization of patients with multiple food allergies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Logsdon SL, Oettgen HC. Anti-IgE therapy: clinical utility and mechanistic insights. Curr. Top. Microbiol. Immunol. 2015;388:39–61. doi: 10.1007/978-3-319-13725-4_3. [DOI] [PubMed] [Google Scholar]
- 136.Assa'ad AH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 137.Spergel JM, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2012;129:456–463. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 138.Cox L, Compalati E, Kundig T, Larche M. New directions in immunotherapy. Curr. Allergy Asthma Rep. 2013;13:178–195. doi: 10.1007/s11882-012-0335-7. [DOI] [PubMed] [Google Scholar]
- 139.Chu DK, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013;131:187–200. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 140.Burton OT, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [In a mouse model, mutations in IL-4Rα impaired Treg cell induction; a mast-cell-specific deletion of Syk, which is essential for FcεRI signalling, restored the induction of tolerance-promoting Treg cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zitnik SE, et al. IL13 variants are associated with total serum IgE and early sensitization to food allergens in children with atopic dermatitis. Pediatr. Allergy Immunol. 2009;20:551–555. doi: 10.1111/j.1399-3038.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- 142.Wenzel S, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 143.Beck LA, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 144.Bae MJ, Shin HS, Kim EK, Kim J, Shon DH. Oral administration of chitin and chitosan prevents peanut-induced anaphylaxis in a murine food allergy model. Int. J. Biol. Macromol. 2013;61:164–168. doi: 10.1016/j.ijbiomac.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 145.Ibañez AE, et al. Unlipidated outer membrane protein Omp16 (U-Omp16) from Brucella spp. as nasal adjuvant induces a Th1 immune response and modulates the Th2 allergic response to cow's milk proteins. PLoS ONE. 2013;8:e69438. doi: 10.1371/journal.pone.0069438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grela F, et al. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-γ. J. Immunol. 2011;186:284–290. doi: 10.4049/jimmunol.1001348. [DOI] [PubMed] [Google Scholar]
- 147.Pali-Scholl I, et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur. J. Pharm. Biopharm. 2013;85:656–664. doi: 10.1016/j.ejpb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 148.Creticos PS, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 149.Patel P, Holdich T, Fischer von Weikersthal-Drachenberg KJ, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J. Allergy Clin. Immunol. 2014;133:121–129. doi: 10.1016/j.jaci.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 150.Hall IP, et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm. Pharmacol. Ther. 2015;32:37–44. doi: 10.1016/j.pupt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Krug N, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2014;133:414–419. doi: 10.1016/j.jaci.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 152.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan — DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 153.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2- epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frossard CP, Asigbetse KE, Burger D, Eigenmann PA. Gut T cell receptor-γδ+ intraepithelial lymphocytes are activated selectively by cholera toxin to break oral tolerance in mice. Clin. Exp. Immunol. 2015;180:118–130. doi: 10.1111/cei.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J. Leukoc. Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 156.Yamada A, et al. Antigen-primed splenic CD8+ T cells impede the development of oral antigen-induced allergic diarrhea. J. Allergy Clin. Immunol. 2009;123:889–894. doi: 10.1016/j.jaci.2008.12.1115. [DOI] [PubMed] [Google Scholar]
- 157.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 159.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 160.Lund S, Walford HH, Doherty TA. Type 2 innate lymphoid cells in allergic disease. Curr. Immunol. Rev. 2013;9:214–221. doi: 10.2174/1573395510666140304235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang Y, et al. Cord blood monocyte-derived inflammatory cytokines suppress IL-2 and induce nonclassic “TH2-type” immunity associated with development of food allergy. Sci. Transl Med. 2016;8:321ra8. doi: 10.1126/scitranslmed.aad4322. [DOI] [PubMed] [Google Scholar]
- 162.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zigmond E, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 164.Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat. Rev. Immunol. 2016;16:90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 166.Frossard CP, Hauser C, Eigenmann PA. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J. Allergy Clin. Immunol. 2004;114:377–382. doi: 10.1016/j.jaci.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 167.Urb M, Sheppard DC. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012;8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol. Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ballesteros-Tato A, et al. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hirota K, et al. Plasticity of TH17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 175.Mirchandani AS, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 176.Sehra S, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J. Allergy Clin. Immunol. 2015;136:433–440. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Blumchen K, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J. Allergy Clin. Immunol. 2010;126:83–91. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 178.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–1463. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 179.James LK, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J. Allergy Clin. Immunol. 2011;127:509–516. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 180.Nouri-Aria KT, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 181.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gri G, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40–OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eigenmann PA, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatr. Allergy Immunol. 2000;11:95–100. doi: 10.1034/j.1399-3038.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 184.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr. Allergy Immunol. 2013;24:476–486. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hruz P. Epidemiology of eosinophilic esophagitis. Dig. Dis. 2014;32:40–47. doi: 10.1159/000357008. [DOI] [PubMed] [Google Scholar]
- 186.Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review. Clin. Rev. Allergy Immunol. 2016;50:159–174. doi: 10.1007/s12016-015-8501-z. [DOI] [PubMed] [Google Scholar]
- 187.Cianferoni A, Spergel JM. Eosinophilic esophagitis and gastroenteritis. Curr. Allergy Asthma Rep. 2015;15:58. doi: 10.1007/s11882-015-0558-5. [DOI] [PubMed] [Google Scholar]
- 188.Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large-scale, prospective population-based study. J. Allergy Clin. Immunol. 2011;127:647–653. doi: 10.1016/j.jaci.2010.12.1105. [DOI] [PubMed] [Google Scholar]
- 189.Elizur A, et al. Cow's milk associated rectal bleeding: a population based prospective study. Pediatr. Allergy Immunol. 2012;23:766–770. doi: 10.1111/pai.12009. [DOI] [PubMed] [Google Scholar]
- 190.Dupont C, et al. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J. Allergy Clin. Immunol. 2010;125:1165–1167. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 191.Wood RA, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–808. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]