Abstract
The developing fetus is vulnerable to low-level exposure to methylmercury (MeHg), an environmental neurotoxicant, but the consequences of exposure during the adolescent period remain virtually unknown. The current experiments were designed to assess the effects of low-level MeHg exposure during adolescence on delay discounting, preference for small, immediate reinforcers over large, delayed ones, using a mouse model. Thirty-six male C57BL/6n mice were exposed to 0, 0.3, or 3.0 ppm mercury (as MeHg) via drinking water from postnatal day 21 through 59, encompassing the murine adolescent period. As adults, mice lever-pressed for a 0.01-cc droplet of milk solution delivered immediately or four 0.01-cc droplets delivered after a delay. Delays ranged from 1.26 to 70.79 seconds, all presented within a session. A model based on the Generalized Matching Law indicated that sensitivity to reinforcer magnitude was lower for MeHg-exposed mice relative to controls; responding in MeHg-exposed mice was relatively indifferent to the larger reinforcer. Sensitivity to reinforcer delay was reduced (delay discounting was decreased) in the 0.3-ppm group, but not in the 3.0-ppm group, compared to controls. Adolescence is a developmental period during which the brain and behavior may be vulnerable to MeHg exposure. As with gestational exposure, the effects are reflected in the impact of reinforcing stimuli.
Keywords: methylmercury, adolescence, delay discounting, impulsive choice, matching law
Human exposure to methylmercury (MeHg), an environmental neurotoxicant, is a major public health concern in the United States and abroad (Sandra Ceccatelli & Aschner, 2012; National Research Council, 2000). Exposure to low doses of MeHg during gestation presents a particular concern because of the development of brain neurocircuitry during this period (Choi, 1991; Rice & Barone, 2000). In laboratory models, gestational MeHg exposure impairs the acquisition of choice (Newland, Reile, & Langston, 2004; Newland, Yezhou, Logdberg, & Berlin, 1994) and spatial- and visual-discrimination reversals (Paletz, Day, Craig-Schmidt, & Newland, 2007; Reed, Paletz, & Newland, 2006). MeHg-induced alterations to the dopamine neurotransmitter system are thought to underlie these behavioral impairments (Newland, Reed, & Rasmussen, 2015). Developmental MeHg exposure increases sensitivity to dopamine transporter inhibitors, such as amphetamine (Rasmussen & Newland, 2001) and cocaine (Reed & Newland, 2009), and increases DA neurotransmitter levels and DA reuptake in the adult rat brain (Bartolome et al., 1982; Bartolome, Whitmore, Seidler, & Slotkin, 1984). Thus, choice and the underlying neurocircuitry that support such behavior (Dalley, Cardinal, & Robbins, 2004) appear especially sensitive to MeHg exposure before birth.
It is unknown whether vulnerability to developmental MeHg exposure includes adolescence, a period during which the brain and behavior continue to mature and develop in humans and nonhumans (Spear, 2000, 2007b). Many consider adolescence to be a period of sensitivity to pharmacological and neurotoxicological insults because of the high degree of neurobehavioral plasticity during this time (Chambers, Taylor, & Potenza, 2003; Spear, 2007a), but specific behavioral effects of adolescent MeHg exposure remain virtually unexplored. Identifying developmental periods that are susceptible to MeHg exposure and the behavioral mechanisms through which MeHg exposure exerts its effects is not only crucial to public health, but also contributes to a greater understanding of the consequences of interfering with cortical development during critical periods.
Human and nonhuman adolescence are both marked by deficits in executive functioning and maladaptive decision making that may persist into adulthood after exposure to drugs or toxicants (Chambers & Potenza, 2003; Hefner & Holmes, 2007; Laviola, Macrì, Morley-Fletcher, & Adriani, 2003; Marco et al., 2011; Pope, Boomhower, Hutsell, Teixeira, & Newland, 2016). In rodent models, the adolescent period is considered to be wholly encompassed by the age range of postnatal day (PND) 21 through 59 (Laviola et al., 2003; Spear, 2007a; Tirelli, Laviola, & Adriani, 2003). During this period there are profound changes in the prefrontal cortex and dopamine neurotransmitter systems, and these changes co-occur with increased risky and impulsive behavior (Brenhouse, Sonntag, & Andersen, 2008; Dreiem, Shan, Okoniewski, Sanchez-Morrissey, & Seegal, 2009; Gelbard, Teicher, Faedda, & Baldessarini, 2000; Teicher, Andersen, & Hostetter, 1995). For example, adolescent mice demonstrate excessive delay discounting (preference for smaller-sooner reinforcers over larger-later ones) relative to adults (Pinkston & Lamb, 2011). High rates of delay discounting are associated with a variety of health-related outcomes in humans and nonhumans, including obesity (Boomhower, Rasmussen, & Doherty, 2013; Lawyer, Boomhower, & Rasmussen, 2015), drug use (de Wit, 2009), and drug addiction (Madden, Petry, Badger, & Bickel, 1997), but the impact of exposure to MeHg on delay discounting is virtually unknown. Though no research has examined the relation between MeHg exposure and delay discounting directly, there is some evidence that early developmental MeHg exposure can affect behavioral processes related to delay discounting and impulsivity, such as decreasing response inhibition in rats (Newland, Hoffman, Heath, & Donlin, 2013) and humans (Stewart et al., 2006). Because delay discounting is highly malleable during adolescence (Adriani et al., 2009; Laviola et al., 2003; Pinkston & Lamb, 2011), exposure to MeHg during the adolescent period could result in permanent alterations in delay discounting that extend into adulthood.
The present experiment was designed to assess the effects of adolescent MeHg exposure on delay discounting in mice. Following adolescent exposure to MeHg via drinking water, adult mice were trained on a rapid acquisition delay-discounting procedure (Pope, Newland, & Hutsell, 2015) in adulthood. Response allocation between a small, immediate reinforcer and a larger reinforcer delivered after a series of six delays were described using a model derived from the generalized matching equation (Baum, 1974; Pope et al., 2015). Called the contextual choice model, this has been applied to delay discounting to estimate sensitivity to both reinforcer magnitude and delay simultaneously (Grace, 1999; Rachlin & Green, 1972).
Methods
Subjects and MeHg exposure
Fifty-seven, 21-day-old, male C57BL/6n mice were purchased from Harlan (Indianapolis, IN) in two separate cohorts. The first cohort was for behavioral testing and comprised thirty-six, 21-day-old, male C57BL/6n mice from twelve litters (three mice/litter). Mice were pair-housed in an AAALAC-accredited animal facility under a 12-hr light/dark cycle (lights on at 0630 h) with temperature and humidity control. Upon arrival, mice were given free access to standard chow and the three littermates were randomly assigned to three exposure groups (n = 12 in each): 0 (tap water), 0.3, and 3 ppm mercury (dissolved as 0, 0.38, and 3.8 ppm MeHgCl2, respectively) in drinking water. These doses were selected because they produce approximately 0, 40, and 400 μg/kg/day of MeHg, respectively, in mice. These exposures cause also irreversible behavioral deficits, but no gross sensorimotor impairment, when exposure occurred during gestation in rats (Newland et al., 2015). MeHg exposure spanned PND 21 through 59. On PND 60, all mice were given tap water (0 ppm MeHg), and MeHg exposure ceased. Beginning two weeks before and throughout experimental sessions, mice were maintained at a body mass of 26 (±1) grams. To accomplish this, mice were weighed daily and the mouse's daily food ration was adjusted to maintain this body mass range.
The second cohort was used for brain-mercury analyses and comprised twenty-one, 21-day-old, male C57BL/6n mice. Mice were exposed to MeHg via drinking water from PND 21 through 59 in an identical fashion as the behavioral cohort. Three mice were given tap water (0 ppm MeHg), nine mice were given 0.3 ppm MeHg, and nine mice were given 3.0 ppm MeHg. Three mice in the 0.3- and 3.0-ppm MeHg groups were euthanized via CO2 on PND 40, 59, and 90. The three control mice were euthanized via CO2 on PND 59. Whole brains were dissected out and stored below 0° C. Total brain mercury was measured via inductively coupled plasma (ICP) mass spectrometry at Michigan State University. All procedures were approved by Auburn University's Institutional Animal Care and Use Committee.
Apparatus
Eleven Med Associates® (St. Albans, VT) operant chambers modified for mice were used for data collection. Each chamber was equipped with two retractable levers on the front wall panel with an LED light above each lever and a houselight centered between two Sonalert® tone generators (high tone: 4500 Hz, low tone: 2700 Hz) at the top of the wall. Centered between each lever was an alcove for liquid-reinforcement delivery, in which one or more 0.01-cc presentations of a 3:1 water and sweetened-condensed milk solution (hereafter, milk) were presented when response criteria were met. Each chamber was enclosed in a sound-attenuating cubicle with an air-circulating fan in the upper left corner of the right wall. A Windows® computer in an adjacent room controlled all experimental contingencies with 0.01-sec resolution. Each mouse was assigned a particular chamber for the duration of the experiment and was assigned to one of four squads that were run in sessions at approximately the same time of day (±15 min) Monday through Friday. The number of mice exposed to 0, 0.3, and 3.0 ppm MeHg in each squad was counterbalanced across squads and chambers.
Procedure
Lever-press training
At PND 90, lever-pressing was established using an autoshaping procedure similar to Reed et al. (2006). Briefly, a lever (left for half of the mice, right for the others) was extended into the chamber with its corresponding stimulus light illuminated for 7 sec or until the lever was pressed. Upon 7 sec or a lever-press, the lever was retracted, the stimulus light was extinguished, and sweetened condensed milk was delivered as the high tone (4500 Hz) sounded. A 70-sec intertrial interval separated each lever presentation. After ten lever-presses occurred, the timed delivery of reinforcement was removed and a fixed-ratio (FR) 1 schedule of reinforcement was imposed. Lever-pressing was considered trained when 40 responses occurred on the FR1 schedule during a single 60-min session. Lever-press training for the opposite lever occurred in a similar manner, and whether the left or right lever was trained first was counterbalanced across subjects. If fewer than 40 responses occurred on a lever for five sessions in a row, hand shaping (i.e., reinforcement of successive approximations) was employed until 40 responses occurred on a lever in a single session.
Delay discounting
After lever-press training, mice began a two-lever choice procedure as described in Pope et al., (2015). Briefly, sessions were structured such that (a) subjects could lever-press for access to a small or large reinforcer, (b) the smaller reinforcer was always delivered after a 1.26-s delay, (c) six different delays to the larger reinforcer were presented in a random order across a session, with each delay signaled by a unique auditory stimulus, and (d) mice experienced the smaller and larger reinforcers an equal number of times—an important variable to control in delay-discounting procedures (Cardinal, Daw, Robbins, & Everitt, 2002; Maguire, Henson, & France, 2014; Tanno, Maguire, Henson, & France, 2014).
Preliminary training began after autoshaping ended. Sessions consisted of six blocks, and each block comprised twelve choice trials during which mice could select one lever that resulted in one 1.2-sec milk presentation and another lever that produced four 1.2-sec milk presentations, each separated by 0.5-sec intervals. The delay interval was 1.26 sec for both alternatives. At the start of a trial, both levers extended into the chamber, and the stimulus lights above each lever illuminated. The computer then selected randomly which lever-press would be reinforced on that trial. The same lever was selected for reinforcement no more than three trials in a row. Assigning which lever was active ensured that a mouse was allowed to respond freely between the two levers, and that it experienced each alternative six times in a twelve-trial block. The mouse could respond on the lever not selected for reinforcement an unlimited number of times. After a single lever-press on the computer-selected lever, the opposite lever retracted and its associated stimulus light extinguished, the stimulus light above the selected lever began blinking (0.5 sec on/off), and one or four presentations of milk occurred after 1.26 sec. Responses on the other lever were recorded but had no other consequences. Following reinforcer delivery, a 3-sec intertrial interval occurred during which both levers retracted and both stimulus lights were extinguished until the next trial. A 10-sec interblock interval separated each block of twelve trials during which both levers were retracted and both stimulus lights were extinguished. A session ended after the completion of six blocks (72 trials) or 75 min, whichever came first. To reduce lever bias, each mouse completed preliminary training with both levers (left and right) being associated with the larger reinforcer for 10 sessions each. The lever that was first associated with the larger reinforcer was counterbalanced across mice and exposure groups. One purpose of preliminary training was to assess the effects of reinforcer magnitude on response allocation when the delay to both reinforcers was equal (1.26 sec).
The delay-discounting procedure began after preliminary training. These sessions were identical to the preliminary-training procedure with the exception that six, differentially signaled, geometrically-spaced delays (1.26, 2.82, 6.31, 14.13, 31.62, and 70.79 sec) to the larger reinforcer were interposed. At the start of a block, the computer selected randomly (without replacement) one of the six delays to the larger reinforcer to be in effect throughout a block. The delay to the smaller reinforcer remained 1.26 sec throughout the session. Each delay to the larger reinforcer had a specific low/high-tone combination pulsating on and off for varying durations associated with it (see Table 1). The delay-specific tones remained present throughout a trial, but not during interblock intervals. The lever associated with the larger reinforcer (left or right) remained constant throughout the experiment. The delay-discounting procedure was in place for 35 sessions to allow behavior to stabilize.
Table 1.
Low/high tone combinations associated with each delay in the delay-discounting procedure
| Delay to larger reinforcer (sec) | Low/high tone durations (sec) |
|---|---|
| 1.26 | 0.15/1.19 |
| 2.82 | 0.74/0.60 |
| 6.31 | 0.92/0.42 |
| 14.13 | 1.04/0.30 |
| 31.62 | 1.13/0.21 |
| 70.79 | 1.19/0.15 |
Data analysis
The primary dependent variable was the ratio of responses on the larger-later reinforcer lever to those on the smaller-sooner reinforcer lever using data taken from the last block of four trials at each delay value. This measure of preference was quantified using the generalized matching equation, which describes the ratio of responses allocated to two alternatives as a function of the ratios of delay to and magnitude of the reinforcers derived from those two alternatives:
| (1) |
where B is the number of responses, M is the magnitude of reinforcement, D is the delay to reinforcement, and the subscripts l and s refer to the larger and smaller alternatives, respectively. The coefficients sM and sD are free parameters representing sensitivity to magnitude (sM) and sensitivity to delay (sD), respectively. sD is the slope of the linear relation between log response ratio and log delay ratio, Dl /1.26. The denominator is a constant because the shorter delay is always 1.26 s. sM affects the Y-intercept of the line because Ml/Ms was always equal to 4/1. From Eq. 1, we would expect preference for the larger reinforcer to decrease as the delay to it increases, hence the negative sign. There is not a separate term for unexplained bias in this equation because bias is incorporated into sM. Position biases were minimized, however, by verifying that both response devices require equivalent amounts of force and by providing a history in which both the left and right levers are associated with the larger reinforcer in preliminary training (Pope et al., 2015).
An information-theoretic approach was used to assess differences in responding among the three MeHg exposure groups (Burnham & Anderson, 2002). This model-comparison analysis has been growing in popularity in the behavioral and neural sciences to model drug and neurotoxicant effects (Avila et al., 2009; Franck, Koffarnus, House, & Bickel, 2015; Sanabria, Acosta, Killeen, Neisewander, & Bizo, 2008) and it has been used in our laboratory for model construction (Hutsell & Newland, 2013). It does not rely on traditional null-hypothesis testing. Instead, a model-comparison analysis allows the user to examine multiple models and identify which is the most likely to fit the data collected. In order to identify the best model it is necessary to test a large number of models and the information theoretical approach encourages, even requires, doing so. Second, it emphasizes the probability of the model given the data, not the probability of the data given the model as in null-hypothesis testing. Therefore, it gives exactly the answer that one typically wants in data analysis. The information-theoretic approach in this regard may be particularly useful for identifying reproducible neurotoxicant effects, which is why we sought to apply a model-comparison analysis to our behavioral data.
In this analysis, the best model given the data is determined by calculating the corrected Akaike information criterion (AICc), which takes into account the degree of fit of a model (i.e., residual sum of squares, RSS) and penalizes additional free parameters (k) in the model for a given number of data points (n) (Burnham & Anderson, 2002). The smallest AICc value indicates the best account of the data after accounting for the degrees of freedom in the model. Because the calculation of the AICc for one model is independent of another model's, multiple models can be tested simultaneously in this approach without the need for corrective measures and, in fact, the testing of multiple models is encouraged to ensure that the one selected is the best of all comparisons.
Response ratios from the final block of four trials were compared across exposure groups. Log10-transformed response ratios, calculated by dividing the responses on the larger-later (L) reinforcer lever by the responses on the smaller-sooner (S) reinforcer lever, from the last five sessions of the baseline period were averaged for each subject. Similarly, log10-transformed delay ratios were calculated by dividing the larger-reinforcer delay by the smaller-reinforcer delay of 1.26 s. Eq. 1 was fit to individual-subject data using least squares regression. First, the full model that included a separate sM and sD for each exposure groups was evaluated. Then, reduced models, in which sM and/or sD remained constant for two or all exposure groups were assessed. The model that resulted in the lowest AICc was considered the best model of those examined. We then calculated the normalized AICc weights (w), which are the probabilities that a given model is the best model of all the ones that were considered given the data. The probabilities are calculated by computing exp(−0.5*ΔAICc)i for each model, i, and then dividing this number by the sum total [exp(−0.5*ΔAICc)] for all models, resulting in a wi (see Burnham & Anderson, 2002 for details). To confirm that the best of the hypothesized models also provided a reasonable fit to the data, the line from that model was plotted against the actual data.
Results
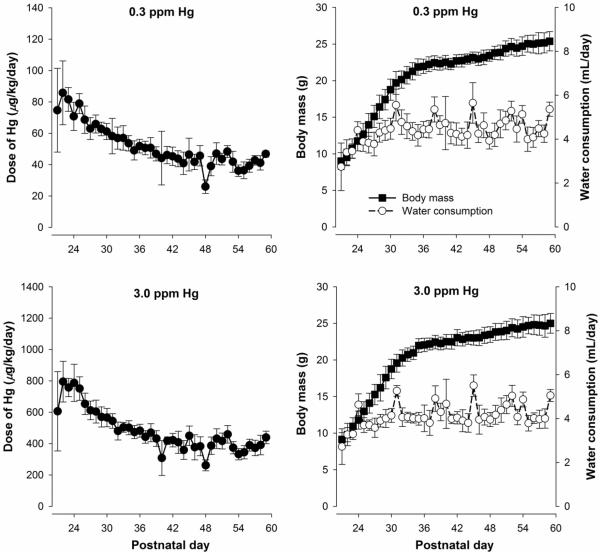
Figure 1 shows the mean estimated dose of mercury, body mass, and water consumption as a function of postnatal day for the two MeHg groups during the adolescent exposure period. Doses were estimated for individual mice, and spillage was accounted for using a sham water bottle. Overall, MeHg dose was highest for both groups at the beginning of adolescence, as has been described in other reports (Adriani, Macrì, Pacifici, & Laviola, 2002; Hefner & Holmes, 2007), and then stabilized to approximately 40 and 400 μg/kg/day MeHg for the 0.3- and 3.0-ppm exposure groups, respectively.
Figure 1.
Left panels: Mean (±SD) dose of MeHg as a function of postnatal day for mice exposed to 0.3 (top) and 3.0 ppm MeHg (bottom) during the adolescent period (postnatal day 21 through 59). Doses were estimated by by averaging the intake of two pair-housed mice. Right panels: Mean (±SD) body mass and water consumption throughout adolescence. Note the y-axes in the left panels differ by an order of magnitude and all error bars represent one SD rather than one SEM.
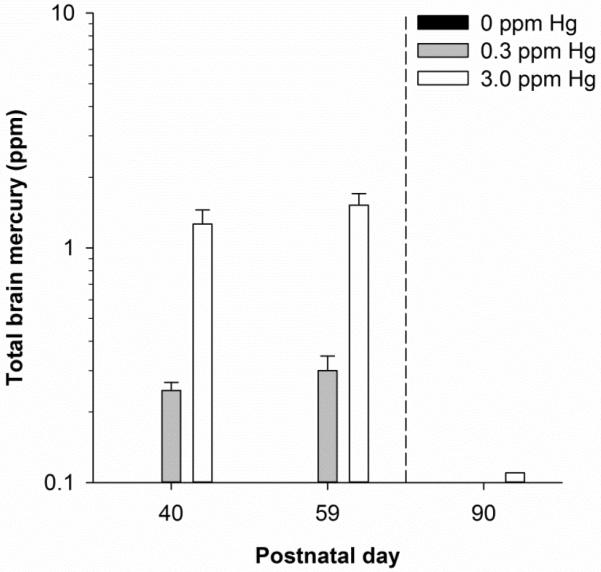
Figure 2 shows mean brain mercury content midway and at the end of the exposure period as well as at the time of behavioral testing, 31 days after exposure ended. Both exposure groups showed an increase in brain mercury across the exposure regimen. At PND 90, brain mercury was undetectable (i.e., <0.1 ppm) in the 0.3-ppm MeHg group and approximately 0.11 ppm in the 3.0-ppm MeHg group. No mercury (<0.1 ppm) was detected in the control group.
Figure 2.
Mean (+SD) total brain mercury at postnatal day (PND) 40, 59, and 90 for 0.3- and 3.0-ppm MeHg exposed mice. Total brain mercury in control animals was assessed at PND 59 only. Exposure lasted from PND 21 through 59. Note errors bars represent one SD rather than one SEM and are too small to visualize in some cases.
Following exposure, all groups acquired lever-pressing at similar rates, with approximately 1–2 mice in each group requiring hand shaping. During preliminary training, estimates of magnitude sensitivity were similar for all groups both before (range of sM: 0.28 – 0.39) and after (range of sM: 0.34 – 0.46) the larger reinforcer was switched to the opposite lever, suggesting there was no bias for responding on one lever over another. Magnitude-sensitivity estimates at the end of preliminary training also were similar across exposure groups (range of sM: .33 – .42), and these are similar to previous studies using C57Bl/6 mice (Pope et al., 2016, 2015). For reference, a magnitude sensitivity of 0.4 corresponds to 100.4 = 2.5 times as many responses on the preferred alternative.
Table 2 lists the seven models that resulted in the lowest AICc values of 25 models that were tested. The full models in which one or both parameters varied across all exposure groups resulted in the lowest AICc values relative to the model in which both sM and sD remained constant (not shown; AICc = −658.33). The best full model was model 3 in which both parameters varied across all exposure groups (AICc = −836.18). The best reduced model was one in which a separate sM was required for each dose of MeHg, but a single sD sufficed for the 0 and 3 ppm condition. A separate sD had to be used for the 0.3 ppm MeHg-exposed mice (model 1). The column wi shows that of all 25 models, the likelihood that model 1 was the best model given the data was 84% with the second-best model only 7%.
Table 2.
Results of the model-comparison approach using the generalized matching equation (Eq. 1). The seven models that resulted in the lowest AICc are shown. The free parameters sM and sD were allowed to vary across all exposure groups (“vary”), vary for one exposure group (e.g., “0.3 ppm”), or remain constant across all exposure groups (“constant”). The best model is bolded.
| Model | RSS | k | n | AICc | Δ AICc | wi |
|---|---|---|---|---|---|---|
| 1. sM (vary), sD (0.3 ppm) | 1.99 | 61 | 216 | −841.52 | 0.00 | 0.84 |
| 2. sM (3.0 ppm), sD (0 ppm) | 2.49 | 49 | 216 | −836.42 | 5.10 | 0.07 |
| 3. sM (vary), sD (vary) | 1.61 | 73 | 216 | −836.18 | 5.34 | 0.06 |
| 4. sM (3.0 ppm), sD (vary) | 2.06 | 61 | 216 | −834.11 | 7.41 | 0.02 |
| 5. sM (vary), sD (3.0 ppm) | 2.07 | 61 | 216 | −833.18 | 8.35 | 0.01 |
| 6. sM (vary), sD (constant) | 2.61 | 49 | 216 | −826.09 | 15.43 | ≈ 0 |
| 7. sM (3.0 ppm), sD (3.0 ppm) | 2.65 | 49 | 216 | −822.86 | 18.67 | ≈ 0 |
RSS = residual sum of squares; k = number of parameters; n = number of data points; AICc = corrected Akaike Information Criterion; Δ AICc = difference between a model's AICc and the smallest AICc, which would be the best model tested; wi = probability that the ith model is the best model given the data.
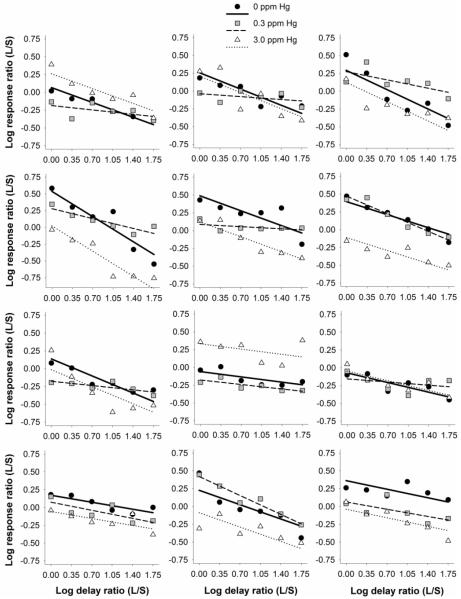
Figure 3 shows log response ratio as a function of log delay ratio for each mouse. Individual panels show responding from three littermates, each experiencing a different exposure. For all mice, larger-reinforcer preference decreased as a function of delay. The best model of Eq. 1 (model 1, shown as lines) was fit to individual-subject data (shown as symbols) and described the data well for 0 ppm (MRSS = 0.06, SEM = 0.01), 0.3 ppm (MRSS = 0.03, SEM = 0.01), and 3.0 ppm MeHg-exposed mice (MRSS = 0.07, SEM = 0.01). The constraints of the best model, which specified that sM vary across dose of MeHg and a separate sD for the 0.3 ppm MeHg-exposed mice, are evident in Figure 3: Y-intercepts vary for each littermate, reflecting changes in sM, and slopes (sD) remain constant (i.e., parallel) for control and 3.0 ppm MeHg-exposed littermates and vary for the 0.3 ppm MeHg-exposed littermate.
Figure 3.
Log response ratio as a function of log delay ratio for individual mice. Each panel shows data from three littermates exposed to 0, 0.3, and 3.0 ppm MeHg during adolescence. Lines represent the best model of Eq. 1 according to the model-comparison analysis in which sM (affecting the line's intercept) varied across all exposure groups, and sD (affecting the line's slope) varied for 0.3 ppm MeHg-exposed mice and remained constant for both controls and 3.0 ppm MeHg-exposed mice. Log response and log delay ratios were calculated by dividing those of the larger-reinforcer lever (L) by the smaller (S).
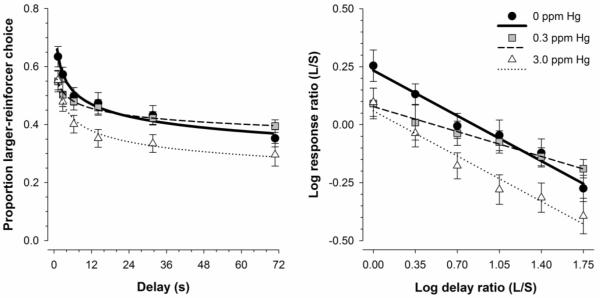
To visualize how group responding changed as a function of delay for the three exposure groups, Figure 4 shows mean larger-reinforcer choice in two formats. The left panel shows preference as a function of delay and the right panel shows log response ratio as a function of log delay ratio. The left panel displays the delay-discounting data in a more traditional format for cross-study comparisons. The right panel shows the data expressed as in Eq. 1, and shows how using the log response ratio and log delay ratios linearizes the function. Mean predictions of Eq. 1 according to the best model are shown as lines. Overall, larger-reinforcer choice decreased as a function of delay for all groups. MeHg-exposed mice on average displayed lower preference for the larger-reinforcer under equal-delay conditions relative to controls.
Figure 4.
Left panel: Mean (±SEM) proportion larger-reinforcer choice as a function of delay for MeHg-exposed mice. Right panel: Mean (±SEM) log response ratio as a function of log delay ratio for MeHg-exposed mice. All lines represent the mean predictions of Eq. 1 according to the best model.
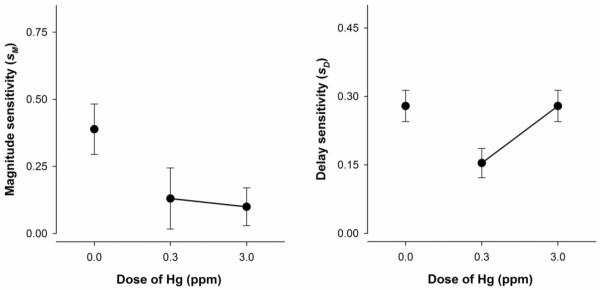
Figure 5 shows mean estimates of magnitude and delay sensitivity for MeHg-exposed mice. According to the best model (model 1), magnitude sensitivity (sM) decreased as MeHg dose increased. Delay sensitivity (sD) was lowest for the 0.3-ppm MeHg-exposed mice and equivalent for controls and 3.0 ppm MeHg-exposed mice.
Figure 5.
Mean (±SEM) parameter estimates of magnitude (left) and delay sensitivity (right) for MeHg-exposed mice. The best model indicated sM varied across all exposure groups, and sD varied for only 0.3 ppm MeHg-exposed mice and remained constant for both controls and 3.0 ppm MeHg-exposed mice.
Discussion
Mice were exposed to MeHg during the adolescent period and delay discounting was measured in adulthood. Mice exposed to either 0.3 or 3.0 ppm MeHg during adolescence displayed an overall similar temporal pattern of exposure (Fig. 1). The dose of MeHg consumed was highest during the first week of exposure and then decreased, stabilizing at approximately 40 and 400 μg/kg/day for the 0.3 and 3.0 ppm groups (respectively) after PND 35. The high dosing at the beginning of adolescence was due to elevated fluid consumption immediately after weaning or during early adolescence (Adriani et al., 2002; Hefner & Holmes, 2007) coupled with low body mass in these young mice. Decreases in dosing, expressed as μg/kg/day, occurred as body mass increased and fluid consumption stabilized across adolescence. The exposure regimen employed here resulted in brain mercury concentrations at the end of exposure that were approximately 0.3 ppm and 1.5 ppm mercury in the 0.3-ppm and 3.0-ppm exposure groups, respectively (Fig. 2). Brain mercury at the time of behavioral testing (PND 90) was undetectable in the 0.3-ppm MeHg exposed mice and approximately 0.11 ppm in the 3.0-ppm MeHg exposed mice. The approximately ten-fold reduction in brain mercury after 31 days without exposure is consistent with a whole-body elimination half-life of approximately nine days in mice (Nielsen & Andersen, 1991; Sundberg, Jönsson, Karlsson, Hallén, & Oskarsson, 1998), although some strains eliminate MeHg much faster (Kostyniak, 1980). The brain mercury concentrations at the end of exposure were slightly lower than those following gestational MeHg exposure in rats (Newland, Reed, LeBlanc, & Donlin, 2006; Newland & Reile, 1999). Further, brain-mercury levels at the end of exposure (PND 59) were similar to brain-mercury levels on PND 40, suggesting that they were beginning to plateau. Steady-states are reached after five or six half-lives so a plateau after 38 days of exposure (59 minus 21 days), too, would be consistent with an elimination half-life of nine days in mice.
The present study employed a model-comparison analysis (Burnham & Anderson, 2002), a robust statistical approach that does not rely on null-hypothesis testing and has been growing in popularity in the neurobehavioral sciences (Avila et al., 2009; Franck et al., 2015; Sanabria et al., 2008), to examine responding following adolescent MeHg exposure. The model that allowed magnitude sensitivity (sM) to vary across all exposure groups and a separate delay sensitivity (sD) for the 0.3 ppm MeHg group was the best model (Table 2.2). Relative to the other model variants tested, the probability that model 1 was the best model for the data obtained was 84%, and Figure 3 and 4 showed that this model fit both individual and group data well. This probability can also be interpreted as the best Bayesian model assuming ideal priors (Anderson, 2008). The next-best models were 7% likely or lower given the data, and these models either drastically misfit the data (e.g., model 2, not shown) or did not justify the additional parameters estimated (i.e., had a more positive AIC value; see model 3). Thus, all behavioral data were interpreted using the constraints of model 1 on parameter estimates.
Adolescent MeHg exposure was associated with reductions in magnitude sensitivity, as evidenced in increased preference for the smaller reinforcer under equal-delay conditions relative to controls. Two accounts of the low magnitude sensitivity in the exposed mice can be offered. First, adolescent MeHg exposure may have diminished the efficacy of reinforcement. Reduced responding for one reinforcer relative to another in a choice arrangement is associated with decreased reinforcer value (Heyman & Monaghan, 1994; Kheramin et al., 2002, 2004). If adolescent MeHg exposure reduced reinforcer efficacy, then that would mean that adolescent MeHg exposure has a different effect than prenatal exposure. Gestational MeHg exposure is associated with enhanced breakpoints and higher response rates for food in rats (Paletz, Craig-Schmidt, & Newland, 2006; Reed, Banna, Donlin, & Newland, 2008), suggesting gestational MeHg exposure increases the impact of reinforcement on responding (Newland et al., 2015). This effect is in the opposite direction of that seen following adolescent exposure here and would represent a distinction between the behavioral effects of gestational and adolescent MeHg exposure. It should be noted though that magnitude sensitivity in the present study describes choice between two reinforcers that differ in their amount, rather than reinforcer efficacy per se. A second possibility is that adolescent MeHg exposure enhanced perseveration and behavioral inflexibility. Because magnitude-sensitivity estimates decreased due to MeHg exposure in the current study, this would suggest increased responding on the smaller-reinforcer lever in exposed mice. Gestational MeHg exposure increases perseverative errors (i.e., enhances responding on a previously reinforced lever) in spatial- and visual-discrimination reversals in rats (Paletz et al., 2007; Reed et al., 2006). In this account, the impact of adolescent MeHg exposure would resemble that of gestational exposure, though a systematic investigation of the effects of adolescent MeHg exposure on reversal learning would be necessary. A third possibility is that adolescent exposure produces a different spectrum of effects than gestational exposure. This would be most likely due to the fact that both gestation and adolescence are associated with different stages of neural development (Rice & Barone, 2000; Spear, 2000), with adolescence primarily comprising continued synaptogenesis, synaptic pruning, and myelination (Bourgeois, Goldman-Rakic, & Rakic, 1994; Giedd et al., 1999). Indeed, many aspects of neural development appear differentially sensitive to MeHg exposure (Ceccatelli, Dare, & Moors, 2010; Rice & Barone, 2000).
Estimates of delay sensitivity were reduced in mice exposed to 0.3 ppm MeHg, indicating a reduction in delay discounting. Stated differently, preference for the larger reinforcer decreased more slowly with increases in delay in that exposure group as compared with the other two groups. This experiment is the first to date to demonstrate that MeHg exposure affects delay discounting in adulthood. Previous reports have shown that gestational MeHg exposure decreases response inhibition—another facet of impulsivity—in adult rats (Newland et al., 2013). It is unclear why delay discounting was reduced in the 0.3-ppm group, but not the 3.0-ppm group; however, some evidence suggests that the effects of different doses of MeHg exposure on behavior are non-monotonic. For example, Bourdineaud et al. (2008) found that diets containing less than 0.3 ppm MeHg enhanced anxiety-like behavior in adult mice exposed for one month beginning on postnatal day 21, but a diet containing 0.52 ppm MeHg resulted in no behavioral change. Bourdineaud et al. (2008) demonstrated this discrepancy may be due to a dose-specific, non-monotonic, MeHg-induced alteration in gene expression; lower levels of MeHg resulted in one pattern of hippocampal gene expression and higher levels of MeHg result in a different pattern.
Many studies have noted that the effects of gestational MeHg exposure on behavior are revealed by challenges to the nervous system, such as aging or acute exposure to stimulants (Newland & Rasmussen, 2000; Rasmussen & Newland, 2001; Reed & Newland, 2009). These challenges might reveal “silent damage” by early-life MeHg exposure and explain why some signs and symptoms of exposure only present themselves in the geriatric period (Weiss, Clarkson, & Simon, 2002). Mice in the present experiment were tested as adults (approximately PND 90 to 140), and it could be that the 3.0-ppm dose would alter delay discounting if subjects were allowed to age further.
Results from the present experiment should be interpreted with some limitations in mind. First, the observed impairments in delay discounting may not have been specific to adolescent exposure but may be a result of chronic exposure to MeHg during the lifespan. No reports to date have systematically compared the effects of adolescent and adult MeHg exposure on behavior. Second, exposure began on PND 21 and continued through 59 in the present study, an age range selected because it is generally considered to encompass the entirety of murine adolescence (Laviola et al., 2003; Spear, 2007a). More nuanced estimates of different periods of rodent adolescence exist (see Adriani & Laviola, 2004), and it is not clear whether MeHg's effects are confined to windows within adolescence, for example, pre- (or late juvenile), mid-, and post-adolescence (Adriani & Laviola, 2004), or whether exposure must span the entire period. Finally, the C57BL/6 strain may be relatively less sensitive to developmental MeHg exposure than other mouse strains. For example, BALB/c mice display less open-field activity following gestational MeHg exposure, whereas C57BL/6J mice were unaffected (Kim, Nakai, Kasanuma, & Satoh, 2000). Thus, the behavioral effects observed in the current study may be larger in a different mouse strain or rodent species.
Limitations aside, the present experiment extends the literature on the behavioral consequences of low-dose MeHg exposure. Specifically, a novel behavioral mechanism (i.e., delay discounting) of MeHg's effects was examined following exposure during adolescence, an underlooked developmental window in the MeHg literature. Results from the present experiment suggest adolescent MeHg exposure alters delay discounting— behavior that underlies executive functioning and decision making—in adult mice, which may have important implications for public health. The current experiment represents a first attempt to assess the behavioral toxicity of adolescent MeHg exposure. More research is needed to determine the extent of this toxicity and the underlying neurobiological mechanisms that permit it.
Highlights.
Mice were exposed to 0, 0.3, and 3.0 ppm methylmercury from postnatal day 21 to 60.
Delay discounting was described using the generalized matching equation.
Brain mercury was eliminated by the time of behavioral testing (postnatal day 90).
Adolescent methylmercury exposure dose-dependently decreased magnitude sensitivity.
0.3 ppm methylmercury, but not 3.0 ppm, reduced delay sensitivity.
Acknowledgements
Funding was provided by the National Science Foundation Graduate Research Fellowship Program (DGE-1414475), ES 024845 from NIEHS, and graduate research grants from Sigma Xi and Psi Chi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Boyer F, Gioiosa L, Macrì S, Dreyer J, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats' nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Information theory and entropy. Springer; New York: 2008. [Google Scholar]
- Avila I, Reilly MP, Sanabria F, Posadas-Sánchez D, Chavez CL, Banerjee N, Castañeda E. Modeling operant behavior in the Parkinsonian rat. Behavioural Brain Research. 2009;198(2):298–305. doi: 10.1016/j.bbr.2008.11.033. http://doi.org/10.1016/j.bbr.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome J, Trepanier P, Chait EA, Seidler FJ, Deskin R, Slotkin TA. Neonatal methylmercury poisoning in the rat: Effects on development of central catecholamine neurotransmitter systems. Toxicology and Applied Pharmacology. 1982;65(1):92–99. doi: 10.1016/0041-008x(82)90366-0. [DOI] [PubMed] [Google Scholar]
- Bartolome J, Whitmore WL, Seidler FJ, Slotkin TA. Exposure to methylmercury in utero: Effects on biochemical development of catecholamine neurotransmitter systems. Life Sciences. 1984;35:657–670. doi: 10.1016/0024-3205(84)90261-3. [DOI] [PubMed] [Google Scholar]
- Baum WM. On two types of deviation from the matching law: Bias and undermatching. Journal of the Experimental Analysis of Behavior. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower SR, Rasmussen EB, Doherty TS. Impulsive-choice patterns for food in genetically lean and obese Zucker rats. Behavioural Brain Research. 2013;241:214–21. doi: 10.1016/j.bbr.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdineaud J-P, Bellance N, Bénard G, Brèthes D, Fujimura M, Gonzalez P, Laclau M. Feeding mice with diets containing mercury-contaminated fish flesh from French Guiana: a model for the mercurial intoxication of the Wayana Amerindians. Environmental Health. 2008;7:1–13. doi: 10.1186/1476-069X-7-53. http://doi.org/10.1186/1476-069X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28(10):2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Ecological Modelling. 2nd ed. Vol. 172. Springer Science & Business Media; 2002. Model selection and multimodel inference: A practical information-theoretic approach. http://doi.org/10.1016/j.ecolmodel.2003.11.004. [Google Scholar]
- Cardinal RN, Daw N, Robbins TW, Everitt BJ. Local analysis of behaviour in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Networks. 2002;15(4–6):617–34. doi: 10.1016/s0893-6080(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Aschner M, editors. Methylmercury and neurotoxicity. Springer; New York: 2012. [Google Scholar]
- Ceccatelli S, Dare E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chemico-Biological Interactions. 2010;188(2):301–308. doi: 10.1016/j.cbi.2010.04.007. http://doi.org/10.1016/j.cbi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. Journal of Gambling Studies. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatriy. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH. Advances in Mercury Toxicology. Springer; US: 1991. Effects of methylmercury on the developing brain; pp. 315–337. [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. http://doi.org/10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Shan M, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicology and Teratology. 2009;31(5):312–7. doi: 10.1016/j.ntt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Franck CT, Koffarnus MN, House LL, Bickel WK. Accurate characterization of delay discounting: A multiple model approach using approximate bayesian model selection and a unified discounting measure. Journal of the Experimental Analysis of Behavior. 2015;103(1):218–233. doi: 10.1002/jeab.128. http://doi.org/10.1002/jeab.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. International Journal of Developmental Neuroscience. 2000;18(1):29–37. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grace R. The matching law and amount-dependent exponential discounting as accounts of self-control choice. Journal of the Experimental Analysis of Behavior. 1999;71:27–44. doi: 10.1901/jeab.1999.71-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191(2):311–22. doi: 10.1007/s00213-006-0646-2. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17206494. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Monaghan MM. Reinforcer magnitude (sucrose concentration) and the matching law theory of response strength. Journal of the Experimental Analysis of Behavior. 1994;61(3):505–16. doi: 10.1901/jeab.1994.61-505. http://doi.org/10.1901/jeab.1994.61-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Newland MC. A quantitative analysis of the effects of qualitatively different reinforcers on fixed ratio responding in inbred strains of mice. Neurobiology of Learning and Memory. 2013;101:85–93. doi: 10.1016/j.nlm.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho M-Y, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, Anderson IM. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2004;175(2):206–14. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Anderson IM. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2002;165(1):9–17. doi: 10.1007/s00213-002-1228-6. http://doi.org/10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Kim CY, Nakai K, Kasanuma Y, Satoh H. Comparison of neurobehavioral changes in three inbred strains of mice prenatally exposed to methylmercury. Neurotoxicology and Teratology. 2000;22(3):397–403. doi: 10.1016/s0892-0362(99)00077-x. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ. Differences in elimination rates of methylmercury between two genetic variant strains of mice. Toxicology Letters. 1980;6:405–410. doi: 10.1016/0378-4274(80)90114-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience and Biobehavioral Reviews. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Boomhower SR, Rasmussen EB. Differential associations between obesity and behavioral measures of impulsivity. Appetite. 2015;95:375–382. doi: 10.1016/j.appet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014:1–7. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Ruocco L. a, Canese R, Sadile AG, Laviola G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neuroscience and Biobehavioral Reviews. 2011;35(8):1722–39. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- National Research Council . Toxicological effects of methylmercury. National Academy Press; Washington, D.C.: 2000. http://doi.org/10.17226/9899. [Google Scholar]
- Newland MC, Hoffman DJ, Heath JC, Donlin WD. Response inhibition is impaired by developmental methylmercury exposure: Acquisition of low-rate lever-pressing. Behavioural Brain Research. 2013;253:196–205. doi: 10.1016/j.bbr.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicology and Teratology. 2000;22(6):819–28. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and blood mercury and selenium after chronic and developmental exposure to methylmercury. Neurotoxicology. 2006;27(5):710–20. doi: 10.1016/j.neuro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reed MN, Rasmussen E. A hypothesis about how early developmental methylmercury exposure disrupts behavior in adulthood. Behavioural Processes. 2015;114:41–51. doi: 10.1016/j.beproc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Reile P. a. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicological Sciences. 1999;50(1):106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology and Teratology. 2004;26(2):179–94. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methylmercury: Reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology and Applied Pharmacology. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Andersen O. Methyl mercuric chloride toxicokinetics in mice. I: Effects of strain, sex, route of administration and dose. Pharmacology & Toxicology. 1991;68(3):201–207. doi: 10.1111/j.1600-0773.1991.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicology and Teratology. 2006;28(1):59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n-3 polyunsaturated fatty acids. Neurotoxicology. 2007;28(4):707–19. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: Adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125(2):194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DA, Boomhower SR, Hutsell BA, Teixeira K, Newland MC. Chronic cocaine exposure in adolescence: Effects on spatial discrimination reversal, delay discounting, and performance on fixed-ratio schedules in mice. Neurobiology of Learning and Memory. 2016;130:93–104. doi: 10.1016/j.nlm.2016.01.017. http://doi.org/10.1016/j.nlm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Pope DA, Newland MC, Hutsell BA. Delay-specific stimuli and genotype interact to determine temporal discounting in a rapid-acquisition procedure. Journal of the Experimental Analysis of Behavior. 2015:1–22. doi: 10.1002/jeab.148. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to D-amphetamine and pentobarbital in adult rats. Neurotoxicology and Teratology. 2001;23(1):45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement efficacy in adulthood. Neurotoxicology and Teratology. 2008;30(1):29–37. doi: 10.1016/j.ntt.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. Behavioral Neuroscience. 2009;123(2):408–17. doi: 10.1037/a0014595. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27(5):721–32. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria F, Acosta JI, Killeen PR, Neisewander JL, Bizo L. a. Modeling the effects of fluoxetine on food-reinforced behavior. Behavioural Pharmacology. 2008;19(1):61–70. doi: 10.1097/FBP.0b013e3282f3df9b. http://doi.org/10.1097/FBP.0b013e3282f3df9b. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: Rationale and methodological considerations. Neurotoxicology and Teratology. 2007a;29(1):1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The developing brain and adolescent-typical behavior patterns: An evolutionary approach. In: Walker E, Bossert J, Romer D, editors. Adolescent Psychopathology and the Developing Brain: Integrating Brain and Prevention Science. Oxford University Press; New York: 2007b. pp. 9–30. [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Pagano J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environmental Health Perspectives. 2006;114(12):1923–1929. doi: 10.1289/ehp.9216. http://doi.org/10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg J, Jönsson S, Karlsson MO, Hallén IP, Oskarsson A. Kinetics of methylmercury and inorganic mercury in lactating and nonlactating mice. Toxicology and Applied Pharmacology. 1998;151:319–329. doi: 10.1006/taap.1998.8456. http://doi.org/10.1006/taap.1998.8456. [DOI] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: Interactions with order of delay presentation. Psychopharmacology. 2014;231(1):85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience & Biobehavioral Reviews. 2003;27(1–2):163–178. doi: 10.1016/s0149-7634(03)00018-6. http://doi.org/10.1016/S0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative diseases. Metal Toxicity. 2002;110:851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]