Abstract
The mammalian target of rapamycin (mTOR) assembles into two different multi-protein complexes, mTORC1 and mTORC2. The mTORC2 complex is distinct due to the unique expression of the specific core regulatory protein Rictor (rapamycin-insensitive companion of mTOR). mTORC2 has been implicated in regulating actin cytoskeletal reorganization but its role in gonadotrope function is unknown. Using the gonadotrope-derived LβT2 cell line, we find that the GnRH agonist buserelin (GnRHa) phosphorylates both mTOR and Rictor. Interestingly, inhibition of mTORC2 blunts GnRHa-induced cyto-architectural rearrangements. Coincident with blunting of actin reorganization, inhibition of mTORC2 also attenuates GnRHa-mediated activation of both protein kinase C (PKC) and extracellular signal regulated kinase (ERK). Collectively, our data suggests that GnRHa-mediated mTORC2 activation is important in facilitating actin reorganization events critical for initiating PKC activity and subsequent ERK phosphorylation in the gonadotrope-derived LβT2 cell line.
Keywords: Gonadotrope, Actin, PKC, ERK, Mammalian Target of Rapamycin Complex 2
1. Introduction
Gonadotropin releasing hormone (GnRH) binding to its cognate receptor (GnRHR) on gonadotrope cells induces coupling to Gαq/11, resulting in activation of phospholipase C (PLC) and cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG). DAG production leads to activation of protein kinase C (PKC) isozymes that initiate Ca2+ influx through activation of voltage-gated L-type Ca2+ channels (VGCCs) in both the gonadotrope derived αT3-1 cell line and primary pituitary gonadotropes (Horn, Bilezikjian, Perrin et al., 1991; McArdle, Bunting and Mason, 1992; Stanislaus, Janovick, Brothers et al., 1997). Recently, exciting work by Dang et al. shows that PKC-mediated Ca2+ influx occurs in specialized microdomains on the plasma membrane and this event is required for extracellular signal regulated kinase (ERK) phosphorylation (Dang, Murtazina, Magee et al., 2014). The ERK pathway consists of a core 3-level signaling module that includes the mitogen activated protein kinase (MAPK)-kinase-kinase Raf-1, the MAPK-kinases MEK1 and 2, and the MAPK’s ERK1 and 2. Once activated, ERKs are essential for regulating many cellular programs including transcription and translation. Our previous work has shown that dynamic remodeling of the actin cytoskeleton is also necessary for ERK activation in gonadotropes (Navratil, Knoll, Whitesell et al., 2007). Taken together, ERK activation by GnRH is dependent on the actin cytoskeleton, PKC, and calcium influx via VGCCs and proceeds through a Raf-1 dependent mechanism.
Upon GnRHR activation, ERK plays a critical role in the transcriptional regulation of the luteinizing hormone beta (LHβ) gene through induction of the immediate early gene early growth response 1 (Egr-1) (Liu, Austin, Mellon et al., 2002; Tremblay and Drouin, 1999; Wolfe and Call, 1999). Female mice deficient in either in Egr-1 or ERK 1/2 are infertile due to deficiencies in LHβ production (Bliss, Miller, Navratil et al., 2009; Lee, Sadovsky, Swirnoff et al., 1996). Thus, GnRHa induction of ERK is essential for proper gonadotrope function by inducing LHβ synthesis necessary for fertility.
Our group has previously demonstrated that primary pituitary cells concentrate LHβ into areas of dynamic membrane reorganization and that disruption of the actin cytoskeleton inhibits luteinizing hormone (LH) release (Navratil, Dozier, Whitesell et al., 2014). Collectively, the actin cytoskeleton is a key regulator of both hormone synthesis and secretion in gonadotropes. Thus, understanding the mechanisms that GnRH utilizes to engage actin has important implications for fertility regulation. Previously, our group found that dynamin and cortactin are two key cell signaling intermediates that associate to engage the actin cytoskeleton following GnRH activation (Edwards, Dang, Murtazina et al., 2016; Navratil et al., 2014). Beyond this work, the full cohort of signaling mechanisms that GnRH utilizes to engage actin remodeling remains unclear.
Recent work suggests that mammalian target of rapamycin (mTOR) can regulate the organization of the actin cytoskeleton in various cells types (Jacinto, Loewith, Schmidt et al., 2004; Manning and Cantley, 2007; Sarbassov, Ali, Kim et al., 2004). mTOR is a serine/threonine protein kinase that forms two distinct complexes, mTORC1 and mTORC2. Both complexes are functionally and structurally different due to their unique complex specific accessory proteins. mTORC1 associates with regulatory associated protein of mTOR (Raptor) and mammalian lethal with SEC13 protein 8 (mLST8) and is sensitive to the inhibitor Rapamycin (RAPA). In gonadotropes, mTORC1 has a well-established role in regulating cap-dependent translational through phosphorylation of both 4E-BP1 and the ribosomal protein S6 kinase (Kim, Do and Lawson, 2014; Nguyen, Santos, Kreidel et al., 2004; Sosnowski, Mellon and Lawson, 2000).
In contrast, mTORC2 associates with the rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1), and mLST8 and is insensitive to the inhibitor RAPA (Jacinto et al., 2004; Peterson, Laplante, Thoreen et al., 2009; Sarbassov et al., 2004). Studying the functional role of mTORC2 has been challenging due to the lack of specific inhibitors. However, the synthesis of a novel selective ATP-competitive inhibitor (PP242) that inhibits both mTORC1 and mTORC2 has increased the ability to differentiate the functionality of the two mTOR complexes when used along side RAPA (Sparks and Guertin, 2010). Accumulating evidence suggests that perturbation of mTORC2 negatively impacts actin polymerization and cell morphology. The proposed mechanism by which mTORC2 mediates actin remodeling events involves downstream activation of PKC to control actin polymerization, although no definitive signaling pathway has emerged (Jacinto et al., 2004; Sarbassov et al., 2004). The precise molecular mechanism of mTORC2 activation utilized by gonadotropes is currently unknown.
Herein, we report that mTOR and the mTORC2 specific protein Rictor are phosphorylated following exposure to the GnRH agonist buserelin (GnRHa) in the gonadotrope-derived LβT2 cell line. Interestingly, when we pharmacologically inhibit mTORC2 using PP242, we blunt GnRHa-induced cyto-architectural rearrangements. The loss of actin reorganization by mTORC2 inhibition also attenuates GnRHa-mediated ERK activation in a dose dependent manner. To define mechanistically the lesion in ERK activity, we also found that inhibition of mTORC2 and actin attenuates PKC activity in response to GnRHa suggesting that PKC resides downstream of actin reorganization. Collectively, our data highlights a novel mechanism where following GnRHa activation, mTORC2 induces actin remodeling which regulates PKC activity to subsequently facilitate downstream ERK activation in gonadotropes.
2. Materials and Methods
2.1 Materials
The anti-phospho-ERK 1/2 (p-ERK), anti-rabbit-horseradish peroxidase (HRP), and anti-mouse-HRP antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-β-tubulin, anti-phospho-Rictor (p-Rictor) and anti-phospho-mTOR (p-mTOR) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Phorbol 12-myristate 13-acetate (PMA), Pierce™ BCA protein assay kit, and all fluorescently labeled Alexa Fluor® secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA). Buserelin (GnRHa) and insulin (Ins) were purchased from Sigma (St. Louis, MO). PP242, rapamycin (RAPA), and jasplakinolide (Jas) were purchased from Merck Millipore (Billerica, MA). Matrigel was purchased from BD Biosciences (San Jose, CA). Glass bottom microwell dishes for confocal studies were obtained through MatTek (Ashland, MA).
2.2 Cell Culture
LβT2 cells (a generous gift from Dr. Pamela Mellon, UCSD) were maintained in high glucose Dulbecco’s Modified Eagle Medium (DMEM) containing 2 mM glutamine, 100 U penicillin/ml, 100 μg streptomycin/ml, 10% FBS by Life Technologies (Grand Island, NY). All cells were grown in 5% CO2 at 37°C in a humidified environment.
2.3 Phalloidin Staining
LβT2 cells were plated in glass-bottom microwell dishes coated with Matrigel (1:100 dilution). Selected cells were pretreated (30 min) with either vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 15 min treatment of either vehicle (0), PMA (10nM), or GnRHa (10nM). Cells were then fixed in 4% paraformaldehyde (PFA) for 20 min and then permeabilized with 0.5% Triton X-100/PBS for 10 min. Cells were then blocked in 1% BSA/PBS for 20 min. For visualization of actin, 5μl of Alexa 488-phalloidin was diluted in 200 μl 1% BSA/PBS and applied to the cells for 20 min at room temperature followed by DAPI staining. Cells were imaged using a Zeiss LSM710 confocal laser-scanning microscope (CLSM) under a 63X oil objective with appropriate fluorescent filters.
2.4 Western Blots
A monolayer of LβT2 cells (2 × 106) were grown overnight in a 6-well culture plate and then serum starved in DMEM for 4–6 h. In selected experiments, after starvation, cells were pretreated with either vehicle (0), RAPA (10nM), PP242 (50μM), or increasing does of PP242 (10μM, 25μM, 50μM, 100μM) for 30 min followed by a 15 min treatment of either vehicle (0), insulin (Ins) (10nM), or GnRHa (10nM). Cells were then washed twice in PBS, lysed in a RIPA buffer and subjected to SDS polyacrylamide gel (10%) electrophoresis (acrylamide:bis-acrylamide ratio of 29:1) and electro-blotted to polyvinylidene difluoride membranes. Membranes were blocked in Tris buffered saline with Tween® 20 (TBST)/2% casein. Membranes were then incubated for 1 h with either an anti-p-ERK antibody (1:1000), an anti-β-tubulin antibody (1:5000), or overnight with an anti-p-mTOR antibody (1:1000) or an anti-p-Rictor antibody (1:1000). Blots were then washed (3 × 10 min) with TBST and then incubated with a 1:10,000 dilution of a HRP-conjugated species-specific secondary antibody for 1 h at room temperature. All blots were washed for 30 min (3 × 10 min) with TBST after secondary antibody and then visualized by chemiluminescence using Pierce SuperSignal reagents, using a Bio-Rad ChemiDoc XRS+ with Image Lab Software. To correct for protein lane loading, all proteins of interest were normalized to β-tubulin.
2.5 PKC Activity Assay
LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), PP242 (50μM), or the actin stabilizing compound Jasplakinolide (Jas) (1μM) followed by a 15 min treatment of either vehicle (0), PMA (10nM), or GnRHa (10nM). Cellular lysates were collected and stored at -80C until assayed. PKC activity was measured using the PKC activity assay kit according to the manufacturer’s instructions (AbCam, Cambridge, U.K.; Cat No. ab139437). Briefly, cells were lysed, sonicated (3 × 20 sec), and LβT2 protein concentration was determined using the BCA method with 10μg of lysate used in the assay. All groups are represented as relative kinase activity (average absorbance(sample) − average absorbance(blank)/quantity of protein used per assay) and data are expressed as mean ± SEM of at least 3 independent experiments.
2.6 Statistical Analysis
All statistical analysis was performed using Graph Pad prism software. Data were analyzed using unpaired Student’s t-test or one-way ANOVA and Tukey’s post-hoc test. Data are presented as mean±SEM of at least 3 independent experiments and P < 0.05 was considered statistically significant.
3. Results
3.1 GnRHa activates the mTORC2 complex in gonadotropes
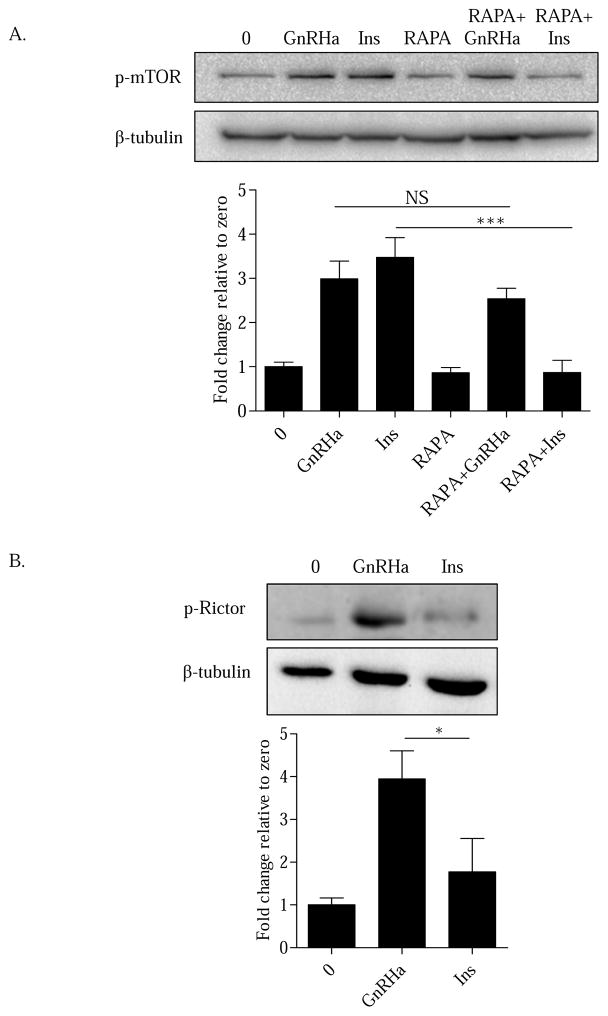
Previous reports have demonstrated that GnRH facilitates cap-dependent translation through mTORC1 in gonadotropes (Nguyen et al., 2004; Sosnowski et al., 2000). At present, it is not clear if GnRH can activate mTORC2. To begin addressing this issue, LβT2 cells were pretreated (30 min) with vehicle (0) or the mTORC1 inhibitor rapamycin (RAPA) (10nM). Cells were then treated with a 15 min treatment of vehicle (0), GnRHa (10nM), or insulin (Ins) (10nM). Consistent with previous work, we found that mTOR is phosphorylated within 15 min of GnRHa treatment (Sosnowski et al., 2000). Insulin signaling is a well-established regulator of mTORC1 activation and was included as a positive control (Figure 1A) (Inoki, Li, Zhu et al., 2002; Manning, Tee, Logsdon et al., 2002). Interestingly, following inhibition of mTORC1 with RAPA, a portion of GnRHa-mediated mTOR phosphorylation remained elevated. This suggests that GnRHa is capable of activating an mTOR complex other than mTORC1. In contrast, insulin activation showed a significant decrease in mTOR phosphorylation (Figure 1A) consistent with its well-established regulation of mTORC1 activity. Thus, our results suggest the possibility that GnRH is capable of mediating mTORC2 phosphorylation in gonadotrope derived LβT2 cells.
Figure 1. GnRHa activates mTORC2 in gonadotropes.
A. LβT2 cells were pretreated (30 min) with vehicle (0) or the mTORC1 inhibitor rapamycin (RAPA) (10nM) followed by a 15 min treatment of either vehicle (0), GnRHa (10nM), or insulin (Ins) (10nM). Cells were lysed in RIPA buffer and Western blotted using an anti-p-mTOR antibody. After probing with an anti-p-mTOR antibody, Western blots were blocked and reprobed with β-tubulin to assess protein lane loading. All groups were normalized to β-tubulin and are represented as fold change relative to zero. GnRHa vs RAPA+GnRHa: non-significant (NS); Ins vs RAPA+Ins: ***P < 0.001. B. LβT2 cells were treated with either vehicle (0), GnRHa (10nM), or Ins (10nM) for 15 min. Cells were lysed in RIPA buffer and Western blotted using an anti-p-Rictor antibody. After probing with an anti-p-Rictor antibody, Western blots were blocked and reprobed with β-tubulin to assess protein lane loading. All groups were normalized to β-tubulin and are represented as fold change relative to zero. GnRHa vs Ins: *P < 0.05.
To validate that GnRHa specifically activates mTORC2, we next addressed whether the complex specific protein Rictor was phosphorylated upon GnRHR activation. To test this, LβT2 cells were treated with either vehicle (0), GnRHa (10nM), or Ins (10nM) for 15 min. Consistent with specific activation of mTORC2, GnRHa treatment increased phosphorylation of Rictor within 15 min (Figure 1B). In contrast, insulin, which works predominately through the mTORC1 complex did not activate Rictor. Taken together, GnRH is capable of mediating mTORC2 activation through the phosphorylation of Rictor.
3.2 Inhibition of mTORC2 decreases ERK activation in a dose dependent manner
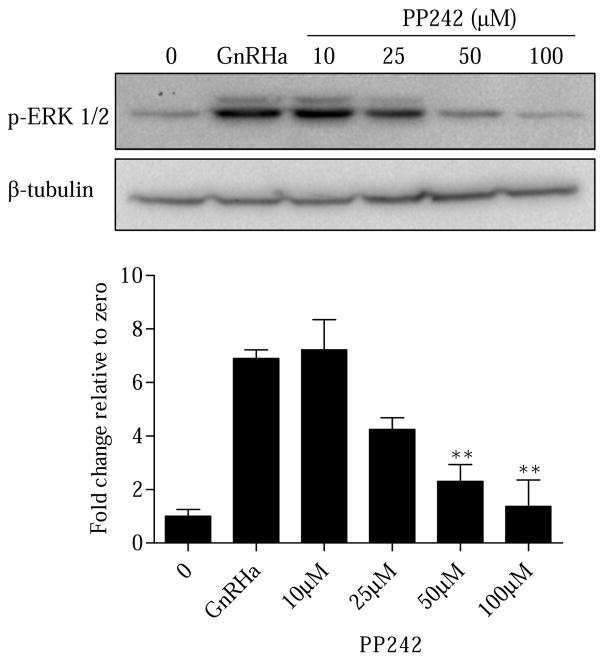
At issue is the functional consequence of GnRHa-induced mTORC2 activation. We were first intrigued with the possibility that inhibition of mTORC2 may impact ERK activation. Our previous work has shown that engagement of the cytoskeleton is necessary for ERK phosphorylation. If mTORC2 is involved in actin signaling, it should be reflected as an inhibition of ERK activity. To test this, LβT2 cells were pretreated (30 min) with vehicle (0) or increasing doses of the pan mTORC inhibitor PP242 (10μM, 25μM, 50μM, 100μM) followed by a 15 min treatment of either vehicle (0) or GnRHa (10nM). ERK phosphorylation was detected 15 min after GnRHa treatment (Figure 2). Following PP242 treatment, GnRHa-mediated ERK phosphorylation remained unchanged at 10μM and 25μM concentrations. However, PP242 treatment was capable of significantly decreasing ERK phosphorylation at 50μM and 100μM doses (Figure 2). The 50μM dose of PP242 was the lowest dose that resulted in a significant decrease in ERK phosphorylation and was subsequently used throughout the rest of our experiments. To confirm that the 50μM dose of PP242 did not affect cell viability in LβT2 cells, we utilized a trypan blue exclusion assay. Our results highlight that a 50μM dose of PP242 for 30 min does not induce an increase in trypan blue staining in LβT2 cells, indicating the cells are viable (Supplemental Figure S1).
Figure 2. Inhibition of mTORC2 results in suppression of p-ERK in a dose dependent manner.
LβT2 cells were pretreated (30 min) with vehicle (0) or increasing doses of the mTOR inhibitor PP242 (10μM, 25μM, 50μM, 100μM) followed by a 15 min treatment of either vehicle (0) or GnRHa (10nM). Cells were lysed in RIPA buffer and Western blotted using an anti-pERK antibody. After probing with an anti-p-ERK antibody, Western blots were blocked and reprobed with β-tubulin to assess protein lane loading. All groups were normalized to β-tubulin and are represented as fold change relative to zero. All groups compared to GnRHa: **P < 0.01.
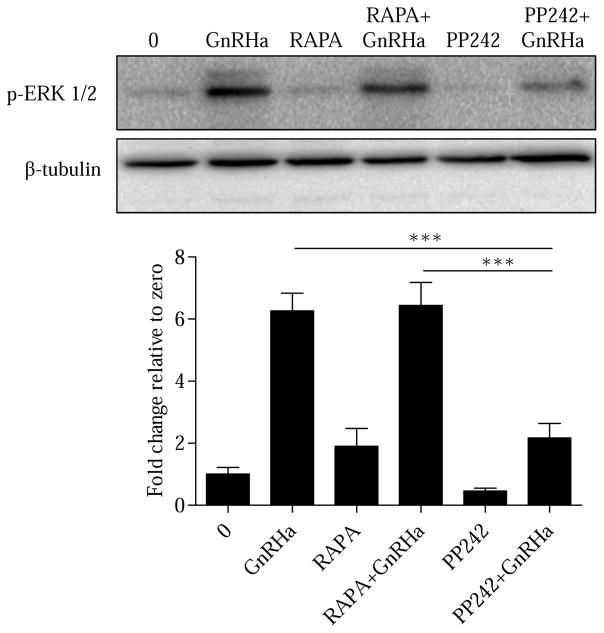
To validate that GnRHa is specifically modulating ERK activation through mTORC2, we used both RAPA and PP242 inhibitors to distinguish the importance of mTORC1 and mTORC2 in the regulation of ERK. LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 15 min treatment of either vehicle (0) or GnRHa (10nM). Selective inhibition of mTORC1 using RAPA had no effect on ERK activation (Figure 3), consistent with previous work (Do, Kim, He et al., 2014). However, PP242 treatment significantly decreased GnRHa-stimulated ERK activation (Figure 3). Taken together, these results suggest that mTORC2 is important in facilitating GnRHa-induced ERK phosphorylation in gonadotropes. To confirm our ERK results, we next examined LHβ mRNA upregulation. It is well known that LHβ mRNA synthesis is highly dependent on ERK activation (Bliss et al., 2009; Liu et al., 2002). LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 3 h treatment of either vehicle (0) or GnRHa (10nM). Our results show that mTORC2 inhibition with PP242 reduces LHβ mRNA following GnRHa (Supplemental Figure S2). This result is consistent with PP242 reducing GnRHa-induced activation of ERK (Figure 3).
Figure 3. GnRHa specifically facilitates ERK activation through mTORC2.
LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 15 min treatment of either vehicle or GnRHa (10nM). Cells were lysed in RIPA buffer and Western blotted using anti-p-ERK antibody. After probing with an anti-p-ERK antibody, Western blots were blocked and reprobed with β-tubulin to assess protein lane loading. All groups were normalized to β-tubulin and are represented as fold change relative to zero. GnRHa vs PP242+GnRHa and RAPA+GnRHa vs PP242+GnRHa: ***P < 0.001.
3.3 mTORC2 inhibition blunts GnRHa-mediated actin reorganization
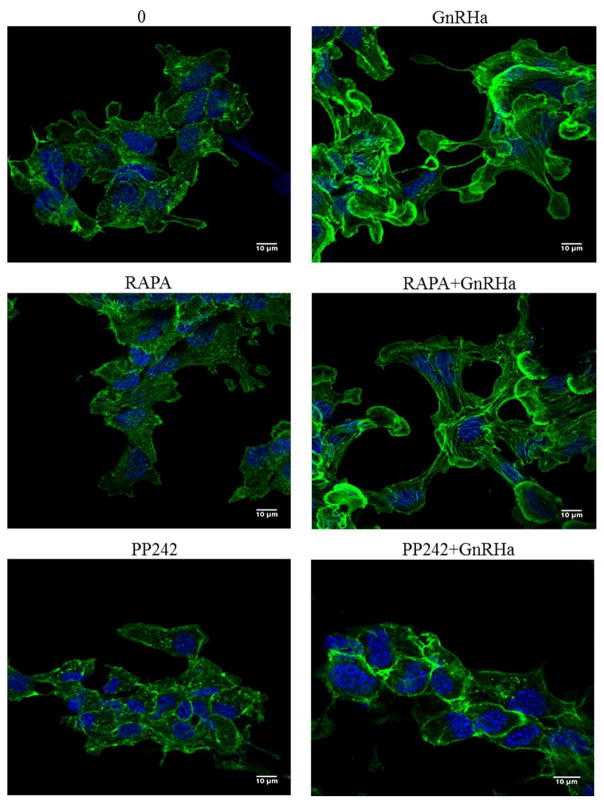
To directly address if mTORC2 can regulate GnRH-induced membrane remodeling events that impact ERK activation, we next examined actin architecture following mTOR inhibition. LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 15 min treatment of either vehicle (0) or GnRHa (10nM). Cells were fixed in 4% PFA and then stained with Alexa 488-conjugated phalloidin and DAPI and imaged by CLSM. Our results confirm our previous studies that actin remodeling occurs following GnRHa stimulation (Edwards et al., 2016; Navratil et al., 2014; Navratil et al., 2007). Consistent with mTORC1 not effecting ERK activity, pretreatment with RAPA did not affect actin reorganization. In contrast, pretreatment with PP242 abrogates GnRHa-mediated actin remodeling events in LβT2 cells (Figure 4). These results suggest that upon GnRHa stimulation, mTORC2 activation plays a key role in regulating gonadotrope actin remodeling events critical for ERK activation.
Figure 4. GnRHa-mediated actin reorganization involves mTORC2.
LβT2 cells were grown on glass-bottom microwell dishes for 24 h. Cells were pretreated (30 min) with vehicle (0), RAPA (10nM), or PP242 (50μM) followed by a 15 min treatment of either vehicle (0) or GnRHa (10nM). Cells were fixed in 4% PFA and then stained with Alexa 488-conjugated phalloidin to label actin and DAPI to label nuclei. All cells were imaged under a 63X oil objective of a Zeiss LSM 710 confocal microscope.
3.4 mTORC2 facilitates GnRH-mediated actin reorganization upstream of PKC
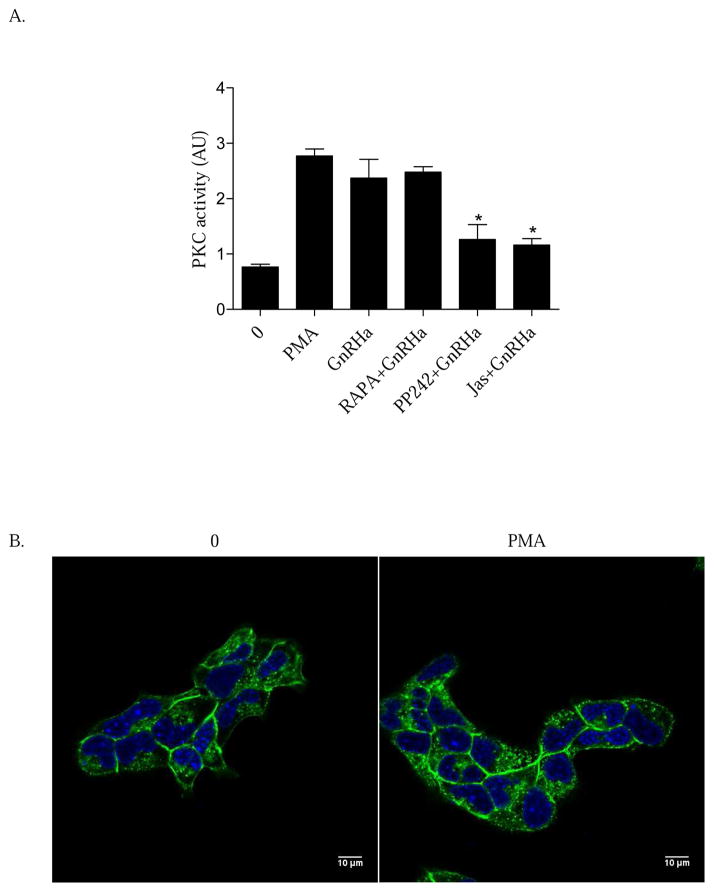
In addition to actin, PKC has also been shown to be key in facilitating ERK activation (Dang et al., 2014). However, it remains unknown whether PKC activity resides upstream or downstream of GnRHa-induced actin remodeling. To elucidate this mechanism, LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), PP242 (50μM), or the actin stabilizing compound Jasplakinolide (Jas) (1μM) followed by a 15 min treatment of either vehicle (0), the PKC activator PMA (10nM), or GnRHa (10nM). Cellular lysates were then collected and measured for PKC activity using an ELISA. Inhibition of mTORC2 suppressed PKC activity in the presence of GnRHa while suppression of mTORC1 with RAPA had no effect (Figure 5A). Additionally, pharmacologically blocking actin polymerization with Jas also suppressed GnRHa-mediated PKC activity (Figure 5A). These data suggest a novel mechanism where mTORC2 engagement of the actin cytoskeleton is occurring upstream of PKC.
Figure 5. GnRHa-mediated PKC activation resides downstream of actin reorganization.
A. LβT2 cells were pretreated (30 min) with vehicle (0), RAPA (10nM), PP242 (50μM), or the actin stabilizing compound Jasplakinolide (Jas) (1μM). Following pretreatment, cells were incubated with a 15 min treatment of either vehicle (0), PMA (10nM), or GnRHa (10nM). Cellular lysates were harvested and PKC activity was assayed using an ELISA kit to assay PKC activity. All groups are represented as relative kinase activity and are compared to GnRHa: *P < 0.05. B. LβT2 cells were grown on glass-bottom microwell dishes for 24 h. Cells were treated with either vehicle (0) or PMA (10nM) for 15 min. Cells were fixed with 4% PFA and then stained for Alexa Fluor 488 phalloidin and DAPI and imaged by CLSM.
If our data is correct, then direct activation of PKC with PMA should reveal an inability to engage membrane remodeling. To test this, LβT2 cells were treated with either vehicle (0) or PMA (10nM) for 15 min. Cells were fixed with 4% PFA and then stained for Alexa Fluor 488 phalloidin and DAPI and imaged with CLSM. Our results show that PMA does not induce cytoskeletal remodeling in gonadotropes, Thus, PKC activity alone is not sufficient to engage the cytoskeleton further highlighting that PKC activation resides downstream of GnRHa actin remodeling. Collectively, we suggest a model were mTORC2-mediated actin remodeling events are critical in regulating PKC activity in gonadotropes. These early signaling events are critical for ERK activation.
4. Discussion
In the current study, we find that both mTOR and the mTORC2 complex specific protein Rictor are phosphorylated following GnRHa treatment. Additionally, we have identified mTORC2 as a key intermediate linking GnRHR signaling to actin remodeling and ERK activation. Little is known about the upstream signaling intermediates regulating activation of mTORC2. In other models, the Rho GTPase, Rac1, is a critical regulator of mTORC2 activation in response to growth factor stimulation (Saci, Cantley and Carpenter, 2011). Rac1 has been shown to directly bind mTOR and facilitate its activation and localization to plasma membrane microdomains (Saci et al., 2011). Previous work by Godoy et al. suggests that GnRH activates Rho family members through microRNA regulation of p250RhoGAP to mediate cell morphology and migration in LβT2 cells (Godoy, Nishimura and Webster, 2011). Other work in heterologous HEK293 cells transfected with GnRHR reveals that GnRH-induced Rac1 activation is important for phosphorylation and recruitment of focal adhesion kinase (FAK) to focal adhesions (Davidson, Pawson, Millar et al., 2004). This recruitment is necessary for proper communication to the cytoskeleton. Taken together, Rac1 is a strong candidate for mTORC2-mediated activation of the actin cytoskeleton in gonadotropes. Ongoing studies in our laboratory are clarifying this possibility.
Understanding the functionality of mTORC2 has been challenging due to the embryonic lethality following deletion of mTORC2 component proteins and the lack of mTORC2 specific inhibitors (Guertin, Stevens, Thoreen et al., 2006). The data that exists suggests mTORC2 can regulate Akt/Protein kinase B activation through phosphorylation at sites Thr450 and Ser473 in various cell models (Frias, Thoreen, Jaffe et al., 2006; Jacinto, Facchinetti, Liu et al., 2006; Sarbassov, Guertin, Ali et al., 2005). The proper activation of Akt is important for regulation of cell proliferation, migration, and survival. Interestingly, GnRH does not activate Akt in gonadotropes (Navratil, Song, Hernandez et al., 2009; Rose, Froment, Perrot et al., 2004). Additionally, GnRH strongly attenuates insulin-mediated Akt phosphorylation (Navratil et al., 2009). Although mTORC2 is a key regulator of Akt activation in other models, it does not appear to play this role in gonadotrope cells.
Evidence also suggests that mTORC2 can regulate the actin cytoskeleton in vitro and in vivo. Specifically, conditional knock out mice devoid of the mTORC2 component Rictor in the central nervous system presented with reductions in cell size and altered cellular morphology (Thomanetz et al., 2013). Furthermore, evidence collected in both yeast and cultured mammalian cells also highlights a role for mTORC2 in the regulation of the actin cytoskeleton (Jacinto et al., 2004; Sarbassov et al., 2004). Consistent with this, our observations that inhibition of mTORC2 blunts membrane remodeling also support a role for mTORC2 in the regulation of the actin cytoskeleton in gonadotropes. Collectively, we suggest the primary functional role of mTORC2 in gonadotropes involves facilitating actin cytoskeletal reorganization in response to GnRH.
The precise mechanism of how actin engagement impacts ERK activation remains unclear. Work by Dang et al. has shown that PKC and the actin cytoskeleton are required to initiate localized VGCC activity that then facilitates ERK phosphorylation in response to GnRH. With this model, we wanted to clarify whether PKC is directly working upstream or downstream of the actin cytoskeleton to affect ERK activation. In many other systems, mTORC2 first mediates the activation of PKCα. It is PKC that then regulates actin cytoskeletal reorganization (Gundlfinger et al., 2003; Metzger, 2010). In contrast with this mechanism, our data shows that direct activation of PKC with PMA was not sufficient to induce cytoskeletal remodeling. Additionally, blunting of actin remodeling either with mTORC2 inhibition or Jas blocked PKC activity. This strongly suggests that PKC is working downstream of the actin cytoskeleton to facilitate activation of ERK in gonadotropes. At issue is how mTORC2-mediated actin cytoskeletal dynamics affect subcellular organization and activation of PKC required for ERK activation. Fractionation experiments have indicated that the α,ε, and ζ PKC isoforms are the major PKCs activated by GnRH (Kratzmeier, Poch, Mukhopadhyay et al., 1996). The novel PKC isoform epsilon (PKCε) has been shown to directly bind actin through an actin-binding motif (Prekeris, Mayhew, Cooper et al., 1996). It should be noted that PKCε is the suggested isoform responsible for initiating VGCC activity in gonadotropes (Dang, Murtazina, Magee et al., 2014). Taken together, the possibility exists that within gonadotropes, the association of PKCε with the actin cytoskeleton may be key in recruiting it to the plasma membrane for activation. Following its recruitment and activation, PKCε then mediates VGCC and ERK activity. Testing this idea is a focus of our future work.
Membrane rafts serve as centers for organizing ligand-mediated communication between the plasma membrane and the intracellular milieu including the actin cytoskeleton. For example, proteomic analysis reveals that many of the molecular components regulating the actin cytoskeleton associate with membrane rafts (Chichili and Rodgers, 2007; Head, Patel and Insel, 2014). Interestingly, the GnRHR, Gαq/11, calmodulin, Raf-1, MEKs, and ERKs themselves are localized within specialized membrane lipid raft microdomains (Bliss, Navratil, Breed et al., 2007; Navratil, Bliss, Berghorn et al., 2003; Navratil, Bliss and Roberson, 2010). In several cell types, mTORC2 is also localized to the plasma membrane and partitions into lipid rafts (Gao, Lowry, Zhou et al., 2011; Hill, Feng and Hemmings, 2002; Partovian, Ju, Zhuang et al., 2008). Thus, within gonadotropes, the potential exists that mTORC2 within membrane rafts is a critical component to initiation of actin polymerization that then recruits GnRH-induced putative raft-complexes/signaling platforms necessary for mitogen activated protein kinase (MAPK) signaling events.
In summary, our results highlight mTOR as a novel intermediate involved in regulating actin dynamics, which is important in mediating ERK activation in gonadotropes. We propose a model where following GnRHa binding, both mTOR and the complex specific protein, Rictor, become activated. Once activated, mTORC2 regulates the actin cytoskeleton resulting in cyto-architectural rearrangements that are important for driving PKC activity. The combination of these GnRHa-induced signaling events is essential in facilitating ERK activation, which is required for enhanced LHβ synthesis to maintain fertility.
Supplementary Material
Highlights.
GnRH activates mTORC2 and the complex specific protein Rictor.
mTORC2 facilitates GnRH-mediated actin cytoskeleton remodeling events
Actin remodeling is upstream of PKC activation
The actin cytoskeleton is key in facilitating both PKC and ERK activation
Acknowledgments
We kindly thank Dr. Pamela Mellon for the LβT2 cell line.
Grant Support
This project was supported by a grant from the National Institute of General Medical Sciences (P20GM103432) from the National Institutes of Health (A.M.N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian S. Edwards, Email: b80@uwyo.edu.
William J. Isom, Email: wyoisom@gmail.com.
References
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21:538–49. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- Chichili GR, Rodgers W. Clustering of membrane raft proteins by the actin cytoskeleton. J Biol Chem. 2007;282:36682–91. doi: 10.1074/jbc.M702959200. [DOI] [PubMed] [Google Scholar]
- Dang AK, Murtazina DA, Magee C, Navratil AM, Clay CM, Amberg GC. GnRH evokes localized subplasmalemmal calcium signaling in gonadotropes. Mol Endocrinol. 2014:me20141208. doi: 10.1210/me.2014-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Pawson AJ, Millar RP, Maudsley S. Cytoskeletal reorganization dependence of signaling by the gonadotropin-releasing hormone receptor. J Biol Chem. 2004;279:1980–93. doi: 10.1074/jbc.M309827200. [DOI] [PubMed] [Google Scholar]
- Do MH, Kim T, He F, Dave H, Intriago RE, Astorga UA, Jain S, Lawson MA. Polyribosome and ribonucleoprotein complex redistribution of mRNA induced by GnRH involves both EIF2AK3 and MAPK signaling. Mol Cell Endocrinol. 2014;382:346–57. doi: 10.1016/j.mce.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BS, Dang AK, Murtazina DA, Dozier MG, Whitesell JD, Khan SA, Cherrington BD, Amberg GC, Clay CM, Navratil AM. Dynamin Is Required for GnRH Signaling to L-Type Calcium Channels and Activation of ERK. Endocrinology. 2016;157:831–43. doi: 10.1210/en.2015-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, Zhang J. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A. 2011;108:14509–14. doi: 10.1073/pnas.1019386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy J, Nishimura M, Webster NJ. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized LbetaT2 pituitary gonadotrope cells. Mol Endocrinol. 2011;25:810–20. doi: 10.1210/me.2010-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–45. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251–5. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- Horn F, Bilezikjian LM, Perrin MH, Bosma MM, Windle JJ, Huber KS, Blount AL, Hille B, Vale W, Mellon PL. Intracellular responses to gonadotropin-releasing hormone in a clonal cell line of the gonadotrope lineage. Mol Endocrinol. 1991;5:347–55. doi: 10.1210/mend-5-3-347. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Kim T, Do MH, Lawson MA. Translational control of gene expression in the gonadotrope. Mol Cell Endocrinol. 2014;385:78–87. doi: 10.1016/j.mce.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–21. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol Endocrinol. 2002;16:419–34. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Bunting R, Mason WT. Dynamic video imaging of cystolic Ca(2+) in the alphaT3-1, gonadotrope-derived cell line. Mol Cell Neurosci. 1992;3:124–32. doi: 10.1016/1044-7431(92)90016-u. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278:31593–602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Bliss SP, Roberson MS. Membrane rafts and GnRH receptor signaling. Brain Res. 2010;1364:53–61. doi: 10.1016/j.brainres.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil AM, Dozier MG, Whitesell JD, Clay CM, Roberson MS. Role of cortactin in dynamic actin remodeling events in gonadotrope cells. Endocrinology. 2014;155:548–57. doi: 10.1210/en.2012-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology. 2007;148:1736–44. doi: 10.1210/en.2006-1153. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Song H, Hernandez JB, Cherrington BD, Santos SJ, Low JM, Do MH, Lawson MA. Insulin augments gonadotropin-releasing hormone induction of translation in LbetaT2 cells. Mol Cell Endocrinol. 2009;311:47–54. doi: 10.1016/j.mce.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2. Mol Endocrinol. 2004;18:1301–12. doi: 10.1210/me.2003-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partovian C, Ju R, Zhuang ZW, Martin KA, Simons M. Syndecan-4 regulates subcellular localization of mTOR Complex2 and Akt activation in a PKCalpha-dependent manner in endothelial cells. Mol Cell. 2008;32:140–9. doi: 10.1016/j.molcel.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A, Froment P, Perrot V, Quon MJ, LeRoith D, Dupont J. The luteinizing hormone-releasing hormone inhibits the anti-apoptotic activity of insulin-like growth factor-1 in pituitary alphaT3 cells by protein kinase Calpha-mediated negative regulation of Akt. J Biol Chem. 2004;279:52500–16. doi: 10.1074/jbc.M404571200. [DOI] [PubMed] [Google Scholar]
- Saci A, Cantley LC, Carpenter CL. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sosnowski R, Mellon PL, Lawson MA. Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol Endocrinol. 2000;14:1811–9. doi: 10.1210/mend.14.11.0550. [DOI] [PubMed] [Google Scholar]
- Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–44. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol Endocrinol. 1997;11:738–46. doi: 10.1210/mend.11.6.0005. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19:2567–76. doi: 10.1128/mcb.19.4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13:752–63. doi: 10.1210/mend.13.5.0276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.