Abstract
Cytomegalovirus vectors are promising delivery vehicles for vaccine strategies that aim to elicit effector CD8+ T cells. To determine how the route of immunization affects CD8+ T cell responses in the lungs of mice vaccinated with a murine cytomegalovirus vector expressing the respiratory syncytial virus matrix (M) protein, we infected CB6F1 mice via the intranasal or intraperitoneal route and evaluated the M-specific CD8+ T cell response at early and late time points. We found that intranasal vaccination generated robust and durable tissue-resident effector and effector memory CD8+ T cell populations that were undetectable after intraperitoneal vaccination. The generation of these antigen-experienced cells by intranasal vaccination resulted in earlier T cell responses, interferon gamma secretion, and viral clearance after respiratory syncytial virus challenge. Collectively, these findings validate a novel approach to vaccination that emphasizes the route of delivery as a key determinant of immune priming at the site of vulnerability.
INTRODUCTION
Tissue-resident memory T (TRM) cells have been found at many barrier sites, including the skin, gastrointestinal tract, and lung, where they are poised to respond quickly to invading pathogens.1, 2, 3, 4 Unlike effector memory T (TEM) cells, which may recirculate, TRM cells remain in the tissue and are often identified by the expression of CD69, an early activation marker, and CD103, an αE integrin, although the latter may vary at different anatomical locations.1, 5 TRM cells act as antigen-specific sentinels and recruit innate and adaptive immune cells into the infected tissue via the secretion of cytokines and chemokines.6, 7 They also proliferate rapidly and kill infected cells, protecting directly against local challenge.3, 4, 5, 8 It may therefore be important to elicit TRM cells as part of an effective vaccination strategy.
Most respiratory virus infections are self-limited, and replication-defective vaccine vectors only express antigen for a short time. Immunization through brief exposure to antigen in these settings typically leads to the generation of central memory T (TCM) cells, which are long-lived but slow to regain effector functions.9, 10, 11 In contrast, persistent vectors such as cytomegalovirus (CMV) produce antigen for longer periods of time and generate high frequencies of TEM cells, which respond quickly to subsequent infection. CMV-based vaccines have proven to be effective against simian immunodeficiency virus (SIV) infection in Rhesus macaques.12 Although vaccination did not lead to sterilizing immunity, challenged animals were able to control viremia to undetectable levels. Protection was attributed to the generation of CD4+ and CD8+ TEM cells, which were able to clear virus from peripheral tissues.13 This approach has also been shown to protect against other infectious diseases, including Ebola and tuberculosis, in various animal models.12, 14, 15, 16, 17, 18 Similar vectors have been evaluated for therapeutic utility against cancer leading to delayed growth or rejection of tumors and even for the purpose of immunocontraception in mice.19, 20, 21
The phenomenon of memory inflation has been observed in murine CMV (MCMV) infection, and large populations of CMV-specific memory T cells are found in elderly humans.22, 23, 24, 25, 26 During MCMV infection, some antigen-specific CD8+ T cells follow canonical memory kinetics, with an early expansion phase followed by rapid contraction after viral containment and the establishment of a low-level stable memory population. In contrast, other antigen-specific CD8+ T cell populations undergo memory inflation and continue to accumulate throughout chronic infection.22, 25, 27 Inflationary T cells typically display an effector (TEFF) phenotype, with low expression of CD127 and CD62L and high expression of the terminal differentiation marker KLRG-1 (KLRG-1+ TEFF).22, 23, 25, 27, 28, 29, 30 This unique phenomenon, which leads to sustained levels of functional MCMV-specific CD8+ T cells, may provide an advantage for vaccination.
In this study, we investigated how the route of administration affects the generation of CD8+ T cell responses following immunization with an MCMV vector expressing the respiratory syncytial virus (RSV) matrix (M) protein (MCMV-M).31, 32 We show that intranasal (IN) vaccination with MCMV-M generates a robust and durable tissue-resident memory population with a TEFF/TEM phenotype that is absent in mice vaccinated via the intraperitoneal (IP) route. Furthermore, tissue-resident memory CD8+ T cells generated by IN vaccination respond rapidly upon antigen re-exposure, leading to lower viral loads after RSV challenge.
RESULTS
Vaccination with MCMV-M induces an inflationary M-specific CD8+ T cell response
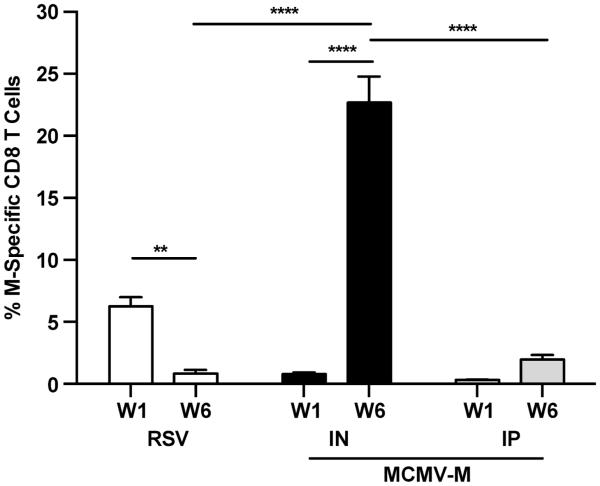
We first evaluated whether vaccination with a recombinant MCMV vector expressing the RSV M protein could generate an M-specific CD8+ T cell response in the lungs. CB6F1 mice were infected with RSV IN or vaccinated with MCMV-M IN or IP. The percentage of CD8+ T cells specific for the DbM187-195 epitope (M-specific) was determined using tetramer staining and flow cytometry at week 1 and week 6 post-infection in the lungs. Mice infected with RSV showed a peak response of 6.25% (Figure 1), which decreased after 6 weeks to 0.84% (p<0.01), consistent with memory contraction. In contrast, mice infected with MCMV-M mounted an M-specific CD8+ T cell response that increased between week 1 and week 6 post-infection, consistent with MCMV-driven memory inflation.27 At 6 weeks post-infection, mice vaccinated IN with MCMV-M had significantly more M-specific CD8+ T cells in the lungs compared to mice infected with RSV (p<0.0001). Interestingly, despite an identical dose of MCMV-M, mice vaccinated IN also had substantially higher frequencies of M-specific CD8+ T cells in the lungs at the same time point compared to mice vaccinated IP (22.72% vs 1.98%, p<0.0001).
Figure 1. MCMV-M generates an M-specific CD8+ T cell population that inflates over time.
Mice were infected with 2×106 PFU of RSV via the intranasal (IN) route or 6×105 PFU of MCMV-M via the IN or intraperitoneal (IP) route. At week 1 (W1) and week 6 (W6) post-infection, the percentage of M-specific CD8+ T cells in the lungs was determined using tetramer staining and flow cytometry. Bars represent mean ± SEM with 5 mice per group. **** p≤0.0001 and **p<0.01 by two-way ANOVA with Tukey’s post-test for multiple comparisons. Data represent two independent experiments.
IN vaccination generates more M-specific CD8+ T cells in the lung parenchyma than IP vaccination
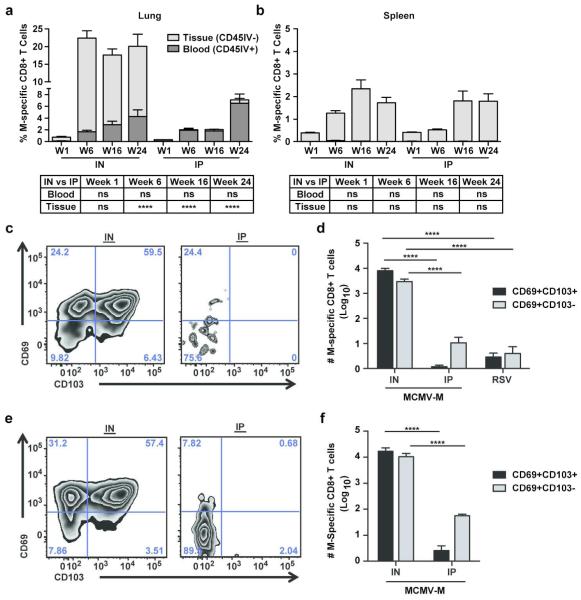
To determine whether IN vaccination generates more tissue-tropic M-specific CD8+ T cells in the lungs, we used an intravascular staining method that exclusively labels leukocytes in the blood, allowing us to distinguish between compartments.33 Mice were vaccinated IN or IP with MCMV-M, and CD8+ T cell responses were evaluated in the lungs and spleen at weeks 1, 6, 16, and 24 post-vaccination. Five minutes prior to sacrifice, mice were injected intravenously with 3μg of anti-CD45 antibody. Cells that stained CD45IV+ were located in the blood and available for immediate labeling following IV antibody administration, and cells that were CD45IV− were protected from IV antibody labeling and categorized as being in the tissue. As expected, IN administration of MCMV-M induced significantly greater frequencies and absolute numbers of M-specific CD8+ T cells in the lung parenchyma at weeks 6, 16, and 24 post-vaccination compared to an equivalent dose of MCMV-M administered IP (p<0.0001, Figure 2a and Supplementary Figure 1a). However, there were no significant differences in the percentages or numbers of M-specific CD8+ T cells in the blood between vaccination groups. In the IN group, M-specific CD8+ T cell frequencies in the blood and tissue increased between week 1 and week 6 post-vaccination, but significance was only achieved in the lung parenchyma (p<0.0001). Similarly, the numbers of M-specific CD8+ T cells in the lung tissue inflated significantly between week 1 and week 6 post-vaccination (p<0.01, Supplementary Figure 1a). As a consequence, more M-specific CD8+ T cells were present in the tissue compared to the blood of mice in the IN group at weeks 6, 16, and 24 post-vaccination (p<0.01). In the IP group, M-specific CD8+ T cell numbers increased between week 1 and week 6 post-vaccination in the blood but not in the tissue (p<0.001, Supplementary Figure 1a). The frequencies of M-specific CD8+ T cells in the spleen were not significantly different between the IN and IP vaccination groups at any time point (Figure 2b). In both groups, significant inflation of the M-specific CD8+ T cell population in the spleen was apparent from week 1 to week 16 post-vaccination (p<0.01). These data demonstrate that IN inoculation with MCMV-M establishes a robust M-specific CD8+ T cell population in the lung parenchyma that is not generated by IP vaccination. In contrast, equivalent systemic responses are induced in the blood and spleen irrespective of the mode of delivery indicating that the increase in local tissue responses by IN vaccination is not at the expense of systemic immunity.
Figure 2. IN vaccination generates more M-specific CD8+ T cells in the lung parenchyma than IP vaccination.
At weeks 1 (W1), 6 (W6), 16 (W16), and 24 (W24) post-vaccination with MCMV-M via the IN or IP route, mice were injected IV with anti-CD45 antibody 5 minutes prior to sacrifice to identify cells in the blood (black) or tissue (grey). M-specific CD8+ T cells were identified by tetramer staining and flow cytometry. (a, b) Percentage of M-specific CD8+ T cells in the tissue and blood of the lungs (a) and spleen (b). (c) Expression of CD69 and CD103 on M-specific CD8+ T cells in the lung tissue at 16 weeks post-vaccination. The percentage of CD8+ T cells in each quadrant is indicated. (d) Total number of CD69+CD103+ and CD69+CD103- M-specific CD8+ T cells in the lung tissue at 16 weeks post-vaccination with MCMV-M or infection with RSV. e) Expression of CD69 and CD103 on M-specific CD8+T cells in the lung tissue at 24 weeks post-vaccination. f) Total number of CD69+CD103+ and CD69+CD103- M-specific CD8+ T cells in the lung tissue at 24 weeks post-vaccination with MCMV-M. Bars represent mean ± SEM with 5 mice per group. **** p≤0.0001 by two-way ANOVA with Tukey’s post-test for multiple comparisons. Data represent two independent experiments.
IN vaccination with MCMV-M induces a tissue-resident memory M-specific CD8+ T cell population
Next, we determined whether the induced tissue-tropic CD8+ T cells expressed CD69, an early activation marker typical of TRM cells, and CD103, an αE integrin commonly used to delineate homing potential. At week 16 and 24 post-vaccination with MCMV-M via the IN or IP route, we evaluated the expression of these markers on M-specific CD8+ T cells identified within the lung parenchyma on the basis of protection from intravascular staining. In the IN group, 61.8% of M-specific CD8+ T cells in the lung parenchyma expressed both CD69 and CD103, while only 0.86% of M-specific CD8+ T cells expressed both markers in the IP group (p<0.0001, Figure 2c). This difference in percentage of TRM was maintained through week 24 post-vaccination (Figure 2e, p<0.0001). We then determined the number of M-specific CD8+ T cells expressing CD69 and CD103 in the lung parenchyma in mice vaccinated IN or IP with MCMV-M and mice infected with RSV. As CD103 is not ubiquitously expressed on TRM cells, we also evaluated CD69 in isolation. We found approximately 1,000-fold more CD69+CD103+ and 100-fold more CD69+CD103− M-specific CD8+ T cells in the lung parenchyma of the IN group compared to the IP group (p<0.0001, Figure 2d). Moreover, IN vaccination with MCMV-M generated significantly greater numbers of CD69+CD103+ and CD69+CD103− M-specific CD8+ T cells compared to RSV infection (p<0.0001, Figure 2d). The number of TRM generated by IN vaccination with MCMV-M was maintained through week 24 post-vaccination (Figure 2f). Similar numbers of these tissue-resident subsets were present after RSV infection and IP vaccination with MCMV-M. These data show that M-specific CD8+ TRM cells in the lung are induced by vaccination with MCMV-M delivered IN but not IP.
IN vaccination with MCMV-M augments the M-specific CD8+ T cell response via the induction of effector and effector memory cells in the lung parenchyma and blood
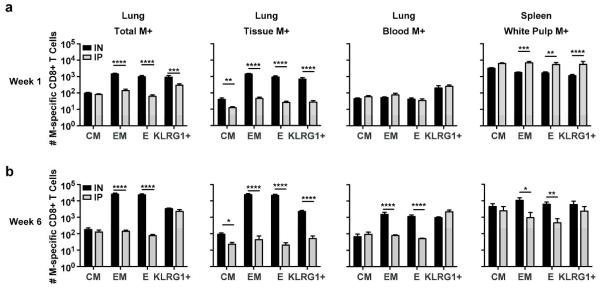
Inflationary memory cells elicited by MCMV are comprised predominantly of KLRG-1+ TEFF cells, together with a smaller subset of TEM cells. We therefore evaluated the memory phenotype of M-specific CD8+ T cells in the tissue and blood of mice vaccinated IN or IP with MCMV-M. At weeks 1, 6, 16, and 24 post-vaccination, M-specific CD8+ T cells were identified by tetramer labeling after intravascular staining and subsequent tissue harvest. Expression of CD44, CD127, KLRG1, and CD62L were determined using flow cytometry. We categorized the M-specific CD8+ T cells into phenotypic subsets as follows: all, CD44+; central memory (CM), CD127+ KLRG1− CD62L+; effector memory (EM), CD127+ KLRG1− CD62L−; effector (E), CD127− KLRG1− CD62L−; and KLRG1+ effector (KLRG1+), CD127− CD62L− KLRG1+. Overall, IN vaccination generated more TEFF and TEM M-specific CD8+ T cells compared to IP vaccination at weeks 1 and 6 (p<0.0001, Figure 3a and b) and weeks 16 and 24 (p<0.01, Supplementary Figure 2). Similar numbers of TCM and KLRG1+ TEFF cells were present at weeks 6, 16, and 24. In the tissue, IN vaccination generated more M-specific CD8+ T cells of all phenotypes at week 1 through week 16 (p<0.05). Although similar numbers of each phenotypic subset were present in the blood at week 1 post-vaccination, there were significantly more TEFF and TEM cells (p<0.0001) in the blood of mice vaccinated via the IN route compared to the IP route at week 6 (Figure 3b) and week 16 (Supplementary Figure 2). IP vaccination generated more TEFF, TEM, and KLRG1+ TEFF cells in the spleen compared to IN vaccination at week 1 (p<0.01). At subsequent time points, however, there were significantly more TEFF and TEM M-specific CD8+ T cells in the spleen in the IN group compared to the IP group (p<0.05). These data indicate that the route of vaccination influences CD8+ T cell phenotype not only in the blood and tissue of the lung, but also in the secondary lymphoid organs.
Figure 3. IN vaccination with MCMV-M augments the M-specific CD8+ T cell response via the induction of effector and effector memory cells in the lung parenchyma and blood.
The memory phenotype of M-specific CD8+ T cells harvested from the indicated sites was determined at week 1 and week 6 post-vaccination with MCMV-M. Mice were injected IV with anti-CD45 antibody 5 minutes prior to sacrifice to identify cells in the blood or tissue. (a) Total number of central memory (CM), effector memory (EM), effector (E), and KLRG1+ effector (KLRG1+) cells at week 1 post-vaccination. (b) Total number of CM, EM, E, and KLRG1+ cells at week 6 post-vaccination. Bars represent mean ± SEM with 5 mice per group. **** p≤0.0001, *** p<0.001, **p<0.01, and *p<0.05 by two-way ANOVA with Tukey’s post-test for multiple comparisons. Data represent two independent experiments.
Inoculation into the lungs is necessary to generate lung-tropic CD8+ T cells
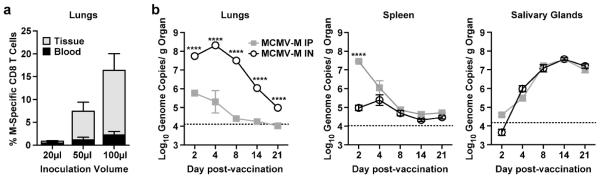
Inoculation volume determines inoculum penetration into the lungs.34, 35, 36 A volume of 20μl will largely remain in the nose, 50μl will enter the upper respiratory tract, and 100μl will enter the lower respiratory tract.34 To determine whether inoculation into the lungs is necessary for the development of tissue-tropic CD8+ T cells, we vaccinated mice IN with 1.2×105 PFU of MCMV-M in a final volume of 20μl, 50μl, or 100μl and determined the percentage of M-specific CD8+ T cells in the lung tissue and blood at week 6 post-vaccination. The tissue-tropic population of M-specific CD8+ T cells was highly dependent on inoculation volume (Figure 4a). Inoculation into the nose (20μl) was not sufficient to generate a response in the lung parenchyma, whereas progressively larger tissue-tropic M-specific CD8+ T cell populations were observed with inoculation volumes of 50μl and 100μl (Figure 4a).
Figure 4. Large volume IN vaccination leads to increased viral replication in the lung and is necessary for the generation of tissue-tropic M-specific CD8+ T cells.
(a) Mice were vaccinated IN with 1.2×105 PFU of MCMV-M in a total volume of 20μl, 50μl, or 100μl. The percentage of M-specific CD8+ T cells in the lung at 6 weeks post-vaccination was determined using tetramer staining and flow cytometry. Bars represent mean ± SEM with 5 mice per group. (b) Quantification of MCMV genome copy number by qPCR in the lung, spleen, and salivary glands of mice infected IN or IP with 6×105 PFU of MCMV-M in 100μl. Error bars represent SEM. Dotted line indicates limit of detection. ****p<0.0001 and ***p<0.001 by two-way ANOVA with Tukey’s post-test for multiple comparisons.
IN vaccination leads to increased MCMV replication in the lung
To test the hypothesis that vector replication is affected by the route of inoculation, we vaccinated mice with 100μl of MCMV-M via the IN or IP route. On days 2, 4, 8, 14, and 21 post-vaccination, DNA was extracted from the lungs, salivary glands, and spleen, and viral loads were determined by qPCR for the IE1 gene.37 Mice vaccinated IN had significantly higher MCMV genome copy numbers in the lungs at all time points (Figure 4b, p<0.0001). IP vaccination was associated with significantly higher viral loads in the spleen on day 2, but no differences were detected on subsequent days. Viral replication in the salivary glands was identical in both vaccination groups. These data demonstrate that the route of administration affects the distribution and kinetics of MCMV replication. As there is greater MCMV replication in the lungs of mice infected via the IN route, it is likely that more antigen is presented at this site during acute infection and that a larger pool of latently MCMV-infected cells persists during chronic infection.
IN vaccination with MCMV-M leads to earlier anti-viral T cell responses after RSV challenge
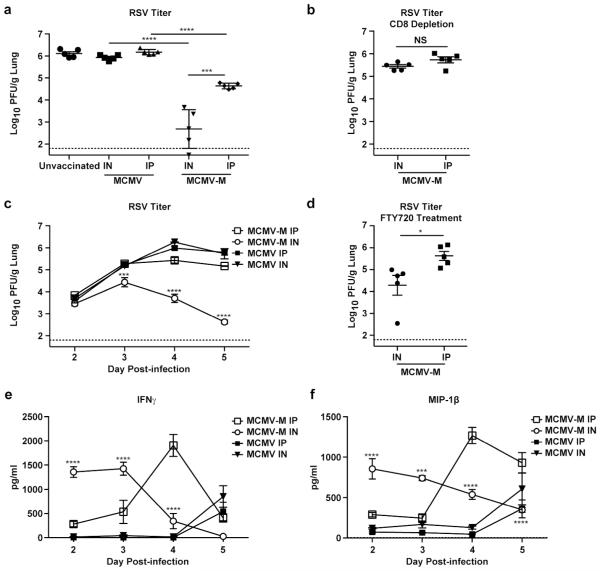
Based on the dramatic differences in tissue localization and CD8+ T cell memory phenotype, we hypothesized that mice vaccinated via the IN route would more readily control RSV infection. At week 16 post-vaccination with MCMV-M or an MCMV control, we challenged mice with RSV IN. On day 5 post-challenge, the lungs were harvested after sacrifice to measure viral load using a plaque assay. Mice infected with the control MCMV via either the IN or IP route had levels of RSV in their lungs similar to those detected in unvaccinated mice. In contrast, vaccination with MCMV-M via either route lead to decreased viral loads compared to controls (p<0.0001, Figure 5a). In addition, mice vaccinated IN with MCMV-M had RSV viral loads that were 100-fold lower compared to mice vaccinated via the IP route (p<0.001). To confirm the role of CD8+ T cells in viral clearance, we injected MCMV-M-vaccinated mice with a depleting anti-CD8 antibody (2.43) on 3 consecutive days prior to RSV challenge. We confirmed depletion of CD8+ T cells in the blood and tissue of the lungs, spleen and the posterior mediastinal lymph node on the day of RSV challenge, and the CD8+ T cell depletion was maintained through 5 days post-RSV challenge (Supplementary Figure 3). When CD8+ T cells were depleted, the difference in viral load between the IN and IP vaccination group was abrogated, indicating the CD8+ T cell responses were responsible for the difference in viral loads between mice vaccinated IN and IP (Figure 5b).
Figure 5. IN vaccination with MCMV-M leads to earlier anti-viral responses compared to IP vaccination.
Mice were challenged with 2×106 PFU of RSV either in the absence of prior vaccination or 16 weeks after IN or IP administration of MCMV or MCMV-M. On days 2-5 post infection, the lungs were harvested for plaque assay and cytokine analysis. (a) Viral loads on day 5 post-infection determined by plaque assay. Dashed line indicates limit of detection. (b) Viral loads on day 5 post-infection after CD8+ T cell depletion. Dashed line indicates limit of detection. NS = not significant. (c) Serial viral loads after challenge with RSV. Dashed line indicates limit of detection. Error bars indicate SEM. Asterisks indicate significant differences between MCMV-M IN and MCMV-M IP. (d) Viral loads on day 5 post-infection after FTY720 treatment. Dashed line indicates limit of detection. (e, f) Concentration of IFNγ (e) and MIP-1β (f) in the lungs determined by multiplex bead-based array after challenge with RSV. Asterisks indicate significant difference between MCMV-M IN and MCMV-M IP. ****p<0.0001, ***p<0.001 and *p<0.05 by one-way ANOVA with Tukey’s post-test for multiple comparisons.
To further support the role of TRM in mediating enhanced protection, we treated mice that had been vaccinated 16 weeks prior with MCMV-M by the IN or IP route with FTY720. FTY720 is a sphingosine-1-phospate receptor 1 agonist and prevents the egress of T cells from lymphoid organs. Treatment with FTY720 reduces the number of recirculating naïve and central memory T cells in the peripheral blood.38 Mice were injected IP with FTY720 daily for 3 days prior to RSV infection and throughout infection. FTY720 treatment significantly reduced the number of CD62L+ M-specific CD8+ T cells in the blood of the lungs, but not in the tissue of mice vaccinated with MCMV-M by the IN or IP route (p<0.05, Supplementary Figure 4b). When vaccinated mice were treated with FTY720 prior to RSV infection, mice vaccinated IN had significantly lower viral titers in lung than mice vaccinated IP further supporting the critical role of tissue-resident M-specific CD8+ T cells in mediating viral control (p<0.05, Figure 5d)
CD8+ TRM cells have been shown to respond rapidly to antigen upon challenge. 6 We therefore hypothesized that IN vaccination, which generates more TRM cells, would lead to earlier anti-viral responses. To evaluate early anti-viral responses, we challenged mice vaccinated 16 weeks previously with MCMV-M or control MCMV. On day 2 through day 5 post-challenge, we harvested the lungs to determine viral load by plaque assay and cytokine levels by bead-based multiplex assay. Mice vaccinated with MCMV-M via the IN route had lower viral loads than mice vaccinated via the IP route at day 3 though day 5 (p<0.001, Figure 5c). In addition, mice vaccinated with MCMV-M via the IP route had lower viral loads at day 4 and day 5 compared to mice vaccinated with control MCMV via either route (p<0.01). However, viral replication relative to controls was suppressed more effectively (p<0.0001) and more rapidly in mice vaccinated IN with MCMV-M. Peak RSV titers were 10-fold lower in mice vaccinated with MCMV-M via the IN route compared to mice vaccinated via the IP route and nearly 100-fold lower compared to mice vaccinated with control MCMV via the IN route (p<0.0001). There were no significant differences in lung RSV titers at any time point after challenge between mice immunized IN or IP with control MCMV.
Next, we evaluated cytokine and chemokine levels in the lungs of vaccinated mice after RSV challenge. Mice vaccinated with MCMV-M via the IN route had significantly higher levels of interferon gamma (IFNγ) and macrophage inflammatory protein-1beta (MIP-1β) on day 2 and day 3 post-challenge compared to mice vaccinated via the IP route (p<0.001, Figure 5e and 5f). Cytokine and chemokine levels decreased by day 4 and day 5 as viral loads decreased. In mice vaccinated with MCMV-M via the IP route, peak levels of IFNγ and MIP-1β occurred on day 4, one day later than IN vaccinated mice. Mice vaccinated with control MCMV did not show increased levels of IFNγ and MIP-1β until day 5 post-challenge with no difference between IN and IP vaccinated groups. These data suggest that the TRM population generated by IN vaccination with MCMV-M responds rapidly on challenge and mediate better control of RSV.
DISCUSSION
In this study, we show that IN administration of an MCMV vector expressing the M protein from RSV generates a robust and durable M-specific CD8+ T cell response. This mode of vaccine delivery drives the accumulation of CD8+ TRM cells in the lung parenchyma, most likely as a consequence of greater antigen expression and viral replication at the site of inoculation. These CD8+ TRM cells mediate early anti-viral responses that rapidly control infection with RSV.
Here we show that local, rather than systemic delivery of a viral antigen, leads to the generation of tissue-resident CD8+ T cells in lung. Pulmonary inoculation has previously been shown to be necessary for the generation of TRM cells in the lung.39, 40 Similar requirements have also been demonstrated for other mucosal tissues, including the intestine and female genital tract.41, 42, 43 Subcutaneous administration of rhCMV elicits CD8+ T cells in bronchoalveolar lavage samples in Rhesus macaques suggesting that other routes of tissue inoculation may also induce TRM.44 IP vaccination with MCMV-M generated an M-specific CD8+ T cell population that was primarily confined to the peripheral circulation, consistent with previously published data showing IP administration of MCMV generates a non-perfusable CD8+ T cell population in the blood supply of the lungs, kidneys, and liver.45 Nonetheless, enhanced local immunity did not occur at the expense of systemic immunity, because equivalent numbers of M-specific CD8+ T cells were located in the spleen after IN vaccination with MCMV-M. Interestingly, most of the M-specific CD8+ T cells in the blood from both IN and IP vaccination do not express CD62L and were not depleted by FTY720 treatment, suggesting that this population does not recirculate, but rather remains resident in the marginating pool. This concept is consistent with a previous study using immune-chimera mice that demonstrated antigen-specific cells located in the vasculature of organs did not appear to recirculate.46
TRM cells are poised to respond quickly to antigen encounter and have been shown to act as immune sentinels via the rapid production of cytokines and chemokines.7, 47 Consistent with these findings, we found that vaccination with MCMV-M via the IN route, which elicited robust populations of TRM cells, led to higher levels of IFNγ and MIP-1β in the lungs of mice at earlier time points post-challenge compared to mice vaccinated via the IP route. These enhanced immune responses mediated earlier clearance of RSV and lower peak viral titers, supporting an important role for CD8+ TRM cells in pathogen control. Although our data demonstrate that CD8+ T cells play a major role in the increased protection seen with IN vaccination, the role of CD4+ T cells in protection has not been elucidated. The RSV M protein also encodes a CD4+ T cell epitope, and the contribution of CD4+ T cells specific for this epitope is currently being evaluated.31
The route of inoculation affected not only the localization but also the memory phenotype of vaccine-induced CD8+ T cells. In mice vaccinated via the IP route, M-specific CD8+ T cells displayed a predominant KLRG1+ TEFF phenotype. These findings are consistent with previously published data showing that inflationary memory populations derive mainly from short-lived KLRG1+ TEFF cells that are constantly replenished.45 However, when we administer the same vector by the intranasal route, there is a significant shift in the phenotype of cells elicited to the same epitope. In mice vaccinated via the IN route, there were similar numbers of KLRG1+ TEFF and TCM cells, but also much larger populations of TEFF and TEM cells across all anatomical locations. These populations may possess a better capacity to respond to antigen re-exposure or longer lifespans since they do not express KLRG1. These data emphasize the critical role of the local environment of antigen-presentation in the character of the immune response.
Steinert et al. recently demonstrated that CD8+ T cell isolation from the tissue is biased by phenotype and location.46 As our data compare two different vaccination routes and all samples were treated identically, it is unlikely that our conclusions will be affected by such technological constraints although we may underestimate the number of M-specific CD8+ T cells. In addition, we were able to evaluate multiple parameters by flow cytometry, which allowed us to probe differences in memory phenotype that may have been missed by microscopy.
While most vaccination schedules require multiple doses or separate prime/boost constructs to minimize anti-vector immunity, CMV vectors encoding inflationary epitopes may require only a single dose to generate robust, durable protection. In particular, TRM populations in the lung have been shown to wane over time.39, 48 In contrast, IN vaccination with a single dose of MCMV-M generated a long-lived TRM population, potentially overcoming the problem of waning immunity in the lung. CD8+ T cells elicited by CMV infection do not assume an exhausted phenotype, but instead remain functional and protect in vivo.25, 26, 49 Moreover, persistent antigen expression leads to the generation of CD8+ TEFF and TEM cells, which respond more rapidly compared to the TCM cells typically generated by acute vectors.
Collectively, we demonstrate that local delivery of a persistent MCMV vector results in a high magnitude of CD8+ effector TRM cells with a single dose leading to early viral control and reduced viral loads. Sustained TRM populations will likely be a key feature of vaccines that protect against infectious diseases that are dependent on T cell-mediated clearance for protection such as tuberculosis, malaria, and HIV.
METHODS
Mice
Age-matched (6-10 weeks) female CB6F1/J mice (Jackson Laboratories, Bar Harbor, ME, USA) were used in all experiments. Mice were housed in the animal care facility at the National Institute of Allergy and Infectious Diseases under specific-pathogen-free conditions and maintained on standard rodent chow and water supplied ad libitum. All studies were reviewed and approved by the National Institutes of Health Animal Care and Use Committee.
Cell lines
MEF (SCRC-1008) cell lines were obtained from the ATCC and cultured in Advanced DMEM (Invitrogen, Grand Island, NY, USA) containing 8% or 10% fetal bovine serum (FBS) or neonatal calf serum, 2mM glutamine, 10U/ml penicillin, 10μg/ml streptomycin, and 0.1M HEPES. HEp-2 cells were grown in Eagle’s minimal essential medium supplemented with 10% FBS, 2mM glutamine, 10U/ml penicillin, 10μg/ml streptomycin, and 0.1M HEPES.
Viruses and infections
Recombinant MCMVs were made using a bacterial artificial chromosome (BAC) system. 21 The RSV M sequence was inserted into the IE2 gene of the K181Δm157 strain of MCMV by two-step allele replacement. BACs were extracted from E. coli using a NucleoBond Xtra Maxi Prep Kit (Macherey-Nagel, Bethlehem, PA, USA). MEF cells were transfected with recombinant BACs via calcium phosphate precipitation (Clonetech, Mountain View, CA, USA). Viruses were passaged and single plaques isolated by serial dilution. Single plaques were selected based on excision of the BAC cassette by loss of GFP, and the presence of the RSV M gene was confirmed by PCR. MCMV stocks were produced by sonication of infected MEF cells. Mice were infected IN or IP with 6×105 PFU of recombinant MCMV-M viruses in 100μl of 10% FBS DMEM, unless otherwise noted. RSV stocks for challenge were generated from the A2 strain of RSV by sonication of infected HEp-2 monolayers.34 Mice were challenged IN with 2×106 PFU of RSV in 100μl of 10% MEM. All IN inoculations were performed following anesthesia with isoflourane (3%). Mice were euthanized by lethal overdose with pentobarbital (250mg/kg).
Intravascular staining and flow cytometry
For intravascular staining, mice were injected intravenously with 3μg of anti-CD45 antibody (BD Biosciences, San Jose, CA, USA). Lungs and spleen were harvested after euthanasia with pentobarbital (250mg/kg). Lymphocytes were isolated by physical disruption of tissue using a GentleMACs machine (Miltenyi Biotec, San Diego, CA, USA) followed by Fico-LITE density gradient centrifugation. Isolated mononuclear cells were washed with PBS and resuspended in FACS staining buffer (PBS + 1%FBS + 0.05% sodium azide). Cells were stained with fluorochrome-labeled antibodies for the lineage markers CD3 (145-2C11) and CD8 (53-6.7) and the phenotypic markers CD44 (IM7), CD62L (MEL-14), CD127 (A7R34), KLRG-1 (2F1/KLRG1), CD69 (H1.2F3), and CD103 (M290) (BD Biosciences or BioLegend, San Diego, CA, USA). The amine viability dye AquaBlue (Invitrogen) was used to identify dead cells. Antigen specificity was determined using DbM187-195 tetramers conjugated to APC (MBL, Woburn, MA, USA). All samples were stained with a pre-mixed antibody cocktail for 20 min at 4°C. Data were collected using an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar San Carlos, CA, USA).
RSV plaque assay and cytokine analysis
Plaque assays were performed as described previously. 50 Lungs were harvested, weighed, and frozen quickly in 10% MEM supplemented with 2mM glutamine, 10U/ml penicillin, and 10μg/ml streptomycin. After thawing, lung tissue was dissociated using the GentleMACs machine on program lung_02 (Miltenyi). Cell suspensions were pelleted to remove cellular debris. Supernatants were serially diluted and inoculated on 80% confluent HEp-2 cell monolayers in triplicate. Cells were incubated for 1 hr at room temperature and overlaid with 1% methyl cellulose in 10% MEM. After 4 days at 37°C, cells were fixed with 10% buffered formalin and stained with hematoxylin and eosin. Plaques were counted and expressed as Log10 PFU/gram of lung tissue. The limit of detection was 1.8 Log10 PFU/gram. For cytokine analysis by multiplex bead-based array, supernatants were sent to AssayGate (Ijamsville, MD, USA).
CD8+ T cell depletion and FTY720 Treatment
Mice were vaccinated with MCMV-M via the IN or IP route. After 16 weeks, 200μg of anti-CD8 antibody (clone 4.23) in a total volume of 200μl was injected IP for 3 consecutive days for CD8+ T cell depletion studies. On the third day, mice were challenged IN with 2×106 PFU of RSV. Five days after challenge, the left lobe of the lung was harvested for plaque assay. For FTY720 experiments, mice which had been vaccinated with MCMV-M via the IN or IP route 16 weeks prior, were given daily IP injections of FTY720 at a dose of 1mg/kg (Sigma-Aldrich, St. Louis, MO, USA) beginning 3 days prior RSV infection until euthanized. On the fourth day of FTY720 treatment, mice were challenged with RSV as described above.
Real-time quantitative PCR
Samples were processed as described above for the plaque assay. DNA was extracted from 200μl of supernatant using a Nucleospin Blood Kit (Clontech). Real-time qPCR was performed for the MCMV IE1 gene as described previously 37.
Statistical analysis
Statistical analyses were performed using one-way or two-way ANOVAs in GraphPad Prism (La Jolla, CA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Brenda Hartman for help with figure preparation. This work was supported by Intramural funding from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. DAP is a Wellcome Trust Senior Investigator.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol. 2014;5:320. doi: 10.3389/fimmu.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 3.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 4.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 5.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol. 2015;195(1):203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11(4 Suppl):S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78(11):5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 12.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney R, Nakai T, Parkins CJ, Caposio P, Fairweather NF, Sesardic D, et al. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine. 2012;30(20):3047–3052. doi: 10.1016/j.vaccine.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S, et al. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine. 2015;33(19):2261–2266. doi: 10.1016/j.vaccine.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Messerle M, Sapinoro R, Santos K, Hocknell PK, Jin X, et al. Murine cytomegalovirus abortively infects human dendritic cells, leading to expression and presentation of virally vectored genes. J Virol. 2003;77(13):7182–7192. doi: 10.1128/JVI.77.13.7182-7192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beverley PC, Ruzsics Z, Hey A, Hutchings C, Boos S, Bolinger B, et al. A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J Immunol. 2014;193(5):2306–2316. doi: 10.4049/jimmunol.1302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis. 2011;5(8):e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, et al. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother. 2012;35(5):390–399. doi: 10.1097/CJI.0b013e3182585d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu G, Smith T, Grey F, Hill AB. Cytomegalovirus-based cancer vaccines expressing TRP2 induce rejection of melanoma in mice. Biochem Biophys Res Commun. 2013;437(2):287–291. doi: 10.1016/j.bbrc.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, et al. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. J Virol. 2005;79(5):2998–3008. doi: 10.1128/JVI.79.5.2998-3008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170(4):2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 23.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35(4):1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 24.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtappels R, Thomas D, Podlech J, Reddehase MJ. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J Virol. 2002;76(1):151–164. doi: 10.1128/JVI.76.1.151-164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, et al. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78(5):2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177(1):450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 28.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 29.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74(24):11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Ruckwardt TJ, Chen M, Johnson TR, Graham BS. Characterization of respiratory syncytial virus M- and M2-specific CD4 T cells in a murine model. J Virol. 2009;83(10):4934–4941. doi: 10.1128/JVI.02140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Haddad EK, Marceau J, Morabito KM, Rao SS, Filali-Mouhim A, et al. A Numerically Subdominant CD8 T Cell Response to Matrix Protein of Respiratory Syncytial Virus Controls Infection with Limited Immunopathology. PLoS Pathog. 2016;12(3):e1005486. doi: 10.1371/journal.ppat.1005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26(2):153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 35.Southam DS, Dolovich M, O'Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L833–839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 36.Miller MA, Stabenow JM, Parvathareddy J, Wodowski AJ, Fabrizio TP, Bina XR, et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One. 2012;7(2):e31359. doi: 10.1371/journal.pone.0031359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang-Feldman YJ, Wojtowicz A, Lochhead GR, Hale MA, Li Y, Pomeroy C. Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection. J Virol Methods. 2006;131(2):122–129. doi: 10.1016/j.jviromet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6(13-14):1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95(2):215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189(6):2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122(12):4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184(5):1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci U S A. 1998;95(4):1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog. 2014;10(7):e1004233. doi: 10.1371/journal.ppat.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161(4):737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14(5):509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson KG, Masopust D. Editorial: Pulmonary resident memory CD8 T cells: here today, gone tomorrow. J Leukoc Biol. 2014;95(2):199–201. doi: 10.1189/jlb.0913493. [DOI] [PubMed] [Google Scholar]
- 49.Pahl-Seibert MF, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, et al. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J Virol. 2005;79(9):5400–5413. doi: 10.1128/JVI.79.9.5400-5413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7(12):e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.