Abstract
We present evidence of a novel form of group hunting. Individual sailfish (Istiophorus platypterus) alternate attacks with other group members on their schooling prey (Sardinella aurita). While only 24% of attacks result in prey capture, multiple prey are injured in 95% of attacks, resulting in an increase of injured fish in the school with the number of attacks. How quickly prey are captured is positively correlated with the level of injury of the school, suggesting that hunters can benefit from other conspecifics' attacks on the prey. To explore this, we built a mathematical model capturing the dynamics of the hunt. We show that group hunting provides major efficiency gains (prey caught per unit time) for individuals in groups of up to 70 members. We also demonstrate that a free riding strategy, where some individuals wait until the prey are sufficiently injured before attacking, is only beneficial if the cost of attacking is high, and only then when waiting times are short. Our findings provide evidence that cooperative benefits can be realized through the facilitative effects of individuals' hunting actions without spatial coordination of attacks. Such ‘proto-cooperation’ may be the pre-cursor to more complex group-hunting strategies.
Keywords: group hunting, sailfish, Istiophorus platypterus, cooperation, proto-cooperation
1. Introduction
Group hunting is a fascinating example of social behaviour that can be observed in taxonomic groups including arthropods [1–3], fishes [4–8], birds [9,10] and mammals [11,12]. The level of coordination between individuals during hunts, both within and between these taxa, varies considerably. In its simplest form, group hunting involves hunters attacking prey together with little or no coordination of attacks while the most complex form, collaborative hunting, involves individuals adopting specific hunting roles to herd and catch their prey [13–15].
Explaining the origins and maintenance of group hunting, however, remains unresolved. Despite group hunting allowing some species to catch considerably larger prey [13,16] as well as increasing the likelihood of making a kill [16], individuals do not necessarily increase the amount of prey they consume when hunting together (compared with when hunting alone). For example, food intake per individual wolf (Canis lupus) can be lower in larger packs compared with smaller hunting groups or lone individuals [17], and lions (Panthera leo) do not always hunt in group sizes that optimize the amount of prey they consume [18]. Other reasons, therefore, may explain the existence of group hunting in some taxa. For example, individuals in groups may be better at limiting the access of kleptoparasites to the kill, may travel less distance, and may have a reduced likelihood of being injured during group hunts, compared with when hunting alone [16,19,20].
When hunters attack smaller grouping prey, the reasons for group hunting appear clearer. In some cases, group hunters use their superior speed and coordinated attacks to disrupt and fragment prey groups [4,21,22]. Groups of piscivorous fishes, for example, have a higher probability of breaking up prey schools and capture more prey than single attackers [21]. Groups of humpback whales (Megaptera novaeangliae) employ bubble-nets to capture schooling fishes [23,24] and various dolphin species have been described to use cooperative hunting strategies [25]. Raptors have similarly been observed to use spatially coordinated attacks to hunt flocking passerines [9,10]. Spatially coordinated attacks appear to break down the collective defences of grouping prey, thereby increasing consumption rates for group hunters. But how did these more complex coordinated attacks evolve from simpler forms of group hunting?
In their simplest form, apparent group hunts may simply be a by-product of clumped prey distribution, when hunters join others by eavesdropping on the cues produced from hunters finding ephemeral food patches [11]. Cattle egrets (Bubulcus ibis), for example, aggregate where prey are highly abundant, with feeding rates and prey density being closely linked [26]. In these cases, it is unclear whether the presence of other hunters benefits individuals' hunting success. In other cases, the presence of other hunters can increase hunting success, even though hunters' attacks are not coordinated in space. Lionfish (Dendrochirus zebra) alternate attacks on schooling prey and catch more prey when hunting in pairs than when alone [5]. Group hunting in a weakly electric fish (Mormyrops anguilloides) does not appear to be spatially coordinated, and instead hunters may benefit from prey fleeing in their direction when prey escape another hunter's failed attack [27]. Black headed-gulls (Larus ridibundus) capture twice as many fishes when hunting in groups of six than when hunting alone, even though attacks are uncoordinated [28]. If individuals can benefit from the hunting actions of others without spatial coordination of attacks, then these group hunts could explain the origins of more complex group hunting strategies. But the mechanisms allowing increased capture rates for individuals with uncoordinated attacks remain unclear. One possibility is that the alternation of attacks gives hunters the opportunity to save energy, while others exhaust and injure the prey. This could allow individuals to benefit from increased capture success during later attacks if it is easier to catch tired, injured prey. Here, we investigate whether such a ‘proto-cooperative’ strategy could benefit individual hunters in groups. We investigated group hunting sailfish (Istiophorus platypterus) that alternate their attacks on schooling sardine prey (Sardinella aurita) [29,30]. Attacks by sailfish appear to be uncoordinated in space, and one sailfish will abandon its attack if another individual attacks the school at the same time.
We first used behavioural observations and image analysis to systematically quantify the group hunting strategies of sailfish, which can only be done in the wild. This puts strong constraints on the type and quantity of data we could record. Therefore, to complement our empirical work, we used a mathematical model to test whether the attack-alternation strategy we observed could be effective at allowing predators to increase their capture success beyond that possible for a solitary sailfish. We hypothesized that group hunting would allow individuals to capture more sardines per unit time using this strategy compared with if they hunted alone. Further, we evaluate the predator group sizes where this attack-alternation strategy is beneficial over solo hunting under different hunting conditions. We also investigate whether this form of group hunting is likely to be exploited by free riders, i.e. individuals that wait until the school is sufficiently injured before attacking.
2. Empirical material and methods
Research was conducted 30–70 km offshore Cancun in the Gulf of Mexico (N 21 28.3–41.15, W 86 38.41–41.30). We observed group-hunting sailfish separating smaller schools of sardines from larger ones containing thousands of fish. The sailfish then herded these smaller schools to the surface where the last stage of the hunt occurred. Under snorkel, we used Casio EX-FH100 cameras (operating at 240 fps) to record these smaller sardine schools that were being attacked by the sailfish. We visited this site once a year for 5 years to record the hunting behaviour of sailfish. However, we could only perform the school injury analyses (see below) in videos when sky conditions were overcast (because we required the light to be evenly distributed across the schools). This restricted the amount of data we could use. In total, our analyses are based on 63 min of video from 2012 documenting these interactions. As we did not observe some of the behaviours and could not calculate some of the measures for all schools (n = 8 in total), we report the number of schools used in each analysis below.
(a). Attack and capture rates
During an attack, sailfish use their rostra to facilitate prey capture by slashing or tapping the sardines [30]. From the videos, we recorded the number of these attack events (n = 210 attacks across all schools) as well as the number of successful prey capture events (n = 51 across all videos). By dividing the total number of captures by the length of the video recordings we had recorded of particular schools, we determined a capture rate for each school (n = 7 schools; note, we did not observe any attacks on one of the schools we recorded). We recorded the number of sardines that the bill hit during these attacks (taps or slashes). This represents the minimum number of fish hit during these attacks because some hits may not have been visible from the camera angle. In 52 out of the 210 attacks, we could not see how many fish were hit and these events were excluded from analysis. We also determined whether we could see if some of the sardines' scales were removed during the attacks. Scale removal indicates injury to the sardines (movie S1). Sometimes it was not clear whether scales were removed or not during an attack (owing to subsequent obstruction by other fish), and therefore these ambiguous events (n = 61 attacks out of 210) were not included when calculating the proportion of fish that were injured during an attack.
(b). Proportion of the school that was injured
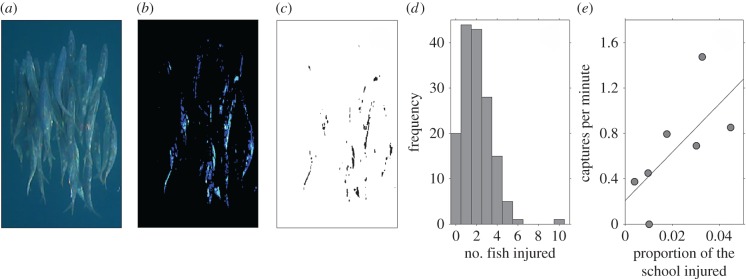
We investigated what proportion of each school had injuries. We selected 39 video stills where light contrast across the schools was minimal. For this analysis, we only selected multiple images from the same video if there was at least 1 min between the two frames of interest (see the electronic supplementary material, table S1 for the number of images used for each school). The marks on the sardines caused by injuries from the bill have a distinctive white/pinkish appearance, different from other parts of the fish's bodies or surrounding water (figure 1a). This allowed us to use image analysis to determine how injured the fish were in the school. To perform this analysis, we marked a polygon around the edge of the school, and then cleared all pixels from outside the marked polygon (setting their grey-scale intensity to 255). We then adjusted the brightness and contrast of each image so that only the injury marks on the fish became pronounced. By adjusting the brightness and contrast for each image appropriately (figure 1b), we could then binary threshold the images to reveal the pixels in each image where the injuries had occurred (figure 1c). Note that because the average intensity of each image differed, we had to adjust the brightness and contrasts of each image manually. We imported the binary converted images into Matlab (2012b). Each image was represented by a matrix where cells equal to zero (black pixels) were injured parts of the school, and cells equal to 255 (white pixels) were uninjured parts of the school. By counting the number of values in the matrix equal to zero, and then by dividing this total by the area of the school calculated by the polygon in ImageJ, we determined the proportion of pixels in the school depicting injuries. We determined the mean proportion of injuries of a school if that school had been measured in multiple images (see the electronic supplementary material, table S1). We note that this semi-automated analysis provides information on a general level of injury, which combines both the frequency and severity of injury into one variable.
Figure 1.
(a) Original frame taken from one of the videos showing the white injury marks on the sardines caused by damage from sailfish bills. (b) The original image has its contrast and brightness adjusted before binary thresholding (c), which reveals the injuries on the sardines. (d) The minimum number of sardines that were hit with the bill during a sailfish's attack (determined for 158 attacks). (e) Relationship between the proportion of the school that was injured and the sailfish capture success rate on the prey schools. Solid line represents the least-squares regression; y = 21.4x + 0.21.
3. Empirical results
(a). Sailfish group hunting behaviour
Sailfish were observed in groups of approximately 6–40 individuals hunting sardine schools (n = 8) that differed in number from approximately 25 to 100–150 fish (electronic supplementary material, S1.1 and figure S1a). Owing to observational limitations, we could not determine the exact number of sailfish that were hunting each sardine school. Different sailfish alternated their attacks on the sardine schools (movie S2). Individual identification of all sailfish was not possible, and therefore we could not determine the order in which individual sailfish attacked the prey school. The median time between consecutive approaches by different sailfish was 6.5 s (electronic supplementary material, S1.2 and figure S1b). There was no relationship between the time between approaches and the sardine school size (Spearman's correlation; ρ = 0.11, n = 7, p = 0.84). The median length of individuals' attacks was 2.6 s, but again, this was not related to school size (Spearman's correlation; ρ = −0.21, n = 7, p = 0.66). A sailfish's attack was interrupted by another sailfish 19% of the time, after which either one or both sailfish would abandon their attack.
During attacks, sailfish used their bills to ‘tap’ or ‘slash’ at the sardines in an attempt to capture individual fish (movie S1) [29]. Only 24% of these attacks resulted in a successful capture and we never observed a sailfish to handle or ingest two or more fish at once. However, both the mean and the median number of sardines that were hit with a sailfish's bill during an attack was 2.0 (figure 1d). While the attacks with the bill were very rarely observed to kill the sardines outright, sardines' scales were removed when contact was made between the sailfish's bills and the sardines' bodies in 95% of cases. Because more fish were injured per attack than were caught, this led to many sardines in the schools having pronounced injuries on their bodies, accumulated from past attacks (figure 1a). The most heavily damaged fish had over 20% of their body covered in injuries (electronic supplementary material, S1.3 and figure S1c).
We observed successful captures (n = 51) on six of the eight sardine schools we recorded. Sailfish caught individual sardines at an average rate (across all the schools) of 0.66 ± 0.17 s.e. sardines per minute. By quantifying the proportion of the school that was injured (figure 1b,c), we found a positive correlation between the school's injury level and the capture rate (Spearman's correlation; ρ = 0.82, n = 7, p = 0.03; figure 1e); sardines in more injured schools were captured more quickly. Given the observational constraints, we could not determine whether it was the most injured fish in the shoal that were captured next; however, we often observed injured sardines breaking off from the prey schools that presumably could not keep pace with the school. These individuals were quickly captured by the sailfish (movie S3). There were non-significant negative correlations between capture success rates and school size (Spearman's correlation; ρ = −0.54, p = 0.24, n = 7), and between the proportion of the school that was injured and school size (Spearman's correlation; ρ = −0.69, p = 0.07, n = 8; see the electronic supplementary material, S1.4 and S1.5 for a discussion of these results).
4. Group hunting model
From the empirical information above, it appears that sailfish increase their capture rates as prey become progressively injured from previous attacks. But this does not explain why they hunt in groups, as a solitary hunter could get these benefits by hunting on its own. To better understand why sailfish hunt in groups, therefore, we built a simple mathematical model to capture the dynamics of the hunt. We chose to model group hunting using a non-spatial, individual-based model. On the one hand, this model effectively accounts for the fundamental temporal ‘mechanics’ of the hunt observed in the field, and on the other hand is open to a full analytical investigation of its dynamics. Our model allows us to systematically investigate the rates at which sailfish catch sardines in different predator group sizes. It also allows us to explore potential differences in the strategies predators could use during the hunt. A general advantage of an individual-based approach is that our model can be easily extended to incorporate more additional features, such as agent heterogeneity or stochastic effects.
We consider a group of N = const. predators (sailfish), hunting a group of initially S(t = 0) = S0 prey (sardines). The number of sailfish observed hunting in groups was N = 6–40; however, group sizes exceeding 50 individuals have been previously reported. Therefore, in our model we studied a range of group sizes from solitary hunters N = 1 up to a group size N = 100. The prey schools from our empirical observations ranged from 25 to 150 sardines. However, we have no information about the number of sardines that were initially separated from the school containing thousands of fish during the initial stages of the hunt. In our model calculations, therefore, we set the initial number of sardines to be larger, but in the same order of magnitude, as the largest groups observed: S0 = 200.
Basic biomechanics predict that small prey (sardine average body length is approximately 19 cm [30]) are more manoeuvrable than larger predators [31,32] (sailfish are approximately 200–250 cm). If the sardines can perform one or more sharp turns, removing a sailfish's potential for attack, then a sailfish is likely to abandon its attack owing to its lower manoeuvrability. Meanwhile, this gives another sailfish an opportunity to initiate its attack sequence. In our model, therefore, each predator needs a finite time to perform an attack, τa, and after an attack it requires a finite time, τr, to be ready for the next attack. τa represents the time where an individual sailfish ‘monopolizes’ the prey school by performing its approach, manoeuvre and attack. Here, we set it to the median attack time observed for hunting sailfish; τa = 2.6 s. τr describes the average time required by an individual hunter to prepare for the next attack sequence, i.e. for the sailfish to assume a suitable position at the rear of the prey school. This time is not available from our observations, as it requires repeated observations of a solitary sailfish hunting a sardine school. However, a reasonable timescale can be estimated from qualitative observations of the hunting process and other timescales, as well as from the assumed cooperative benefits of the hunt. Here, we reasonably assume that τr is larger than τa, and significantly shorter than 1 min. Therefore, we use as a default parameter τr = 20 s. Note that while the attack and preparation times may vary, only their average values, τa and τr, determine the conditions where group hunting is beneficial (see the electronic supplementary material, figure S2 for an exploration of how τa and τr determine these conditions).
A single predator requires the time Δtsingle = τa + τr for a full attack cycle: ‘perform attack’ (τa) and ‘prepare for next attack’ (τr). Thus, it attacks on average only once during this time interval and the number of attacks scales linearly with time na(t) = t/Δtsingle. If we have more than one predator, the average time interval between two attacks by a focal individual depends on the number of predators N, whereby two cases have to be distinguished: (i) if N is small, then on average all other hunters can perform their attacks within the time required by the focal individual to prepare for the next attack, and the average time interval between initiation of subsequent attacks for the focal individual is simply τa + τr. (ii) If N is larger than 1 + τr/τa, then the focal individual will typically have to wait until other, better prepared individuals have performed their attacks. If we assume that at any time the individual with the longest waiting time will attack next, then typically all other hunters will perform their attacks between two subsequent attacks of the focal individual and the corresponding time interval becomes Nτa. In summary, therefore, the average waiting time between two attacks for an individual predator can be expressed as
Using this we can calculate the average number of attacks na,s(T, N) an individual predator performs until time T in a group of size N (electronic supplementary material, figure S3). Note that, T, can be interpreted simply as the time available for hunting, and is different from the actual time required to hunt down a school of sardines, Ttot (see the electronic supplementary material, figure S4 for an exploration of how Ttot changes depending on the hunters' group size). The average number of attacks performed by single hunter at time T in a group of N is given by
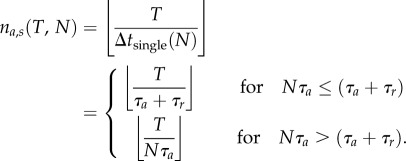 |
2.6 |
Here,  indicates the floor function as
indicates the floor function as 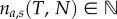 .
.
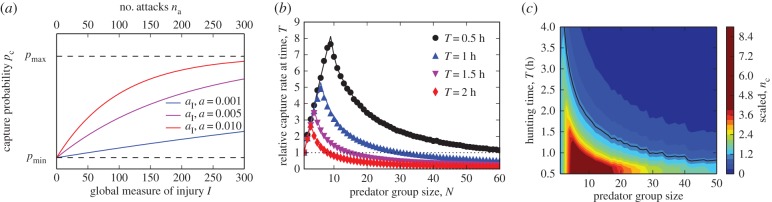
An attacking predator has the probability pc to catch a single prey. During each attack, there is also a chance that prey are injured. As it is unclear how the injuries are distributed among individuals, we introduce a global measure of injury in the prey school I. In the empirical data, we found a correlation between the level of injury of a school and the capture success rate (see Results). This suggests that the capture probability is a monotonically increasing function of the injury level in the prey school, pc = pc(I) (figure 2a). The capture probability pc(I) can never exceed 1, thus it has to approach pmax ≤ 1 for I → ∞. Using this, and assuming that the global injury level increases linearly with the number of attacks, we may rewrite the probability of capture as a function of the number of attacks na: pc(I) → pc(na) (see figure 2a, and the electronic supplementary material, S2.1). We have also checked that a nonlinear dependence of I on na does not qualitatively affect our findings (see the electronic supplementary material, S2.2).
Figure 2.
(a) Capture probability versus the global measure of injury of the prey school and the number of attacks for different values of the injury growth constants aI, a (see electronic supplementary material, equations S1 and S2 for details) assuming ΔI = 1. The dashed lines indicate pmin and pmax. (b) Group hunting model: number of prey captured per individual as a function of N scaled by the number of prey captured for a solitary predator (horizontal dotted line). The largest group sizes, which offer an advantage to solitary hunting, are typically observed for short times T ≤ 1 h and decrease for longer times (or small prey schools). (c) Theoretical prediction on number of fish captured per predator versus predator group size for varying hunting times T. Again, nc is normalized by the number of prey captured by a solitary predator. Solid line shows the contour corresponding to the value of nc = 1 (same as solitary hunter) and represents therefore the border of the region where group hunting is beneficial. Default model parameters in all panels if not varied or otherwise stated: τa = 2.6 s, τa = 20 s, a = 5 × 10−4, pmin = 0, pmax = 1, S0 = 200.
During the full cycle of the focal individual (‘attack sequence’ + ‘preparation/waiting time’) on average, N attacks take place, which increases the injury of the prey and therefore the capture probability. The number of attacks performed by all hunters can thus be expressed as na = iN, with i being the number of the attacks by a focal individual and N being the group size. We can calculate the expected number of prey captured by a focal predator at time T by summing over all the capture probabilities pc(iN) during its subsequent attacks i. Here, we have to take into account that the total school size imposes an upper limit on the possible number of fish caught, which is simply the average number of prey per predator S0/N. Thus, the expected number of prey captured per predator is
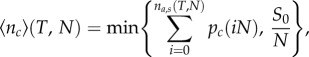 |
4.1 |
with na,s(T, N) being the number of attacks performed by the focal individual in a group of N hunters, up to T (the time available for hunting). We have explored the model's behaviour with different parameters, and whereas quantitative results might differ, the overall results appear surprisingly robust and the qualitative predictions remain unchanged.
(a). Group hunt simulations
In order to test our theoretical predictions, we performed numerical simulations of a simple individual-based model. N hunters perform subsequent attacks on a school of S(t) sardines, with the initial school size being S(t = 0) = S0. The attack sequence of each hunter has a fixed duration τa. The attack may lead to a successful capture of a single prey with probability pc(na), which is a function of the number of all previous attacks on the school according to equation S2 in the electronic supplementary material. A simulation run is terminated when all prey are captured S(t) = 0. The preparation time for the next attack for each hunter is τr. The initial attack order is set randomly. As time progresses, the next attack is performed by the individual with the longest waiting time. For a fixed attack duration τa and preparation time τr, the initial attack order of the hunters remains unchanged. All results are obtained by averaging 100 independent simulation runs. We confirmed that our results are robust with respect to random attacks and preparation times, which introduces additional stochasticity and randomizes the order of the hunters within a single run (see the electronic supplementary material, S2.3 for details).
(b). Are there benefits for free riders?
This form of group hunting immediately raises questions surrounding the existence of producers and free riders in groups. Producers (hunters that begin attacking from the start of the hunt) generate a public good where higher levels of prey injury leads to higher capture success rates. There is the potential, therefore, for free riders (individuals that delay their attacks for some time until the school is sufficiently injured) to avoid paying the costs of attacking at the beginning of the hunt where the initial capture probability is low, and profit from the higher capture probability at later stages of the hunt.
In order to explore possible fitness trade-offs in terms of the energy expenditure versus energy uptake, we combined the stochastic individual-based model with an energetic balance equation (see the electronic supplementary material, S2.4). We consider an ‘optimal’ situation of being a single free rider hunting with N − 1 producers. The free rider refrains from attacking prey at the beginning of the hunt for a time Tfr (attack delay time). Using the energy payoffs an individual receives during the hunt, Δetotal,i, we can calculate the relative energy payoff of an individual fi within a population:
| 4.2 |
which scales between 0 for minimal energy payoff and 1 for maximal energy payoff. Here, Δetotal,i is a function of a sailfish's base energy expenditure (the energy needed to simply remain with the prey school), the energy required to perform attacks, and the energy received by the captures it makes during a hunt (see the electronic supplementary material, S2.4).
In order to assess possible energy benefits of free riders, we calculated the difference between the average relative energy payoff of free riders and producers:
| 4.3 |
Positive values indicate an advantage for the free riding strategy, whereas negative values indicate on average higher energy payoffs for the producers. All results discussed were obtained by simulating 100 independent runs for each group size N and attack delay time Tfr.
5. Modelling results
If the time available for hunting T → ∞, the expected number of sardines caught 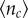 is always equal to S0/N, and always has a maximum at N = 1. Hence, if time is not a limiting factor, then it is always better for a predator to hunt alone because it would not have to share prey with conspecifics. However, predators may attempt to maximize how many prey they catch per unit time (i.e. the capture rate), and not just the absolute amount of prey they catch (see the electronic supplementary material, S1.6). Under this scenario, it may not be beneficial to hunt alone. By performing numerical simulations of the model, we determined the conditions where group hunting can improve capture rates for individual sailfish. Figure 2b,c shows the number of fish captured per predator as a function of group size N, scaled by the number of prey a solitary hunter (N = 1) would have caught at that time (see the electronic supplementary material, figure S5 for unscaled values). In this way, we can identify the maximum group size Nm, where each individual outperforms a solitary hunter. This depends strongly on the available hunting time. While the optimal group sizes that maximize prey intake rates per hunter are small (10 when hunting times are short (T = 0.5 h) to 3 when hunting times are long (T = 2 h)), the group sizes where group hunting outweighs hunting alone are typically much larger. For T = 0.5 h, we observe Nm = 70, which then quickly decreases to Nm = 30 for T = 1 h and Nm = 13 for T = 1.5 h. Eventually, for T → ∞, Nm will always converge to 1 owing to the finite size of the school. At short times T, Nm is always larger than 1 + τr/τa, which is the group size at which the individual hunters start to pay temporal costs of group hunting (electronic supplementary material, figure S3). We checked how changing the initial number of sardines in the prey school, S0, affected the conditions under which group hunting was beneficial. Smaller (or larger) initial prey group sizes shifted the hunting times so that shorter (or longer) times T made group hunters outperform solitary hunters.
is always equal to S0/N, and always has a maximum at N = 1. Hence, if time is not a limiting factor, then it is always better for a predator to hunt alone because it would not have to share prey with conspecifics. However, predators may attempt to maximize how many prey they catch per unit time (i.e. the capture rate), and not just the absolute amount of prey they catch (see the electronic supplementary material, S1.6). Under this scenario, it may not be beneficial to hunt alone. By performing numerical simulations of the model, we determined the conditions where group hunting can improve capture rates for individual sailfish. Figure 2b,c shows the number of fish captured per predator as a function of group size N, scaled by the number of prey a solitary hunter (N = 1) would have caught at that time (see the electronic supplementary material, figure S5 for unscaled values). In this way, we can identify the maximum group size Nm, where each individual outperforms a solitary hunter. This depends strongly on the available hunting time. While the optimal group sizes that maximize prey intake rates per hunter are small (10 when hunting times are short (T = 0.5 h) to 3 when hunting times are long (T = 2 h)), the group sizes where group hunting outweighs hunting alone are typically much larger. For T = 0.5 h, we observe Nm = 70, which then quickly decreases to Nm = 30 for T = 1 h and Nm = 13 for T = 1.5 h. Eventually, for T → ∞, Nm will always converge to 1 owing to the finite size of the school. At short times T, Nm is always larger than 1 + τr/τa, which is the group size at which the individual hunters start to pay temporal costs of group hunting (electronic supplementary material, figure S3). We checked how changing the initial number of sardines in the prey school, S0, affected the conditions under which group hunting was beneficial. Smaller (or larger) initial prey group sizes shifted the hunting times so that shorter (or longer) times T made group hunters outperform solitary hunters.
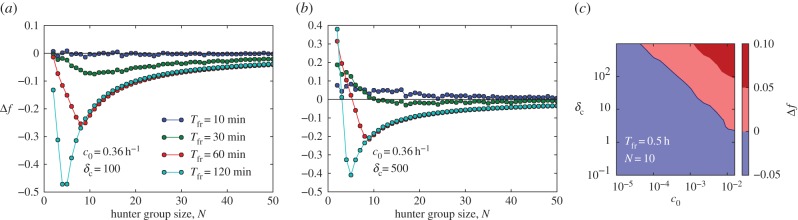
We also investigated whether a free riding strategy could be beneficial for some individuals in groups. One key parameter, δc, predominantly controls whether there is an advantage for the free riding strategy with respect to energy payoffs (see the electronic supplementary material, S2.4 for details). δc is a dimensionless number that represents the effective increase of the energy expenditure during an attack relative to the base energy expenditure. A value of δc = 1, would correspond to a doubling of the energy consumption rate during an attack sequence. An advantage for the free riding strategy can only be observed for very large values of 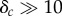 (figure 3a,b). Even then, this advantage only becomes significant for small hunter group sizes N < 10 or small attack delay times Tfr (figure 3b). Decreasing values of δc and the base energy consumption rate, c0, make free riding increasingly unlikely for a given group size and hunting time (figure 3c). Free riding remains disadvantageous for large regions of parameter space if we allow for nonlinear dependence of the injury level on the number of attacks (electronic supplementary material, S2.2), or if there is the potential for the hunt to be interrupted (electronic supplementary material, S2.5).
(figure 3a,b). Even then, this advantage only becomes significant for small hunter group sizes N < 10 or small attack delay times Tfr (figure 3b). Decreasing values of δc and the base energy consumption rate, c0, make free riding increasingly unlikely for a given group size and hunting time (figure 3c). Free riding remains disadvantageous for large regions of parameter space if we allow for nonlinear dependence of the injury level on the number of attacks (electronic supplementary material, S2.2), or if there is the potential for the hunt to be interrupted (electronic supplementary material, S2.5).
Figure 3.
(a,b) Relative energy payoff difference Δf versus hunter group size N for different attack delay times Tfr with c0 = 0.0001 s−1 = 0.36 h−1 and different values of the relative energetic costs of attacks δc = 100 (a) and δc = 500 (b). (c) Δf versus c0 and δc for fixed Tfr = 0.5 h and N = 10 (blue region indicates Δf < 0; i.e. where free riding is not beneficial). All other simulation parameters as in the main text and electronic supplementary material, S2.4.
6. Discussion
We have proposed a simple mechanism that can explain why sailfish hunt in groups. During a sailfish's attack, more fish are injured than are caught. Injuries can simply result as a by-product of the sailfish attempting to catch sardines, and we do not suggest that the sailfish are attempting to injure but not catch the sardines during attacks. Sailfish bills are covered in small denticles or micro-teeth [29,33], which probably facilitates this injury. Because more fish are injured than caught per attack, this necessarily leads to more injured fish in the school as the number of attacks increases. We found a positive correlation between the injury level of a school and the rate at which prey in that school were caught. Our modelling approach demonstrated that individuals using an attack-alternation strategy while hunting in groups can achieve increased per capita capture rates compared to if hunting alone. This strategy does not require spatial coordination of attacks between hunters. Simply hunting in a group can improve capture success rates, even though individuals do not need to change how they attack prey whether alone, or in groups.
Like other systems [27,28], sailfish do not appear to spatially coordinate their attacks. In fact, sailfish predominantly attack the prey schools when no other individual is doing so (presumably to reduce the risk of individuals injuring themselves during attacks). This suggests that sailfish time their attacks to generally take place after another hunter has departed the prey school. Indeed, temporally coordinated attacks have been observed in other species that hunt grouping prey and have been shown to improve capture success rates [5,34]. Modelling studies have also indicated that temporally coordinated attacks act to improve capture success rates for group hunters [35]. While temporal, and not spatial coordination occurs during individuals' attacks, spatial coordination may occur in other aspects of the hunt. Sailfish herd and chase their prey, which may involve individuals moving to positions around the prey school that are dependent on the positions of other hunters. Alternatively, this herding behaviour may simply be a by-product of predators occupying empty space around the prey school, without direct coordination between predators' movements, unlike other group hunting species [25]. Fine resolution sonar data will be needed to investigate these herding dynamics further. In any case, we have demonstrated that group hunting can benefit individual sailfish without spatially coordinated attacks or individuals adopting specific hunting roles. Our results also highlight that temporal, rather than spatial coordination of attacks, may allow for simpler forms of cooperative behaviour to evolve.
We found a correlation between the level of injury of the prey school and the capture success rates of sailfish. While we interpret this as a causal link, there are other explanations which could lead to this correlation. For example, if some groups of predators are more efficient at catching prey (and as a by-product injure more sardines in the school) than other predator groups, this may lead to higher capture rates on more injured schools. Little is known about the social organization of group-hunting marine fishes [36]. The traditional assumption has been that these predators live in fission–fusion systems with little social cohesion [37]. However, novel tracking technology and interest in social networks have provided a fresh methodological and conceptual approach to this topic, producing some evidence for significant co-occurrence of particular individuals [38–40]. Understanding the social organization of sailfish groups, perhaps by identifying individuals using unique markings or sail patterns would greatly improve our understanding of this system.
More work is needed to determine the causal mechanism between increased capture success and prey injury level. While we observed injured fish breaking off from the shoal that were quickly captured, it may not always be the most injured fish in groups that are captured next. Injured fish may have reduced ability to transfer directional information about a predators' location, which in turn could affect the school's escape manoeuvres [41,42]. This may lead to non-injured fish being at greater risk in injured, versus non-injured prey schools. It is also likely that multiple attacks can have internal physiological effects on prey behaviour. For example, attacks over time are likely to reduce the energy stores in prey, reducing their ability to perform escape manoeuvres or sustain high escape speeds through fatigue. Hence sustained attacks, even without predators actively injuring their prey, could lead to increased capture success rates for group hunters [43,44]. This may explain why in other systems, prey intake rate increases as a function of group size, without predators coordinating their attacks [28]. Indeed, the attack and success rates of other marine predators that attack schooling prey are in the same order of magnitude as our study [45,46]. In theory, our model can be applied to any system where the likelihood of capturing prey increases as a function of the number of attacks of previous predators.
Cooperation through turn-taking strategies has been described in other systems, for example, in predator-inspection behaviour in fishes [47,48]. But the exploration of turn-taking has usually been assessed in dyads and the role of turn-taking is not well understood in larger groups. Indeed, turn-taking strategies in larger groups raise interesting questions regarding the potential for cheating [49]. It has previously been proposed that when hunters attack small grouping prey that cannot be shared, there is no temptation to cheat, as not participating in the hunt returns no payoffs [50]. However, this approach did not consider that it may be easier to catch prey over time as they receive injuries or become exhausted. The increase in injured prey over time can be interpreted as a public good [51], which may be susceptible to exploitation by cheaters that delay the onset of their attacks. In microbial communities with diffusing public goods, the partial monopolization of resources owing to spatial localization may promote cooperation by denying non-producers access to resources [52,53]. While such an explicit spatial effect is likely to be absent in the highly dynamic turn-taking hunting process of sailfish, a direct analogy can be drawn via the intrinsic coupling of the production of the public good and the capture of individual prey. Producers (attackers) have access to the prey school from the onset of the hunt, albeit with low initial capture probabilities. Our investigation of the potential energetic trade-offs during the hunt suggests that individuals who delay their onset of attacks (free riders) would only benefit from such a strategy if the cost of attacking was 10 times higher than simply remaining with the prey school. Future work, with observations on the behaviour of individually identifiable predators, will be needed to determine if this strategy exists. Nevertheless, opportunistic access to the prey school, combined with the by-product of injuring prey during attempted captures, can promote individual hunting success in groups. We regard this form of group hunting, which does not require explicit cooperation, as ‘proto-cooperation’.
Our results demonstrate that individuals can benefit from group hunting without specific hunting roles (as in collaborative hunting), higher social organization or complex cognition. While hunting in groups potentially reduces the total amount of prey an individual predator is likely to catch, sailfish can offset this by collectively catching more prey per unit time when hunting together. This facilitative hunting method raises new questions surrounding the evolution of cooperative behaviour in group living animals.
Supplementary Material
Acknowledgements
We thank Rodrigo Friscione Wyssmann and the staff of Solo Buceo for their help in the field. We thank two anonymous referees, B. Taborsky, I. D. Couzin, D. J. T. Sumpter, C. E. Tarnita and M. Wolf for helpful comments or discussions on the manuscript.
Ethics
All research was conducted in line with the laws and legislation of Secretaría de Medio Ambiente y Recursos Naturales, Mexico.
Authors' contributions
All authors except P.R., D.S. and S.K. collected field data. J.E.H.-R. and D.S. analysed the empirical data and performed statistical analyses. P.R. designed the modelling component of the paper. All authors wrote the paper. All authors gave final approval for publication.
Data accessibility
All data accompanying this paper are available at Dryad Digital Repository http://dx.doi.org/10.5061/dryad.t9m6c [54].
Competing interests
We have no competing interests.
Funding
J.K. and P.R. acknowledge funding from Leibniz-Institute of Freshwater Ecology and Inland Fisheries. P.R. acknowledges the funding via the P.R.I.M.E. Fellowship by the German Academic Exchange Service. J.E.H.-R. was supported by a Knut and Alice Wallenberg Foundation Grant awarded to D. J. T. Sumpter.
References
- 1.Wilson EO. 1958. The beginnings of nomadic and group-predatory behavior in the ponerine ants. Evolution 12, 24–31. [Google Scholar]
- 2.Duncan FD, Crewe RM. 1994. Group hunting in a ponerine ant, Leptogenys nitida Smith. Oecologia 97, 118–123. ( 10.1007/BF00317915) [DOI] [PubMed] [Google Scholar]
- 3.Harwood G, Avilés L. 2013. Differences in group size and the extent of individual participation in group hunting may contribute to differential prey-size use among social spiders. Biol. Lett. 9, 20130621 ( 10.1098/rsbl.2013.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handegard NO, Boswell KM, Ioannou CC, Leblanc SP, Tjøstheim DB, Couzin ID. 2012. The dynamics of coordinated group hunting and collective information transfer among schooling prey. Curr. Biol. 22, 1213–1217. ( 10.1016/j.cub.2012.04.050) [DOI] [PubMed] [Google Scholar]
- 5.Lönnstedt OM, Ferrari MC, Chivers DP. 2014. Lionfish predators use flared fin displays to initiate cooperative hunting. Biol. Lett. 10, 20140281 ( 10.1098/rsbl.2014.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strübin C, Steinegger M, Bshary R. 2011. On group living and collaborative hunting in the yellow saddle goatfish (Parupeneus cyclostomus) 1. Ethology 117, 961–969. ( 10.1111/j.1439-0310.2011.01966.x) [DOI] [Google Scholar]
- 7.Vail AL, Manica A, Bshary R. 2013. Referential gestures in fish collaborative hunting. Nat. Comm. 4, 1765 ( 10.1038/ncomms2781) [DOI] [PubMed] [Google Scholar]
- 8.Vail AL, Manica A, Bshary R. 2014. Fish choose appropriately when and with whom to collaborate. Curr. Biol. 24, R791–R793. ( 10.1016/j.cub.2014.07.033) [DOI] [PubMed] [Google Scholar]
- 9.Hector DP. 1986. Cooperative hunting and its relationship to foraging success and prey size in an avian predator. Ethology 73, 247–257. ( 10.1111/j.1439-0310.1986.tb00915.x) [DOI] [Google Scholar]
- 10.Ellis DH, Bednarz JC, Smith DG, Flemming SP. 1993. Social foraging classes in raptorial birds. Bioscience 43, 14–20. ( 10.2307/1312102) [DOI] [Google Scholar]
- 11.Dechmann DK, Heucke SL, Giuggioli L, Safi K, Voigt CC, Wikelski M. 2009. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc. B 276, 2721–2728. ( 10.1098/rspb.2009.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel D, Packer C. 1991. Group hunting behaviour of lions: a search for cooperation. Anim. Behav. 41, 697–709. ( 10.1016/S0003-3472(05)80907-8) [DOI] [Google Scholar]
- 13.Bailey I, Myatt JP, Wilson AM. 2013. Group hunting within the carnivora: physiological, cognitive and environmental influences on strategy and cooperation. Behav. Ecol. Sociobiol. 67, 1–17. ( 10.1007/s00265-012-1423-3) [DOI] [Google Scholar]
- 14.Gazda SK, Connor RC, Edgar RK, Cox F. 2005. A division of labour with role specialization in group hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc. R. Soc. B 272, 135–140. ( 10.1098/rspb.2004.2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stander PE. 1992. Cooperative hunting in lions: the role of the individual. Behav. Ecol. Sociobiol. 29, 445–454. ( 10.1007/BF00170175) [DOI] [Google Scholar]
- 16.Creel S, Creel NM. 1995. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339. ( 10.1016/0003-3472(95)80048-4) [DOI] [Google Scholar]
- 17.Schmidt PA, Mech LD. 1997. Wolf pack size and food acquisition. Am. Nat. 150, 513–517. ( 10.1086/286079) [DOI] [PubMed] [Google Scholar]
- 18.Packer C, Scheel D, Pusey AE. 1990. Why lions form groups: food is not enough. Am. Nat. 136, 1–19. ( 10.1086/285079) [DOI] [Google Scholar]
- 19.Vucetich JA, Peterson RO, Waite TA. 2004. Raven scavenging favours group foraging in wolves. Anim. Behav. 67, 1117–1126. ( 10.1016/j.anbehav.2003.06.018) [DOI] [Google Scholar]
- 20.Carbone C, Du Toit J, Gordon I. 1997. Feeding success in African wild dogs: does kleptoparasitism by spotted hyenas influence hunting group size? J. Anim. Ecol. 66, 318–326. ( 10.2307/5978) [DOI] [Google Scholar]
- 21.Major PF. 1978. Predator-prey interactions in two schooling fishes, Caranx ignobilis and Stolephorus purpureus. Anim. Behav. 26, 760–777. ( 10.1016/0003-3472(78)90142-2) [DOI] [Google Scholar]
- 22.Eklöv P, Diehl S. 1994. Piscivore efficiency and refuging prey: the importance of predator search mode. Oecologia 98, 344–353. ( 10.1007/BF00324223) [DOI] [PubMed] [Google Scholar]
- 23.Clapham PJ. 2000. Functional aspects of cetacean communication. In Cetacean societies, field studies of dolphins and whales (eds Mann J, Connor R, Tyack P, Whitehead H), pp. 173–196. Chicago, IL: University of Chicago Press. [Google Scholar]
- 24.Leighton TG, Richards SD, White PR. 2004. Trapped within a wall of sound. Acoust. Bull. 29, 24–29. [Google Scholar]
- 25.Würsig B. 1986. Delphinid foraging strategies. In Dolphin cognition and behavior: a comparative approach (eds Schusterman R, Thomas JA, Wood FC), pp. 347–359. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 26.Scott D. 1984. The feeding success of cattle egrets in flocks. Anim. Behav. 32, 1089–1100. ( 10.1016/S0003-3472(84)80225-0) [DOI] [Google Scholar]
- 27.Arnegard ME, Carlson BA. 2005. Electric organ discharge patterns during group hunting by a mormyrid fish. Proc. R. Soc. B 272, 1305–1314. ( 10.1098/rspb.2005.3101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Götmark F, Winkler DW, Andersson M. 1986. Flock-feeding on fish schools increases individual success in gulls. Nature 319, 589–591. ( 10.1038/319589a0) [DOI] [PubMed] [Google Scholar]
- 29.Domenici P, et al. 2014. How sailfish use their bills to capture schooling prey. Proc. R. Soc. B 281, 20140444 ( 10.1098/rspb.2014.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marras S, et al. 2015. Not so fast: swimming behavior of sailfish during predator–prey interactions using high-speed video and accelerometry. Integr. Comp. Biol. 55, 719–727. ( 10.1093/icb/icv017) [DOI] [PubMed] [Google Scholar]
- 31.Webb PW, De Buffrénil V. 1990. Locomotion in the biology of large aquatic vertebrates. Trans. Am. Fish Soc. 119, 629–641. ( 10.1577/1548-8659(1990)119%3C0629:LITBOL%3E2.3.CO;2) [DOI] [Google Scholar]
- 32.Domenici P. 2001. The scaling of locomotor performance in predator–prey encounters: from fish to killer whales. Comp. Biochem. Phys. A 131, 169–182. ( 10.1016/S1095-6433(01)00465-2) [DOI] [PubMed] [Google Scholar]
- 33.Fierstine HL, Voigt NL. 1996. Use of rostral characters for identifying adult billfishes (Teleostei: Perciformes: Istiophoridae and Xiphiidae). Copeia 1996, 148–161. ( 10.2307/1446950) [DOI] [Google Scholar]
- 34.Thiebault A, Semeria M, Lett C, Tremblay Y. 2016. How to capture fish in a school? Effect of successive predator attacks on seabird feeding success. J. Anim. Ecol. 85, 157–167. ( 10.1111/1365-2656.12455) [DOI] [PubMed] [Google Scholar]
- 35.Lett C, Semeria M, Thiebault A, Tremblay Y. 2014. Effects of successive predator attacks on prey aggregations. Theor. Ecol. 7, 239–252. ( 10.1007/s12080-014-0213-0) [DOI] [PubMed] [Google Scholar]
- 36.Fréon P, Dagorn L. 2000. Review of fish associative behaviour: toward a generalisation of the meeting point hypothesis. Rev. Fish. Biol. Fish. 10, 183–207. ( 10.1023/A:1016666108540) [DOI] [Google Scholar]
- 37.Krause J, Butlin RK, Peuhkuri N, Pritchard VL. 2000. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol. Rev. 75, 477–501. ( 10.1111/j.1469-185X.2000.tb00052.x) [DOI] [PubMed] [Google Scholar]
- 38.Wilson ADM, Croft DP, Krause J. 2014. Social networks in elasmobranchs and teleost fishes. Fish Fish. 15, 676–689. ( 10.1111/faf.12046) [DOI] [Google Scholar]
- 39.Bayliff WH. 1988. Integrity of schools of skipjack tuna, Katsuwonus pelamis, in the eastern Pacific Ocean, as determined from tagging data. Fish. B-NOAA 86, 631–643. [Google Scholar]
- 40.Klimley AP, Holloway CF. 1999. School fidelity and homing synchronicity of yellowfin tuna, Thunnus albacares. Mar. Biol. 133, 307–317. ( 10.1007/s002270050469) [DOI] [Google Scholar]
- 41.Gerlotto F, Bertrand S, Bez N, Gutierrez M. 2006. Waves of agitation inside anchovy schools observed with multibeam sonar: a way to transmit information in response to predation. ICES J. Mar. Sci. 63, 1405–1417. ( 10.1016/j.icesjms.2006.04.023) [DOI] [Google Scholar]
- 42.Nøttestad L, Axelsen BE. 1999. Herring schooling manoeuvres in response to killer whale attacks. Can. J. Zool. 77, 1540–1546. ( 10.1139/z99-124) [DOI] [Google Scholar]
- 43.Domenici P, Batty R, Similä T. 2000. Spacing of wild schooling herring while encircled by killer whales. J. Fish Biol. 57, 831–836. ( 10.1111/j.1095-8649.2000.tb00278.x) [DOI] [Google Scholar]
- 44.Guinet C, Domenici P, De Stephanis R, Barrett-Lennard L, Ford JKB, Verborgh P. 2007. Killer whale predation on bluefin tuna: exploring the hypothesis of the endurance-exhaustion technique. Mar. Ecol. Prog. Ser. 347, 111–119. ( 10.3354/meps07035) [DOI] [Google Scholar]
- 45.Parrish JK. 1992. Levels of diurnal predation on a school of flat-iron herring, Harengula thrissina. Environ. Biol. Fishes 34, 257–263. ( 10.1007/BF00004771) [DOI] [Google Scholar]
- 46.Parrish JK, Strand SW, Lott JL. 1989. Predation on a school of flat-iron herring, Harengula thrissina. Copeia 1989, 1089–1091. ( 10.2307/1446009) [DOI] [Google Scholar]
- 47.Dugatkin LA. 1997. Cooperation among animals: an evolutionary perspective. New York, NY: Oxford University Press. [Google Scholar]
- 48.Milinski M. 1987. Tit for tat in sticklebacks and the evolution of cooperation. Nature 325, 433–435. ( 10.1038/325433a0) [DOI] [PubMed] [Google Scholar]
- 49.Dugatkin LA. 1997. The evolution of cooperation. Bioscience 47, 355–362. ( 10.2307/1313150) [DOI] [Google Scholar]
- 50.Packer C, Ruttan L. 1988. The evolution of cooperative hunting. Am. Nat. 132, 159–198. ( 10.1086/284844) [DOI] [Google Scholar]
- 51.Levin SA. 2014. Public goods in relation to competition, cooperation, and spite. Proc. Natl Acad. Sci USA 111, 10 838–10 845. ( 10.1073/pnas.1400830111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen B, Gore J, Nowak MA. 2013. Spatial dilemmas of diffusible public goods. Elife 2, e01169 ( 10.7554/eLife.01169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. 2014. Solutions to the public goods dilemma in bacterial biofilms. Curr. Biol. 24, 50–55. ( 10.1016/j.cub.2013.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbert-Read JE, et al. 2016. Data from: Proto-cooperation: group hunting sailfish improve hunting success by alternating attacks on grouping prey. Dryad Digital Repository. ( 10.5061/dryad.t9m6c) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data accompanying this paper are available at Dryad Digital Repository http://dx.doi.org/10.5061/dryad.t9m6c [54].