Abstract
The question of why males of many species produce elaborate mating displays has now been largely resolved: females prefer to mate with males that produce such displays. However, the question of why females prefer such displays has been controversial, with an emerging consensus that such displays often provide information to females about the direct fitness benefits that males provide to females and/or the indirect fitness benefits provided to offspring. Alternative explanations, such as production of arbitrarily attractive sons or innate pre-existing female sensory or perceptual bias, have also received support in certain taxa. Here, we describe multivariate female preference functions for male acoustic traits in two chirping species of field crickets with slow pulse rates; our data reveal cryptic female preferences for long trills that have not previously been observed in other chirping species. The trill preferences are evolutionarily pre-existing in the sense that males have not (yet?) exploited them, and they coexist with chirp preferences as alternative stable states within female song preference space. We discuss escape from neuronal adaptation as a possible mechanism underlying such latent preferences.
Keywords: sensory bias, neuronal adaptation, preference functions, Gryllus
1. Introduction
Nearly 150 years after being proposed by Darwin [1], the idea that female preferences have driven the evolution of male mating display characters is now supported by a large and diverse scientific literature [2]. Whether or not female preferences are adaptive has been the subject of contentious debate [3]. Adaptive explanations have broad support in diverse taxa and include both direct (i.e. fecundity) [4] and indirect (i.e. genetic) benefits [5]. Arbitrary ‘Fisherian’ or sexy-son models have also received support [6], as have models in which female sensory or perceptual biases evolutionarily pre-date male display traits [7]. Taxa for which pre-existing biases have been well characterized often reveal that the biases themselves were under selection in other contexts, e.g. foraging [7]; examples in which the bias is likely a by-product of basic principles of neural processing are relatively rare [8,9]. This may reflect the fact that neural mechanisms of signal processing in females are generally not well characterized in most kinds of organisms.
The mechanisms underlying female processing of male sexual signals are only partially understood in crickets. Nonetheless, crickets have become a model system for understanding the neurological basis of signal processing, and signal processing in crickets is among the best understood of all taxa [10–12]. Female crickets process male song signals both at the peripheral auditory tympana and centrally in the brain. Amplitude and frequency are coded similarly as numbers of neural spikes per unit time: louder sounds result in more neural spikes, and frequencies that best match cricket hearing are encoded as louder, i.e. with more spikes, than frequencies that less closely match cricket hearing [13]. Unlike in anurans, there is no separate central nervous system processing of frequency information [13,14], thus acoustic multivariate female preference functions in crickets are multivariate in the temporal domain only, without complex interactions between frequency and temporal pattern as observed in Hyla frogs [15]. The resultant spike trains are evaluated in brain circuits for temporal structure on both the pulse and chirp timescales [16]. Individual neurons and their connections have been identified which collectively represent a neural filter tuned to male pulse rates [17]. The output of such a pulse rate filter can be integrated over longer timescales, i.e. chirps or trills, such that overall signal attractiveness reflects the proportion of sound in the signal space (i.e. chirp or trill duty cycle) conditional on an attractive pulse rate. Such integration across pulse and chirp timescales can often successfully be modelled with linear–nonlinear models using Gabor functions, and such models can often predict female phonotactic responses across diverse species [16].
Multivariate tests of female preference functions are well suited to reveal the complexity of signal processing [15,18–21]. As examples, multivariate preference functions can test: (i) the relative weighting of different traits (i.e. importance of trait A versus trait B), (ii) non-additive effects (i.e. attractiveness of variants of trait A conditional on specific values of trait B) and (iii) signal integration across timescales and/or sensory modalities. Although univariate preference tests can reveal unexpected properties of female preference space [22], given the multivariate and often multimodal nature of many sexual signalling systems [23,24], a multivariate approach is more likely to reveal otherwise hidden features in female preference space. Here we explore the signal recognition space of female crickets in an effort to better understand signal processing and the integration of pulse and chirp timescales, building upon our prior work with both chirping and trilling species [19,20].
We characterized female preference functions in two chirping species of Gryllus field crickets with unusually slow pulse rates (electronic supplementary material, figure S1a,b): G. firmus Scudder 1902, and an undescribed species, we refer to here as G#13. As an aid to continuity in the literature we note that G#13 will be named ‘G. longicercus’ in an upcoming revision of North American Gryllus (DB Weissman, DA Gray 2001–2016, unpublished data); per Article 8.3 of the ICZN the manuscript name ‘G. longicercus’ is disclaimed as ‘not available’. Gryllus firmus occurs along the southeastern USA Atlantic and Gulf coast regions [25,26]; G#13 occurs in portions of the Sonoran and Chihuahuan deserts of the western USA and Mexico (DB Weissman, DA Gray 2001–2016, unpublished data). Within Gryllus, the two species are not close relatives (electronic supplementary material, figure S2), but both typically call with a three to five pulse chirp at unusually slow pulse rates: at 24°C, G. firmus average about 18 p s−1 [27], whereas G#13 average about 10 p s−1 (DA Gray 2005, unpublished data); these are among the slowest pulse rates in North American Gryllus. Because pulse rate is consistently a principal song feature important in cricket song recognition [16,28], examination of species with low pulse rates might reveal unexpected effects of signal processing.
2. Material and methods
(a). Animals
Experiments were performed using primarily first- and second-generation virgin adult female crickets from laboratory cultures held at 25 ± 2°C with ad libitum food and water. The G. firmus culture was initiated with ca 20–30 females collected in Gainesville, Lake City, and Live Oak, FL, USA; the G#13 culture was initiated with ca 15–20 females collected in the KOFA National Wildlife Refuge, Arizona, USA.
(b). Female preference functions
Female preferences were tested at 24–26°C, using a trackball system as described previously [19,20]. In brief, the trackball was a hollow 1.2–1.8 g sphere (100 mm diam.) supported by an air column in an acoustically insulated box. Two loudspeakers (Piezo Horntweeter PH8) placed 90° apart played custom synthetic stimuli at 80 dB (re. 2 × 10−5 Pa) as measured at the female crickets' position (Bruel & Kjaer 2231 meter with Bruel & Kjaer 4133 half-inch condenser microphone).
As the crickets turned on the sphere, the x–y movements of the sphere were recorded by optical sensors. All test series included four controls (silent, tone and an attractive stimulus at the beginning and end of a test series) and eight test patterns in random order. For each test signal, the lateral deviation of a female relative to each of the two speakers was averaged and normalized with respect to an attractive control signal in order to obtain comparable data across test series. Phonotaxis scores were therefore typically between 0 (no orientation towards the sound signal) and 1 (strong orientation towards the signal), although negative scores (orientation away from the test signal) and scores higher than 1 (orientation towards the test signal stronger than towards the control) were possible (see [19] for additional details).
Sample sizes ranged from six to 30 individual females for any given test pattern (mean ± s.d. = 19 ± 6.5). Test patterns were designed to assess female response to temporal features of male song, given an attractive frequency. To determine those song frequencies, we first tested female response to songs with species-typical pulse patterns but with frequencies ranging from 2.5 to 7 kHz. We then used attractive frequencies to create stimuli that tested responses over a large parameter space for pulse and chirp timescales.
Integration across timescales was tested with a ‘transfer function’ that assesses response to stimuli at the short (pulse) timescale in the absence of information at the longer (chirp) timescale, and vice versa. For these tests, females were presented with a continuous series of sound pulses separated by silent intervals at a constant duty cycle. Very slow rates thus correspond to the temporal structure of chirps in the absence of pulse rate information (e.g. 0.5 s sound pulse followed by 0.5 s silence, repeated continuously), whereas faster rates correspond to the temporal structure of pulses in the absence of chirp rate information (e.g. 50 ms sound pulse followed by 50 ms silence, repeated continuously).
3. Results
(a). Frequency
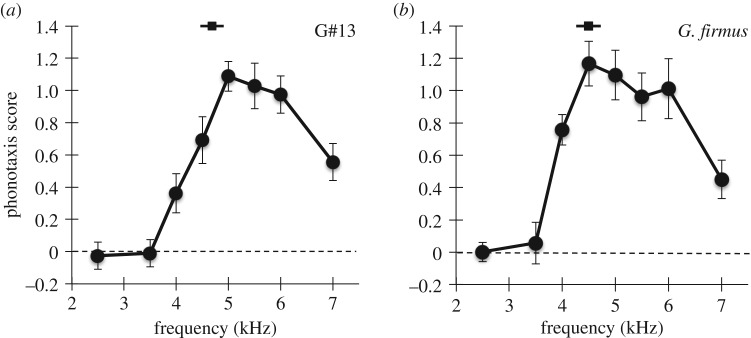
Both species showed clear preferences for 4.5–5 kHz song (figure 1a,b) and responded well to frequencies up to 6 kHz. Based on these results, subsequent tests used either 5 or 5.5 kHz for G#13 and either 4 or 4.5 kHz for G. firmus.
Figure 1.
Female response as a function of carrier frequency in G#13 (a) and G. firmus (b). Error bars are s.e. (N = 27 G#13 and 28 G. firmus females). Inset black squares with lines represent typical male song values.
(b). Pulse and chirp profiles
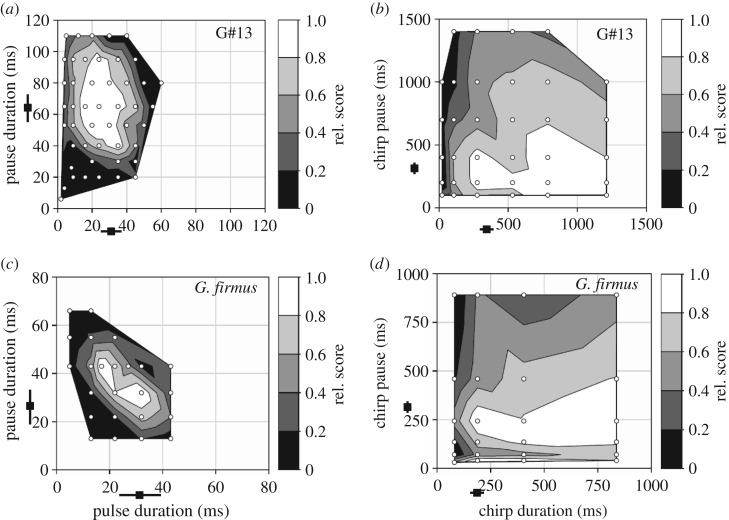
Bivariate preference functions for both species are presented in figure 2a–d. Each panel shows female response as a function of the duration of pauses between stimuli (pulse or chirp) and as a function of the duration of the stimulus itself (pulse or chirp). Transects from the upper left to the lower right thus increase in duty cycle (i.e. sound energy) for a given rate, whereas transects from the upper right to the lower left increase in rate for a given duty cycle.
Figure 2.
Bivariate preference functions at the pulse (a,c) and chirp (b,d) timescales for G#13 (a,b) and G. firmus (c,d). Black squares with lines at the axes represent typical male song values.
Both species exhibit short timescale pulse filters closely matched to male song pulse rates and pulse duty cycles (figure 2a,c and electronic supplementary material, figure S3a–d). Both species also respond well to conspecific chirp patterns; however, the overall profiles of the chirp filters of both species are unusual (figure 2b,d). The G#13 chirp filter (figure 2b) appears to favour high chirp duty cycles, i.e. long chirps and short pauses, with the greatest response to discrete chirps 787 ms in duration with 400 ms pauses between chirps (corresponding to 10 pulses chirp−1 with 22 ms pulses and 63 ms pulse pauses, i.e. 11.8 p s−1) or to chirp pause durations approaching zero (i.e. a continuous trill). The G. firmus chirp filter (figure 2d) more closely matches male song but is clearly bimodal with strongest responses to either ca 189 ms chirps coupled with ca 243 ms chirp pauses or to chirp pauses approaching zero, i.e. a continuous trill. Both chirp duration and chirp pause contribute to stimulus attractiveness. This can be visualized when the bivariate chirp preference surfaces are decomposed to univariate preference functions (electronic supplementary material, figure S4a,b).
(c). Transfer function
Both species failed to respond to continuous trills with pulse rates faster or slower than their normal pulse rate (figure 3). However, both species responded strongly to continuous trills given the correct pulse rate, pulse duty cycle and frequency. G#13 response to continuous trills of 5.5 kHz pulses at 12 p s−1 was very strong (figure 3a), provided the pulse duty cycle was 25% or 50%. The mean ± s.e. response to 5.5 kHz, 25% DC, 12 p s−1 was 1.11 ± 0.12, significantly different from zero, Z = 9.57, p < 0.00001, but not from 1 (i.e. the positive control consisting of species-typical pulse and chirp pattern), Z = 0.92, 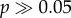 . However, G#13 response was not different from zero even for attractive pulse rates if the DC was increased to 75%. Gryllus firmus likewise showed a strong response to continuously presented pulses (figure 3b), with maximal response to 4.5 kHz, 50% DC, and 14 p s−1. Mean ± s.e. response was 0.82 ± 0.10, significantly different from zero, Z = 8.37, p < 0.00001, and nearly as strong as to the positive control, Z = −1.89, p = 0.06. Neither species responded to longer sound pulses presented at low rates (i.e. < 5 p s−1) representative of a continuous series of chirps in the absence of pulse rate modulation (figure 3). The dependence of response upon pulse duty cycle matches univariate pulse duty cycle preferences (electronic supplementary material, figure S3b,d).
. However, G#13 response was not different from zero even for attractive pulse rates if the DC was increased to 75%. Gryllus firmus likewise showed a strong response to continuously presented pulses (figure 3b), with maximal response to 4.5 kHz, 50% DC, and 14 p s−1. Mean ± s.e. response was 0.82 ± 0.10, significantly different from zero, Z = 8.37, p < 0.00001, and nearly as strong as to the positive control, Z = −1.89, p = 0.06. Neither species responded to longer sound pulses presented at low rates (i.e. < 5 p s−1) representative of a continuous series of chirps in the absence of pulse rate modulation (figure 3). The dependence of response upon pulse duty cycle matches univariate pulse duty cycle preferences (electronic supplementary material, figure S3b,d).
Figure 3.
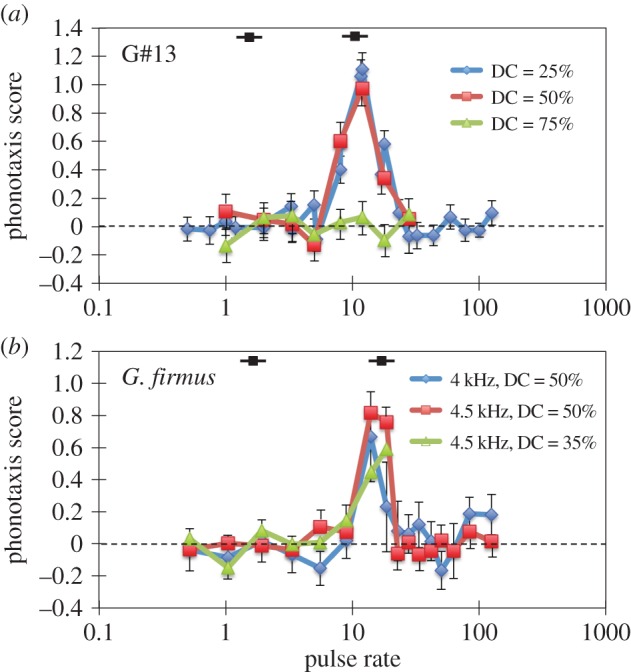
Transfer function: female response as a function of the rate of continuously presented sound tones alternating with pauses at a given duty cycle (DC) and frequency; G#13 (a) and G. firmus (b). Inset black squares with lines represent typical male song values for pulse and chirp rate. Error bars are s.e.; for different test series, N females range from 14–20 for G#13, and from 6–27 for G. firmus. (Online version in colour.)
4. Discussion
These two species have unusually slow pulse rates for Gryllus crickets yet the pulse rate filters documented here correspond to the pulse rate filters known in congeners [19,20,29,30]. What is highly unusual about these two species is their responses to song pattern variation on the chirp timescale. Gryllus crickets evidently evaluate the chirp duty cycle given a correct pulse rate. This is effectively an integration step across timescales: positive pulse recognition output (short timescale) is evaluated over a given time window corresponding to chirp evaluation (long timescale) [12,16]. Females of several chirping and trilling species show preferences for chirp (or trill) duty cycles which are greater than conspecific males tend to produce, with duty cycle preference functions in chirping species often ‘closed’ (i.e. unimodal convex) [31,32], whereas duty cycle preference functions in trilling species are often ‘open-ended’ [19]. In transfer function tests, the trilling species respond well to continuous pulses at an appropriate pulse rate, whereas chirping species do not or only to a limited extent [19,20,31–34]. Gryllus firmus and G#13 studied here are the first chirping Gryllus known to respond strongly to continuous trills with species-typical pulse rates, although females of both Hyla and Tettigonia have been shown to respond well to pulse and interpulse durations well beyond natural male variation [35]. The strong female response to trills can be seen in our results both for the chirp profile (figure 2b,d) and the transfer function (figure 3a,b), which are independent test series. Responses to continuous trills nearly equal (G. firmus) or even slightly exceed (G#13) responses to their respective species-specific (chirped) control songs. These responses thus represent strong latent biases within the female signal recognition system of these species.
If we consider the bivariate chirp preference surface as a fitness landscape, it is apparent that males of both species currently occupy one of several possible fitness peaks, i.e. alternative stable states coexist within female preference space. Transitions to an alternative peak might occur rapidly if the fitness valley were crossed, i.e. if males evolved to exploit the pre-existing female perceptual biases identified here. G#13 appears to have a relatively shallow fitness valley (figure 2b and electronic supplementary material, figure S4a). In contrast, G. firmus appears to show a steeper fitness valley as preference with respect to chirp pause is strongly bimodal with a sharp decrease in attractiveness at 70–135 ms pauses (figure 2d and electronic supplementary material, figure S4b). Could males evolve to exploit the female bias and evolve long trills? This would require sufficient phenotypic and genetic variation, often observed in crickets [36,37], and available acoustic niche space. Both G. firmus and G#13 are sympatric with other Gryllus that produce long trills, which in theory could limit the sound space available to them. However, G. rubens and G. texensis (sympatric with G. firmus) call with ca 50–60 and ca 60–80 p s−1 trills, respectively [19,38,39], and an undescribed regular trilling species (‘G#14’), sympatric with G#13, calls with ca 35–45 p s−1 [19,40], all well faster than G. firmus and G#13.
The question arises, why these chirping species exhibit latent preference profiles for chirp patterns (figure 2b,d) more similar to those of trilling species [19] when compared with chirping species [20,29,31,32]. In terms of physiological mechanisms, the phenomenon of neuronal adaptation (NA) appears as a prime candidate (‘adaptation’ used here in the neurobiological sense, indicating a decreased response to repeated stimuli, without any implications regarding evolutionary fitness or history of selection). NA is a fundamental feature of neural systems, and a common property of both vertebrate and invertebrate sensory processing [41]. Despite its pervasive nature, the negative feedback imposed by NA has significant complexity and variability as the time constants characterizing the rate of decreased neural firing can range from tens of milliseconds to several seconds [41,42].
The contribution of NA to song pattern selectivity is supported by direct neurophysiological evidence in the cricket Gryllus bimaculatus, in which pulse rate recognition is based on a small neuronal network consisting of five well characterized neurons [10,17]. Three of these five neurons show the signatures of NA as their response levels decrease with consecutive pulses in a chirp (see figure 2 in [17]). Therefore, a system already equipped with NA at the level of pulse recognition will cease to respond to longer trill-like pulse trains as it requires recovery from adaptation for a full response. Our results with these two species show that female phonotactic response does not diminish with repeated pulses presented as continuous trills, therefore, we conclude that NA does not eliminate neural spiking for these species. Potentially, the pulse rates of these species are sufficiently low as to escape the 40–60 ms NA time constants described in the auditory pathway of crickets [43]. Interestingly, in the katydid Neoconocephalus triops, Prešern et al. [44] have recently shown strong NA at the neuronal level to fast (140 p s−1) sound pulses but a lack of NA to slow (7 p s−1) sound pulses. It is also possible that the slow pulse rate does not play an important role, and these species simply show reduced or absent NA for other unknown reasons. Regardless of the mechanism for diminished NA, in the two species examined here, NA seems not to dampen female response to long trills.
Our results differ in some important ways from the original pre-existing sensory bias model [45]. That model originally focused on two central criteria: the female preference for the novel trait phylogenetically pre-dating the origin of the trait, and the preference for the novel trait exceeding the preference for the standard trait. With respect to the origin of the bias, in our Gryllus system, a strong female response to trills could be an evolutionary holdover from an ancestral preference for trills in ancestral species with trills, rather than representing a derived bias. We cannot completely rule out this possibility, however given what is known of the phylogeny of Gryllus, the distribution of chirping and trilling species, as well as evidence from five species of chirping Gryllus which do not respond strongly to continuous trills (see electronic supplementary material, figure S2), we conclude that this is unlikely. With respect to the strength of the bias, here we show that the preference for trills is equivalent to the preference for species-typical chirps, but does not significantly exceed it; that is, we identify alternative stable states, or preference surface peaks, that coexist within the female perceptual system. Anagenesis as envisioned by the original pre-existing sensory bias model would likely require the preference based on the pre-existing bias to exceed the preference based on the current male trait. However, with alternative but equivalent preference states, the potential for latent perceptual biases to contribute to cladogenesis is highlighted. In our view, this significantly enriches the sensory bias model, already significantly broadened from its original formulation [7].
Finally, we note that many animal mating signals are comprised of simple units repeated at a characteristic rate and often at a more complex, higher-order temporal pattern. Such signals are found in some birds, certain fish, most anurans and many divergent insect groups, e.g. crickets and katydids, lacewings, cicadas, treehoppers, even some true flies and wasps. Such repetitive mating signals are likely subject to diminishing female responses, i.e. habituation at the organismal level [46,47] or NA at the neural level [48]. The presence, strength or absence of NA may therefore account for some of the higher-order pattern selectivity in a wide range of communication systems across different modalities; however, NA alone is unlikely to explain all aspects of complex signal responses, e.g. differences in efficacy of signal components when presented in different temporal order [49]. Nonetheless, evolutionary changes in NA may have profound effects on signal evolution in diverse taxa, and might promote signal diversification, especially if changes in NA cause female signal preference space to have multiple pre-existing fitness peaks.
Supplementary Material
Acknowledgements
We are grateful to the reviewers and to the Associate Editor, William Searcy, who provided a great deal of constructive feedback, which improved the manuscript considerably. We gratefully acknowledge the support of Hans-Jürgen Dahmen with the trackball system and the help of Elisa Becker, Kolja Haß and Daria Ivanova in some of the trackball experiments.
Ethics
All research was conducted under appropriate animal care; permits were not required for cricket collection.
Data accessibility
Data are deposited in the Dryad Digital Repository subject to a 1 year embargo [50] at http://dx.doi.org/10.5061/dryad.66bh6.
Authors' contributions
D.A.G. collected crickets, analysed data and was primary author of the manuscript; E.G. collected phonotaxis data and contributed to the manuscript; T.B. collected crickets, collected phonotaxis data and contributed to the manuscript; R.M.H. designed experiments, analysed data and contributed to the manuscript.
Competing interests
We have no competing interests.
Funding
D.A.G. has been supported by grants from the CSUN Science and Mathematics Dean's Office and the Office of Research and Graduate Studies. R.M.H. received support from the DFG he2812/4-1. This study was part of the GENART project funded by the Leibniz Association (SAW-2012-MfN-3).
References
- 1.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 2.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 3.Jones AG, Ratterman NL. 2009. Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl Acad. Sci. USA 106(Suppl 1), 10 001–10 008. ( 10.1073/pnas.0901129106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Møller A, Jennions M. 2001. How important are direct fitness benefits of sexual selection? Die Naturwissenschaften 88, 401–415. ( 10.1007/s001140100255) [DOI] [PubMed] [Google Scholar]
- 5.Puurtinen M, Ketola T, Kotiaho JS. 2009. The good-genes and compatible-genes benefits of mate choice. Am. Nat. 174, 741–752. ( 10.1086/606024) [DOI] [PubMed] [Google Scholar]
- 6.Prokop ZM, Michalczyk Ł, Drobniak SM, Herdegen M, Radwan J. 2012. Meta-analysis suggests choosy females get sexy sons more than ‘good genes’. Evolution 66, 2665–2673. ( 10.1111/j.1558-5646.2012.01654.x) [DOI] [PubMed] [Google Scholar]
- 7.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol Syst 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 8.Wilczynski W, Rand AS, Ryan MJ. 2001. Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behav. Evol. 58, 137–151. ( 10.1159/000047268) [DOI] [PubMed] [Google Scholar]
- 9.Grant AJ, Mayer MS, Mankin RW. 1989. Responses from sensilla on antennae of male Heliothis zea to its major pheromone component and two analogs. J. Chem. Ecol. 15, 2625–2634. ( 10.1007/BF01014721) [DOI] [PubMed] [Google Scholar]
- 10.Kostarakos K, Hedwig B. 2014. Pattern recognition in field crickets: concepts and neural evidence. J. Comp. Physiol. A 201, 73–85. ( 10.1007/s00359-014-0949-4) [DOI] [PubMed] [Google Scholar]
- 11.Ronacher B, Hennig RM, Clemens J. 2015. Computational principles underlying recognition of acoustic signals in grasshoppers and crickets. J. Comp. Physiol. A 201, 61–71. ( 10.1007/s00359-014-0946-7) [DOI] [PubMed] [Google Scholar]
- 12.Clemens J, Hennig RM. 2013. Computational principles underlying the recognition of acoustic signals in insects. J. Comput. Neurosci. 35, 75–85. ( 10.1007/s10827-013-0441-0) [DOI] [PubMed] [Google Scholar]
- 13.Imaizumi K, Pollack GS. 1999. Neural coding of sound frequency by cricket auditory receptors. J. Neurosci. 19, 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 15.Gerhardt HC, Brooks R. 2009. Experimental analysis of multivariate female choice in gray treefrogs (Hyla versicolor): evidence for directional and stabilizing selection. Evolution 63, 2504–2512. ( 10.1111/j.1558-5646.2009.00746.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennig RM, Heller K-G, Clemens J. 2014. Time and timing in the acoustic recognition system of crickets. Front. Physiol. 5, 286 ( 10.3389/fphys.2014.00286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schöneich S, Kostarakos K, Hedwig B. 2015. An auditory feature detection circuit for sound pattern recognition. Sci. Adv. 1, e1500325 ( 10.1126/sciadv.1500325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentsen CL, Hunt J, Jennions MD, Brooks R. 2006. Complex multivariate sexual selection on male acoustic signaling in a wild population of Teleogryllus commodus. Am. Nat. 167, E102–E116. ( 10.1086/501376) [DOI] [PubMed] [Google Scholar]
- 19.Blankers T, Hennig MR, Gray DA. 2015. Conservation of multivariate female preference functions and preference mechanisms in three species of trilling field crickets. J. Evol. Biol. 28, 630–641. ( 10.1111/jeb.12599) [DOI] [PubMed] [Google Scholar]
- 20.Hennig MR, Blankers T, Gray DA. 2016. Divergence in male cricket song and multivariate female preference functions in three allopatric sister species. J. Comp. Physiol. A 202, 347–360. ( 10.1007/s00359-016-1083-2) [DOI] [PubMed] [Google Scholar]
- 21.Ower GD, Judge KA, Steiger S, Caron KJ, Smith RA, Hunt J, Sakaluk SK. 2013. Multivariate sexual selection on male song structure in wild populations of sagebrush crickets, Cyphoderris strepitans (Orthoptera: Haglidae). Ecol. Evol. 3, 3590–3603. ( 10.1002/ece3.736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basolo AL. 1998. Evolutionary change in a receiver bias: a comparison of female preference functions. Proc R. Soc. Lond. B 265, 2223–2228. ( 10.1098/rspb.1998.0563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bro-Jørgensen J, Beeston J. 2015. Multimodal signalling in an antelope: fluctuating facemasks and knee-clicks reveal the social status of eland bulls. Anim. Behav. 102, 231–239. ( 10.1016/j.anbehav.2015.01.027) [DOI] [Google Scholar]
- 24.Girard MB, Elias DO, Kasumovic MM. 2015. Female preference for multi-modal courtship: multiple signals are important for male mating success in peacock spiders. Proc. R. Soc. B 282, 2015222 ( 10.1098/rspb.2015.2222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker TJ.2014. Crickets in singing insects of North America. See http://entnemdept.ifas.ufl.edu/walker/Buzz/crickets.htm .
- 26.Harrison RG, Arnold J. 1982. A narrow hybrid zone between closely related cricket species. Evolution 36, 535–552. ( 10.2307/2408099) [DOI] [PubMed] [Google Scholar]
- 27.Doherty JA, Storz MM. 1992. Calling song and selective phonotaxis in the field crickets, Gryllus firmus and G. pennsylvanicus (Orthoptera: Gryllidae). J. Insect Behav. 5, 555–569. ( 10.1007/BF01048004) [DOI] [Google Scholar]
- 28.Gabel E, Gray DA, Hennig MR. 2016. How females of chirping and trilling field crickets integrate the 'what' and 'where' of male acoustic signals during decision making. J. Comp. Physiol. A 202, 823–837. ( 10.1007/s00359-016-1124-x) [DOI] [PubMed] [Google Scholar]
- 29.Grobe B, Rothbart MM, Hanschke A, Hennig RM. 2012. Auditory processing at two time scales by the cricket Gryllus bimaculatus. J. Exp. Biol. 215, 1681–1690. ( 10.1242/jeb.065466) [DOI] [PubMed] [Google Scholar]
- 30.Hennig RM, Weber T. 1997. Filtering of temporal parameters of the calling song by cricket females of two closely related species: a behavioral analysis. J. Comp. Physiol. A 180, 621–630. ( 10.1007/s003590050078) [DOI] [Google Scholar]
- 31.Rothbart MM, Hennig RM. 2012. Calling song signals and temporal preference functions in the cricket Teleogryllus leo. J. Comp. Physiol. A 198, 817–825. ( 10.1007/s00359-012-0751-0) [DOI] [PubMed] [Google Scholar]
- 32.Rothbart MM, Hennig RM. 2012. The Steppengrille (Gryllus spec./assimilis): selective filters and signal mismatch on two time scales. PLoS ONE 7, e43975 ( 10.1371/journal.pone.0043975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennig RM. 2009. Walking in Fourier's space: algorithms for the computation of periodicities in song patterns by the cricket Gryllus bimaculatus. J. Comp. Physiol. A 195, 971–987. ( 10.1007/s00359-009-0473-0) [DOI] [PubMed] [Google Scholar]
- 34.Beckers OM, Wagner WE Jr. 2012. Divergent preferences for song structure between a field cricket and its phonotactic parasitoid. J. Insect Behav. 25, 467–477. ( 10.1007/s10905-011-9312-6) [DOI] [Google Scholar]
- 35.Schul J, Bush SL. 2002. Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc. R. Soc. Lond B 269, 1847–1852. ( 10.1098/rspb.2002.2092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blankers T, Lübke A, Hennig R. 2015. Phenotypic variation and covariation indicate high evolvability of acoustic communication in crickets. J. Evol. Biol. 28, 1656–1669. ( 10.1111/jeb.12686) [DOI] [PubMed] [Google Scholar]
- 37.Gray DA, Cade WH. 1999. Quantitative genetics of sexual selection in the field cricket, Gryllus integer. Evolution 53, 848–854. ( 10.2307/2640724) [DOI] [PubMed] [Google Scholar]
- 38.Gray DA, Cade WH. 2000. Sexual selection and speciation in field crickets. Proc. Natl Acad. Sci. USA 97, 14 449–14 454. ( 10.1073/pnas.97.26.14449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izzo AS, Gray DA. 2004. Cricket song in sympatry: species specificity of song without reproductive character displacement in Gryllus rubens. Ann. Entomol. Soc. Am. 97, 831–837. ( 10.1603/0013-8746(2004)097%5B0831:CSISSS%5D2.0.CO;2) [DOI] [Google Scholar]
- 40.Sakaguchi KM, Gray DA. 2011. Host song selection by an acoustically-orienting parasitoid fly exploiting a multi-species assemblage of cricket hosts. Anim. Behav. 81, 851–858. ( 10.1016/j.anbehav.2011.01.024) [DOI] [Google Scholar]
- 41.Benda J, Herz AVM. 2003. A universal model for spike-frequency adaptation. Neural Comput. 15, 2523–2564. ( 10.1162/089976603322385063) [DOI] [PubMed] [Google Scholar]
- 42.Hildebrandt KJ, Ronacher B, Hennig RM, Benda J. 2015. A neural mechanism for time-window separation resolves ambiguity of adaptive coding. PLoS Biol. 13, e1002096 ( 10.1371/journal.pbio.1002096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benda J, Hennig RM. 2008. Spike-frequency adaptation generates intensity invariance in a primary auditory interneuron. J. Comput. Neurosci. 24, 113–136. ( 10.1007/s10827-007-0044-8) [DOI] [PubMed] [Google Scholar]
- 44.Prešern J, Triblehorn JD, Schul J. 2015. Dynamic dendritic compartmentalization underlies stimulus-specific adaptation in an insect neuron. J. Neurophysiol. 113, 3787–3797. ( 10.1152/jn.00945.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan MJ, Fox JH, Wilczynski W, Rand AS. 1990. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343, 66–67. ( 10.1038/343066a0) [DOI] [PubMed] [Google Scholar]
- 46.Searcy WA. 1992. Song repertoire and mate choice in birds. Am. Zool. 32, 71–80. ( 10.1093/icb/32.1.71) [DOI] [Google Scholar]
- 47.Collins SA. 1999. Is female preference for male repertoires due to sensory bias? Proc. R. Soc. Lond. B 266, 2309–2314. ( 10.1098/rspb.1999.0924) [DOI] [Google Scholar]
- 48.Schul J. 1997. Neuronal basis of phonotactic behaviour in Tettigonia viridissima: processing of behaviourally relevant signals by auditory afferents and thoracic interneurons. J. Comp. Physiol. A 180, 573–583. ( 10.1007/s003590050074) [DOI] [Google Scholar]
- 49.Gerhardt HC, Humfeld SC, Marshall VT. 2007. Temporal order and the evolution of complex acoustic signals. Proc. R. Soc. B 274, 1789–1794. ( 10.1098/rspb.2007.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray DA, Gabel E, Blankers T, Hennig RM. Data from: Multivariate female preference tests reveal latent perceptual biases. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in the Dryad Digital Repository subject to a 1 year embargo [50] at http://dx.doi.org/10.5061/dryad.66bh6.