Abstract
Although the lung is one of the least studied organs in diabetes, increasing evidence indicates that it is an inevitable target of diabetic complications. Nevertheless, the underlying biochemical mechanisms of lung injury in diabetes remain largely unexplored. Given that redox imbalance, oxidative stress, and mitochondrial dysfunction have been implicated in diabetic tissue injury, we set out to investigate mechanisms of lung injury in diabetes. The objective of this study was to evaluate NADH/NAD+ redox status, oxidative stress, and mitochondrial abnormalities in the diabetic lung. Using STZ induced diabetes in rat as a model, we measured redox-imbalance related parameters including aldose reductase activity, level of poly ADP ribose polymerase (PAPR-1), NAD+ content, NADPH content, reduced form of glutathione (GSH), and glucose 6-phophate dehydrogenase (G6PD) activity. For assessment of mitochondrial abnormalities in the diabetic lung, we measured the activities of mitochondrial electron transport chain complexes I to IV and complex V as well as dihydrolipoamide dehydrogenase (DLDH) content and activity. We also measured the protein content of NAD+ dependent enzymes such as sirtuin3 (sirt3) and NAD(P)H: quinone oxidoreductase 1 (NQO1). Our results demonstrate that NADH/NAD+ redox imbalance occurs in the diabetic lung. This redox imbalance upregulates the activities of complexes I to IV, but not complex V; and this upregulation is likely the source of increased mitochondrial ROS production, oxidative stress, and cell death in the diabetic lung. These results, together with the findings that the protein contents of DLDH, sirt3, and NQO1 all are decreased in the diabetic lung, demonstrate that redox imbalance, mitochondrial abnormality, and oxidative stress contribute to lung injury in diabetes.
Keywords: Diabetes, Lung injury, Mitochondrial abnormalities, Oxidative stress, Redox imbalance
1. Introduction
Diabetes is a problem of glucose metabolism and diabetes complications is the outcome of glucose toxicity, which is often manifested by increased protein glycation, activation of the polyol pathway and poly ADP ribose polymerase (PARP), and protein kinase C activation [1], [2], [3], [4], [5]. Mechanistically, all these hyperglycemia upregulated pathways can eventually lead to production of reactive oxygen species (ROS) that then induce oxidative stress, mitochondrial dysfunction, and cell death [6], [7]. Although the lung is one of the least studied organs in diabetes complications, increasing evidence has indicated that the lung is a target of diabetic injury [8], [9], [10], [11]. Nevertheless, the underlying mechanisms remain largely unknown.
As glucose is one of the major sources of NADH, its excess can often lead to excess NADH production and NAD+ deficiency, thereby causing NADH/NAD+ redox imbalance [12]. The major source of this redox imbalance is thought to come from the activation of the polyol pathway and poly ADP ribose polymerase (PARP) [13], [14], [15], [16]. On one hand, the polyol pathway converts NADPH to NADH when it transforms glucose to fructose via a two–reaction mechanism [17], resulting in NADH overproduction at the consumption of glucose [18], [19]. On the other hand, as PARP uses NAD+ as its substrate and is usually over-activated by DNA oxidative damage in diabetes [20], cellular NAD+ could be potentially depleted [21], [22], [23]. Therefore, the overall outcome of the two activated pathways is NADH/NAD+ redox imbalance with diminished levels of NAD+ and increased levels of NADH, leading to reductive stress that gradually progresses to oxidative stress [24].
Oxidative stress occurs when cellular antioxidative system is defeated by ROS that are overproduced under a variety of disease conditions including diabetes [25]. As mitochondrion is a major source of ROS and a target of ROS [26], [27], its abnormalities have been thought to contribute to diabetic pathogenesis [28]. However, whether mitochondrial abnormalities also occur in the diabetic lung remains to be evaluated. In the present study, using STZ induced diabetes in rat as a model; we characterized pulmonary redox imbalance and its associated pathways. Specifically, we measured the activities of mitochondrial membrane complexes I to V. We also measured the enzyme activities of mitochondrial dihydrolipoamide dehydrogenase (DLDH) and its possible modifications by protein acetylation. Additionally, lung mitochondrial ROS production and overall protein oxidative damage were quantified. NAD(P)H: quinone oxidoreductase 1 (NQO1) protein content and activity and sirtuin 3 (sirt3) protein content were also evaluated in the context of redox imbalance and mitochondrial abnormalities in the diabetic lung.
2. Materials and methods
2.1. Chemicals
Biotin-linked aldehyde reactive probe ARP) for protein carbonyl assay was from Cayman Chemical (Ann Arbor, MI). Dihydrolipoamide was synthesized from lipoamide in our own laboratory using sodium borohydride as previously described [29], [30]. ε-amino-N-caproic acid was obtained from MP Biochemicals. Acrylamide/bisacrylamide, ammonium persulfate, Bradford protein assay solution, coomassie brilliant blue (CBB) R-250, immunoblotting membrane, and an ECL immunochemical detection kit were from Bio-Rad laboratories (Richmond, CA, USA). NADH, BSA, lipoamide, EDTA, ATP, and NBT chloride tablets were obtained from Sigma (St. Louis, MO, USA). Serva Blue G was purchased from Serva (Heidelberg, Germany). Anti-PARP antibody was purchased from Trevigen (Gaithers burg, MD). Anti-NQO1 antibodies were from Sigma. Rabbit anti-DLDH polyclonal antibodies (IgG) and goat anti-rabbit IgG conjugated with horseradish peroxidase were purchased from US Biological (Swampscott, MA, USA) and Invitrogen (San Diego, CA, USA), respectively. Other antibodies were from Abcam (Cambridge, MA).
2.2. Diabetes induction in rats
Young adult male Sprague Dawley rats obtained from Charles River were used in this study. Diabetes was induced by a single intraperitoneal injection of STZ (60 mg/kg body weight) into rats weighing 220–250 g after overnight fasting as previously described [31]. STZ solutions were made fresh by dissolving in 0.1 M sodium citrate buffer (pH 4.5) and control animals received sodium citrate buffer only. Blood glucose concentration was monitored once a week using blood glucose test strips (FreeStyle lite from Abbott Diabetes Care Inc., Alameda, California). Rats with blood glucose contents exceeding 200 mg/dl were deemed diabetic. Four weeks post STZ injections, rats were sacrificed and tissues were collected. All animal studies procedures were approved by the UNTHSC committee for research.
2.3. Isolation of lung mitochondria
Mitochondria from the lung were isolated according to a previously described method [32] with slight modifications. Essentially, lung tissues were homogenized (1g/10 ml isolation buffer) in mitochondrial isolation buffer containing 15 mM MOPS (pH 7.2), 70 mM sucrose, 230 mM mannitol, and 1 mM K+-EDTA. The homogenates were centrifuged at 800g for 10 min at 4 °C. The resulting supernatant was further centrifuged at 8,000g for 10 min also at 4 °C. The resulting pellet containing crude mitochondria was washed with 10 ml of the isolation buffer followed by centrifugation under the same conditions. The obtained mitochondrial pellet was either used immediately or frozen at −80 °C until use.
2.4. Measurement of H2O2 and protein carbonyls
Lung tissue homogenate H2O2 was measured by the Amplex Red method [33] using a kit purchased from Invitrogen (catalog number A22188). Protein carbonyls of whole mitochondrial preparation were measured by derivatization with biotin-linked aldehyde reactive probe (ARP) [34] followed by SDS-PAGE resolution of the carbonylated proteins and Western blot assay and densitometric quantification of each gel lane.
2.5. Measurement of NAD+/NADH ratio, NADPH, and ATP
Lung tissue homogenate NAD+/NADH ratio was measured spectrophotometrically by following the changes at 340 nm using a kit from BioAssay (Hayward, CA). NADPH content was measured by a kit from BioVision (Milpitas, CA, Catalog number: K347-100) according to the manufacturer's instructions. ATP content was determined colorimetrically by the ATP Colorimetric/Fluorometric Assay kit that is also from BioVision (Milpitas, CA, catalog number K354-100). This method quantifies phosphorylated glycerol that can be easily monitored at 570 nm.
2.6. Measurement of enzyme activities
Aldose reductase activity was measured spectrophotometrically by following the decrease of NADPH's absorption at 340 nm as previously described [35]. DLDH dehydrogenase activity was measured in the forward reaction as previously described [36], [37]. Measurement of mitochondrial complexes I, IV and V activities was also conducted as previously described using in-gel based assays or spectrophotometric assays [38]. Activities for complexes II and III were measured spectrophotometrically as previously described [39], [40]. NQO1 activity was measured according to the method of Lind et al. [41] and G6PD activity was measured by monitoring NADPH production at 340 nm as previously described [42]. Caspase-3 activity was measured using a kit also from BioAssay (Hayward, CA). Mitochondrial membrane potential was measured by a kit purchased from BioVision (Milpitas, CA) according to the manufacturer's instruction manual.
2.7. Polyacrylamide gel electrophoresis and Western blot analysis
SDS-PAGE (typically 10% resolving gel) was performed according to standard procedures [43]. One of the resulting gels was stained with Coomassie colloid blue [38], and the other gel was subjected to electrophoretic transfer to membrane for immunoblotting [44]. Signals on the immunoblotting membrane were visualized with an enhanced chemiluminescence kit. Nongradient blue native gel electrophoresis (BN-PAGE) was performed as previously described [36]. All images were scanned by an EPSON PERFECTION 1670 scanner. All densitometric quantifications of gel images were analyzed by AlphaEaseFC software.
2.8. Data analysis
Where appropriate, all values were presented as mean±SEM. Statistical data analysis was performed using GraphPad's 2-tailed unpaired t-test (GraphPad, San Diego, CA). A p value less than 0.05 (p<0.05) was deemed statistically significant.
3. Results
3.1. Redox imbalance in the diabetic lung
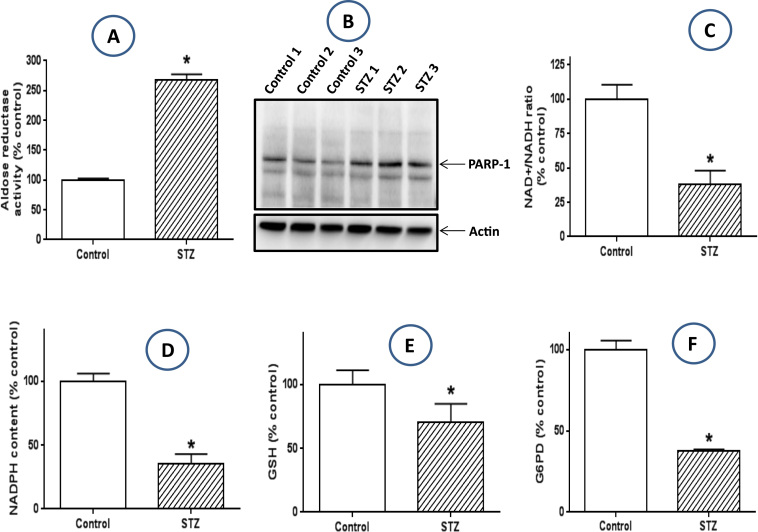
In many diabetic tissues that have been well studied, redox imbalance between NADH and NAD+ is the primary driving force for ROS production and oxidative stress [12], [13]. This redox imbalance is believed to mainly originate from two enzyme systems activated by persistent hyperglycemia. One reaction is the polyol pathway including aldose reductase and sorbitol dehydrogenase [45]. This pathway converts glucose to fructose and NADPH to NADH, resulting in overproduction of NADH [46]. Another pathway is poly ADP ribose polymerase (PARP) that uses NAD+ as its substrate [47]. This enzyme can be over-activated by hyperglycemia induced DNA oxidative damage, resulting in potential depletion of NAD+[48]. Therefore an overall outcome of NADH/NAD+ redox imbalance occurs in diabetic tissues [13]. To test whether this redox imbalance mechanism takes place in the diabetic lung, we measured aldose reductase activity and the protein content of PARP-1, results in Fig. 1A demonstrate that the activity of aldose reductase, the rate-limiting enzyme of the polyol pathway [49], was indeed higher than that in the control lung. Similarly, results in Fig. 1B demonstrate that PARP-1 protein content was upregulated in the diabetic lung. Together, the upregulation of these two pathways contributed to a much lower level of NAD+ in the diabetic lung than in the healthy lung as observed in Fig. 1C. Moreover, we also found that in the diabetic lung, NADPH content was lower, so was GSH content, the normal level of the latter depends on a normal level of NADPH that is used by glutathione reductase to make GSH from GSSG [50]. Additionally, we also found that the activity of glucose-6 phosphate dehydrogenase (G6PD) was also lower in the diabetic lungs than in the healthy lungs, suggesting that a decreased level of NADPH could be partly driven by a low activity of G6PD that is responsible for NADPH production from glucose [51], [52].
Fig. 1.
: Redox imbalance parameters in the diabetic lung. When compared with the lungs from non-diabetic rats, the diabetic lungs show an increased aldose reductase activity (A), an increased protein expression of PARP-1, a significantly decreased level of NAD+(C), a decreased level of NADPH (D), a decreased level of reduced from of glutathione (GSH) (E), and a decreased activity of G6PD (F).
3.2. Increased activities of mitochondrial electron transport chain complexes
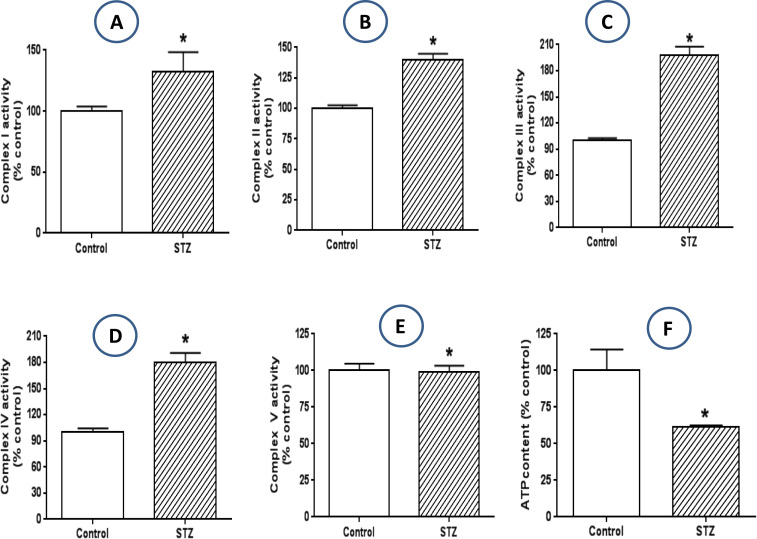
The excess NADH in the diabetic lung as measured in Fig. 1C suggests that there is an oversupply of electrons to mitochondrial electron transport chain. Therefore we next determined the effects of this excess NADH on the enzyme activities of mitochondrial electron transport chain components complexes I to IV. Results shown in Fig. 2(A-D) indicate that the enzyme activities of all the four complexes were elevated in the diabetic lung, suggesting an enhanced electron transport imposed by excess NADH on the mitochondrial electron transport chain. We also measured complex V activity but did not detect any different between control and diabetes (Fig. 2E). Nonetheless, ATP content was much lower in the diabetic lung than in the healthy lungs (Fig. 2F). These results suggested that the upregulated electron transport chain activities (I to IV) are not for ATP production. Rather, the increased electron transport chain function may contribute to increased ROS production, given that majority of ROS can be produced by mitochondria in diabetes [53].
Fig. 2.
: Activities of mitochondrial membrane complexes. The activities of mitochondrial electron transport chain complexes I to IV (A to D) all exhibited decreases in the diabetic lung when compared with those in the controls. No difference in complex V activity between control and STZ-diabetes could be detected (E). Cellular ATP content was lower in the diabetic lung than in the non-diabetic lung (F).
3.3. Increased oxidative stress in the diabetic lung
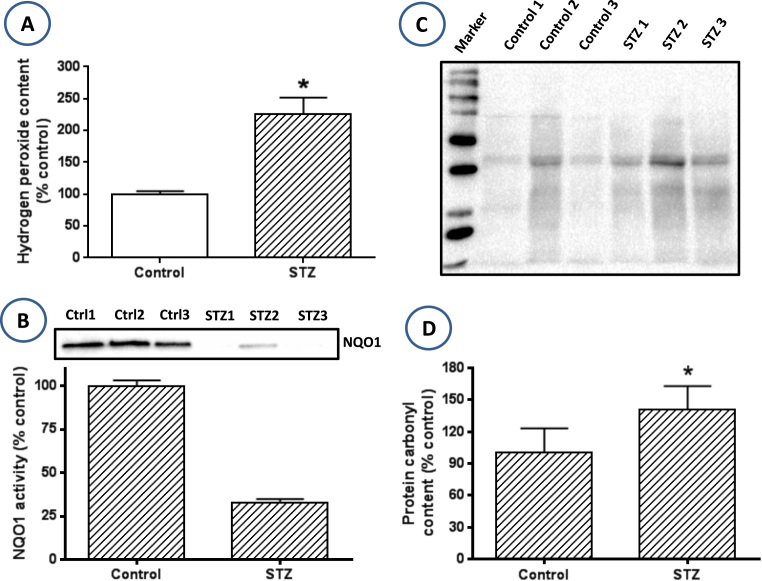
To test the above hypothesis that the upregulated mitochondrial electron transport chain leads to increased ROS production, we then measured hydrogen peroxide in the lung homogenate. A comparison between control and diabetic lungs indicates that H2O2 content was much higher in the diabetic lung than in the healthy lung (Fig. 3A). Moreover, we also measured lung mitochondrial protein carbonylation using western blot assay. Results indicate increased total protein carbonylation in the diabetic group than in the control group (Fig. 3, C and D). Moreover, we also measured the activity and content of NQO1, a second phase antioxidant enzyme that is usually upregulated by the Nrf2 transcription factor signaling pathway [54]. Result in Fig. 3B indicates that both NQO1 content and activity were much lower in the diabetic lung than in the control lung. Taken together, our results indicate that NADH/NAD+ redox imbalance in the diabetic lung induces oxidative stress that may be implicated in lung dysfunction in diabetes.
Fig. 3.
: Elevated level of oxidative stress and attenuated antioxidative capacity in the diabetic lung. (A) ROS production reflected by the level of H2O2 was increased in the diabetic lung. (B) NQO1 protein content was lower in the diabetic lung than in the non-diabetic lung. (C) Lung mitochondrial protein carbonylation assessed by Western blot assay using aldehyde-reactive probe as the labeling reagent. (D) Densitometric analysis of protein carbonyl content between control and diabetes. Data are derived from (C).
3.4. Upregulation of complex I activity is likely mediated by increased expression of nicotinamide N-methyltransferase (NNMT) and NDUFS3
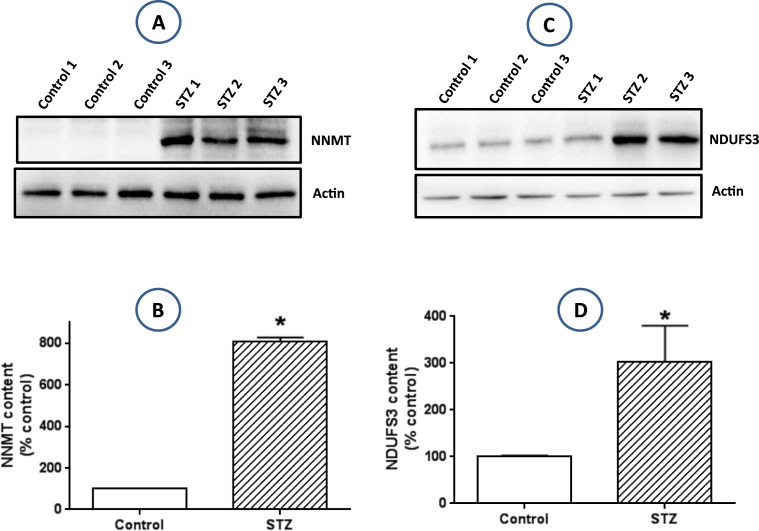
As complex I is the entry point of electrons into the electron transport chain, we next determine whether hyperglycemia upregulates complex I. While complex I upregulation could be an adaptive response to excess NADH, this upregulation could also increase mitochondrial ROS production given that the more NADH oxidized by complex I, the more ROS produced by complex I [55], [56]. Based on reports in the literature that NNMT can be upregulated by overnutrition and hyperglycemia and that this upregulation elevates the expression of the complex I subunit NDUFS3 [57], [58], [59] thereby increasing complex I activity, we measured by western blot methods the protein content of both NNMT and NDUFS3 (Fig. 4A and C). Densitometric analysis of these western blot results indicated that the protein content of both NNMT and NDUFS3 were significantly increased (Fig. 4B and D), demonstrating that NNMT upregulation by diabetic hyperglycemia can increase complex I activity by increasing the expression of complex I NDUFS3 subunit.
Fig. 4.
: Evaluation of protein expression of NNMT and NDUFS3. (A) Increased expression of NNMT in the diabetic lung measured by Western blot assay. (B) Densitometric quantitation of Western blot band intensity shown in (A). (C) Increased expression of NDUFS3 in the diabetic lung measured by Western blot assay. (D) Densitometric quantitation of Western blot band intensity as shown in (C).
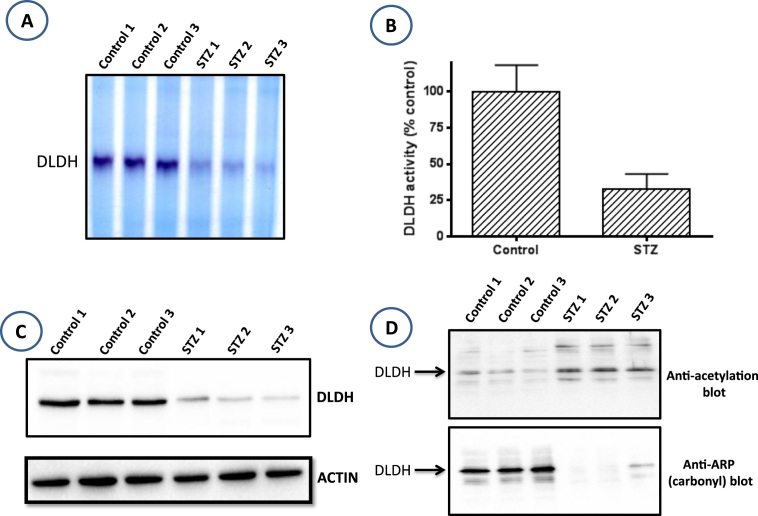
3.5. Attenuated expression of dihydrolipoamide dehydrogenase (DLDH) in the diabetic lung
Dihydrolipoamide dehydrogenase (DLDH) is a component of three mitochondrial alpha keto acid dehydrogenase complexes [37]. It is a key enzyme in the production of acetyl-CoA from pyruvate and branched chain amino acids [60]. DLDH uses NAD+ as one of its two substrates and makes NADH [61]. It is known that the level of NAD+ could affect either the level or the activity of DLDH [62], [63]. To test whether a low NAD+ content in the diabetic lung leads to an attenuated DLDH function or a low DLDH protein content, we measured DLDH content and enzyme activity. Enzymatic activity was measured by both blue native gel electrophoresis and spectrometry. Results in Fig. 5A and B indicate that DLDH activity was indeed significantly lower in the diabetic lung than in the healthy lung. Moreover, data in Fig. 5C indicates that DLDH content was remarkably decreased in the diabetic lung, indicating that a lower DLDH activity in the diabetic lung is contributed by a lower DLDH content. Furthermore, when the DLDH activity bands on the blue native gel were analyzed by mass spectrometry, 7 DLDH peptides were recovered in the control samples while none could be recovered in the STZ-treated samples (data not shown), confirming that DLDH is indeed down-regulated in the diabetic lung.
Fig. 5.
Comparison of DLDH activity and expression between control and diabetic lungs. (A) Decreased DLDH activity in the diabetic lung assessed by BN-PAGE. (B) Decreased DLDH activity in the diabetic lung measured spectrophotometrically. (C) Decreased DLDH protein content in the diabetic lung assessed by Western bot assay. (D) DLDH acetylation (Upper), but not DLDH protein carbonylation (lower) was increased in the diabetic lung. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article)
It should be pointed out that the low DLDH activity observed in A and B could also be contributed by DLDH acetylation as DLDH from the diabetic lung was found to exhibit elevated levels of protein acetylation (Fig. 5D, upper panel). Interestingly, DLDH protein was not found to be damaged via carbonylation (Fig. 5D, lower panel), a parameter used to quantitate protein oxidative damage [44], [64]. This finding is in agreement with our previous findings that DLDH is not an apparent target for carbonylation during aging [61], a process associated with increased oxidative stress [65].
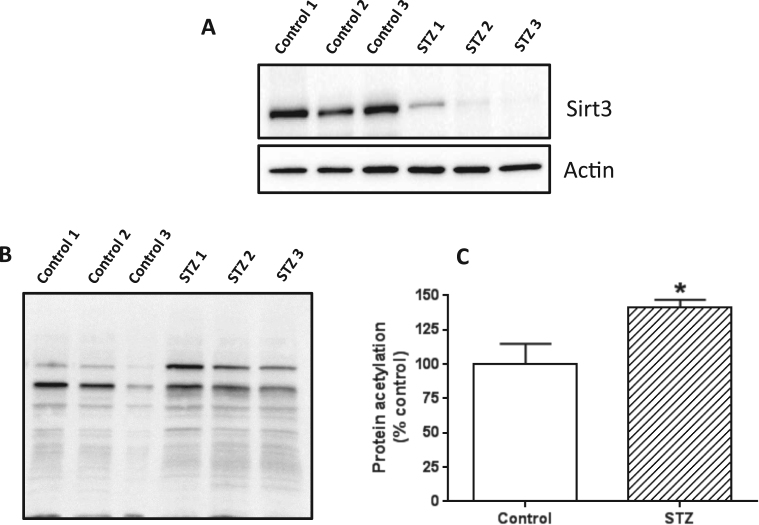
3.6. Decreased mitochondrial sirtuin 3 (sirt3) content in the diabetic lung
It has been well established that the level of NAD+ in a cell can dictate the level of sirtuin proteins [66], which are deacetylation enzymes that are known to be involved in redox signaling and metabolic control [67]. Our finding that NAD+ content is decreased in the diabetic lung suggests that sirt3 level could be attenuated as well given that sirtuin protein expression is NAD+ dependent [66]. To test this likelihood, we measured mitochondrial sirt3 protein content by western blot. Result in Fig. 6A demonstrates that sirt3 protein content was severely decreased in the diabetic lung than in the control lung. Consequently, total mitochondrial protein acetylation in the diabetic lung was greater than that in the control lung (Fig. 6B and C), which is in agreement with the observation that DLDH acetylation was increased in the diabetic lung (Fig. 5D, upper panel).
Fig. 6.
: (A) Sirt3 protein content was decreased in the diabetic lung. Anti-sirt3 antibodies were used for this evaluation with actin as the loading control. (B) Western blot detection of mitochondrial protein acetylation; shown are lung mitochondria isolated from three control rats and 3 diabetic rats, respectively. (C) Densitometric quantification of mitochondrial protein acetylation. Data were derived from (B).
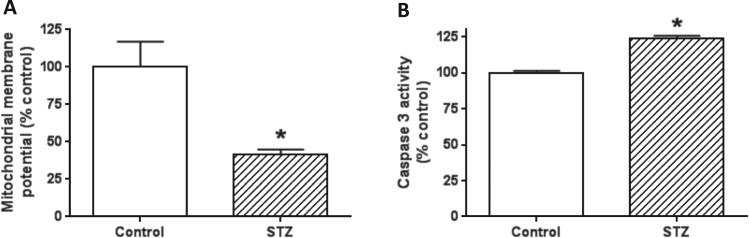
3.7. Impaired mitochondrial membrane potential and increased cell death in the diabetic lung
Our findings that enhanced mitochondrial electron transport did not lead to increased mitochondrial ATP production suggest that mitochondria in the diabetic lung are not well-coupled. Therefore, we next measured mitochondrial membrane potential. Results in Fig. 7A demonstrate that diabetic pulmonary mitochondrial membrane potential was lower than that in control, which could lead to enhanced electron leakage and oxidative stress as shown in Fig. 5. Moreover, less mitochondrial ATP production can also suggest an increased cell death. Indeed, measurement of caspase-3 activity, a parameter reflecting the magnitude of cell death, demonstrates an increased level of apoptosis as caspase-3 activity was much higher in diabetes than in control (Fig. 7B).
Fig. 7.
: Determination of mitochondrial membrane potential and cell death. (A) Mitochondrial membrane potential in the diabetic lung was significantly lower than that in the healthy lung. (B) Increased caspase-3 activity in the diabetic lung than in the healthy lung, indicating increased cell death in the diabetic lung (N =3 for each measurement).
4. Discussion
The major findings of the present study are the following: 1) NADH/NAD+ redox imbalance occurred in the diabetic lung and was likely contributed by activation of the polyol pathway and PARP. 2) The activities of mitochondrial complexes I to IV were upregulated while there were no changes in complex V activity. 3) Mitochondrial ATP output was lower in the diabetic lung than in the non-diabetic lung. 4) Mitochondrial ROS production and protein carbonylation were increased, which was accompanied by decreased protein content and activity of NQO1, an inducible antioxidant enzyme involved in cellular defense against oxidative stress [68]. 5) Complex I upregulation by diabetic hyperglycemia was likely achieved by upregulation of NNMT that in turn upregulates NDUFS3, a key complex I subunit involved in complex I assembly and function [69]. 6) DLDH protein content was decreased in the diabetic lung, which impaired DLDH activity that may also be accentuated by the observation that DLDH acetylation was increased in the diabetic lung, a process that can be enhanced by down regulation of mitochondrial sirt3 that is responsible for protein deacetylation [70]. 7) Mitochondrial membrane potential in the diabetic lung was decreased and cell death was increased. Taken together, results of the present study shed insights into the biochemical mechanisms of lung injury in diabetes. It should be pointed out that one caveat of the study is that we did not study whether the redox imbalance occurs to all the cellular populations in the lung that is composed of nearly 40 different cell types.
Our study presents strong evidence that there occurs also redox imbalance in the diabetic lung with NADH being in excess. Excess NADH could over burden the electron transport chain and cause more mitochondrial ROS production. As an adaptive response to handle NADH pressure complex I was found to be upregulated (Fig. 2A), together with other electron transport chain complexes II to IV (Fig. 2B-D). The adverse effect of this upregulation, unfortunately, would be increased ROS production given that the more NADH oxidized by complex I, the more ROS produced by complex I [26], [71]. Indeed, excess NADH appears to be used for ROS generation as complex V was not upregulated and ATP output by mitochondria was decreased (Fig. 2E and F), indicating an uncoupling effect of diabetic hyperglycemia on pulmonary mitochondrial oxidative phosphorylation as shown in Fig. 7A and increased cell death as shown in Fig. 7B. These results demonstrate overall mitochondrial abnormalities in the presence of glucose oversupply in that excess NADH is not completely used for ATP production but rather diverted for production of mitochondrial ROS that could be involved in cell death and lung injury in diabetes.
Our study also demonstrates that NADPH-related signaling pathways in the diabetic lungs were also compromised. Not only NADPH level was found to be attenuated, activity of G6PD and levels of GSH (reduced glutathione) were also found to be suppressed. It seems that the decrease in NADPH content could be contributed by two pathways that are deregulated in diabetes by hyperglycemia. One pathway is NADPH consumption by the polyol pathway for the generation of NADH; another pathway is functional impairment of G6PD that could lead to less NADPH production. As NADPH is required for GSH formation from GSSG by glutathione reductase [42], [72], less NADPH would lead to less GSH formation. This is indeed what we have found in the diabetic lung whereby GSH content was low (Fig. 1E). Our results are similar to what have been found in the diabetic kidneys in which G6PD activity, NADPH and GSH levels were all found to be lower in diabetic animals than in non-diabetic controls [73]. It should be noted that the alterations in G6PD activities in diabetes are likely tissue dependent. For example, in the brain of STZ diabetic rats, G6PD activity was markedly increased [74]. G6PD activity was also found to be increased in the liver of Zucker diabetic rats [75]. Similarly, NADPH content in certain diabetic tissues has been reported to be elevated when compared with that in non-diabetic conditions [76], [77], [78]. Nonetheless, in our present study, we found that NADPH level declined in diabetic lung (Fig. 1D). It should be pointed out that the level of NADH can increase dramatically in the presence of a decreased NADPH content is due to the fact that cellular NADPH concentration is usually much higher than that of NADH. For example, in red blood cells, NDAPH content is approximately 10 times higher than that of NADH [79].
In the present study, we also analyzed NAD+-dependent enzymes such as NQO1, DLDH, and sirt3. Our results show that the functions of all the three enzymes were impaired in the diabetic lung, either through down regulation of protein expression or posttranslational modifications or both. With respect to NQO1, as its expression is controlled by Nrf2 transcription factor [80], our observation that NQO1 content showed a decrease in the diabetic lung suggests that the Nrf2 signaling pathway is suppressed in the diabetic lung; which needs to be further evaluated in future studies. Nonetheless it has been reported that the Nrf2 signaling pathway is down regulated in other diabetic tissues such as liver and heart [81], [82]. It should be noted, however, that how Nrf2 is regulated in diabetes may be tissue dependent as it has been shown that in the kidney, Nrf2 could be upregulated by diabetic hyperglycemia [83].
With respect to DLDH, this protein is also known to be able to generate ROS [84], [85], [86]. This enzyme uses NAD+ as its substrate and makes NADH that can be fed into the electron transport chain. In the presence of excess NADH, DLDH can be inhibited via a feedback inhibitory mechanism [87]. In the present study, we found that DLDH in the diabetic lungs was down regulated with a diminished DLDH protein content (Fig. 5C). This diminution would certainly impair the role of DLDH in mitochondrial bioenergetics. Functional impairment of DLDH in the diabetic lung could also partially originate from its cysteine acetylation; a process regulated by sirt3 and is known to affect protein functions [70], [88], [89]. Our findings that DLDH underwent protein acetylation in the diabetic lung are in agreement with previous reports that DLDH is a target of protein acetylation [88], [90], [91]. We think that DLDH acetylation in diabetic lung could be governed by two mechanisms. One is due to oversupply of acetyl-CoA that can chemically modify a protein cysteine residues [92], [93], [94], another mechanism would be the down regulation of mitochondrial sirt3 (Fig. 6) detected in the diabetic lung, which would lead to less deacetylation of DLDH. Our data suggest that DLDH dysfunction might be a pathogenic mechanism for diabetic lung injury.
With respect to sirt3, our observation that sirt3 is down regulated in the diabetic lung mitochondria (Fig. 6) agrees with the results of previous studies whereby sirt3 shows a decreased expression in diabetic tissues [95], [96], [97], [98]. The reason for this is likely due to the fact that NAD+ is decreased in diabetes [99], [100]. It is known that the level of sirtuin expression is dependent on NAD+ content [66], [101]. As sirt3 is responsible for protein deacetylation, its decreased expression would certainly increase protein acetylation, as was in the case for DLDH and other mitochondrial proteins (Fig. 6B and C). Therefore, impaired sirt3 signaling pathway could provide another mechanism of mitochondrial abnormalities associated with lung injury in diabetes.
5. Summary
In this study, we have presented evidence that in the diabetic lung, NADH/NAD+ redox balance was perturbed with NADH being in excess and NAD+ being deficient. Consequently, mitochondrial electron transport chain could be under NADH electron pressure and was indeed found to be upregulated in response to this pressure. However, this upregulation does not seem to lead to enhanced mitochondrial ATP production as complex V activity was not upregulated and ATP content was suppressed. Conversely, the upregulation of electron transport chain function was found to be associated with decreased mitochondrial membrane potential, increased ROS generation, elevated oxidative stress, and increased cell death. Moreover, the function of NAD+-dependent enzymes such as NQO1, DLDH, and sirt3 were all found to be impaired in the diabetic lung. Taken together, the present study has elucidated mechanisms by which lung function can be impaired in diabetes.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported in part by a UNTHSC seed grant RI10015. LJY was also supported in part by a National Institutes of Health grant R01NS079792.
References
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 3.Luo X., Wu J., Jing S., Yan L.J. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016;7:90–110. doi: 10.14336/AD.2015.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng H., Wu J., Jin Z., Yan L.J. Protein Modifications as Manifestations of Hyperglycemic Glucotoxicity in Diabetes and Its Complications. Biochem Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H., Wu J., Jin Z., Yan L.-J. Potential biochemical mechanisms of lung injury in diabetes. Aging Dis. 2017;8 doi: 10.14336/AD.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 7.Kassab A., Piwowar A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie. 2012;94:1837–1848. doi: 10.1016/j.biochi.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Kuitert L.M. The lung in diabetes--yet another target organ? Chron. Respir. Dis. 2008;5:67–68. doi: 10.1177/1479972308091408. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Ma Z., Guo Z., Zhao F., Wang Y., Cai L., Yang J. Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochem Biophys. 2014;70:1385–1391. doi: 10.1007/s12013-014-0068-4. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Tan Y., Zhao F., Ma Z., Wang Y., Zheng S., Epstein P.N., Yu J., Yin X., Zheng Y., Li X., Miao L., Cai L. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am. J. Physiol. Endocrinol. Metab. 2011;301:E132–E144. doi: 10.1152/ajpendo.00629.2010. [DOI] [PubMed] [Google Scholar]
- 11.Pitocco D., Fuso L., Conte E.G., Zaccardi F., Condoluci C., Scavone G., Incalzi R.A., Ghirlanda G. The diabetic lung--a new target organ? Rev. Diabet. Stud. 2012;9:23–35. doi: 10.1900/RDS.2012.9.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Jin Z., Zheng H., Yan L.J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016;9:145–153. doi: 10.2147/DMSO.S106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boesten D.M., von Ungern-Sternberg S.N., den Hartog G.J., Bast A. Protective pleiotropic effect of flavonoids on nad(+) levels in endothelial cells exposed to high glucose. Oxid. Med Cell Longev. 2015. 2015:894597. doi: 10.1155/2015/894597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W.H., Martin K.A., Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharm. 2012;3:87. doi: 10.3389/fphar.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masutani M., Suzuki H., Kamada N., Watanabe M., Ueda O., Nozaki T., Jishage K., Watanabe T., Sugimoto T., Nakagama H., Ochiya T., Sugimura T. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieper A.A., Brat D.J., Krug D.K., Watkins C.C., Gupta A., Blackshaw S., Verma A., Wang Z.Q., Snyder S.H. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kador P.F., Kinoshita J.H. Role of aldose reductase in the development of diabetes-associated complications. Am. J. Med. 1985;79:8–12. doi: 10.1016/0002-9343(85)90504-2. [DOI] [PubMed] [Google Scholar]
- 18.Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharm. Rev. 1998;50:21–33. [PubMed] [Google Scholar]
- 19.Hodgkinson A.D., Sondergaard K.L., Yang B., Cross D.F., Millward B.A., Demaine A.G. Aldose reductase expression is induced by hyperglycemia in diabetic nephropathy. Kidney Int. 2001;60:211–218. doi: 10.1046/j.1523-1755.2001.00788.x. [DOI] [PubMed] [Google Scholar]
- 20.Obrosova I.G., Drel V.R., Pacher P., Ilnytska O., Wang Z.Q., Stevens M.J., Yorek M.A. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo C., Zanchi A., Komjati K., Pacher P., Krolewski A.S., Quist W.C., LoGerfo F.W., Horton E.S., Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 22.Puthanveetil P., Zhang D., Wang Y., Wang F., Wan A., Abrahani A., Rodrigues B. Diabetes triggers a PARP1 mediated death pathway in the heart through participation of FoxO1. J. Mol. Cell Cardiol. 2012;53:677–686. doi: 10.1016/j.yjmcc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Mouchiroud L., Houtkooper R.H., Auwerx J. NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol. 2013;48:397–408. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L.J. Pathogenesis of Chronic Hyperglycemia: from Reductive Stress to Oxidative Stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson M.J., Papa S., Bolanos J., Bruckdorfer R., Carlsen H., Elliott R.M., Flier J., Griffiths H.R., Heales S., Holst B., Lorusso M., Lund E., Oivind Moskaug J., Moser U., Di Paola M., Polidori M.C., Signorile A., Stahl W., Vina-Ribes J., Astley S.B. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Asp. Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 26.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z., Wu J., Yan L.J. Chemical Conditioning as an Approach to Ischemic Stroke Tolerance: Mitochondria as the Target. Int J. Mol. Sci. 2016;17 doi: 10.3390/ijms17030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koliaki C., Roden M. Alterations of Mitochondrial Function and Insulin Sensitivity in Human Obesity and Diabetes Mellitus. Annu Rev. Nutr. 2016 doi: 10.1146/annurev-nutr-071715-050656. [DOI] [PubMed] [Google Scholar]
- 29.Patel M.S., Vettakkorumakankav N.N., Liu T.C. Dihydrolipoamide dehydrogenase: activity assays. Methods Enzym. 1995;252:186–195. doi: 10.1016/0076-6879(95)52022-8. [DOI] [PubMed] [Google Scholar]
- 30.Patel M.S., Hong Y.S. Lipoic acid as an antioxidant: the role of dihydrolipoamide dehydrogenase. In: Armstrong D., editor. Free Radical and Antioxidant Protocols. Humana Press; Totowa, NJ: 1998. pp. 337–346. [DOI] [PubMed] [Google Scholar]
- 31.Wu J., Luo X., Yan L.J. Two dimensional blue native/SDS-PAGE to identify mitochondrial complex I subunits modified by 4-hydroxynonenal (HNE) Front. Physiol. 2015;6 doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro A., Gomez C., Lopez-Cepero J.M., Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 34.Yan L.J. Analysis of oxidative modification of proteins. Curr. Protoc. Protein Sci. Chapter. 2009;14 doi: 10.1002/0471140864.ps1404s55. (Unit14 14) [DOI] [PubMed] [Google Scholar]
- 35.Bagnasco S.M., Uchida S., Balaban R.S., Kador P.F., Burg M.B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc. Natl. Acad. Sci. USA. 1987;84:1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan L.J., Yang S.H., Shu H., Prokai L., Forster M.J. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE. Electrophoresis. 2007;28:1036–1045. doi: 10.1002/elps.200600574. [DOI] [PubMed] [Google Scholar]
- 37.Yan L.J., Thangthaeng N., Forster M.J. Changes in dihydrolipoamide dehydrogenase expression and activity during postnatal development and aging in the rat brain. Mech. Ageing Dev. 2008;129:282–290. doi: 10.1016/j.mad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan L.J., Forster M.J. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal. Biochem. 2009;389:143–149. doi: 10.1016/j.ab.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripathy M.K., Mitra D. Differential modulation of mitochondrial OXPHOS system during HIV-1 induced T-cell apoptosis: up regulation of Complex-IV subunit COX-II and its possible implications. Apoptosis. 2010;15:28–40. doi: 10.1007/s10495-009-0408-9. [DOI] [PubMed] [Google Scholar]
- 40.Gusdon A.M., Votyakova T.V., Reynolds I.J., Mathews C.E. Nuclear and mitochondrial interaction involving mt-Nd2 leads to increased mitochondrial reactive oxygen species production. J. Biol. Chem. 2007;282:5171–5179. doi: 10.1074/jbc.M609367200. [DOI] [PubMed] [Google Scholar]
- 41.Lind C., Cadenas E., Hochstein P., Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzym. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 42.Yan L.J., Christians E.S., Liu L., Xiao X., Sohal R.S., Benjamin I.J. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan L.J., Levine R.L., Sohal R.S. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan L.J., Orr W.C., Sohal R.S. Identification of oxidized proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunochemical detection, isoelectric focusing, and microsequencing. Anal. Biochem. 1998;263:67–71. doi: 10.1006/abio.1998.2799. [DOI] [PubMed] [Google Scholar]
- 45.Ng T.F., Lee F.K., Song Z.T., Calcutt N.A., Lee A.Y., Chung S.S., Chung S.K. Effects of sorbitol dehydrogenase deficiency on nerve conduction in experimental diabetic mice. Diabetes. 1998;47:961–966. doi: 10.2337/diabetes.47.6.961. [DOI] [PubMed] [Google Scholar]
- 46.Lee A.Y., Chung S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 47.Pacher P., Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid. Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabo C. Roles of poly(ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharm. Res. 2005;52:60–71. doi: 10.1016/j.phrs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Chung S.S., Chung S.K. Aldose reductase in diabetic microvascular complications. Curr. Drug Targets. 2005;6:475–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 50.Kirsch M., De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 51.Sato T., Sasaki H., Watanabe R., Yoshinaga K. Enhancement of pentose phosphate pathway in vascular intima from diabetic rabbit. Tohoku J. Exp. Med. 1988;155:97–100. doi: 10.1620/tjem.155.97. [DOI] [PubMed] [Google Scholar]
- 52.Wamelink M.M., Struys E.A., Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 2008;31:703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 53.Panigrahy S.K., Bhatt R., Kumar A. Reactive oxygen species: sources, consequences and targeted therapy in type-II diabetes. J. Drug Target. 2016:1–36. doi: 10.1080/1061186X.2016.1207650. [DOI] [PubMed] [Google Scholar]
- 54.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 55.Treberg J.R., Quinlan C.L., Brand M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2011;286:27103–27110. doi: 10.1074/jbc.M111.252502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper J.M., Mann V.M., Krige D., Schapira A.H. Human mitochondrial complex I dysfunction. Biochim Biophys. Acta. 1992;1101:198–203. doi: 10.1016/s0005-2728(05)80019-2. [DOI] [PubMed] [Google Scholar]
- 57.Kraus D., Yang Q., Kong D., Banks A.S., Zhang L., Rodgers J.T., Pirinen E., Pulinilkunnil T.C., Gong F., Wang Y.C., Cen Y., Sauve A.A., Asara J.M., Peroni O.D., Monia B.P., Bhanot S., Alhonen L., Puigserver P., Kahn B.B. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parsons R.B., Aravindan S., Kadampeswaran A., Evans E.A., Sandhu K.K., Levy E.R., Thomas M.G., Austen B.M., Ramsden D.B. The expression of nicotinamide N-methyltransferase increases ATP synthesis and protects SH-SY5Y neuroblastoma cells against the toxicity of complex I inhibitors. Biochem. J. 2011;436:145–155. doi: 10.1042/BJ20101685. [DOI] [PubMed] [Google Scholar]
- 59.Milani Z.H., Ramsden D.B., Parsons R.B. Neuroprotective effects of nicotinamide N-methyltransferase and its metabolite 1-methylnicotinamide. J. Biochem. Mol. Toxicol. 2013;27:451–456. doi: 10.1002/jbt.21508. [DOI] [PubMed] [Google Scholar]
- 60.Yan L.J., Thangthaeng N., Sumien N., Forster M.J. Serum dihydrolipoamide dehydrogenase Is a labile enzyme. J. Biochem. Pharm. Res. 2013;1:30–42. [PMC free article] [PubMed] [Google Scholar]
- 61.Williams C.H., Jr. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase-a family of flavoenzyme transhydrogenases. In: Muller F., editor. Chemistry and Biochemistry of Flavoenzymes. CRC Press; Boca Raton: 1992. pp. 121–212. [Google Scholar]
- 62.Bajotto G., Murakami T., Nagasaki M., Sato Y., Shimomura Y. Decreased enzyme activity and contents of hepatic branched-chain alpha-keto acid dehydrogenase complex subunits in a rat model for type 2 diabetes mellitus. Metabolism. 2009;58:1489–1495. doi: 10.1016/j.metabol.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 63.Moxley M.A., Beard D.A., Bazil J.N. Global kinetic analysis of mammalian E3 reveals ph-dependent NAD+/NADH regulation, physiological kinetic reversibility, and catalytic optimum. J. Biol. Chem. 2016;291:2712–2730. doi: 10.1074/jbc.M115.676619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzym. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 65.Yan L.J., Sohal R.S. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vedantham S., Thiagarajan D., Ananthakrishnan R., Wang L., Rosario R., Zou Y.S., Goldberg I., Yan S.F., Schmidt A.M., Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes. 2014;63:761–774. doi: 10.2337/db13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013;56:133–171. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- 68.Dinkova-Kostova A.T., Talalay P. NAD(P)H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaokar T.M., Patil D.P., Shouche Y.S., Gaikwad S.M., Suresh C.G. Human mitochondrial NDUFS3 protein bearing Leigh syndrome mutation is more prone to aggregation than its wild-type. Biochimie. 2013;95:2392–2403. doi: 10.1016/j.biochi.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Hirschey M.D., Shimazu T., Jing E., Grueter C.A., Collins A.M., Aouizerat B., Stancakova A., Goetzman E., Lam M.M., Schwer B., Stevens R.D., Muehlbauer M.J., Kakar S., Bass N.M., Kuusisto J., Laakso M., Alt F.W., Newgard C.B., Farese R.V., Jr., Kahn C.R., Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirst J., King M.S., Pryde K.R. The production of reactive oxygen species by complex I. Biochem Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 72.Luo X., Li R., Yan L.J. Roles of pyruvate, NADH, and mitochondrial complex I in redox balance and imbalance in β cell function and dysfunction. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/512618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y., Osborne B.W., Stanton R.C. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am. J. Physiol. Ren. Physiol. 2005;289:F1040–F1047. doi: 10.1152/ajprenal.00076.2005. [DOI] [PubMed] [Google Scholar]
- 74.Ulusu N.N., Sahilli M., Avci A., Canbolat O., Ozansoy G., Ari N., Bali M., Stefek M., Stolc S., Gajdosik A., Karasu C. Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem. Res. 2003;28:815–823. doi: 10.1023/a:1023202805255. [DOI] [PubMed] [Google Scholar]
- 75.Gupte R.S., Floyd B.C., Kozicky M., George S., Ungvari Z.I., Neito V., Wolin M.S., Gupte S.A. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 2009;47:219–228. doi: 10.1016/j.freeradbiomed.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ido Y., Nyengaard J.R., Chang K., Tilton R.G., Kilo C., Mylari B.L., Oates P.J., Williamson J.R. Early neural and vascular dysfunctions in diabetic rats are largely sequelae of increased sorbitol oxidation. Antioxid. Redox Signal. 2010;12:39–51. doi: 10.1089/ars.2009.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obrosova I.G. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid. Redox Signal. 2005;7:1543–1552. doi: 10.1089/ars.2005.7.1543. [DOI] [PubMed] [Google Scholar]
- 78.Williamson J.R., Ido Y. Linking diabetic complications to sorbitol oxidation, oxidative stress and metabolic suppression. J. Diabetes Metab. 2012;3:1000219. [Google Scholar]
- 79.Sander B.J., Oelshlegel F.J., Jr., Brewer G.J. Quantitative analysis of pyridine nucleotides in red blood cells: a single-step extraction procedure. Anal. Biochem. 1976;71:29–36. doi: 10.1016/0003-2697(76)90006-3. [DOI] [PubMed] [Google Scholar]
- 80.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 81.Gao S., Yang Z., Shi R., Xu D., Li H., Xia Z., Wu Q.P., Yao S., Wang T., Yuan S. Diabetes blocks the cardioprotective effects of sevoflurane postconditioning by impairing Nrf2/Brg1/HO-1 signaling. Eur. J. Pharm. 2016;779:111–121. doi: 10.1016/j.ejphar.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 82.Bhakkiyalakshmi E., Sireesh D., Sakthivadivel M., Sivasubramanian S., Gunasekaran P., Ramkumar K.M. Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur. J. Pharm. 2016;777:9–16. doi: 10.1016/j.ejphar.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 83.Zhou X., Feng Y., Zhan Z., Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J. Biol. Chem. 2014;289:28827–28834. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tahara E.B., Barros M.H., Oliveira G.A., Netto L.E., Kowaltowski A.J. Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J. 2007;21:274–283. doi: 10.1096/fj.06-6686com. [DOI] [PubMed] [Google Scholar]
- 85.Bando Y., Aki K. Mechanisms of generation of oxygen radicals and reductive mobilization of ferritin iron by lipoamide dehydrogenase. J. Biochem. 1991;109:450–454. doi: 10.1093/oxfordjournals.jbchem.a123402. [DOI] [PubMed] [Google Scholar]
- 86.Kareyeva A.V., Grivennikova V.G., Cecchini G., Vinogradov A.D. Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production. FEBS Lett. 2011;585:385–389. doi: 10.1016/j.febslet.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ide S., Hayakawa T., Okabe K., Koike M. Lipoamide dehydrogenase from human liver. J. Biol. Chem. 1967;242:54–60. [PubMed] [Google Scholar]
- 88.Sol E.M., Wagner S.A., Weinert B.T., Kumar A., Kim H.S., Deng C.X., Choudhary C. Proteomic investigations of lysine acetylation identify diverse substrates of mitochondrial deacetylase sirt3. PLoS One. 2012;7:e50545. doi: 10.1371/journal.pone.0050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kosanam H., Thai K., Zhang Y., Advani A., Connelly K., Diamandis E.P., Gilbert R.E. iabetes induces lysine acetylation of intermediary metabolism enzymes in the kidney. Diabetes. 2014;63:2432–2439. doi: 10.2337/db12-1770. [DOI] [PubMed] [Google Scholar]
- 90.Rardin M.J., Newman J.C., Held J.M., Cusack M.P., Sorensen D.J., Li B., Schilling B., Mooney S.D., Kahn C.R., Verdin E., Gibson B.W. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc. Natl. Acad. Sci. USA. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vazquez E.J., Berthiaume J.M., Kamath V., Achike O., Buchanan E., Montano M.M., Chandler M.P., Miyagi M., Rosca M.G. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc. Res. 2015;107:453–465. doi: 10.1093/cvr/cvv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paik W.K., Pearson D., Lee H.W., Kim S. Nonenzymatic acetylation of histones with acetyl-CoA. Biochim Biophys. Acta. 1970;213:513–522. doi: 10.1016/0005-2787(70)90058-4. [DOI] [PubMed] [Google Scholar]
- 93.Ramponi G., Manao G., Camici G. Nonenzymatic acetylation of histones with acetyl phosphate and acetyl adenylate. Biochemistry. 1975;14:2681–2685. doi: 10.1021/bi00683a018. [DOI] [PubMed] [Google Scholar]
- 94.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 95.Jing E., Emanuelli B., Hirschey M.D., Boucher J., Lee K.Y., Lombard D., Verdin E.M., Kahn C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hou X., Zeng H., He X., Chen J.X. Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J. Cell Mol. Med. 2015;19:53–61. doi: 10.1111/jcmm.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caton P.W., Richardson S.J., Kieswich J., Bugliani M., Holland M.L., Marchetti P., Morgan N.G., Yaqoob M.M., Holness M.J., Sugden M.C. Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia. 2013;56:1068–1077. doi: 10.1007/s00125-013-2851-y. [DOI] [PubMed] [Google Scholar]
- 98.Turkmen K., Karagoz A., Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J. Diabetes. 2014;5:894–900. doi: 10.4239/wjd.v5.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obrosova I.G., Stevens M.J., Lang H.J. Diabetes-induced changes in retinal NAD-redox status: pharmacological modulation and implications for pathogenesis of diabetic retinopathy. Pharmacology. 2001;62:172–180. doi: 10.1159/000056091. [DOI] [PubMed] [Google Scholar]
- 100.Canto C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A.A., Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Camacho-Pereira J., Tarrago M.G., Chini C.C., Nin V., Escande C., Warner G.M., Puranik A.S., Schoon R.A., Reid J.M., Galina A., Chini E.N. CD38 dictates Age-Related NAD Decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]