Abstract
Transition metals are required trace elements for all forms of life. Due to their unique inorganic and redox properties, transition metals serve as cofactors for enzymes and other proteins. In bacterial pathogenesis, the vertebrate host represents a rich source of nutrient metals, and bacteria have evolved diverse metal acquisition strategies. Host metal homeostasis changes dramatically in response to bacterial infections, including production of metal sequestering proteins and the bombardment of bacteria with toxic levels of metals. Presumably, in response, bacteria have evolved systems to subvert metal sequestration and toxicity. The coevolution of hosts and their bacterial pathogens in the battle for metals has uncovered emerging paradigms in social microbiology, rapid evolution, host specificity, and metal homeostasis across domains. This review focuses on recent advances and open questions in our understanding of the complex role of transition metals at the host-pathogen interface.
Keywords: host-pathogen interface, metal acquisition, metal homeostasis, nutritional immunity, transition metal
INTRODUCTION
Metals are essential for all forms of life. Metal cofactors serve structural and catalytic roles in biological processes, including precursor biosynthesis, DNA replication, transcription, respiration, and responses to oxidative stress. Most organisms require the first-row transition metals manganese, iron, cobalt, nickel, copper, and zinc. Excepting zinc, these metals have unfilled d-orbitals and thus are redox active. Their ability to easily cycle between oxidation states contributes to both their catalytic properties and their toxicity. Their divalent cations bind ligands similarly, but each metal has different properties such that they are only occasionally functional substitutes. Thus, protein mismetallation often ablates function, necessitating exquisitely sensitive metal homeostasis systems. In bacterial pathogens, homeostasis strategies include complex metal-responsive transcriptional regulatory networks, as reviewed elsewhere (20, 62, 78, 130). Understanding how metal homeostasis proteins recognize and respond to the correct metals is an active area of research in bioinorganic chemistry with implications for bacterial and host metal metabolism during infection.
In the context of infection, bacterial and host metal metabolism determine disease pathogenesis with three emerging principles. First, although the host represents a rich nutrient metal source for bacteria, the host immune system can block bacterial metal acquisition in a process termed nutritional immunity. Second, hosts barrage bacteria with toxic concentrations of metals. Finally, dysregulation of host metal homeostasis—through genetic mutation or nutrition—alters susceptibility to infection. This review summarizes findings, highlights recent work, and discusses questions to be pursued in the study of metals at the interface of bacterial pathogens and their hosts.
BACTERIAL METAL ACQUISITION AND HOST NUTRITIONAL IMMUNITY
In order to obtain their required nutrient metals, bacteria have evolved three main classes of metal acquisition systems: elemental metal import, extracellular metal capture by siderophores, and metal acquisition from host proteins (including metal piracy from host nutritional immunity proteins). In response, mammalian hosts have evolved strategies to restrict bacterial metal acquisition. The protein transferrin was first reported to inhibit microbial growth by iron chelation in egg whites and human plasma in the 1940s, and lactoferrin was later shown to have similar properties at mucosal surfaces (105, 120, 121) (Figure 1) (see sidebar, Nutritional Immunity Protein Moonlights as an Antimicrobial). Thirty years later, Eugene Weinberg popularized the term nutritional immunity to describe the phenomenon, which has since been expanded to include host restriction of zinc, manganese, and nonmetal nutrients such as amino acids (65, 140, 149). Growing evidence suggests that this battle for metals represents an interface for coevolution of hosts and bacteria. As posited by the Red Queen hypothesis in evolutionary biology, with each development in nutritional immunity defense, pathogens evolve a new offense (11, 133). The following sections discuss our understanding of nutrient transition metals at the host-pathogen interface, focusing on iron, zinc, and manganese.
Figure 1.
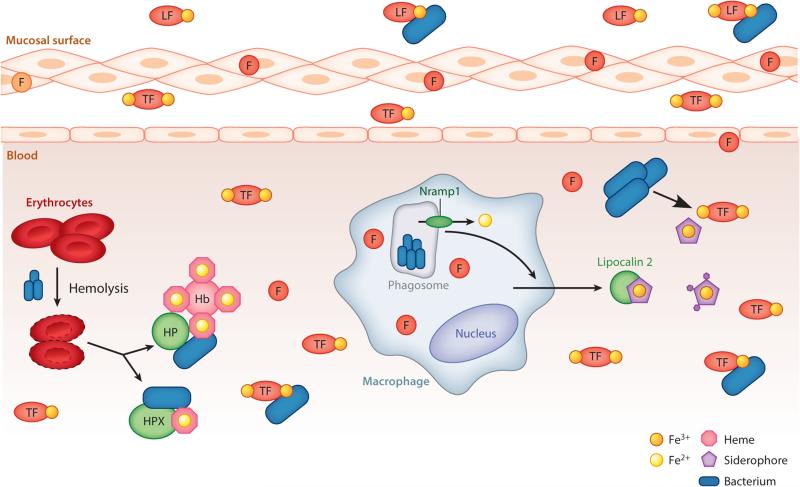
Iron at the host-pathogen interface. In the host, Fe3+ is limited because of chelation by lactoferrin (LF) at the mucosal surface, transferrin (TF) in blood and tissue, and ferritin (F) in the cellular cytoplasm and at low levels in serum. Some bacteria can acquire iron from these host ferroproteins. In the bloodstream, bacteria can liberate iron from erythrocytes by hemolysis followed by extraction of heme from hemoprotein complexes, including hemoglobin (Hb), hemoglobin-haptoglobin (HP), and hemopexin (HPX). Intracellular pathogens are iron starved by Nramp1-mediated iron efflux from the phagosome. Nramp1 expression further induces lipocalin 2 production to bind and sequester some bacterial siderophores, whereas stealth siderophores evade binding.
Iron and the Host-Pathogen Battle: The Vanguard in Understanding
Iron is the most widely used transition metal in biological systems, presumably because it is the most abundant element on Earth, making up 80% of the Earth's core. Bacterial pathogens require iron for essential processes, including the pentose phosphate pathway, DNA replication, transcription, and cofactor biosynthesis. For example, biosynthesis of the cofactor heme (Fe2+-protoporphyrin IX) is important in Brucella abortus systemic infection and Staphylococcus aureus systemic and bone infection (5, 57, 58). Additionally, the iron-sulfur cluster chaperone Nfu was found to be important in mouse and moth models of infection by S. aureus and Acinetobacter baumannii, respectively (86, 150). Iron is incorporated into cofactors in its soluble ferrous form (Fe2+). Many bacteria import Fe2+ with the ferrous transporter Feo, which is important for colonization and/or virulence in a number of bacteria, including the gastrointestinal pathogens Helicobacter pylori, Salmonella enterica, and Campylobacter jejuni (77) (Figure 2a). However, Fe2+ is largely unavailable for bacterial pathogens due to host restriction, and the physiochemical properties of iron make Fe3+ the predominant form in the host environment, as describe below.
Figure 2.
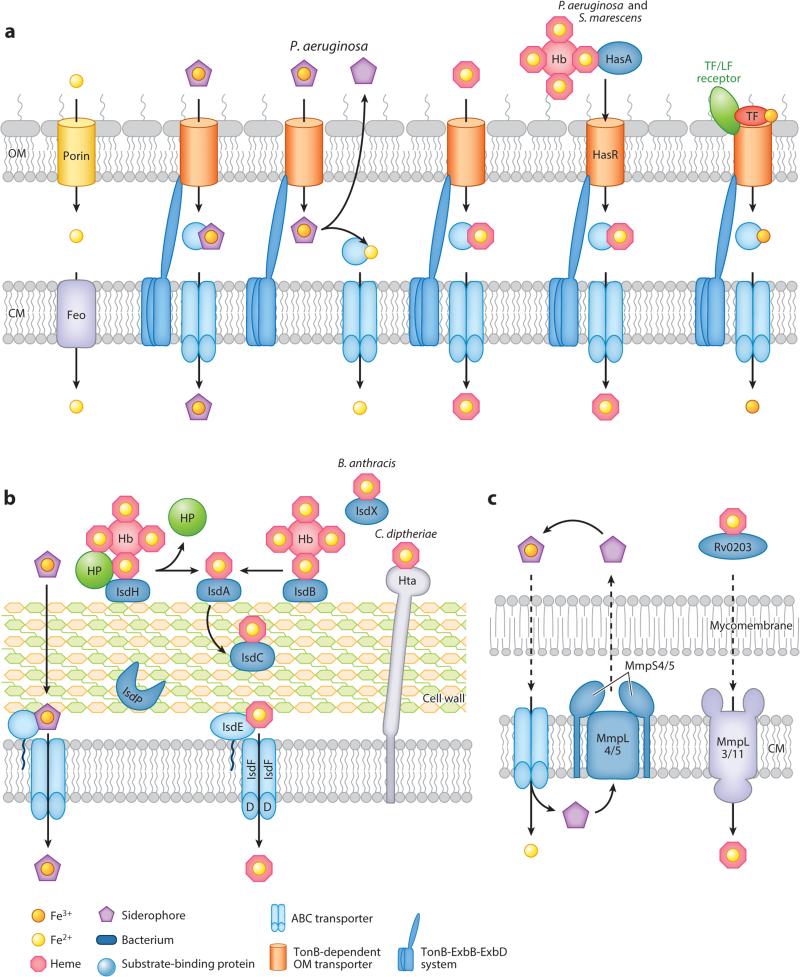
Iron acquisition in bacterial pathogens. (a) Gram-negative pathogens use porins or TonB-dependent outer membrane (OM) transporters to mediate the passage of iron complexes to the periplasm and, subsequently, the Fe2+ transporter Feo or ABC transporters to transport iron complexes into the cytoplasm. Pseudomonas aeruginosa reduces Fe3+-siderophores in the periplasm and exports deferrated siderophores from the periplasm via an ABC transporter (process not shown). P. aeruginosa and Serratia marescens use the secreted hemophore HasA and its receptor HasR for heme acquisition. Some bacteria also encode transferrin or lactoferrin OM receptors to actively pirate Fe3+ from host nutritional immunity proteins. (b) Gram-positive pathogens acquire iron using siderophores or dedicated heme/hemoprotein uptake systems. Pathogenic Staphylococcus spp. and Bacillus spp. use the Isd system to mediate the passage of heme through the cell wall to a dedicated transporter. Additional species-specific features include the Staphylococcus lugdunensis autolysin IsdP and Bacillus anthracis–secreted hemophores IsdX1 and IsdX2. Corynebacterium diptheriae uses the lipoprotein heme transporters HtaA/HtaB. (c) Portions of the mycobacterial iron acquisition process have been elucidated. Mycobacteria couple Fe3+-siderophore import with reduction and Fe2+ release; the deferrated siderophores can then be exported by the MmpL4/5-MmpS4/5 system for recycling. Heme import requires the secreted hemophore Rv0203 and the CM importers MmpL3/11. Abbreviations: ABC, ATP-binding cassette; CM, cytoplasmic membrane; Hb, hemoglobin; HP, hemoglobin-haptoglobin; LF, lactoferrin; TF, transferrin.
Two-thirds of iron in humans is sequestered in erythrocytes as heme bound to the oxygen-carrying protein hemoglobin (24). Under oxic conditions and at physiological pH in serum, free iron is insoluble as Fe3+. In vertebrates, nearly all Fe3+ is bound by transferrin in serum, by ferritin in cells, or by lactoferrin in milk, saliva, tears, mucus, and neutrophil secondary granules. These factors combined result in a low free-iron concentration in human serum, which is further restricted in response to bacterial infection (24). Bacteria have evolved mechanisms to circumvent host iron restriction. For the simplest solution, the Lyme disease pathogen Borrelia burgdorferi evolved a complete lack of iron requirement by exploiting the interchangeability of transition metals and replacing iron with manganese in many metalloenzymes (110). Pathogens that have not weaned themselves off iron often rely on cheating, thievery, and piracy to acquire iron in spite of nutritional immunity.
Siderophores, Evasion of Host Interference, and Cheating
Many bacterial pathogens use siderophores to overcome iron limitation in the host environment. Siderophores are low molecular weight, high-affinity Fe3+-binding compounds secreted and imported by bacteria for iron acquisition. Siderophores have exceptionally high-iron binding affinity, which allows some siderophores to steal iron from host proteins. For example, enterobactin produced by Enterobacteriaceae binds Fe3+ (Kd 10−51–10−49 M) more tightly than transferrin (Kd 10−24–10−20 M), thus outcompeting transferrin chelation (23, 81). In Gram-negative bacteria, siderophores are transported across the outer membrane by a TonB-dependent system, then trafficked through the periplasm and imported into the cytoplasm by ATP-binding cassette (ABC) transporters (93) (Figure 2a). Gram-positive bacteria import Fe3+-siderophores with lipoprotein substrate-binding proteins and ABC transporters (93) (Figure 2b).
Iron is released from the siderophores by one of two main mechanisms. Siderophores with high Fe3+ binding affinity, such as enterobactin or bacillibactin produced by Bacillus anthracis, are hydrolyzed by specific esterases before Fe3+ is reduced to Fe2+. The fate of the hydrolyzed siderophores is not well understood; they may be further degraded or recycled (93). For other siderophores, ferric reductases reduce Fe3+ to Fe2+ that is then released due to its lower binding affinity or by competitive sequestration (93). Deferrated siderophores can then be recycled. Pseudomonas aeruginosa uses a dedicated periplasmic export system to recycle siderophores (66). Recent studies in Mycobacterium tuberculosis determined that one system exports both de novo synthesized and recycled siderophores from the cytoplasm and is required to prevent cytoplasmic siderophore accumulation and poisoning (69) (Figure 2c).
Siderophore iron acquisition and host interference illustrate an evolutionary arms race in bacterial pathogenesis. The vertebrate host produces the siderophore-binding protein lipocalin 2, also known as siderocalin or neutrophil gelatinase–associated lipocalin, during the innate immune response to bacterial infection to sequester siderophores such as enterobactin or bacillibactin (1, 44). In response, bacteria evolved to elaborate stealth siderophores that evade lipocalin 2 binding, including the highly glycosylated salmochelin produced by pathogenic enterobacteria and petrobactin produced by B. anthracis (1, 43). Remarkably, expression of the salmochelin biosynthetic locus confers virulence to a nonpathogenic Escherichia coli, implying stealth siderophore production is a key virulence determinant (43).
Many pathogens encode multiple siderophore biosynthetic systems, suggesting that diverse siderophores allow context-specific replicative advantages. Studies on siderophore production in enterobacterial pathogens have provided insight into these fitness benefits. During S. enterica gastrointestinal infection, S. enterica uses salmochelin to gain a competitive advantage over commensals but is outcompeted for siderophores by the probiotic E. coli strain Nissle, which reduces S. enterica colonization (33, 113). Although enterobactin can be sequestered by lipocalin 2, it has a higher binding affinity for Fe3+ than stealth siderophores salmochelin and yersiniabactin. This high Fe3+ binding efficiency renders enterobactin essential in certain host niches. In the lung, enterobactin is not required for Klebsiella pneumoniae pneumonia but is required for invasion into surrounding tissue; its higher Fe3+ binding affinity enables K. pneumoniae to acquire iron from transferrin in the perivascular space (9). Counterintuitively, uropathogenic E. coli use enterobactin to resist lipocalin 2 in human urine, apparently by extracting Fe3+ from Fe3+-catechol-lipocalin 2 complexes (122). Human urine varies in characteristics such as pH and aryl metabolite (catechol) content. In donors with high urine pH, lipocalin 2 complexes with catechols and Fe3+ to sequester iron from invading pathogens. Uropathogenic E. coli can overcome this sequestration owing to the exceptionally high Fe3+ binding affinity of enterobactin (9). Given the large number of siderophores identified thus far, understanding mechanisms driving siderophore diversity warrants further investigation.
In addition to selective pressures associated with maintaining multiple siderophore biosynthetic systems, siderophore production in P. aeruginosa serves as a model to understand how cheating drives evolution. Because siderophores are secreted, bacteria can import siderophores for iron acquisition without bearing the metabolic cost of production. In this way, siderophores are public goods. Experimental evolution in P. aeruginosa showed that relatedness and low competition selects for cooperation (i.e., siderophore production), whereas local competition selects for cheaters (53). Additional research found that the nutritional environment and regulatory cross talk between social behaviors (i.e., quorum sensing and production of multiple siderophores) further modulate the selection for cooperation versus cheating in P. aeruginosa (49, 117). Importantly, long-term P. aeruginosa infection in the cystic fibrosis lung was recently shown to select for siderophore cheating, eventually driving some populations to lose siderophore production and thus reduce fitness in the iron-limited host (7). The competitive dynamics leading to complete loss of siderophore production demonstrate that bacterial communities are susceptible to the tragedy of the commons, an economics term first applied to evolutionary biology by Garrett Hardin in 1968 to describe each individual seeking maximum benefit from common goods at the expense of population-wide fitness (60). The P. aeruginosa findings suggest that the intensity of competition in the host environment can drive bacterial populations to extinction.
Iron Piracy Subverts Nutritional Immunity
In addition to acquiring iron from heme, some pathogens have developed specialized systems to obtain iron from nutritional immunity proteins through a process termed iron piracy. The transferrin-binding proteins TbpA/TbpB that are present in species, including Neisseria spp., Haemophilus influenzae, and Moraxella catarrhalis, are TonB-dependent receptors that bind transferrin and extract Fe3+ for transport into the cell (96). Additionally, some pathogens express lactoferrin receptors, including pathogenic Neisseria spp., Treponema pallidum, H. pylori, and Streptococcus pneumoniae (34, 59, 96). With these systems, enterprising bacteria convert host proteins that evolved to restrict bacterial growth into nutrient iron sources.
Selective pressure at the receptor-ligand binding sites between host transferrin and bacterial transferrin-binding proteins has driven evolution at the host-pathogen interface. Like the hemoglobin-IsdB interaction, transferrin-binding proteins restrict host range for numerous pathogens. The obligate human pathogens of Neisseria spp. are host restricted based on their transferrin-binding proteins, a fact that has been exploited to develop murine models for meningitis by introducing or expressing human transferrin (102, 148). Structural analysis of TbpB from the porcine pathogen Actinobacillus pleuropneumoniae revealed a single conserved residue required for transferrin binding, whereas surrounding residues varied extensively, presumably due to host selective pressure (95). Elegant evolutionary work showed that transferrin genetic variation among apes is the result of rapid evolution to counteract bacterial iron piracy (10). This work emphasizes the utility of metal acquisition by bacterial pathogens as a model to uncover rapid evolution at protein interfaces between host and pathogen.
Iron Thievery: Bacterial Heme Acquisition
Because the majority of iron in the human body is sequestered in erythrocytes, many bacterial pathogens evolved mechanisms to access heme iron. These pathogens first lyse erythrocytes, bind hemoglobin or other host heme proteins, and then extract heme for direct use or degradation for liberation of free iron (27). Following erythrocyte lysis, mammalian hosts employ hemopexin and haptoglobin to bind and sequester free heme and hemoglobin, respectively, to protect against their oxidative activities. Bacterial heme acquisition systems have surface-associated receptors that bind heme or hemoproteins and pass heme through the periplasm and/or cell wall to a membrane heme transporter.
Heme acquisition systems are well described for many Gram-negative pathogens and rely on an outer membrane receptor such as HmuR that binds heme or hemoproteins, transporting heme through the periplasm by a TonB-ExbB-ExbD system (19) (Figure 2a). Heme is then transported into the cytoplasm by an ABC transporter. Some bacteria additionally release hemophores, such as heme assimilation system HasA in Serratia marescens and P. aeruginosa, which extracts heme from hemoglobin with 10−11 M binding affinity and relays it to the outer membrane hemophore receptor HasR (76, 144). M. tuberculosis uses a secreted hemophore with a unique fold, demonstrating the diversity of these systems (131). Many Gram-positive pathogens use the iron-regulated surface determinant Isd heme uptake system with dedicated cell wall sortase and heme passage proteins to acquire iron from free heme or heme complexed to hemoglobin or hemoglobin-haptoglobin (87) (Figure 2b). B. anthracis additionally encodes secreted hemophores IsdX1 and IsdX2 that bind heme extracellularly for import but are not required for virulence, implying that there are functional redundancies in B. anthracis heme acquisition (84). The recent discovery of an iron-regulated autolysin in the opportunistic pathogen Staphylococcus lugdunensis suggests that Gram-positive bacteria remodel their cell walls to accommodate heme acquisition proteins (42). Heme acquisition is required for virulence in Staphylococcus aureus, B. anthracis, and Streptococcus pyogenes (27). In Corynebacterium diphtheriae, one of at least two redundant heme transport–associated Hta uptake systems is tethered to the cytoplasmic membrane rather than relying on cell wall sortases. The roles that the two Hta systems play in virulence remain unknown (4).
The importance of heme as an iron source during infection has driven evolution at the host-pathogen interface. S. aureus evolved to use hemoglobin as its preferred iron source in infection, and its IsdB hemoglobin-binding protein evolved to preferentially bind human hemoglobin over hemoglobin from other animals (109, 123). Additionally, P. aeruginosa lineages colonized in cystic fibrosis patients evolved to prefer heme over the siderophore pyoverdine as an iron source (85). These examples suggest that the interaction between bacterial heme acquisition proteins and host hemoglobin is also susceptible to selective pressure in the host-pathogen arms race. Therefore, bacterial heme acquisition may offer opportunities for further understanding host-pathogen evolution.
Zinc Turf Wars: Sequestration and Potential for Piracy
Following iron, zinc is the second most abundant transition metal cofactor. Zinc binding by proteins can be structural, regulatory, or catalytic, and zinc is required for many metalloproteases. Bacterial zinc uptake (Znu) systems are ABC transporters required for virulence in a variety of pathogens, including S. pneumoniae, Acinetobacter baumannii, Listeria monocytogenes, and P. aeruginosa (29, 36, 64) (Figure 3a). Recent work uncovered two novel zinc acquisition systems in Yersinia spp. In Yersinia pseudotuberculosis, a type VI secretion system (T6SS) imports zinc by transporting a zinc-binding protein effector bound to zinc, and maximal virulence requires both the Znu and T6SS systems, thus expanding known roles for T6SS (139). In Y. pestis, Bobrov et al. (16) demonstrated that the siderophore yersiniabactin binds zinc and is transported into the cell using a dedicated Zn2+-yersiniabactin importer, YbtX. Importantly, in a model of septicemic plague, Y. pestis displays a virulence defect only in strains lacking both the znu transporter and yersiniabactin biosynthesis genes. This pivotal finding established the importance of siderophore noniron metal acquisition in bacterial pathogenesis.
Figure 3.
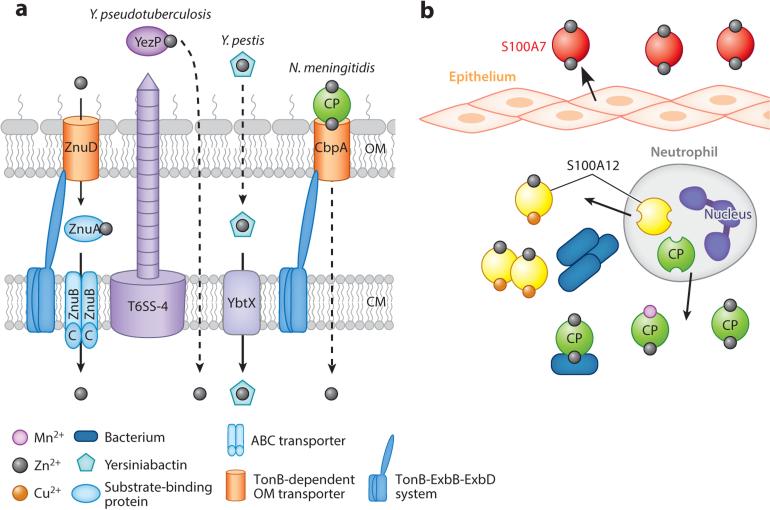
Zinc sequestration and bacterial zinc import. (a) Pathogenic bacteria acquire zinc using ABC transporters ZnuABC (Gram-negative bacteria) and AdcABC (Gram-positive bacteria; not shown). Zinc is transported through the outer membrane (OM) using the TonB-dependent transporter ZnuD. Additional zinc acquisition systems include a T6SS-secreted zincophore protein YezP in Yersinia pseudotuberculosis, transport of yersiniabactin-Zn2+ in Y. pestis, and an OM receptor CbpA that binds calprotectin (CP) in Neisseria meningitidis. (b) During infection, zinc is limited by secretion of zinc-chelating S100 proteins: S100A7 is secreted at epithelial surfaces, whereas S100A12 and CP are secreted by innate immune proteins such as neutrophils. Some bacteria can acquire zinc from CP. Abbreviations: ABC, ATP-binding cassette; CM, cytoplasmic membrane; T6SS, type VI secretion system.
Bacterial zinc acquisition systems are required during infection to counteract limitation by nutritional immunity. During the acute phase response to inflammation, serum zinc drops due to enhanced zinc uptake and sequestration by metallothionein in liver cells (55). Additionally, members of the helix-loop-helix (EF-hand type) Ca2+-binding S100 protein family of the vertebrate innate immune system exert nutrient limitation through extracellular zinc chelation (Figure 3b). The importance of metal chelation by S100 proteins for innate immunity was first established with the demonstration that S100A7 (psoriasin) protects human skin from infection by zinc chelation (51). Psoriasin also contributes to mucosal immunity on the tongue and in the vagina (92, 94). Similarly, S100A12 (calgranulin C), which binds both Zn2+ and Cu2+, is induced in H. pylori infection and limits bacterial growth through zinc chelation (56).
Molecular imaging by mass spectrometry demonstrated that Staphylococcus aureus liver abscesses are severely depleted of zinc and manganese and depletion depends on heterodimers of S100A8/S100A9 (calprotectin) (30). Calprotectin is an abundant neutrophil protein that can chelate Zn2+ and Mn2+ and efficiently competes away these nutrient metals from numerous bacterial pathogens in vitro (32). In addition to S. aureus, calprotectin zinc limitation is important for controlling infection by bacterial pathogens, including L. monocytogenes, A. baumannii, K. pneumoniae, and H. pylori (3, 47, 64, 147). Recent work showed that Fe2+ binding by calprotectin contributes to inhibition of bacterial growth in vitro, an activity that remains to be investigated during infection (98). Counterintuitively, calprotectin enhances pathogenesis of some bacteria by diverse mechanisms. For example, in pneumococcal pneumonia, calprotectin appears to protect S. pneumoniae from host-induced zinc toxicity in the lung (2). Calprotectin enhances S. enterica growth in the inflamed gut, presumably because S. enterica can outcompete the gut microbiota for limited zinc (79). Subinhibitory concentrations of calprotectin also increase H. pylori biofilm formation (46). In addition, calprotectin promotes pneumonia co-infection of P. aeruginosa and S. aureus by abrogating production of anti-staphylococcal factors by P. aeruginosa (137). This finding offers a potential explanation for co-infection by P. aeruginosa and S. aureus in cystic fibrosis patients, where calprotectin is abundant. The differential effects of calprotectin zinc sequestration likely reflect differences in host environments, bacterial zinc requirements, and acquisition systems.
Recent work identified the potential for bacterial zinc piracy from S100 proteins during infection. The Neisseria meningitidis outer membrane receptor CbpA was shown to bind calprotectin and extract zinc in a TonB-dependent manner (127). This finding suggests that bacteria can exploit host zinc nutritional immunity proteins analogously to iron acquisition from transferrin and lactoferrin. In this case, interactions between CpbA and calprotectin may also be susceptible to rapid evolution, establishing a new front in the study of the evolutionary battle for nutrient metals.
Manganese Restriction: A War in Two Theaters
Manganese is an essential trace element for all forms of life and serves as a cofactor for enzymes involved in oxidative stress, DNA replication, and central metabolism, including manganese superoxide dismutase and pyruvate kinase. Bacterial manganese import systems generally belong to the MntH/Nramp or ABC transporter families and are important during infection in a number of pathogenic bacteria, including S. enterica, S. aureus, Neisseria gonorrhoeae, B. abortus, Streptococcus spp., and Yersinia spp. (71) (Figure 4a,b). In the Lyme disease pathogen B. burgdorferi, the BmtA manganese transporter is required for virulence. Curiously, BmtA is not related to bacterial manganese importers but, rather, to the ZIP (Zrt/Irt-like protein) family transporters in eukaryotes (106). The divergence of B. burgdorferi manganese importers from other pathogens may reflect its unique evolutionary strategy to substitute its iron requirement with manganese. Interestingly, the requirement for manganese acquisition systems can depend on the mode of infection or the infected organ. For S. aureus, mutants lacking MntH and ABC transport systems have a lower burden in the liver during systemic infection but maintain their burden in the kidney (73). In Y. pestis, mutants lacking both transport systems display no defect in pneumonic plague but are attenuated in bubonic plague (108). These findings contribute to the understanding that the requirement for metal import depends on the specific host environment.
Figure 4.
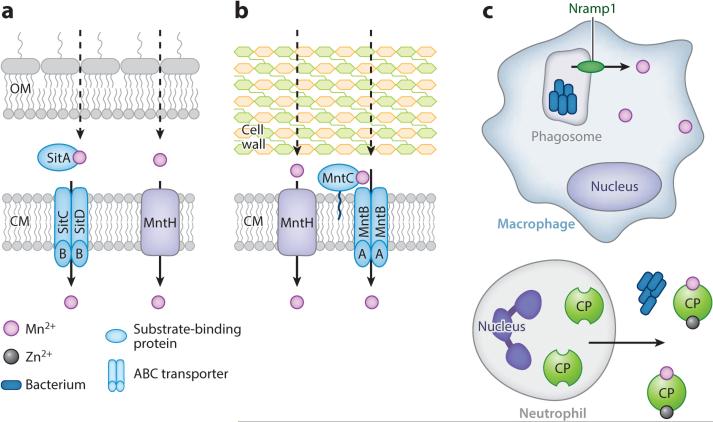
Intra- and extracellular manganese restriction. (a) Gram-negative pathogens import manganese with the SitABCD or MntH transporters; the mechanism of transport across the outer membrane (OM) is unknown. (b) Gram-positive pathogens import manganese with the MntABC or MntH transporter. (c) The phagosome membrane protein Nramp1 effluxes manganese from the phagosome, limiting availability for intracellular pathogens. Extracellularly, manganese is sequestered by calprotectin (CP), which is secreted by innate immune cells, including neutrophils, in response to infection. Abbreviations: ABC transporter, ATP-binding cassette transporter; CM, cytoplasmic membrane.
The host innate immune system has evolved both extra- and intracellular manganese restriction mechanisms (71) (Figure 4c). The Mn2+-binding activity of calprotectin limits available manganese for many bacterial pathogens (32). In S. aureus, calprotectin-mediated manganese restriction inhibits the bacterium's oxidative stress defense program by limiting manganese superoxide dismutase activity (72). In turn, the S. aureus high-affinity manganese import systems help combat calprotectin limitation during infection (73). In contrast to what occurs in the liver, S. aureus kidney abscesses remain manganese depleted in calprotectin-deficient animals, implicating additional host measures of manganese sequestration (73). During S. enterica infection of the gut, manganese acquisition is required for infection, where manganese restriction is IL-22 dependent but calprotectin-independent (35). Therefore, open areas for investigation include the role of calprotectin-dependent manganese sequestration in other infections and the remaining calprotectin-independent mechanisms of host manganese chelation.
Intracellular pathogens also face host manganese limitation in the phagosome. In response to infection, neutrophils and macrophages express the natural resistance–associated macrophage protein 1 (Nramp1), which depletes the phagosome of manganese and, to a lesser extent, iron (67, 142). Bacterial manganese acquisition systems are important for resisting Nramp1-mediated manganese restriction and may contribute differentially, such as in S. enterica infection of stimulated macrophages. The fact that polymorphisms in nramp1 affect host susceptibility to infection emphasizes the importance of manganese limitation. The commonly used laboratory mouse strains C57BL/6 and BALB/c are Nramp1-deficient and rapidly succumb to S. enterica infection, in contrast to Nramp1-sufficient mouse strains (135). Human nramp1 polymorphisms affect susceptibility to tuberculosis, meningococcal diseases, and leprosy (14). Additionally, nramp1 expression in macrophages induces the host siderophore-sequestering protein lipocalin 2, uncovering a regulatory link between multiple mechanisms of nutritional immunity (45). Therefore, continued study of nutrient-limiting proteins may expose additional multimetal regulatory networks involved in nutritional immunity.
Cobalt, Nickel, and Copper: Neglected Transition Metals in Nutritional Immunity
Less is known about the roles of cobalt, nickel, and copper acquisition in bacterial pathogenesis. Nickel is well established as an essential cofactor for virulence factors, including nickel-iron hydrogenase and urease in H. pylori, where the NixA nickel transporter is required for virulence (41, 100). Urease is also expressed by Proteus mirabilis and Staphylococcus saprophyticus, other causative agents of urinary tract infections (21). Cobalt is an enzymatic cofactor for virulence determinants, including S. pneumoniae GlcNAc deacetylase and Helicobacter pylori arginase (15, 89). Dedicated cobalt transporters have not been identified, but many bacterial pathogens encode nickel-cobalt transporters (115). In S. aureus, the nickel transporter Nik is required for urease activity and efficient kidney colonization, and the cobalt and nickel transporter Cnt is additionally required for infection in systemic and urinary tract infections in mice (63, 114). Recent work demonstrated that Cnt transports metals in complex with staphylopine, a nicotianamine-like metallophore capable of binding nickel, cobalt, zinc, copper, and iron (50). Characterization of staphylopine marks the first description of a nicotianamine-like molecule in bacteria and the first broad-spectrum metallophore.
The role of copper in bacterial pathogenesis is an exciting area of current investigation. Copper is important during aerobic growth as a cofactor of cytochrome c oxidase and copper-zinc superoxide dismutase. It is unclear whether bacterial pathogens actively transport copper and whether copper is restricted by host nutritional immunity. In mycobacteria, porins in the outer membrane are known to be important for copper transport (124). Mounting evidence demonstrates that the siderophore yersiniabactin binds Cu2+ in physiologically relevant environments and that Cu2+-yersiniabactin can be imported in uropathogenic E. coli using the TonB-dependent siderophore importer FyuA (26, 75). Copper acquisition by the fungal pathogen Cryptococcus neoformans is critical for meningoencephalitis virulence but not pneumonia, demonstrating that some host niches are copper limited (129). These findings raise questions as to the mechanism of copper limitation in these niches (e.g., cerebrospinal fluid). Although the host immune protein S100A12 binds copper in addition to zinc, its antimicrobial activity against H. pylori appears to be restricted to zinc chelation (56). Improved understanding of the importance of cobalt, nickel, and copper for bacterial pathogenesis will determine whether the host actively limits these metals through nutritional immunity and whether bacterial pathogens are affected by their limitation or can counteract host-mediated limitation.
HOST-IMPOSED METAL TOXICITY
Although the first-row transition metals are required trace elements for biological systems, they are also inherently toxic. One potential mechanism of toxicity is Fenton chemistry by the redox-active transition metals iron, copper, and manganese. In the Haber-Weiss reaction of Fenton chemistry, Fe2+ reacts with hydrogen peroxide to form Fe3+, a hydroxyl radical, and hydroxide; Fe3+ can then react with hydrogen peroxide to regenerate Fe2+ in addition to a hydroperoxyl radical and a proton. These oxidative species inhibit cell growth by damaging proteins, DNA, and lipids. Another mechanism of toxicity is protein mismetallation. The prevalence of metalloproteins in the bacterial cell and their relatively compatible binding ligands necessitate tight control of metal homeostasis for continued metabolism. Complementary to nutrient metal limitation, hosts have evolved mechanisms to deploy toxic levels of metal ions for bacterial control, and bacteria use multiple metal exporters to resist host intoxication (20, 39, 54).
Copper Ions as Ammunition
Copper has long been recognized as antimicrobial and is often embedded in textiles and medical devices as a microbicide. Although copper can carry out Fenton chemistry, its toxicity is caused by mismetallation in bacterial species investigated thus far. The primary targets of its toxicity in E. coli and N. gonorrhoeae are iron-sulfur cluster proteins in branched chain amino acid and heme biosynthesis, respectively; in S. pneumoniae, the primary target is the manganese-containing aerobic ribonucleotide reductase (38, 68, 83) (Figure 5a). In a possible twist on Fenton chemistry, copper was also found to potentiate nitrosative stress in N. gonorrhoeae (37).
Figure 5.
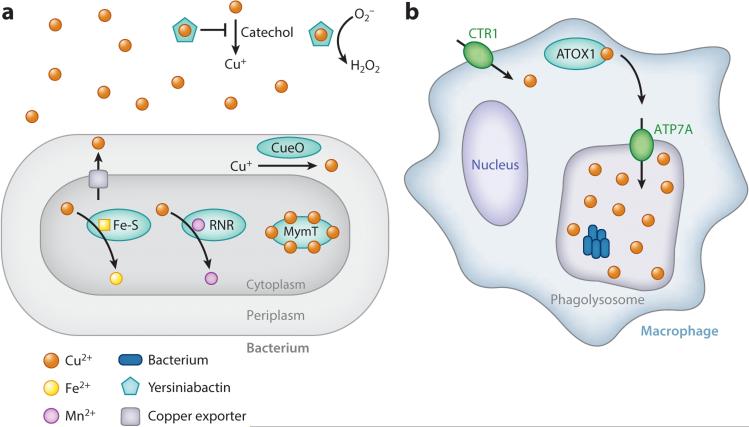
Copper intoxication and bacterial resistance. (a) Evidence thus far suggests that copper is toxic because it displaces metal cofactors in certain proteins, including iron-sulfur clusters and manganese in ribonucleotide reductase (RNR). Bacteria have evolved diverse mechanisms to withstand copper toxicity, including copper efflux. In uropathogenic Escherichia coli, extracellular yersiniabactin-Cu2+ prevents copper reduction to the more reactive Cu+ and can convert superoxide to hydrogen peroxide. E. coli also utilizes the periplasmic copper oxidase CueO to detoxify Cu+. Mycobacteria also produce a metallothionein, MymT, to sequester cytoplasmic copper. (b) In response to infection, phagocytic cells, including macrophages, import copper into the cytosol with the transporter CTR1. Copper is then shuttled by ATOX1 to the phagolysosomal membrane, where it is transported into the phagolysosome by ATP7A to intoxicate bacterial cells.
Host bactericidal mechanisms take advantage of copper toxicity to create poisonous microenvironments for intracellular bacteria. During bacterial infection, interferon-γ induces expression of copper transport protein 1 (CTR1) to import copper from the environment to ATOX1, which then shuttles copper to the ATP7A transporter at the membrane of the phagolysosome for copper accumulation (74, 143, 145) (Figure 5b). Accordingly, copper resistance is required for virulence of many intracellular pathogens. N. gonorrhoeae, S. enterica, and mycobacteria employ copper exporters and extracellular copper oxidation (39, 119) (Figure 5a).
Investigation of copper resistance has uncovered surprising mechanisms. For example, in response to copper toxicity, mycobacteria produce the bacterial metallothionein MymT to sequester copper in the cytoplasm (52). Uropathogenic E. coli use Cu2+-yersiniabactin to protect against copper toxicity and oxidative stress through at least two mechanisms: (a) binding to yersiniabactin prevents catechol-mediated Cu2+ reduction to Cu+, which could participate in Fenton chemistry, and (b) Cu2+-yersiniabactin exhibits extracellular and catalytic superoxide dismutase–like activity (25, 26). Our understanding of metal resistance beyond export mechanisms is in its infancy, but findings thus far suggest it may be fertile ground for discovery.
Zinc Toxicity Expands the Host Armory
Zinc cannot participate in Fenton chemistry and its mechanism of toxicity likely involves mismetallation. Zinc intoxication competitively inhibits manganese binding to an importer in S. pneumoniae and the glycolytic enzymes phosphofructokinase and glyceraldehyde-3-phosphate dehydrogenase in S. pyogenes (88, 104) (Figure 6). Like copper toxicity, zinc intoxication as a host defense mechanism was first described during mycobacterial infection. Transcriptional profiling of M. tuberculosis in human macrophages identified signs of metal intoxication in bacteria and in the host, revealing a burst of free zinc in macrophages and intraphagosomal zinc accumulation (18).
Figure 6.
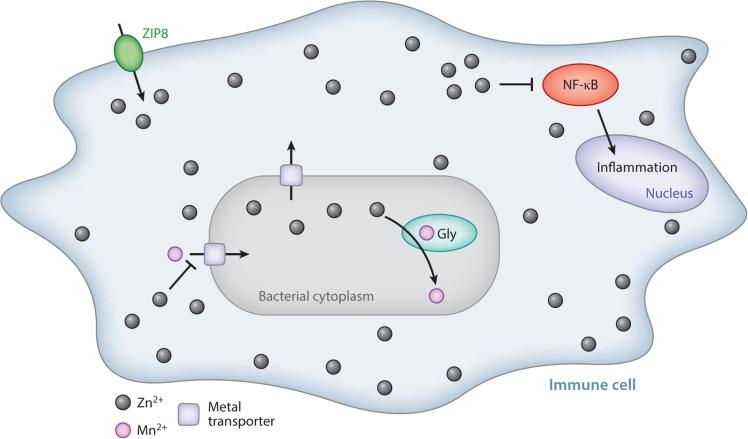
Zinc intoxication in innate immune cells. In response to infection, innate immune cells accumulate zinc in the cytoplasm through ZIP8-mediated import and in the phagosome by an unknown mechanism. Zinc accumulation in the cytoplasm modulates nuclear factor (NF)-κB activation, dampening proinflammatory responses. Bacterial zinc poisoning inhibits manganese import and the manganese-utilizing glycolytic enzymes (Gly) phosphofructokinase and glyceraldehyde-3-phosphate dehydrogenase. Bacteria express zinc efflux proteins in response.
Zinc efflux by the cadmium-zinc-cobalt (Czc) family exporter was also found to be important for S. pyogenes in a mouse model of infection (103). Human neutrophils respond to S. pyogenes internalization by mobilizing zinc, apparently increasing free cytosolic zinc (103). Interestingly, an increase in cytosolic zinc due to the transporter ZIP8 is an innate immune signaling mechanism in macrophages and lung epithelial cells. Specifically, zinc accumulation modulates nuclear factor (NF)-κB activation and inflammation (80). It is therefore unclear whether S. pyogenes zinc poisoning in the cytosol of neutrophils is an evolved mechanism of zinc intoxication or a side effect of the host using zinc as an immune signal. S. pneumoniae also likely experiences zinc intoxication during infection, based on the protective effect of zinc-chelating calprotectin and the increase in zinc/manganese ratios in the lung, brain, blood, and nasopharynx that occur during infection (2, 88). Further research may determine whether the rise in cytosolic zinc in neutrophils and macrophages is the main mechanism for zinc intoxication.
Potential Intoxicants: Manganese, Iron, Nickel, and Cobalt
There are currently no described mechanisms of host intoxication using the remaining first-row transition metals, but all are toxic at high levels and therefore could be harnessed by the host for intoxication. In a Mongolian gerbil model of stomach colonization, H. pylori requires resistance to the cadmium, zinc, and nickel (Czn) exporter, suggesting that H. pylori encounters metal stress in the host (125). In addition to elemental iron, Fe2+ in heme is toxic to bacterial cells (27). One could imagine host-imposed heme toxicity as a response to infection; consistent with this, B. anthracis induces heme efflux pumps during infection (126).
Manganese is often considered to be protective against toxicity. However, manganese efflux is important in multiple pathogens, revealing distinct metal niches in the host. For example, resistance to manganese toxicity by the manganese exporter MntX is conserved in N. meningitidis but not N. gonorrhoeae, suggesting that manganese homeostasis differs in their infectious niches (134). MntX appears to be important for maintaining the appropriate manganese/iron ratio, which is particularly important in low-iron environments (134). Similarly, the manganese efflux protein MntE is required for S. pneumoniae pathogenesis in murine nasal passages and dissemination to blood (116). S. pyogenes also requires MntE for virulence and resistance to manganese toxicity and hydrogen peroxide stress, apparently due to dysregulation of metal homeostasis (132). The maintenance of manganese efflux systems suggests that although the host may not impose manganese intoxication per se, it may manipulate metal ratios to disrupt bacterial metal homeostasis.
HOST METAL HOMEOSTASIS AND BACTERIAL INFECTIONS
Host metal status can dramatically affect susceptibility to bacterial infections. Changes in host metal homeostasis can cause increased metal availability for bacteria, which is thought to be particularly important for opportunistic pathogens that have not evolved the host-specific metal acquisition systems described above (141). Data establishing the link between host metal homeostasis and infection are often correlative and do not address bacterial mechanisms of increased pathogenicity; therefore, animal models of genetic and environmental variation in metal homeostasis offer potential avenues for molecular characterization. This section briefly reviews how genetic and dietary differences in host metal homeostasis can affect bacterial infection.
Iron Overload Lowers Defenses to Infection
Human genetic polymorphisms in iron homeostasis can confer increased susceptibility or resistance to bacterial infection. The hereditary blood disorders β-thalassemia and hemochromatosis can cause iron overload and are associated with increased rates of infection (141). In patients with β-thalassemia, which is caused by mutations in the hemoglobin β-chain, infectious disease is the second most common cause of death (>50% caused by Enterobacteriaceae) (138). Although iron overload is a consistent feature in β-thalassemia, its relationship to infection is muddied due to the prevalence of splenectomy and blood transfusion. However, in a murine model of β-thalassemia, the iron overload feature is isolated and confers increased susceptibility to infection by S. enterica and L. monocytogenes (6) (Table 1).
Table 1.
Selected bacterial species with improved virulence in host environments with metal dysregulation
| Bacterial species | Host metal dysregulation | Host | Effect on infection | Reference |
|---|---|---|---|---|
| Acinetobacter calcoaceticus | Occupational inhaled iron exposure | Human | Cluster of A. calcoaceticus pneumonia cases with two deaths | 31 |
| Enteroaggregative Escherichia coli | Dietary zinc deficiency | Mouse | Enhanced disease | 17 |
| Helicobacter pylori | Dietary iron deficiency | Gerbil | Enhanced bacterial burdens and carcinogenesis | 101 |
| Listeria monocytogenes | Iron overload from β-thalassemia | Mouse | Increased mortality and bacterial burdens | 6 |
| Dietary zinc deficiency | Rat | Reduced immune response | 22 | |
| Mannheimia hemolytica | Dietary copper deficiency | Mouse | Reduced lethal dose | 70 |
| Mycobacterium bovis | Dietary zinc deficiency | Guinea pig | Enhanced bacterial burdens early in infection | 90 |
| Mycobacterium tuberculosis | Dietary iron overload | Human | Shift from latent to active infection | 48 |
| Salmonella enterica | Iron overload from β-thalassemia | Mouse | Increased mortality | 6 |
| Dietary zinc deficiency | Rat | Increased mortality | 99 | |
| Streptococcus pneumoniae | Dietary zinc deficiency | Mouse | Increased mortality and bacterial burdens | 128 |
| Vibrio vulnificus | Iron overload by injection | Mouse | Reduced lethal dose | 146 |
| Hepcidin deficiency | Mouse | Increased bacteremia and mortality, partially rescued by dietary iron depletion | 8 | |
| Yersinia enterocolitica | Dietary iron overload | Human | Infection by commensal species | 91 |
| Yersinia pestis | Iron overload from hemochromatosis | Human, mouse | Restored virulence of attenuated vaccine strain | 112 |
Hemochromatosis is also associated with increased infection by multiple, normally noninvasive pathogens. The importance of iron has been corroborated in mouse models of infection by Vibrio vulnificus and nonpigmented Y. pestis (plague vaccine strains) (112, 146). Until recently, it was unclear which aspect of hemochromatosis caused this increase in infection: liver injury, high basal iron overload, or the inability to reduce serum iron in response to infection. Using a mouse model of hemochromatosis by hepcidin deficiency, acute hypoferremia in response to infection was isolated as the critical mechanism of increased infection by V. vulnificus (8). Increased susceptibility could be partially rescued by dietary iron depletion or administration of hepcidin agonists, suggesting therapeutic potential to treat patients with hereditary iron overload and infection (8).
Excess iron can also develop from environmental exposure through occupational hazards and diet. For example, workers exposed to high levels of iron-containing dust have increased rates of respiratory tract infection (107). A cluster of pneumonia cases caused by the opportunistic pathogen Acinetobacter calcoaceticus was linked to iron inhalation by foundry workers (31). Excess iron can be induced by dietary iron overload, generally due to inappropriate iron supplementation. Dietary iron overload has caused shifts from commensalism to pathogenesis by Yersinia enterocolitica, and from latent to acute forms of tuberculosis (48, 91). The fact that zinc and manganese are similarly restricted by nutritional immunity suggests that dietary overload may also increase susceptibility to infection.
Dietary Metal Deficiencies and Infection
There are well-established links between infection and dietary deficiencies of iron, copper, and zinc. Iron deficiency is specifically associated with H. pylori infection, which is a leading cause of gastric cancer. Work in a gerbil model showed that iron restriction by host dietary deficiency or limitation in vitro enhanced H. pylori pathogenesis via increased expression and elaboration of virulence factors (101). H. pylori infection can also induce iron deficiency, highlighting the complex nature of trace metals at the host-pathogen interface.
In contrast, sufficient dietary copper and zinc are required to resist infection generally, which has been the subject of previous reviews (39, 55). Copper and zinc are important for immune development and can serve as bacterial intoxicants, and intracellular zinc levels serve as an immune signal. Copper deficiency is associated with neutropenia and increases susceptibility to infection by Mannheimia haemolytica and S. enterica in animal models (70, 99). In addition to neutrophil development, dietary copper is important for phagosomal copper intoxication: Copper supplementation of guinea pig food led to increased Mycobacterium tuberculosis killing (145).
Approximately one-third of the world's population suffers from zinc deficiency, which is associated with increased rates of diarrhea and pneumonia (111). Prophylactic zinc supplementation was found to reduce incidence of persistent diarrhea by 33% and pneumonia by 41% in a pooled analysis; zinc as a therapeutic reduced prevalence of diarrhea by 34% but had no effect on pneumonia (13). Because zinc is critical for immune function, the role of enhanced bacterial virulence in a zinc-deficient host is unclear. Although rodent studies addressing the effect of zinc deficiency on pneumonia and diarrheal infection are limited, zinc deficiency was shown to increase susceptibility to L. monocytogenes, pneumococcal pneumonia, and diarrheal infection by enteroaggregative E. coli (EAEC), and to alter the time course of Mycobacterium bovis infection (17, 22, 90, 128). Notably, EAEC had increased expression of putative virulence factors in the cecal contents of zinc-deficient mice, suggesting that host zinc deficiency can alter bacterial pathogenesis (17).
CONCLUDING REMARKS
Transition metal biology at the host-pathogen interface is an active area of cross-disciplinary research spanning chemistry, biochemistry, microbiology, immunology, human genetics, and environmental and nutrition sciences. The field presents a number of opportunities for therapeutic potential. As secreted public goods, bacterial siderophores present a promising therapeutic target with reduced selective pressure for resistance, because the bacterium that evolves resistance does not necessarily benefit. For example, gallium (Ga2+) quenching of P. aeruginosa siderophores reduced pathogenesis in a Galleria mellonella model and retained efficacy longer than the conventional antibiotics gentamicin and ciprofloxacin (118). If a bacterium were to evolve production of gallium-resistant siderophore, it would be secreted and available to the entire bacterial population. Therefore, the producer of the gallium-resistant siderophore would not gain a selective advantage. In this way, targeting bacterial public goods decouples resistance and selective pressure. The fact that gallium siderophore quenching takes advantage of its interchangeability with biologically relevant transition metals suggests that gallium may also be toxic to the host due to host protein mismetallation. Therefore, other methods to target siderophores present attractive therapeutic opportunities. For example, vaccines against bacterial siderophores could similarly decouple resistance and selective pressure without causing metal intoxication of the host.
Additional areas of interest include bacterial metal homeostasis, evolution of bacterial pathogenesis, the role of nutritional immunity in modulating bacterial social behavior, and the effect of host metal nutrition on bacterial virulence. Recent work defining a low molecular weight thiol as the labile zinc buffer in Bacillus subtilis has expanded our understanding of metal homeostasis in bacteria, but the role of metal buffering and metallochaperones in pathogenesis remains largely unexplored (82). Recent work on the Acinetobacter baumanii response to zinc starvation identified a putative Zn metallochaperone and that intracellular histidine contributes to the labile zinc pool, expanding our understanding of zinc homeostasis in this opportunistic pathogen (97). We expect that additional targets of metal piracy will open new avenues for the study of rapid evolution at the host-pathogen interface. Additionally, polymicrobial infections can modulate the metal environment in the host. Infection by respiratory syncytial virus causes transferrin release, increasing available iron and promoting P. aeruginosa infection and biofilm formation (61). Future work will likely continue to uncover the role of metal homeostasis in bacterial pathogenesis and additional mechanisms by which metals influence coinfections.
The effect of host dietary metals on bacterial virulence also remains open for exploration. For example, future work could expand the effect of nutrient metals on gut microbial ecology and colonization resistance to pathogens. Transposon mutagenesis followed by selection and high-throughput sequencing of insertion sites could help determine whether bacterial pathogens can take advantage of altered nutrient metal landscapes in copper- and zinc-deficient hosts. Although the requirement of transition metals for enzyme catalysis likely predates life itself, the battle for metals at the host-pathogen interface remains a hotbed of evolution and continues to present opportunities for bettering human health.
NUTRITIONAL IMMUNITY PROTEIN MOONLIGHTS AS AN ANTIMICROBIAL.
Beyond its role in nutritional immunity, lactoferrin can inhibit bacterial growth by a variety of mechanisms including generation of antimicrobial peptides and binding of bacterial cell wall components (136). Bacterial pathogens have evolved systems to protect against these activities. Lactoferrin generates the antimicrobial peptides lactoferricin and lactoferrampin during bacterial infection. Recent work revealed rapid evolution in primates at the binding interface of lactoferricin and bacterial proteins that prevent lactoferrin-mediated killing (12). Additionally, the Staphylococcus aureus heme acquisition protein IsdA protects against lactoferrin-mediated killing by inhibiting lactoferrin's serine protease activity (28). Thus, resistance to a host nutritional immunity protein moonlighting as an antimicrobial agent relies on a bacterial metal acquisition protein moonlighting as a protease inhibitor. These evolutions/counter-revolutions exemplify the Red Queen hypothesis in bacterial pathogenesis.
SUMMARY POINTS.
Although bacterial pathogens use vertebrate hosts as nutrient metal sources, hosts possess multiple mechanisms to restrict bacterial access to transition metals through nutritional immunity. Bacteria evade host metal sequestration through metal acquisition strategies, including stealth siderophores and metal piracy from host proteins. The coevolution of pathogens and vertebrate hosts at the nutrient metal interface illustrates the Red Queen hypothesis in evolutionary biology.
Vertebrate hosts barrage bacteria with metals during intracellular infection, exploiting the toxicity of high concentrations of copper and zinc. The host intoxicates intraphagosomal bacteria by transporting high levels of copper with ATP7A. The mechanism of zinc intoxication and its relationship to zinc innate immune signaling is still being elucidated. Resistance to copper and zinc toxicity is important for virulence in a number of pathogens.
Host metal dysregulation through genetic disease or dietary metal intake affects susceptibility to infection by many bacterial pathogens. In general, iron overload, zinc deficiency, and copper deficiency increase susceptibility to infection. During H. pylori infection, iron deficiency accelerates production of bacterial virulence factors and carcinogenesis, demonstrating that changes in dietary metals can affect bacterial virulence.
The interchangeability of transition metal binding requires all organisms, including bacterial pathogens and their vertebrate hosts, to maintain appropriate metal homeostasis to prevent toxicity and mismetallation. The role of metal homeostasis at the host-pathogen interface remains an important area of investigation.
Transition metals: d-block elements in rows 3–12 of the periodic table that generally have partially filled d-orbitals and produce many oxidation states
Siderophores: small molecules that bind Fe3+ with high affinity secreted by microbes to acquire iron from the environment
Transferrin: a protein that sequesters iron in blood, transporting iron to the rest of the body following absorption in the duodenum
Red Queen hypothesis: a concept referencing Lewis Carroll's Through the Looking Glass to describe evolutionary pressures on organisms in close association
Heme: Fe2+-protoporphyrin IX, a cofactor for cytochrome oxidases used for respiration in bacteria and eukaryotes
ATP-binding cassette (ABC) transporters: a family of proteins that transport molecules across the cytoplasmic membrane via ATP hydrolysis
Public goods: a term originating from economics to describe resources that may be costly to produce but are freely accessible to all
Tragedy of the commons: refers to loss of a public resource from overuse, from economist William Forster Lloyd's phrase describing overgrazing of common lands
Hemopexin: a protein that binds free heme to protect against its oxidative effects and recycle the body's iron
Haptoglobin: a protein that binds free hemoglobin to protect against its oxidative effects; most hemoglobin-haptoglobin is then removed by the spleen
TonB-ExbB-ExbD system: a protein complex that harnesses the proton motive force for active transport across the outer membrane in Gram-negative bacteria
Type VI secretion system (T6SS): a system used by Gram-negative bacteria to transport effector proteins into host or bacterial target cells
Metallothioneins: a family of low molecular weight cytosolic proteins rich in cysteines that bind heavy metals on their thiol groups
S100 protein family: Ca2+-binding proteins with a helix-loop-helix (EF-hand type) conformation that often bind additional metals and serve as nutritional immunity proteins
Natural resistance–associated macrophage protein 1 (Nramp1): a protein that exports iron and manganese from the phagosome and enhances resistance to intracellular pathogens
β-thalassemia: a hereditary blood disorder caused by defects in synthesis of the hemoglobin β-chain that can cause iron overload
Hemochromatosis: hereditary blood disorder caused by mutations in transferrin receptor–interacting genes and characterized by iron overload
Iron overload: an accumulation of iron in the body due to genetic diseases, repeated blood transfusion, or excess iron consumption
ACKNOWLEDGMENTS
We thank members of the Skaar laboratory for critical reading of the manuscript and Sarah A. Marcus for helpful discussion. The writing of this manuscript was supported through fellowships to L.D.P. from the National Institutes of Health (NIH) (5T32HL094296-08/1F32AI122516-01) and research grants to E.P.S. from the NIH (R01 AI069233, R01 AI107233, R01 AI101171), US Department of Veterans Affairs (I01 BX002482/BX/BLRD), Defense Advanced Research Projects Agency, and American Asthma Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED/RELATED RESOURCES
- 1.Abergel RJ, Wilson MK, Arceneaux JE, Hoette TM, Strong RK, et al. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. PNAS. 2006;103:18499–503. doi: 10.1073/pnas.0607055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achouiti A, Vogl T, Endeman H, Mortensen BL, Laterre PF, et al. Myeloid-related protein-8/14 facilitates bacterial growth during pneumococcal pneumonia. Thorax. 2014;69:1034–42. doi: 10.1136/thoraxjnl-2014-205668. [DOI] [PubMed] [Google Scholar]
- 3.Achouiti A, Vogl T, Urban CF, Rohm M, Hommes TJ, et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLOS Pathog. 2012;8:e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen CE, Schmitt MP. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J. Bacteriol. 2009;191:2638–48. doi: 10.1128/JB.01784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almiron M, Martinez M, Sanjuan N, Ugalde RA. Ferrochelatase is present in Brucella abortus and is critical for its intracellular survival and virulence. Infect. Immun. 2001;69:6225–30. doi: 10.1128/IAI.69.10.6225-6230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ampel NM, van Wyck DB, Aguirre ML, Willis DG, Popp RA. Resistance to infection in murine β-thalassemia. Infect. Immun. 1989;57:1011–17. doi: 10.1128/iai.57.4.1011-1017.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen SB, Marvig RL, Molin S, Johansen HK, Griffin AS. Long-term social dynamics drive loss of function in pathogenic bacteria. PNAS. 2015;112:10756–61. doi: 10.1073/pnas.1508324112. [Evolutionary analysis of long-term infection showed that local competition drives siderophore cheating and community loss-of-function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, et al. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe. 17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. mBio. 2012;3:e00224–11. doi: 10.1128/mBio.00224-11. [Evolutionary and biochemical analyses showed that rapid evolution drives host-pathogen interactions in metal acquisition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber MF, Elde NC. Escape from bacterial iron piracy through rapid evolution of transferrin. Science. 2014;346:1362–66. doi: 10.1126/science.1259329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber MF, Elde NC. Buried treasure: evolutionary perspectives on microbial iron piracy. Trends Genet. 2015;31:627–36. doi: 10.1016/j.tig.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber MF, Kronenberg Z, Yandell M, Elde NC. Antimicrobial functions of lactoferrin promote genetic conflicts in ancient primates and modern humans. PLOS Genet. 2016;12(5):e1006063. doi: 10.1371/journal.pgen.1006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J. Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell JM, Searle S, Mohamed H, White JK. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol. Lett. 2003;85:197–203. doi: 10.1016/s0165-2478(02)00231-6. [DOI] [PubMed] [Google Scholar]
- 15.Blair DE, Schuttelkopf AW, MacRae JI, van Aalten DM. Structure and metal-dependent mechanism of peptidoglycan deacetylase, a streptococcal virulence factor. PNAS. 2005;102:15429–34. doi: 10.1073/pnas.0504339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobrov AG, Kirillina O, Fetherston JD, Miller MC, Burlison JA, Perry RD. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol. Microbiol. 2014;93:759–75. doi: 10.1111/mmi.12693. [This study discovered a dedicated Zn2+-siderophore importer and established its importance during infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolick DT, Kolling GL, Moore JH, Jr, de Oliveira LA, Tung K, et al. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli–induced diarrhea. Gut Microbes. 2014;5:618–27. doi: 10.4161/19490976.2014.969642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, et al. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–59. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun V, Hantke K. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 2011;15:328–34. doi: 10.1016/j.cbpa.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Braymer JJ, Giedroc DP. Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr. Opin. Chem. Biol. 2014;19:59–66. doi: 10.1016/j.cbpa.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burne RA, Chen YY. Bacterial ureases in infectious diseases. Microbes Infect. 2000;2:533–42. doi: 10.1016/s1286-4579(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 22.Carlomagno MA, Coghlan LG, McMurray DN. Chronic zinc deficiency and listeriosis in rats: acquired cellular resistance and response to vaccination. Med. Microbiol. Immunol. 1986;175:271–80. doi: 10.1007/BF02126048. [DOI] [PubMed] [Google Scholar]
- 23.Carrano CJ, Raymond KN. Ferric ion sequestering agents. 2. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J. Am. Chem. Soc. 1979;101:5401–4. [Google Scholar]
- 24.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–19. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi KS, Henderson JP. Pathogenic adaptations to host-derived antibacterial copper. Front. Cell Infect. Microbiol. 2014;4:3. doi: 10.3389/fcimb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012;8:731–36. doi: 10.1038/nchembio.1020. [Demonstrated that yersiniabactin secreted by uropathogenic Escherichia coli binds copper to resist toxicity during infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choby JE, Skaar EP. Heme acquisition and synthesis in bacterial pathogens. J. Mol. Biol. 2016 doi: 10.1016/j.jmb.2016.03.018. In press. doi: 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke SR, Foster SJ. IsdA protects Staphylococcus aureus against the bactericidal protease activity of apolactoferrin. Infect. Immun. 2008;76:1518–26. doi: 10.1128/IAI.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett D, Wang J, Schuler S, Lopez-Castejon G, Glenn S, et al. Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect. Immun. 2012;80:14–21. doi: 10.1128/IAI.05904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–65. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 31.Cordes LG, Brink EW, Checko PJ, Lentnek A, Lyons RW, et al. A cluster of Acinetobacter pneumonia in foundry workers. Ann. Intern. Med. 1981;95:688–93. doi: 10.7326/0003-4819-95-6-688. [DOI] [PubMed] [Google Scholar]
- 32.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. PNAS. 2013;110:3841–46. doi: 10.1073/pnas.1220341110. [This structural analysis of calprotectin established the molecular basis for high-affinity manganese binding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, et al. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhaenens L, Szczebara F, Husson MO. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori. Infect. Immun. 1997;65:514–18. doi: 10.1128/iai.65.2.514-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, et al. Salmonella mediates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe. 2016;19:814–25. doi: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dintilhac A, Alloing G, Granadel C, Claverys JP. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 1997;25:727–39. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 37.Djoko KY, Franiek JA, Edwards JL, Falsetta ML, Kidd SP, et al. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect. Immun. 2012;80:1065–71. doi: 10.1128/IAI.06163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djoko KY, McEwan AG. Antimicrobial action of copper is amplified via inhibition of heme biosynthesis. ACS Chem. Biol. 2013;8:2217–23. doi: 10.1021/cb4002443. [DOI] [PubMed] [Google Scholar]
- 39.Djoko KY, Ong CL, Walker MJ, McEwan AG. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015;290:18954–61. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Orazio M, Mastropasqua MC, Cerasi M, Pacello F, Consalvo A, et al. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics. 2015;7:1023–35. doi: 10.1039/c5mt00017c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect. Immun. 1994;62:3604–7. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrand AJ, Haley KP, Lareau NM, Heilbronner S, McLean JA, et al. An iron-regulated autolysin remodels the cell wall to facilitate heme acquisition in Staphylococcus lugdunensis. Infect. Immun. 2015;83:3578–89. doi: 10.1128/IAI.00397-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. PNAS. 2006;103:16502–7. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 45.Fritsche G, Nairz M, Libby SJ, Fang FC, Weiss G. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J. Leukoc. Biol. 2012;92:353–59. doi: 10.1189/jlb.1111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaddy JA, Radin JN, Cullen TW, Chazin WJ, Skaar EP, et al. Helicobacter pylori resists the antimicrobial activity of calprotectin via lipid A modification and associated biofilm formation. mBio. 2015;6:e01349–15. doi: 10.1128/mBio.01349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaddy JA, Radin JN, Loh JT, Piazuelo MB, Kehl-Fie TE, et al. The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLOS Pathog. 2014;10:e1004450. doi: 10.1371/journal.ppat.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gangaidzo IT, Moyo VM, Mvundura E, Aggrey G, Murphree NL, et al. Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 2001;184:936–39. doi: 10.1086/323203. [DOI] [PubMed] [Google Scholar]
- 49.Ghoul M, West SA, Diggle SP, Griffin AS. An experimental test of whether cheating is context dependent. J. Evol. Biol. 2014;27:551–56. doi: 10.1111/jeb.12319. [DOI] [PubMed] [Google Scholar]
- 50.Ghssein G, Brutesco C, Ouerdane L, Fojcik C, Izaute A, Wang S, et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science. 2016;352(6289):1105–9. doi: 10.1126/science.aaf1018. [DOI] [PubMed] [Google Scholar]
- 51.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 52.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, et al. Identification of a copper-binding metal-lothionein in pathogenic mycobacteria. Nat. Chem. Biol. 2008;4:609–16. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–27. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 54.Guilhen C, Taha MK, Veyrier FJ. Role of transition metal exporters in virulence: the example of Neisseria meningitidis. Front. Cell Infect. Microbiol. 2013;3:102. doi: 10.3389/fcimb.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175–80. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 56.Haley KP, Delgado AG, Piazuelo MB, Mortensen BL, Correa P, et al. The human antimicrobial protein calgranulin C participates in control of Helicobacter pylori growth and regulation of virulence. Infect. Immun. 2015;83:2944–56. doi: 10.1128/IAI.00544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammer ND, Cassat JE, Noto MJ, Lojek LJ, Chadha AD, et al. Inter- and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus. Cell Host Microbe. 2014;16:531–37. doi: 10.1016/j.chom.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, et al. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. mBio. 2013;4:e00241–13. doi: 10.1128/mBio.00241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammerschmidt S, Bethe G, Remane PH, Chhatwal GS. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 1999;67:1683–87. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardin G. The tragedy of the commons. Science. 1968;162:1243–48. [PubMed] [Google Scholar]
- 61.Hendricks MR, Lashua LP, Fischer DK, Flitter BA, Eichinger KM, et al. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. PNAS. 2016;113:1642–47. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgins KA, Giedroc D. Insights into protein allostery in the CsoR/RcnR family of transcriptional repressors. Chem. Lett. 2014;43:20–25. doi: 10.1246/cl.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiron A, Posteraro B, Carriere M, Remy L, Delporte C, et al. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol. Microbiol. 2010;77:1246–60. doi: 10.1111/j.1365-2958.2010.07287.x. [DOI] [PubMed] [Google Scholar]
- 64.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLOS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012;10:525–37. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imperi F, Tiburzi F, Visca P. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. PNAS. 2009;106:20440–45. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance–associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 2000;192:1237–48. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson MD, Kehl-Fie TE, Rosch JW. Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumoniae. Metallomics. 2015;7:786–94. doi: 10.1039/c5mt00011d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones CM, Wells RM, Madduri AV, Renfrow MB, Ratledge C, et al. Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. PNAS. 2014;111:1945–50. doi: 10.1073/pnas.1311402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones DG, Suttle NF. The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. J. Comp. Pathol. 1983;93:143–49. doi: 10.1016/0021-9975(83)90052-x. [DOI] [PubMed] [Google Scholar]
- 71.Juttukonda LJ, Skaar EP. Manganese homeostasis and utilization in pathogenic bacteria. Mol. Microbiol. 2015;97:216–28. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–64. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, et al. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun. 2013;81:3395–405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim HW, Chan Q, Afton SE, Caruso JA, Lai B, et al. Human macrophage ATP7A is localized in the trans-Golgi apparatus, controls intracellular copper levels, and mediates macrophage responses to dermal wounds. Inflammation. 2012;35:167–75. doi: 10.1007/s10753-011-9302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koh EI, Hung CS, Parker KS, Crowley JR, Giblin DE, Henderson JP. Metal selectivity by the virulence-associated yersiniabactin metallophore system. Metallomics. 2015;7:1011–22. doi: 10.1039/c4mt00341a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krieg S, Huche F, Diederichs K, Izadi-Pruneyre N, Lecroisey A, et al. Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. PNAS. 2009;106:1045–50. doi: 10.1073/pnas.0809406106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau CK, Krewulak KD, Vogel HJ. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol. Rev. 2015;40:273–98. doi: 10.1093/femsre/fuv049. [DOI] [PubMed] [Google Scholar]
- 78.Lisher JP, Giedroc DP. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell Infect. Microbiol. 2013;3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–39. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loomis LD, Raymond KN. Solution equilibria of enterobactin and metal-enterobactin complexes. Inorg. Chem. 1991;30:906–11. [Google Scholar]
- 82.Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol. Microbiol. 2014;94:756–70. doi: 10.1111/mmi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. PNAS. 2009;106:8344–49. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maresso AW, Garufi G, Schneewind O. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLOS Pathog. 2008;4:e1000132. doi: 10.1371/journal.ppat.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marvig RL, Damkiaer S, Khademi SM, Markussen TM, Molin S, Jelsbak L. Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. mBio. 2014;5:e00966–14. doi: 10.1128/mBio.00966-14. [Independent Pseudomonas aeruginosa isolates from cystic fibrosis patients lost siderophore acquisition, evolving a heme iron preference.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mashruwala AA, Pang YY, Rosario-Cruz Z, Chahal HK, Benson MA, et al. Nfu facilitates the maturation of iron-sulfur proteins and participates in virulence in Staphylococcus aureus. Mol. Microbiol. 2015;95:383–409. doi: 10.1111/mmi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–9. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 88.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, et al. A molecular mechanism for bacterial susceptibility to zinc. PLOS Pathog. 2011;7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGee DJ, Zabaleta J, Viator RJ, Testerman TL, Ochoa AC, Mendz GL. Purification and characterization of Helicobacter pylori arginase, RocF: unique features among the arginase superfamily. Eur. J. Biochem. 2004;271:1952–62. doi: 10.1111/j.1432-1033.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- 90.McMurray DN, Yetley EA. Response to Mycobacterium bovis BCG vaccination in protein- and zinc-deficient guinea pigs. Infect. Immun. 1983;39:755–61. doi: 10.1128/iai.39.2.755-761.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melby K, Slordahl S, Gutteberg TJ, Nordbo SA. Septicaemia due to Yersinia enterocolitica after oral overdoses of iron. BMJ (Clin. Res. Ed.) 1982;285:467–68. doi: 10.1136/bmj.285.6340.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyer JE, Harder J, Sipos B, Maune S, Kloppel G, et al. Psoriasin (S100A7) is a principal antimicrobial peptide of the human tongue. Mucosal Immunol. 2008;1:239–43. doi: 10.1038/mi.2008.3. [DOI] [PubMed] [Google Scholar]
- 93.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007;71:413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mildner M, Stichenwirth M, Abtin A, Eckhart L, Sam C, et al. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol. 2010;3:602–9. doi: 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- 95.Moraes TF, Yu RH, Strynadka NC, Schryvers AB. Insights into the bacterial transferrin receptor: the structure of transferrin-binding protein B from Actinobacillus pleuropneumoniae. Mol. Cell. 2009;35:523–33. doi: 10.1016/j.molcel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 96.Morgenthau A, Pogoutse A, Adamiak P, Moraes TF, Schryvers AB. Bacterial receptors for host transferrin and lactoferrin: molecular mechanisms and role in host-microbe interactions. Future Microbiol. 2013;8:1575–85. doi: 10.2217/fmb.13.125. [DOI] [PubMed] [Google Scholar]
- 97.Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe. 2016;19:826–36. doi: 10.1016/j.chom.2016.05.007. [Identified a putative zinc metallochaperone and the contribution of histidine to the labile zinc pool.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015;11:765–71. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Newberne PM, Hunt CE, Young VR. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br. J. Exp. Pathol. 1968;49:448–57. [PMC free article] [PubMed] [Google Scholar]
- 100.Nolan KJ, McGee DJ, Mitchell HM, Kolesnikow T, Harro JM, et al. In vivo behavior of a Helicobacter pylori SS1 nixA mutant with reduced urease activity. Infect. Immun. 2002;70:685–91. doi: 10.1128/iai.70.2.685-691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, et al. Iron deficiency accelerates Helicobacter pylori–induced carcinogenesis in rodents and humans. J. Clin. Invest. 2013;123:479–92. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oftung F, Lovik M, Andersen SR, Froholm LO, Bjune G. A mouse model utilising human transferrin to study protection against Neisseria meningitidis serogroup B induced by outer membrane vesicle vaccination. FEMS Immunol. Med. Microbiol. 1999;26:75–82. doi: 10.1111/j.1574-695X.1999.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 103.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J. Infect. Dis. 2014;209:1500–8. doi: 10.1093/infdis/jiu053. [Showed zinc mobilization in the neutrophil cytoplasm as one mechanism for host zinc poisoning of pathogens.] [DOI] [PubMed] [Google Scholar]
- 104.Ong CL, Walker MJ, McEwan AG. Zinc disrupts central carbon metabolism and capsule biosyn-thesis in Streptococcus pyogenes. Sci. Rep. 2015;5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oram JD, Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta. 1968;170:351–65. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- 106.Ouyang Z, He M, Oman T, Yang XF, Norgard MV. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. PNAS. 2009;106:3449–54. doi: 10.1073/pnas.0812999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmer K, Coggon D. Does occupational exposure to iron promote infection? Occup. Environ. Med. 1997;54:529–34. doi: 10.1136/oem.54.8.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perry RD, Craig SK, Abney J, Bobrov AG, Kirillina O, et al. Manganese transporters Yfe and MntH are Fur-regulated and important for the virulence of Yersinia pestis. Microbiology. 2012;158:804–15. doi: 10.1099/mic.0.053710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pishchany G, McCoy AL, Torres VJ, Krause JC, Crowe JE, Jr, et al. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe. 2010;8:544–50. doi: 10.1016/j.chom.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–53. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 111.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 2013;4:176–90. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quenee LE, Hermanas TM, Ciletti N, Louvel H, Miller NC, et al. Hereditary hemochromatosis restores the virulence of plague vaccine strains. J. Infect. Dis. 2012;206:1050–58. doi: 10.1093/infdis/jis433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–86. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Remy L, Carriere M, Derre-Bobillot A, Martini C, Sanguinetti M, Borezee-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol. Microbiol. 2013;87:730–43. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 115.Rodionov DA, Hebbeln P, Gelfand MS, Eitinger T. Comparative and functional genomic analysis of prokaryotic nickel and cobalt uptake transporters: evidence for a novel group of ATP-binding cassette transporters. J. Bacteriol. 2006;188:317–27. doi: 10.1128/JB.188.1.317-327.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signaling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 2009;72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ross-Gillespie A, Dumas Z, Kummerli R. Evolutionary dynamics of interlinked public goods traits: an experimental study of siderophore production in Pseudomonas aeruginosa. J. Evol. Biol. 2015;28:29–39. doi: 10.1111/jeb.12559. [DOI] [PubMed] [Google Scholar]
- 118.Ross-Gillespie A, Weigert M, Brown SP, Kummerli R. Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol. Med. Public Health. 2014;2014:18–29. doi: 10.1093/emph/eou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rowland JL, Niederweis M. A multicopper oxidase is required for copper resistance in Mycobacterium tuberculosis. J. Bacteriol. 2013;195:3724–33. doi: 10.1128/JB.00546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schade AL, Caroline L. Raw hen egg white and the role of iron in growth inhibition of Shigella dysenteriae, Staphylococcus aureus, Escherichia coli and Saccharomyces cerevisiae. Science. 1944;100:14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- 121.Schade AL, Caroline L. An iron-binding component in human blood plasma. Science. 1946;104:340. [PubMed] [Google Scholar]
- 122.Shields-Cutler RR, Crowley JR, Hung CS, Stapleton AE, Aldrich CC, et al. Human urinary composition controls antibacterial activity of siderocalin. J. Biol. Chem. 2015;290:15949–60. doi: 10.1074/jbc.M115.645812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–28. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 124.Speer A, Rowland JL, Haeili M, Niederweis M, Wolschendorf F. Porins increase copper susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 2013;195:5133–40. doi: 10.1128/JB.00763-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stahler FN, Odenbreit S, Haas R, Wilrich J, van Vliet AH, et al. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 2006;74:3845–52. doi: 10.1128/IAI.02025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stauff DL, Skaar EP. Bacillus anthracis HssRS signalling to HrtAB regulates haem resistance during infection. Mol. Microbiol. 2009;72:763–78. doi: 10.1111/j.1365-2958.2009.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stork M, Grijpstra J, Bos MP, Manas Torres C, Devos N, et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLOS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [Neisseria meningitidis CbpA binds calprotectin and supports zinc-dependent growth, suggesting a potential for zinc piracy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strand TA, Hollingshead SK, Julshamn K, Briles DE, Blomberg B, Sommerfelt H. Effects of zinc deficiency and pneumococcal surface protein A immunization on zinc status and the risk of severe infection in mice. Infect. Immun. 2003;71:2009–13. doi: 10.1128/IAI.71.4.2009-2013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun TS, Ju X, Gao HL, Wang T, Thiele DJ, et al. Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat. Commun. 2014;5:5550. doi: 10.1038/ncomms6550. [DOI] [PubMed] [Google Scholar]
- 130.Troxell B, Hassan HM. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell Infect. Microbiol. 2013;3:59. doi: 10.3389/fcimb.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, et al. Discovery and characterization of a unique mycobacterial heme acquisition system. PNAS. 2011;108:5051–56. doi: 10.1073/pnas.1009516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, et al. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio. 2015;6:e00278–15. doi: 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Valen L. A new evolutionary law. Evol. Theory. 1973;1:1–30. [Google Scholar]
- 134.Veyrier FJ, Boneca IG, Cellier MF, Taha MK. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLOS Pathog. 2011;7:e1002261. doi: 10.1371/journal.ppat.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vidal SM, Pinner E, Lepage P, Gauthier S, Gros P. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 1996;157:3559–68. [PubMed] [Google Scholar]
- 136.Vogel HJ. Lactoferrin, a bird's eye view. Biochem. Cell Biol. 2012;90:233–44. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 137.Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 2016;7:11951. doi: 10.1038/ncomms11951. [Zinc sequestration by calprotectin promotes co-infection by Pseudomonas aeruginosa and Staphylococcus aureus..] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wanachiwanawin W. Infections in E-β thalassemia. J. Pediatr. Hematol. Oncol. 2000;22:581–87. doi: 10.1097/00043426-200011000-00027. [DOI] [PubMed] [Google Scholar]
- 139.Wang T, Si M, Song Y, Zhu W, Gao F, et al. Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLOS Pathog. 2015;11:e1005020. doi: 10.1371/journal.ppat.1005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weinberg ED. Nutritional immunity: host's attempt to withhold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 141.Weinberg ED. Iron availability and infection. Biochim. Biophys. Acta. 2009;1790:600–5. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Wessling-Resnick M. Nramp1 and other transporters involved in metal withholding during infection. J. Biol. Chem. 2015;290:18984–90. doi: 10.1074/jbc.R115.643973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 2009;284:33949–56. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wolff N, Izadi-Pruneyre N, Couprie J, Habeck M, Linge J, et al. Comparative analysis of structural and dynamic properties of the loaded and unloaded hemophore HasA: functional implications. J. Mol. Biol. 2008;376:517–25. doi: 10.1016/j.jmb.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 145.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, et al. Copper resistance is essential for virulence of Mycobacterium tuberculosis. PNAS. 2011;108:1621–26. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 1981;34:503–7. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zaia AA, Sappington KJ, Nisapakultorn K, Chazin WJ, Dietrich EA, et al. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009;2:43–53. doi: 10.1038/mi.2008.63. [DOI] [PubMed] [Google Scholar]
- 148.Zarantonelli ML, Szatanik M, Giorgini D, Hong E, Huerre M, et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect. Immun. 2007;75:5609–14. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang YJ, Rubin EJ. Feast or famine: the host-pathogen battle over amino acids. Cell Microbiol. 2013;15:1079–87. doi: 10.1111/cmi.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zimbler DL, Park TM, Arivett BA, Penwell WF, Greer SM, et al. Stress response and virulence functions of the Acinetobacter baumannii NfuA Fe-S scaffold protein. J. Bacteriol. 2012;194:2884–93. doi: 10.1128/JB.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
RELATED RESOURCES
- 1.Nriagu JO, Skaar EP. Trace Metals and Infectious Diseases. MIT Press; Cambridge, MA: 2015. [PubMed] [Google Scholar]
- 2.Skaar EP, Raffatellu M. Metals in infectious diseases and nutritional immunity. Metallomics. 2015;7:926–28. doi: 10.1039/c5mt90021b. [DOI] [PubMed] [Google Scholar]