Abstract
Galloway et al. recently described a method to alter vectors to include Type IIS restriction enzymes for high efficiency cloning. Utilizing this method, the multiple cloning sites of complementation and overexpression vectors commonly used in our laboratory were altered to contain recognition sequences of the Type IIS restriction enzyme, BspQI. Use of this enzyme increased the rate of cloning success to >97% efficiency. L(+)-Arabinose-inducible complementation vectors and overexpression vectors encoding N-terminal recombinant tobacco etch virus protease (rTEV)-cleavable H6-tags were altered to contain BspQI sites that allowed for cloning into all vectors using identical primer overhangs. Additionally, a vector used for directing the synthesis of proteins with a C-terminal, rTEV-cleavable H6-tag was engineered to contain BspQI sites, albeit with different over-hangs from that of the previously mentioned vectors. Here we apply a method used to engineer cloning vectors to contain BspQI sites and the use of each vector in either in vivo complementation studies or in vitro protein purifications.
Keywords: Type IIS restriction cloning, High-efficiency cloning vectors, Complementation vectors, Overexpression vectors, Escherichia coli, S. enterica
1. Introduction
Increasing cloning efficiency is desirable for the rapid completion of experiments where complementation of function or protein overproduction is the objective. Engler et al. developed a method termed Golden Gate cloning; in which a Type IIS restriction enzyme (BsaI) is utilized for high efficiency cloning into expression constructs (Engler et al., 2008). Additionally, Galloway et al. (Galloway et al., 2013) reported an approach for the introduction of BspQI sites into a vector’s multiple-cloning site (MCS). Type IIS restriction enzymes (e.g., BspQI, BsaI, BpiI) have been used in cloning methods for the construction of gene expression reporters (Oster and Phillips, 2011), recombinant protein expression (Engler et al., 2008; Galloway et al., 2013), assembly of multiple DNA fragments (Engler et al., 2009) or gene fusions and promoter shuffling (Engler and Marillonnet, 2014).
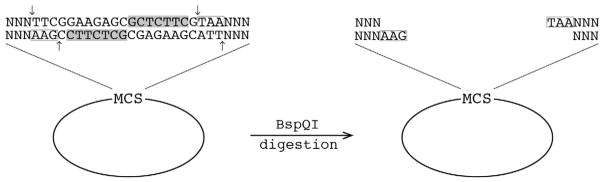
Type IIS restriction enzymes cleave at least one strand of DNA located outside of the recognition sequence, with BspQI cleaving both strands of DNA (Roberts et al., 2003). Type IIS restriction enzymes act as dimers, with one domain binding to a 4–7 base pair recognition sequence and another domain interacting with a cleavage site 1–20 nucleotides away from the recognition site (Pingoud et al., 2005; Szybalski et al., 1991). Having a separate cut site from the recognition sequence is valuable for vector design because the cleavage site can be engineered to have its own unique overhangs. An enzyme, such as BspQI, has a recognition sequence [GCTCTTC (1/4)] where the first number in parentheses corresponds to the position of cleavage on the coding strand and the second number to the cleavage site on the complementary strand (Roberts et al., 2003). This creates a three base pair overhang, resulting in 81 different possible overhangs (Fig. 1).
Fig. 1.
Visual representation of BspQI vector digestion. BspQI binds to the recognition site [GCTCTTC (1/4), highlighted in grey] and will cut both strands at indicated arrows. This creates a three base pair overhang. The cut site does not interfere with recognition and can be custom designed. Shown is an example of vectors constructed in this study (pCV1–3, pTEV18, 19). For a more detailed figure, see supplemental.
The method described by Galloway et al. simplifies cloning efforts because digestion and ligation occur in the same reaction mixture, substantially reducing the time needed to clone genes of interest (Galloway et al., 2013). Additionally, because Type IIS restriction enzymes cut outside of their recognition sequence, ligated insert-vector products no longer contain the recognition site, and any empty vector remaining in the reaction mixture can be linearized by addition of the Type IIS restriction enzyme being used, substantially reducing false-positive background (Engler et al., 2008).
In this study, we used the method described by Galloway et al. to introduce BspQI sites into the MCSs of plasmids used for arabinose-inducible expression of genes of interest, or for the overproduction of proteins. Here, we modified the MCSs of three complementation vectors (pBAD24, pBAD30, and pBAD33-SD1 (Guzman et al., 1995a)). Specifically, each MCS was modified to contain two BspQI sites. We also modified two overexpression vectors to contain BspQI sites matching those present in the complementation vectors. The resulting vectors directed the synthesis of proteins of interest fused to an N-terminal, re-combinant tobacco etch virus (rTEV)-cleavable hexahistidine (H6-tag (pTEV5, (Rocco et al., 2008)) or an N-terminal, rTEV-cleavable H6, maltose binding protein tag (pTEV6, (Rocco et al., 2008)). Conveniently, cloning into all of the above-mentioned vectors was done using the same primer overhangs. Lastly, an overexpression vector with a pTEV backbone was also altered to contain BspQI sites. In this case, however, the protein whose synthesis was directed by this vector was fused to a C-terminal, rTEV-cleavable H6-tag. Cloning into all of the BspQI-containing vectors was ≥97% efficient. The main goal of this work is to provide investigators with information regarding the specific aforementioned vectors.
2. Materials and methods
2.1. Bacterial strains, culture media, chemicals, and sequencing methods
All strains used in this study are listed in Table S1. Escherichia coli C41 (λDE3) (Miroux and Walker, 1996) and DH5α (New England Biolabs) strains were grown at 37 °C in lysogeny broth (LB, Difco). Strains used for growth analysis were derivatives of Salmonella enterica sv Typhimurium LT2 and grown at 37 °C in nutrient broth (NB, Difco) containing NaCl (85 mM), or no-carbon essential (NCE) minimal medium (Berkowitz et al., 1968) supplemented with sodium acetate (10 mM), L-methionine (0.5 mM), MgSO4 (1 mM), and trace minerals (Atlas, 1995). Antibiotics were used at the following concentrations: ampicillin, 100 μg mL−1; chloramphenicol, 20 μg mL−1. All chemicals were purchased from Fischer unless noted otherwise; chloramphenicol, L(+)-arabinose (Sigma-Aldrich); ethylenediaminetetra-acetic acid (EDTA, VWR); and isopropylβ-D-1-thiogalactopyranoside (IPTG, IBI Scientific). All restriction enzymes were purchased from Thermo Scientific™ with the exception of BspQI (New England Biolabs). DNA sequencing was performed using Big Dye® Terminator v3.1 protocols (Applied Biosystems). DNA sequencing was performed at the Georgia Genomics Facility.
2.2. Construction of vectors containing BspQI sites
Native BspQI sites (GCTCTTC) in the backbone of pBAD24, pBAD30, pBAD33-SD1, pTEV5, and pTEV6 were eliminated via site-directed mutagenesis and new BspQI sites were introduced into the MCS as described previously (Galloway et al., 2013). For a detailed procedure, see Supplemental Information or reference 2. Newly engineered vectors are listed in Table S3 and are hereafter referred to as pTEV16 (derived from pTEV5), pTEV17 (derived from pTEV6), and pCV1 (for Complementation Vector, derived from pBAD24), pCV2 (derived from pBAD30), and pCV3 (derived pBAD33-SD1).
2.3. Site-directed mutagenesis of pTEV16 and pTEV17
To reduce the number of primers and to facilitate cloning into over-expression and complementation vectors, DNA containing the cut site (pTEV16: AGC, pTEV17: ACC) was mutated to the pCV cut site (i.e., TTC) by site-directed mutagenesis. Polymerase chain reaction was performed using Pfu Ultra II DNA polymerase using primers listed in Table S2. Modifications included an anneal time of 60 s, an extension temperature of 68 °C, and an extension time of 2.5 min kb−1. DNA changes were confirmed by sequencing.
2.4. Construction of C-terminal rTEV cleavable H6-tag BspQI vector
The pTEV18 vector was amplified with Pfu Ultra II DNA polymerase using primers outside of the N-terminal H6-tag and MCS (Table S2). PCR product was purified using Wizard® SV DNA Clean-Up System (Promega). Linear blunt-end fragments were ligated using the Fast-Link-DNA Ligation Kit (Epicentre), and transformed into DH5α chemically competent cells (Maniatis et al., 1982). Cells were plated on LB + ampicillin, incubated overnight at 37 °C, and 10 individual ApR colonies were used to inoculate fresh LB + ampicillin medium. Plasmids were isolated from each overnight culture using the Wizard® Plus SV Miniprep DNA Purification System (Promega). The presence of BspQI sites in the MCS was determined by cutting with BspQI and an enzyme outside of the MCS (e.g., ScaI). The resulting MCS was sequenced to confirm the presence of the BspQI site and C-terminal H6 rTEV cleavable tag.
2.5. Cloning of SeAcs into newly designed BspQI vectors
To illustrate the efficiency of the new vectors, the S. enterica acs+ gene encoding acetyl-CoA synthetase was cloned. For this purpose, primers were designed with BspQI sites on the 5′-ends such that when amplicons were cut, a three base pair overhang was complementary to overhangs in the corresponding digested vector (Table S2). The acs+ gene was PCR amplified from S. enterica LT2 genomic DNA using Pfu Ultra II DNA polymerase. PCR products corresponding to the correct size were verified via gel electrophoresis and extracted as described above. Gene products were cloned into pCV1, pCV2, pCV3 pTEV16, pTEV17, pTEV18, pTEV19 and pTEV20 as described elsewhere (Galloway et al., 2013). For a more detailed procedure, refer to supplemental materials or reference 2. A sample of the ligation reaction (1 μL) was used to transform E. coli DH5α competent cells (Maniatis et al., 1982), and cells were plated on LB containing the appropriate antibiotic. The presence of inserts in the cloning vectors was confirmed by colony PCR with Go Taq® Green Master Mix (Promega) using primers that annealed to the plasmid outside of the MCS (Table S2). PCR products were analyzed on a 1% agarose gel with Tris/Borate/EDTA buffer for 40 min at 115 V. Cells harboring plasmids containing the expected inserts were grown overnight in LB supplemented with the appropriate antibiotic, and plasmids were isolated as described above. The presence of acs+ in each plasmid was confirmed by DNA sequencing.
2.6. Growth studies
Complementation vectors carrying the acs+ allele or empty vector were electroporated into acs+ and Δacs strains (O’Toole et al., 1993) (Table S1). Starter cultures of each strain were grown overnight at 37 °C with shaking in NB containing the appropriate antibiotic. Fresh minimal medium (198 μl) containing sodium acetate (10 mM), appropriate antibiotics, and L(+)arabinose (250 μM) was dispensed into each well of a 96-well microtiter dish. Each well was inoculated with 2 μl (1% v/v) of the aforementioned overnight cultures. Arabinose was included in the medium to induce expression of acs+ from the ParaBAD promoter. Growth was monitored at 630 nm with shaking at 37 °C for 24 h (BioTek ELx808-1 Ultra microplate reader). Three technical and three biological replicates were analyzed; a representative growth curve is shown. Data were analyzed using Prism v6 software (GraphPad) and error bars represent standard deviation.
2.7. Purification of SeAcs from pTEV16-20
Plasmids encoding H6-SeAcs (pACS65 and pACS67), H6-MBP-tagged SeAcs (pACS66 and pACS68), and SeAcs-H6 (pACS69) were electroporated using a protocol described elsewhere (Seidman et al., 1997) into Escherichia coli strain C41 (λDE3) (Miroux and Walker, 1996) Δpat (strain JE9314). Cultures of cells containing plasmids were grown to stationary phase (OD650 ~ 1.3) and sub-cultured (1:100 v/v) into 1 L of LB + ampicillin. Cultures were grown shaking at 37 °C to an OD650 of 0.5, after which ectopic gene expression was induced with IPTG (0.5 mM). Cultures were grown overnight at 25 °C, cells were harvested by centrifugation at 6000 ×g for 15 min at 4 °C, and cell pellets were stored at −80 °C until used Acs proteins were purified from cell pellets as described (Hentchel and Escalante-Semerena, 2015). The amount of Acs in fractions was quantified on a NanoDrop™ 1000 Spectrophotometer (Thermo Scientific) using the molecular weight (72.15 kDa) and extinction coefficient (138,770 M−1 cm−1; ExPASy ProtParam) of the protein. Percent purity was calculated using ImageQuant™ TL.
3. Results
3.1. Cloning efficiencies of newly designed vectors
The S. enterica acs+ gene was cloned into each vector (Materials and methods Section 2.6) to test complementation (pCV1–3) and overexpression (pTEV16-20) using primers listed in Table S2. For each vector, 20 colonies were screened using colony PCR and cloning efficiencies were calculated (Table 1). A 2.2-kb band was indicative of a positive result, as shown in Fig. S2. The cloning efficiency varied between 95 and 100%. Information on troubleshooting cloning issues can be found in Table 3 of the Conclusions section.
Table 1.
Cloning efficiencies of newly designed vectors.
| Vector | GenBank accession # | No. of positive clones (20 screened)a | Efficiency (%)b |
|---|---|---|---|
| pCV1 | KU974153 | 19 | 95 |
| pCV2 | KU974154 | 20 | 100 |
| pCV3 | KU974155 | 19 | 95 |
| pTEV16 | KU974156 | 20 | 100 |
| pTEV17 | KU974157 | 20 | 100 |
| pTEV18 | KU974158 | 18 | 90 |
| pTEV19 | KU974159 | 19 | 95 |
| pTEV20 | KU974160 | 20 | 100 |
A positive result was indicated by a 2.2 kbband as shown in Fig. S2.
Colony PCR was used to screen 20 colonies for each vector.
Cloning efficiencies were calculated as described under Materials & methods Section 2.6.
Table 3.
Troubleshooting.
| Problem | Potential explanations or solutions |
|---|---|
| Cloning yielding all empty vector |
|
| Cloning yielding incorrect insert sizes |
|
| Cloning yielding zero colonies |
|
3.2. Description of complementation vectors (pCVs)
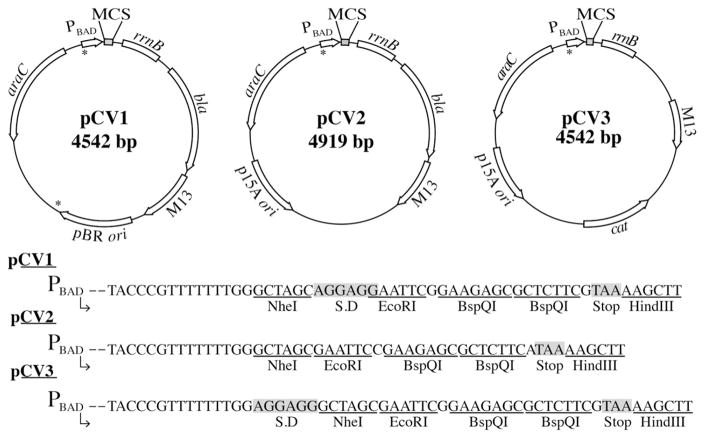
Plasmids pCV1, pCV2, and pCV3 contained the promoter region of the E. coli araBAD operon (ParaBAD) for the purpose of inducing expression of genes of interest by the addition of L(+)-arabinose (Cronan, 2006; Guzman et al., 1995a). All vectors contained an rrnB terminator, and either a bla+ gene for ampicillin resistance (pCV1, pCV2) or a cat+ gene for chloramphenicol resistance (pCV3) (Guzman et al., 1995b). Plasmid pCV1 contained a pBR322 origin of replication, while pCV2 and pCV3 contain a p15A origin of replication, making pCV1 compatible in vivo with either pCV2 or pCV3. All plasmids contained M13 intergenic regions for ssDNA packaging into phage capsids. The MCS and plasmid maps of each complementation vector are shown in Fig. 2. Noteworthy is the presence of the Shine Dalgarno (SD) sequence in plasmids pCV1 and pCV3. Plasmid pCV2 did not contain a SD sequence, allowing for the cloning of a gene and its native ribosome-binding site. A stop codon was added after the second BspQI site and when combined with a gene’s native stop codon, the error of read through was reduced. NheI, EcoRI and HindIII restriction sites surrounded the BspQI sites for verification of inserts by restriction analysis. It was necessary to add BspQI recognition and cut sites to both forward and reverse primers. This allowed for cutting of amplified gene products so they could anneal to the corresponding digested overhangs for each vector. In the case of pCV plasmids, the primer overhangs were identical for all three vectors. A simplified list of overhangs to be added to primers for each vector can be found in Table 2.
Fig. 2.
Plasmid maps and MCSs of complementation vectors. Plasmid maps show notable genetic features. Plasmid MCS sequences of each vector are shown between NheI and HindIII restriction sites. S.D. in MCSs represents Shine-Dalgarno (ribosome-binding site) sequence. Asterisks identify location of BspQI sites in original vectors that were mutated from GCTCTTC to WCTWTTC, as explained in Materials and methods Section 2.3.
Table 2.
Designing primers for vectors used in this study.
| Plasmid | Sequence to add to 5′ of primer |
|---|---|
| pTEV16 forward primer | 5′NNGCTCTTCNAGC |
| pTEV17 forward primer | 5′NNGCTCTTCNACC |
| pCV1, pCV2, pCV3, pTEV18, pTEV19 forward primer | 5′NNGCTCTTCNTTC |
| pCV1, pCV2, pCV3, pTEV16, pTEV17, pTEV18, pTEV19 reverse primer | 5′NNGCTCTTCNTAA |
| pTEV20 forward primer | 5′NNGCTCTTCNTAC |
| pTEV20 reverse primer | 5′NNGCTCTTCNTTC |
3.3. Use of complementation vectors for growth behavior analysis
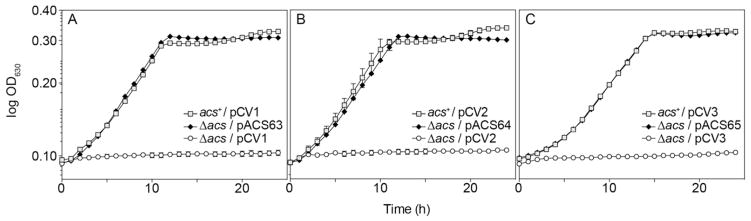
S. enterica Δacs strains grow poorly on 10 mM acetate as the sole source of carbon and energy, and such growth defect can be corrected by the ectopic expression of the acs+ allele (Chan et al., 2011). We used the above-mentioned phenotype of S. enterica acs strains to verify the functionality of the acs+ allele in the newly described complementation vectors as described in the Materials and Methods Section 2.7. Plasmids pCV1, pCV2, or pCV3 encoding S.e. acs+ (pACS62-pACS64) were induced with L(+)-arabinose (250 μM), a concentration of inducer that effectively compensated for the absence of the Acs protein in a S. enterica Δacs strain (Fig. 3). This result indicated that introduction of BspQI sites into the MCS did not disrupt the intended function of these plasmids.
Fig. 3.
Growth analysis and complementation of S. enterica acs phenotype. S.e. acs+ was cloned into pCV1-pCV3 and transformed into a acs strain to assess the effectiveness of the vectors. Cells were grown in NCE medium with acetate (10 mM) and transcription of acs+ in each vector was induced with L(+)-arabinose (250 μM). Growth curves were obtained using a microplate reader (BioTek Instruments).
3.4. Overexpression vectors containing rTEV protease cleaving sites
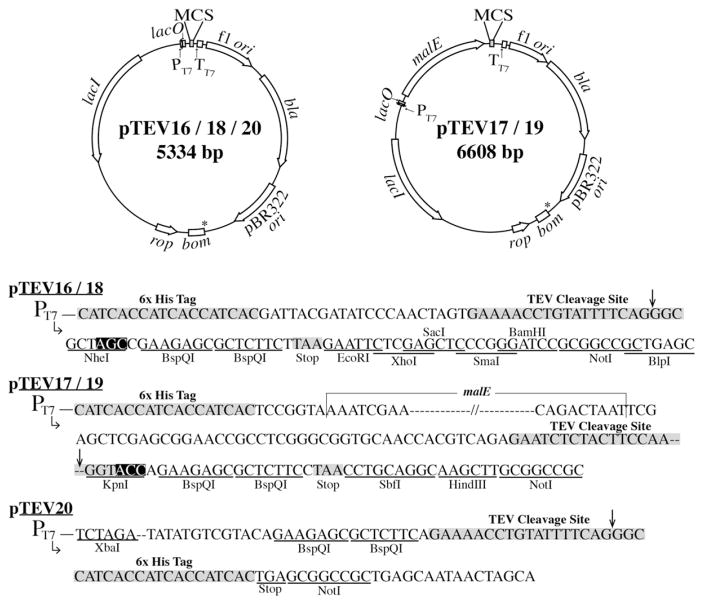
All of the TEV vectors described herein contained the E. coli lacI+ allele, whose protein product acts as a repressor to the T7 promoter of the plasmids. Addition of IPTG had the dual effect of relieving LacI repression and inducing transcription of genome-encoded T7 RNA polymerase. All vectors included a bla+ gene for the synthesis of β-lactamase, which provided resistance to ampicillin, an f1 origin for ssDNA packaging into phage capsids, and a pBR322 origin of replication (Bolivar, 1978; Soberon et al., 1980). The plasmid maps and MCSs of plasmids pTEV16-20 are shown in Fig. 4.
Fig. 4.
Plasmid maps and MCSs of overexpression vectors. Plasmid maps show notable genetic features. Plasmid MCS sequences of each vector are shown. Nucleotides inside black boxes of pTEV16 were changed to TTC resulting in pTEV18 and nucleotides in black boxes in pTEV17 were changed to TTC resulting in pTEV19. Therefore pTEV18 lacks an NheI site and pTEV19 lacks a KpnI site. An XbaI site lies upstream of the H6-tag. Plasmid pTEV20 was constructed by amplifying plasmid pTEV18 outside of the H6-tag and NotI restriction site, resulting in an rTEV-cleavable C-terminal H6-tag. Arrows mark the rTEV cleavage site. Asterisks identify location of BspQI sites in original vectors that were mutated from GCTCTTC to WCTWTTC, as explained in Materials and methods Section 2.3.
3.4.1. Plasmids pTEV16, pTEV18
Genes expressed from plasmids pTEV16 and pTEV18 resulted in proteins with an N-terminal, rTEV-cleavable H6-tag. Plasmid pTEV18 was constructed by mutagenizing the NheI site of plasmid pTEV16 to GCTTTC, rendering this site inactive, but allowed for the design of one set of primers that could be used for cloning into the complementation and overexpression vectors. Tag removal resulted in proteins with three additional residues (i.e., Gly-Ala-Ser for pTEV16; or Gly-Ala-Phe for pTEV18) on the N-terminus. A stop codon was engineered immediately after the BspQI sites to reduce read through when combined with the stop codon of the gene of interest. Plasmids pTEV16 and pTEV18 also contained multiple restriction sites in the MCS for alternative cloning methods or verification via restriction analysis. An XbaI site in plasmid pTEV18 is present upstream of the H6-tag, if needed for insert verification by restriction analysis.
3.4.2. Plasmids pTEV17, pTEV19
Genes expressed from plasmids pTEV17 and pTEV19 directed the synthesis of proteins with an N-terminal rTEV-cleavable H6-tag fused to a maltose binding protein (MBP). The MBP-tag was included with the goal of increasing protein solubility. Plasmid pTEV19 was constructed by mutagenizing the KpnI site of pTEV17 to GGTTTC, rendering this site inactive but allowing for the design of primers that could be used with complementation and overexpression vectors in this study. A stop codon was also engineered in these vectors to reduce read through. Tag removal resulted in proteins with two additional residues (i.e., Gly-Thr for pTEV17; or Gly-Phe for pTEV19). Additional restriction sites different from those found in plasmids pTEV16 and pTEV18 were present in plasmids pTEV17 and pTEV19 for alternative cloning options or verification via restriction analysis. An XbaI site in pTEV19 lies upstream of the H6-tag, if needed for insert verification by restriction analysis.
3.4.3. Vectors engineered for the synthesis of C-terminally tagged proteins
Plasmid pTEV20 was also engineered for the overproduction of proteins. However, pTEV20 differed from the aforementioned overexpression vectors by the location of the rTEV-cleavage site and the primer overhangs. Proteins overproduced in cells harboring plasmid pTEV20 have a C-terminal H6-tag that can be removed by rTEV protease. Plasmid pTEV20 was constructed by amplifying plasmid pTEV18 outside of the H6-tag and MCS; consequently pTEV20 maintained the backbone features of plasmids pTEV16 and pTEV18. Differences in the MCS of pTEV20 can be seen in Fig. 4. Designed primer overhangs also differed for this vector due to the fact that there is no stop codon engineered immediately downstream of the cloned gene. When designing primers for pTEV20, it was necessary to remove the stop codon of the native gene to ensure read through of the rTEV cleavage site and the H6-tag. After cleavage, proteins obtained from pTEV20 retained six additional amino acids (i.e., Glu-Asn-Leu-Tyr-Phe-Gln) at the C-terminus. A simplified list of overhangs to add to primers for each TEV-vector can be seen in Table 2.
3.5. Isolation of Salmonella enterica acetyl-CoA synthetase (Acs) from pTEV16-20
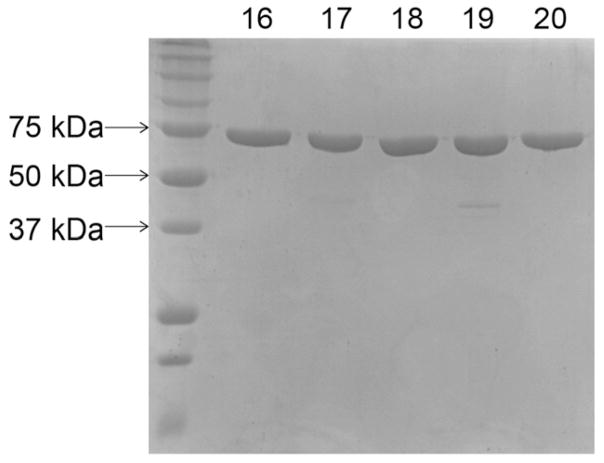
To assess the efficacy of the overexpression vectors, S.e. acs+ was cloned into pTEV16-20, overexpressed in E. coli strain C41 (ΔDE3), and proteins were purified as described in the Materials and Methods Section 2.8. Purity of protein purified from cells overexpressing acs+ from pTEV16, pTEV17, pTEV18, pTEV19, and pTEV20 was assessed and final protein products are shown in Fig. 5. Yields of SeAcs protein purified from overexpression from each vector were 8.35 mg L−1 (pTEV16), 0.9 mg L−1 (pTEV17), 8.55 mg L−1 (pTEV18), 0.9 mg L−1 (pTEV19), 5.32 mg L−1 (pTEV20).
Fig. 5. SDS-PAGE gel of purified Acs.
A 12.5% SDS-PAGE gel shows molecular weight standards (BioRad Precision Plus Protein™, left) and Acs (72 kDa) purified from cells harboring pACS65 (pTEV16), pACS66 (pTEV17), pACS67 (pTEV18), pACS68 (pTEV19), and pACS69 (pTEV20). Numbers above each well correspond to protein purified from cells harboring respective pTEV vectors (e.g., well labeled 16 corresponds to pTEV16).
4. Conclusions
Here we describe modified cloning vectors containing BspQI restriction sites that allow for rapid and efficient cloning of genes of interest. Common issues that may arise during cloning may be resolved by solutions provided in Table 3. Using these vectors paired with a previously described cloning method (Galloway et al., 2013), the number of cells carrying empty vectors is greatly reduced. We also have designed the complementation vectors (pCV1–3) and N-terminal rTEV cleavable H6-tag vectors (pTEV18–19) to carry the same primer overhangs so that only one primer set is needed for in vivo and in vitro studies. Because MBP is a 42-kDA protein, yields of the cleaved protein once fused to MBP were lower. For this reason, it may be necessary to increase expression volumes to obtain similar yields compared to the pTEV16/18 vectors. It is important to note that when purifying proteins encoded by plasmids pTEV17 or pTEV19, some MBP contamination may occur, which if necessary may be removed using an MBPTrap HP column (GE Healthcare Life Sciences). Additionally, an N-terminal tag may be problematic for some proteins due to folding issues or interference with the protein’s activity. For this reason we have provided a C-terminal rTEV-cleavable H6-tag vector (pTEV20). Due to the nature of the rTEV cleavage site, recombinant proteins purified and cleaved from the cells harboring this vector will contain additional amino acids compared to the N-terminally tagged vectors. Overall, we have established a set of vectors that should facilitate complementation studies or protein production.
Supplementary Material
Acknowledgments
This work was supported by USPHS grant R01 GM062203 to J.C.E.-S. The authors thank Nicholas Galloway in the laboratory of Dr. Cory Momany for the technical assistance.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.plasmid.2016.05.001.
References
- Atlas R. Handbook of Media for Environmental Microbiology. CRC Press; Boca Raton: 1995. [Google Scholar]
- Berkowitz D, et al. Procedure for identifying nonsense mutations. J Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978;4:121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Chan CH, et al. In Salmonella enterica, the sirtuin-dependent protein acylation/ deacylation system (SDPADS) maintains energy homeostasis during growth on low concentrations of acetate. Mol Microbiol. 2011;80:168–183. doi: 10.1111/j.1365-2958.2011.07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE. A family of arabinose-inducible Escherichia coli expression vectors having pBR322 copy control. Plasmid. 2006;55:152–157. doi: 10.1016/j.plasmid.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Engler C, Marillonnet S. Golden gate cloning. Methods Mol Biol. 2014;1116:119–131. doi: 10.1007/978-1-62703-764-8_9. [DOI] [PubMed] [Google Scholar]
- Engler C, et al. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, et al. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE. 2009;4:e5553. doi: 10.1371/journal.pone.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway NR, et al. Rapid cloning for protein crystallography using type IIS restriction enzymes. Crystal Growth & Design. 2013;13:2833–2839. [Google Scholar]
- Guzman LM, et al. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995a;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, et al. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995b;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentchel KL, Escalante-Semerena JC. In Salmonella enterica, the Gcn5-related acetyltransferase MddA (formerly YncA) acetylates methionine sulfoximine and methionine sulfone, blocking their toxic effects. J Bacteriol. 2015;197:314–325. doi: 10.1128/JB.02311-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, et al. Introduction of plasmid and bacteriophage lambda into Escherichia coli. In: Maniatis T, et al., editors. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 1982. [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Oster CJ, Phillips GJ. Vectors for ligation-independent construction of lacZ gene fusions and cloning of PCR products using a nicking endonuclease. Plasmid. 2011;66:180–185. doi: 10.1016/j.plasmid.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, et al. Analysis of mutants of defective in the synthesis of the nucleotide loop of cobalamin. J Bacteriol. 1993;175:3317–3326. doi: 10.1128/jb.175.11.3317-3326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingoud A, et al. Type II restriction endonucleases: structure and mechanism. Cell Mol Life Sci. 2005;62:685–707. doi: 10.1007/s00018-004-4513-1. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco CJ, et al. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid. 2008;59:231–237. doi: 10.1016/j.plasmid.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman CG, et al. Introduction of plasmid DNA into cells. In: Ausubel FM, et al., editors. Current Protocols in Molecular Biology. Vol. 1. Wiley Interscience; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Soberon X, et al. Construction and characterization of new cloning vehicles. IV Deletion derivatives of pBR322 and pBR325. Gene. 1980;9:287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Szybalski W, et al. Class-IIS restriction enzymes—a review. Gene. 1991;100:13–26. doi: 10.1016/0378-1119(91)90345-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.