Abstract
Leptin has been implicated as a key upstream mediator of pathways associated with coronary vascular dysfunction and disease. The purpose of this investigation was to test the hypothesis that leptin modifies the coronary artery proteome and promotes increases in coronary smooth muscle contraction and proliferation via influences on Rho kinase signaling. Global proteomic assessment of coronary arteries from lean swine cultured with obese concentrations of leptin (30 ng/mL) for 3 days revealed significant alterations in the coronary artery proteome (68 proteins) and identified an association between leptin treatment and calcium signaling/contraction (4 proteins) and cellular growth and proliferation (35 proteins). Isometric tension studies demonstrated that both acute (30 min) and chronic (3 day, serum-free media) exposure to obese concentrations of leptin potentiated depolarization-induced contraction of coronary arteries. Inhibition of Rho kinase significantly reduced leptin-mediated increases in coronary artery contractions. The effects of leptin on the functional expression of Rho kinase were time-dependent, as acute treatment increased Rho kinase activity while chronic (3 day) exposure was associated with increases in Rho kinase protein abundance. Proliferation assays following chronic leptin administration (8 day, serum-containing media) demonstrated that leptin augmented coronary vascular smooth muscle proliferation and increased Rho kinase activity. Inhibition of Rho kinase significantly reduced these effects of leptin. Taken together, these findings demonstrate that leptin promotes increases in coronary vasoconstriction and smooth muscle proliferation and indicate that these phenotypic effects are associated with alterations in the coronary artery proteome and dynamic effects on Rho kinase pathway.
Keywords: Leptin, Rho kinase, coronary
INTRODUCTION
Numerous studies have suggested that factors derived from adipose tissue have the potential to influence several key mechanisms of obesity-induced coronary disease including the promotion of vascular dysfunction and smooth muscle proliferation [22;29;36;44]. For example, recent studies support that alterations in the adipokine secretion profile of coronary perivascular adipose tissue (PVAT), which surrounds the large conduit arteries of the heart, contribute to impaired endothelial-dependent dilation and augmented smooth muscle contraction in the setting of obesity [11;26;30;34-36]. Specifically, coronary PVAT has been shown to potentiate coronary artery contractions and attenuate vasodilation via effects on Rho kinase signaling and smooth muscle CaV1.2 and K+ channels [4;27;34;36]. While these effects are in contrast with the anti-contractile effects of peripheral PVAT [8;16;51;53], they are consistent with reported increases in pro-inflammatory and pro-atherogenic factors in coronary PVAT of subjects with documented atherosclerotic disease [9;10;17;22]. However, the specific adipokines and pathways responsible for these deleterious influences remain ill defined.
A growing body of evidence implicates a role for the adipose tissue hormone, leptin, as a key upstream mediator of pathways associated with coronary vascular dysfunction and the initiation and progression of coronary disease in obesity [2;3]. Plasma concentrations and expression of leptin in coronary PVAT are markedly elevated in obese subjects [11;13;15;36;47] and leptin receptors (ObR) are highly expressed throughout the wall of diseased coronary arteries [6;14;20;36]. Increases in leptin levels (30-90 ng/mL) have been associated with the activation of a number of pro-inflammatory pathways (e.g. monocyte chemoattractant protein-1; tumor necrosis factor-α [7;18]), attenuation of endothelial-dependent dilation, and further impairment of obesity-induced endothelial dysfunction [20;21;36]. However, the effects of leptin on coronary vascular smooth muscle remain equivocal, as leptin either attenuates or has no effect on contractile responses to a variety of agonists in isolated rat aorta [14;38;39]. Importantly, the majority of these studies were conducted following acute, short-term exposure to leptin (30-60 min). Therefore, understanding of the vascular effects of longer-term leptin exposure is rather limited.
Several studies suggest an interrelationship between leptin signaling and the RhoA/Rho kinase pathway, a known regulator of vascular smooth muscle contraction [54-56]. Recent findings demonstrate that perivascular overexpression of leptin promotes neointima formation after carotid artery injury [44] and that exogenous administration of leptin stimulates proliferation of isolated vascular smooth muscle cells from rodents [28;42;45]. Interestingly, activation of the RhoA/Rho kinase pathway has been implicated in leptin-mediated increases in vascular smooth muscle cell proliferation and hypertrophy [56]. However, contrasting studies have found that leptin produces dose-dependent decreases in proliferation [6] and have failed to support a role for RhoA/Rho kinase in mediating vascular smooth muscle proliferation [24;48]. Thus, further studies are required to identify the effects of short-term and long-term leptin administration on coronary vascular smooth muscle contraction and proliferation and to elucidate the precise mechanisms involved.
Accordingly, the purpose of this investigation was to test the hypothesis that leptin promotes (1) marked alterations in the coronary proteomic expression profile that favor pathways associated with vascular smooth muscle contraction and proliferation; and (2) increases in coronary smooth muscle contraction and proliferation via a Rho kinase dependent pathway. Findings from this investigation provide novel evidence that leptin contributes to mechanistic alterations in coronary vascular function and support the growing paradigm that leptin acts as an upstream mediator in the development of obesity-induced coronary disease.
MATERIALS AND METHODS
All experimental procedures and protocols in this investigation were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals. Upon sacrifice, hearts from domestic swine (body weight ~50 kg) were excised and perfused via aortic cannulation with 4°C, Ca2+-free Krebs buffer (131.5 mM NaCl, 5mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 25mM NaHCO3, 10 mM glucose). Coronary arteries were grossly dissected from the heart, placed under a dissecting scope, and cleaned of surrounding myocardium and adventitia. Arteries were then cut into 3 mm rings and subjected to the protocols outlined below.
Proteomics
Coronary arteries were cut into 3 mm rings and were placed in 12-well tissue culture dishes with serum-free, low glucose (100 mg/dL) Dulbecco's Modified Eagle Medium (DMEM: Corning Cellgro, Manassas, VA, 10014CM) containing penicillin (100 U/mL) and streptomycin (100 μg/mL) (MP Biomedicals, 1670249). Arteries were maintained in a 5% CO2 atmosphere at 37°C for three days of incubation without (control) or with leptin (30 ng/mL: Sigma Aldrich, St. Louis, MO, L4146) for 3 days. Following the culture period, arteries were frozen in liquid N2 and delivered on dry ice to the Ohio State University Proteomics Core for protein extraction. Tissues were homogenized in 1:10 w/v in ice cold Buffer A (1% digitonin, 0.05% NP-40, NaCl 150 mM, Tris 50 mM, pH 7.4) with Complete Protease Inhibitors and PhosSTOP (Roche Diagnostics) using a polytron homogenizer (Power Gen 700, Fisher Scientific). Proteins were extracted on ice for 1 hr, centrifuged at 80,000g for 30 min, and protein concentration of supernatant was determined with the Dc Protein Assay (Bio-Rad). Proteins were eluted in Laemmli Reducing Sample Buffer for 1D gel electrophoresis.
In-Gel Digestion
Each band was cut into 8 fractions based on relative protein abundance and placed in 96 well plates for in-gel digestion. Briefly, gel pieces were washed in 100 μl of 50% methanol/5% acetic acid for 30 min. The wash step was repeated a total of 3 times and slices were left in a storage solution of 50 μl of 50% methanol/5% acetic acid until digestion. Digestion was carried out by adding 100 μl 50 mM ammonium bicarbonate (ABC) for 10 min followed by 100 μl acetonitrile for 10 min. The gel bands were rehydrated with dithiothreitol (DTT) (prepared as 5 mg/ml in 50 mM ABC) and incubated for 30 min followed by a 30 min incubation with iodoacetamide (prepared as 15 mg/ml iodoacetamide in 50 mM ABC) in the dark. The gel bands were washed again with 2 cycles of acetonitrile and 50 mM ABC in 10 and 5 min increments, respectively and thendried for 10 min. The protease was driven into the gel pieces by rehydrating them in 50 μL of sequencing grade modified trypsin from Promega (Madison, WI, prepared at 5 μg/ml with 0.01% ProteaseMAX Surfactant in 50 mM ABC) and incubated at room temperature overnight. The peptides were extracted from the polyacrylamide with 50 μl 50% acetonitrile and 5% formic acid for 10 min a total of 3 times and a final extraction with 50 μl of acetonitrile for 10 min and then pooled together. The extracted pools were dried completely and resuspended in 20 μl of 50 mM acetic acid.
Mass Spectrometry
The final digests were analyzed using capillary-liquid chromatography-nanospray tandem mass spectrometry (Capillary-LC/MS/MS) of global protein. Identification was performed on a Thermo Finnigan LTQ orbitrap mass spectrometer equipped with a microspray source (Michrom Bioresources Inc, Auburn, CA) operated in positive ion mode. Samples (6.4 μl from each fraction) were separated on a capillary column (0.2X150mm Magic C18AQ 3μ 200A, Michrom Bioresources Inc, Auburn, CA) using an UltiMate™ 3000 HPLC system (LC-Packings A Dionex Co, Sunnyvale, CA). Each sample was injected into the μ-Precolumn Cartridge (Dionex, Sunnyvale, CA) and desalted with 50 mM acetic acid for 5 min. The injector port was then switched to injection mode and the peptides were eluted off of the trap onto the column. Mobile phase A was 50mM acetic acid in water and acetonitrile was used as mobile phase B. Flow rate was set at 2μl/min. Mobile phase B was increased from 2% to 5% in 5 min and again from 5% to 30% in 30 min, then from 30% to 50% in 8 min. The gradient was increased again from 50% to 85% in 3 min and then kept at 85% for another 1 min before being brought back to 2% in 0.1 min. The column was equilibrated at 2% of mobile phase B (or 98% A) for 10 min before the next sample injection. MS/MS data was acquired with a spray voltage of 2.2 kV and a capillary temperature of 175 °C. The scan sequence of the mass spectrometer was based on the data dependent TopTen™ method in preview mode; the analysis was programmed for a full scan recorded between 350-2,000 Da and a MS/MS scan to generate product ion spectra to determine amino acid sequence in consecutive scans of the ten most abundant peaks in the spectrum. The full scan resolution was set at 30,000 to achieve high mass accuracy MS determination. The CID fragmentation energy was set to 35%. Dynamic exclusion is enabled with a repeat count of 1 within 18 s, a mass list size limit of 500, exclusion duration of 10 s and a low mass width and high mass width were set at 30ppm.
Protein Identification and Quantitation
Sequence information from the MS/MS data was processed by converting the .raw files into a mgf files using MsConvert (ProteoWizard) and later merged into a merged file (.mgf) using an in-house program, RAW2MZXML_n_MGF_batch (merge.pl, a Perl script) and searched using Mascot Daemon by Matrix Science version 2.3.2 (Boston, MA) against the NCBInr Other Mammalia Database (version 20150104, 1,412,788 sequences). Trypsin was used as the enzyme and four missed cleavages were permitted. Considered variable modifications were oxidation (Met), carbamidomethylation (Cys) and deamination (Asn, Gln). The mass accuracy of the precursor ions were set to 20ppm and the fragment mass accuracy was set to 0.8 Da. One 13C peak was included in the search in case of the accidental pick of 13C peaks. A decoy database was also searched to determine the false discovery rate (FDR) and peptides were filtered according to the FDR. The significance threshold was set at P < 0.05. Percolator score was used to further validate the search results and the actual FDR was less than 1% after using percolator scores.
Label-free quantitation was performed using the spectral count approach, in which the relative protein quantitation is measured by comparing the number of MS/MS spectra identified from the same protein in each of the multiple LC/MSMS datasets. Scaffold was used for quantitation analysis. The protein filter was set at 99% to ensure the false discovery rate is less than 1% and the peptide filter was set at 95%.
Functional Assessment of Isolated Coronary Arteries
Isometric tension studies on coronary artery rings were performed as previously described [27;30]. Briefly, 3 mm coronary artery rings (without PVAT) were mounted in organ baths filled with Ca2+-containing Krebs buffer (131.5 mM NaCl, 5mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 25mM NaHCO3, 10 mM glucose, 4mM CaCl2) and maintained at 37°C. Once stabilized at optimal passive tension (~4 g), arteries were incubated without (control) or with leptin (30 ng/mL: Sigma Aldrich, L4146) and/or fasudil (1 μM: Sigma Aldrich, H139) for 30 minutes (acute exposure) and then exposed to increasing concentrations of KCl (10 – 60 mM) and to the thromboxane A2 receptor agonist, U46619 (1 μM: Tocris, 1932). Coronary arteries cultured in serum-free media for 3 days with or without leptin (30 ng/mL: Sigma Aldrich, L4146) and/or fasudil (1 μM: Sigma Aldrich, H139) (chronic exposure) were also mounted in organ baths and exposed to increasing concentrations of KCl (10 – 60 mM) and to U46619 (1 μM: Tocris, 1932). Acute and chronic studies were also conducted in endothelium denuded coronary arteries in which the endothelium was removed by gently rubbing fine-tip forceps along the lumen. Active tension development (peak tension minus baseline tension) was recorded at each concentration for each group. Endothelial denudation was confirmed by <15% relaxation to bradykinin (1 μM: Sigma Aldrich, B3259).
Western Analysis
Following acute (30 minute) or chronic (3 day culture) incubation with or without leptin, coronary arteries were frozen in liquid N2 and stored at −80°C. Arteries were homogenized in 70 μL of Tissue Protein Extraction Reagent (Thermo Scientific, 78510) and total protein was quantified as previously described [41]. Equivalent amounts of protein (50 μg) were loaded onto 10% polyacrylamide gels (Life Technologies, NP0302) for electrophoresis and blotting. Membranes were incubated with primary antibody directed against Rho kinase (Rock-2, 1:200, Santa Cruz Biotechnology, sc-1851) overnight at 4°C and donkey anti-goat IRDye 800CW secondary antibody (1:15,000, Li-Cor, 926-32214) for 1 hour at room temperature. To verify equal protein loading, membranes were washed and incubated with antibody to β-actin (1:200, Santa Cruz Biotechnology, sc-1616). Immunoreactivity was visualized using a Li-Cor Odyssey CLx imaging system. Chameleon Duo (Li-Cor) was used as a protein ladder. Densitometry analyses were conducted using Li-Cor Image Studio Lite, version 5.2. Protein levels were normalized to levels of β-actin and reported as “% control,” i.e. protein levels from each sample were normalized to the average level of the protein in control arteries within the same condition.
Rho Kinase Activity Assay
Protein homogenates of the samples outlined above were subjected to a commercially available enzyme immunoassay for the detection of active Rho kinase (Rho-associated Kinase (ROCK) Activity Assay, Millipore, CSA001). Briefly, equal amounts of protein (50 μg) from each sample were added to plates pre-coated with recombinant MYPT1. A detection antibody specific for phosphorylated MYPT1 and a HRP-conjugated secondary antibody were added, respectively. The amount of phosphorylated substrate was measured by adding the chromogenic substrate tetramethylbenzidine and reading the absorbance signal at 450 nm. Absorbance values were then normalized to a standard curve of active recombinant ROCK-II enzyme.
Proliferation Assays
Additional culture studies were conducted in which arteries were incubated in serum-containing (30% fetal bovine serum, Glibco, 10437-028), low glucose (100 mg/dL) Dulbecco's Modified Eagle Medium (DMEM: Corning Cellgro, 10014CM) containing penicillin (100 U/mL) and streptomycin (100 μg/mL) (MP Biomedicals, 1670249) at 37°C in a 5% CO2 atmosphere. Arteries were incubated with or without leptin (30 ng/mL: Sigma Aldrich, L4146) and/or fasudil (1 μM: Sigma Aldrich, H139) for 8 days, with media changes conducted every 2 days. To confirm functional responses at this time point, both intact and denuded arteries were subjected to the isometric tension studies outlined above. In a subset of untreated and leptin treated arteries, 5-Bromo-2’-deoxyuridine (BrdU; 20 μmol/L) was added to the culture medium for the final 6 hours of culture. Arteries were then fixed in 10% formalin, paraffin embedded, and processed for BrdU labeling in nuclei utilizing an immunohistochemical detection assay (BrdU Labeling and Detection Kit II, Roche, 11299964001). Positive staining for BrdU-labeled nuclei indicates DNA synthesis, a marker of cellular proliferation. To specifically investigate vascular smooth muscle proliferation, arteries from all treatment groups were formalin fixed, paraffin embedded, and processed for co-immunostaining with anti-α smooth muscle actin (1:50, Abcam, ab5694) and anti-proliferating cell nuclear antigen (PCNA, 1:2,000, Abcam, ab29). Mach 2 Double Stain 2 (Biocare Medical, MRCT525) was used as secondary with chromogens Vulcan Fast Red and 3,3′-Diaminobenzidine, respectively. Immunohistochemistry was performed in conjunction with the Indiana University Health Pathology Laboratory (Indianapolis, IN). Slides were imaged at 20X magnification. Four distinct fields of view were captured per artery and data were averaged for n=1. Quantitation of positive staining was performed using the open source modification of Image J (Fiji) [43] and a custom modification of the trichrome quantification macro (The University of Chicago Integrated Light Microscopy Core Facility). Positive PCNA staining was quantitated in artery regions also staining positively for α-smooth muscle actin. Data are reported as “% control,” i.e. percent positive staining from each sample was normalized to the average level of positive staining in untreated, control arteries.
Statistical Analyses
Statistical comparisons of proteomic results were performed on proteins which met Scaffold false discovery rate (FDR) criterion by Student's t-test. Proteins with P < 0.05 were considered significantly different between treatment groups. Protein lists and corresponding expression values were uploaded onto the Ingenuity Pathway Analysis software server (content version: 24718999) and analyzed to interpret cellular functions and canonical pathways associated with alterations in the proteomic profile between treatment groups. For isometric tension studies, a two-way ANOVA was used to test the effects of leptin and/or inhibitors (Factor A) relative to concentrations of specific agonists (Factor B). When statistical differences were found with ANOVA (P < 0.05), a Student-Newman-Keuls multiple comparison test was performed. A Student's t-test was used to compare the results of Western blot, Rho Kinase activity, and proliferation assays. Data are presented as mean ± SE with “n” equal to number of pigs studied. SigmaPlot version 11.0 (Systat Software Inc, San Jose, CA) was used for all statistical analyses and generation of figures.
RESULTS
Effect of leptin exposure on the coronary proteomic expression profile
To investigate potential factors and pathways affected by leptin exposure, global proteomic profiling was performed on coronary arteries cultured in the presence or absence of leptin (30 ng/mL) for 3 days. This non-biased target discovery approach detected significant alterations in 69 proteins (P ≤ 0.05) in leptin treated arteries. A complete list of the 793 detected proteins is provided in Online Resource 1. The top 15 unique upregulated and downregulated proteins are listed in Table 1. Ingenuity Pathway Analysis (IPA) software identified significant associations (P<0.001) between leptin treatment and numerous cellular functions, namely calcium signaling (4 proteins) and cellular growth and proliferation (35 proteins). These findings are of interest, as previous studies have suggested a potential link between leptin and vascular reactivity [14;20;36;38] and proliferation [28;44]. Additional cellular pathways/processes identified include cellular movement, migration, and invasion (25 proteins), cell spreading (6 proteins) and quantity of smooth muscle cells (4 proteins).
Table 1.
Protein Expression Profile of Leptin-treated Coronary Arteries
| Gene Name | Protein Name | Fold Change | P Value | IPA |
|---|---|---|---|---|
| Upregulated Proteins | ||||
| MYOZ2 | Calsarcin-1 (myozenin-2) | 5.8 | 0.02 | 4 |
| RAB21 | Ras-related protein, Rab-21 | 4.8 | 0.0002 | |
| SORBS1 | Sorbin and SH3 domain-containing protein 1, isoform 1 | 4.4 | 0.007 | 5 |
| LASP1 | LIM and SH3 domain protein 1 | 3.2 | 0.05 | 2,3 |
| EIF6 | Eukaryotic translation inititation factor 6, isoform X1 | 1.6 | 0.05 | 2 |
| ADIRF* | Adipogenesis regulatory factor | 1.5 | 0.02 | |
| Cald1 | Non-muscle caldesmon, isoform X1 | 1.5 | 0.005 | |
| MTPN | Myotrophin | 1.5 | 0.03 | 2 |
| S100A11 | Protein S100-A11 | 1.4 | 0.04 | 2,3 |
| RPL12 | 60S ribosomal protein L12 | 1.3 | 0.01 | |
| TPM4 | Tropomyosin alpha-4 chain, isoform X3 | 1.3 | 0.02 | 1 |
| TPM4 | Tropomyosin alpha-4 chain, isoform X2 | 1.3 | 0.03 | 1 |
| TPM4 | Tropomyosin alpha-4 chain, isoform X1 | 1.3 | 0.02 | 1 |
| TUBA1C | Tubulin alpha chain-like | 1.2 | 0.02 | 2,3 |
| TPM2 | Tropomyosin beta chain, isoform X1 | 1.2 | 0.03 | 1 |
| Downregulated Proteins | ||||
| MYOF | Myoferlin | 7.6 | 0.03 | 2,4 |
| ALDH4A1 | Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial | 6.1 | 0.009 | |
| ATPIF1 | ATPase inhibitor, mitochondrial precursor | 4.9 | 0.00003 | 2 |
| SRI | Sorcin | 4.8 | 0.00003 | |
| SSR4 | Translocon-associated protein subunit delta, isoform X1 | 2.6 | 0.04 | |
| PRKAR2A | cAMP-dependent protein kinase type II-alpha regulatory subunit | 2.6 | 0.04 | 1,2,3 |
| CSPG4 | Chondroitin sulfate proteoglycan 4 | 2.4 | 0.03 | 2,3,5 |
| HSP90AA1 | Heat shock protein HSP 90-alpha | 2.3 | 0.02 | 2,3 |
| SLC25A3 | Phosphate carrier protein, mitochondrial | 2.3 | 0.02 | |
| FBN1 | Fibrillin-1 | 2.2 | 0.02 | 2,3,5 |
| PDIA4 | Protein disulfide-isomerase A4 precursor | 2.1 | 0.04 | |
| RNH1 | Ribonuclease inhibitor | 2.1 | 0.03 | 2,3 |
| RPN2 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 2 precursor | 2.0 | 0.02 | |
| GLUD1 | Glutamate dehydrogenase 1, mitochondrial precursor | 1.8 | 0.0004 | |
| IGTA1* | Integrin alpha 1 | 1.8 | 0.04 | |
Values for fold change in expression of coronary arteries cultured for three days with leptin (30 ng/mL) versus untreated controls (n=4 each group). Ingenuity Pathway Analysis (IPA)
Calcium signaling
Cell proliferation
Cell movement, migration, invasion
Quantity of smooth muscle cells
Cell spreading.
Bos taurus homolog.
Acute and chronic leptin administration augments coronary artery contractions
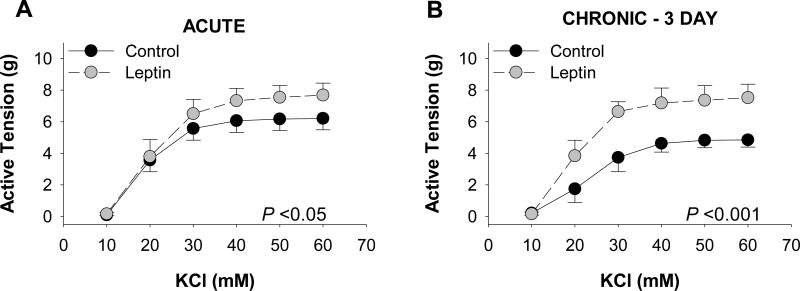
The effects of leptin on coronary vascular reactivity were examined by comparing KCl-induced contractions of coronary arteries following short-term, acute (30 min) and long-term, chronic (3 day, serum-free culture) exposure to “obese” concentrations of leptin (30 ng/mL), i.e., plasma concentrations typically reported in obese subjects [33;46]. Overall contractile responses of acute leptin treated arteries were significantly augmented (P = 0.04), with maximal tension development increased by 1.5 ± 0.2 g at 60 mM KCl (Figure 1A). Chronic exposure to leptin also augmented overall active tension development to KCl (P<0.001), with an increase of 2.7 ± 0.49 g at 60 mM (Figure 1B). Control responses to KCl were reduced following 3 days of culture (P = 0.006, Figure 1B vs 1A). This reduction in contraction was also observed in response to the thromboxane A2 receptor agonist, U46619 (1 μM, P = 0.04), although leptin had no effect on U46619 contractions following acute (P = 0.58) or chronic (P = 0.34) exposure (Online Resource 2, Figure I). KCl contractions in control and leptin treated arteries were unaffected by removal of the endothelium, and similar effects of leptin were observed in endothelium denuded arteries (confirmed by <30% relaxation to 1μM bradykinin) following both acute (Online Resource 2, Figure IIA) and chronic (Online Resource 2, Figure IID) exposure.
Fig. 1.
Leptin augments depolarization-induced coronary artery contractions. Acute (30 min) exposure to leptin (30 ng/mL) increased KCl-induced contractions ~1.3 g at doses >40 mM (n=9 [A]). Chronic (3 day culture in serum-free media) leptin administration (30 ng/mL) increased tension development ~2.5 g at doses >40 mM (n=4 [B])
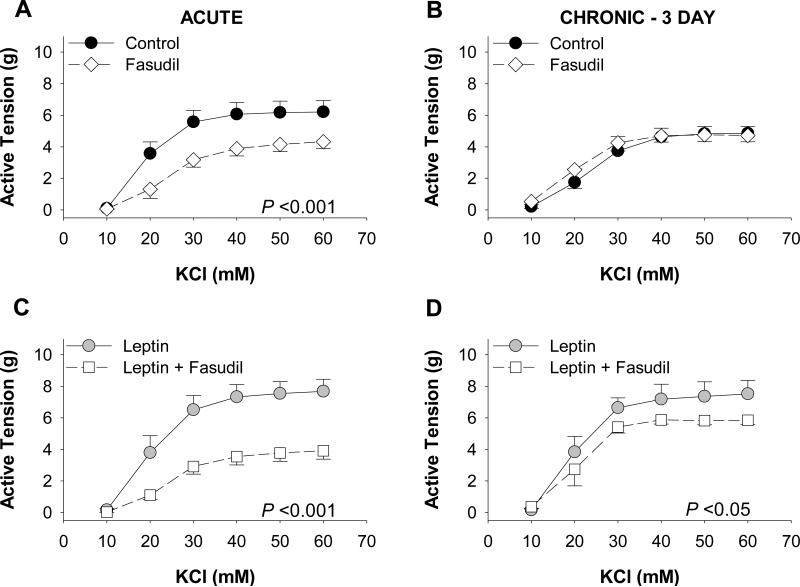
Role of Rho kinase in leptin-mediated coronary contraction
To investigate the role of Rho kinase signaling in mediating the functional effects of leptin, coronary arteries were co-incubated with/without leptin (30 ng/mL) and/or the Rho kinase inhibitor, fasudil (1 μM). Acute fasudil treatment significantly reduced vasoconstriction to KCl (10 – 60 mM) compared to untreated (control) arteries (P < 0.001, Figure 2A). In contrast, fasudil treatment had no effect on contractile responses to KCl (10 – 60 mM) in untreated (control) arteries cultured for 3 days (P = 0.50, Figure 2B). Fasudil administration significantly decreased the effect of acute (P < 0.001, Figure 2C) and chronic (P = 0.01, Figure 2D) leptin exposure on KCl-induced contractions. However, this effect of fasudil was markedly greater following acute (~3.8 g at concentrations >40 mM) versus chronic exposure (~1.4 g at concentrations >40 mM) to leptin (P < 0.001). KCl contractions in arteries treated with leptin and/or fasudil were unaffected by removal of the endothelium, and similar effects of fasudil were observed in endothelium denuded arteries following both acute (Online Resource 2, Figure IIB and IIC) and chronic (Online Resource 2, Figure IIE and IIF) exposure.
Fig. 2.
Role of Rho kinase in leptin-mediated coronary contraction. In the absence of leptin, acute (n=9 [A]), but not chronic (n=4 [B]) treatment with the Rho kinase inhibitor, fasudil (1 μM), diminished vasoconstriction to KCl. Inhibition of Rho kinase reduced the effect of both acute (n=9 [C]) and chronic (n=4 [D]) leptin administration on KCl-induced contractions. However, the fasudil-mediated reduction in tension was greater following acute (C) versus chronic (D) leptin exposure
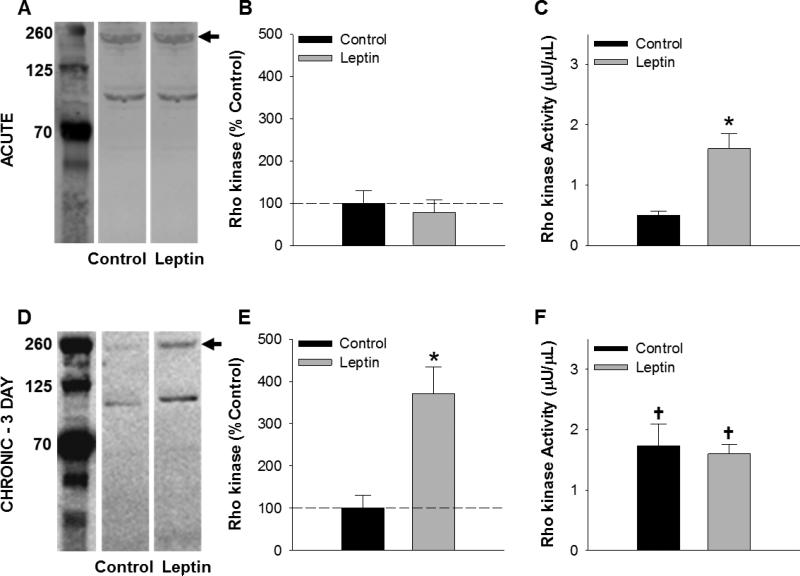
Effects of leptin on Rho kinase expression and activity
Further analyses were conducted to examine the effects of leptin on Rho kinase abundance and activity. Western blot analyses revealed no difference in normalized protein abundance of Rho kinase in acute leptin treated arteries (P = 0.61, Figure 3A and 3B). A commercially available Rho-associated kinase (ROCK) activity assay demonstrated that acute leptin exposure significantly increased the level of Rho kinase activity from 0.50 ± 0.07 μU/μL in untreated arteries to 1.61 ± 0.25 μU/μL in leptin treated arteries (P = 0.01, Figure 3C). Following chronic exposure to leptin (3 day, serum-free culture), the abundance of Rock-2 protein was increased ~3.5-fold relative to untreated cultured arteries (P = 0.009, Figure 3D and 3E). While Rho kinase activity level was increased relative to acute untreated arteries following 3 days of culture, chronic exposure to leptin did not affect overall Rho kinase activity, relative to untreated time-control (1.73 ± 0.36 μU/μL in untreated versus 1.60 ± 0.16 μU/μL in leptin treated arteries; P = 0.92, Figure 3F).
Fig. 3.
Effects of acute versus chronic leptin treatment on Rho kinase. Representative blots of Rho kinase protein abundance in coronary arteries following acute (A) and chronic (3 day culture in serum free media) (D) leptin exposure. Average data are expressed as % relative to control. Acute leptin treatment increased Rho kinase activity (C) in the absence of a change in protein abundance (B). Following chronic exposure, protein abundance was significantly elevated in leptin treated arteries (E), while no difference in overall Rho kinase activity was detected relative to untreated control arteries (F). All groups n = 3. *P<0.05, leptin versus control. †P<0.05, versus acute control
Leptin and vascular smooth muscle cell proliferation
Additional studies were conducted to directly investigate the effects of leptin on coronary vascular proliferation. In initial experiments, coronary arteries were cultured in serum-containing media for 8 days in the presence or absence of leptin, with 5-Bromo-2’-deoxyuridine (BrdU) added to the media for the final six hours of culture. Systematic quantitation of the BrdU staining pattern using ImageJ revealed a ~32% increase in BrdU-positive nuclei in leptin treated relative to untreated, control arteries (P = 0.02, Online Resource 2, Figure III). Based on these findings, further studies were conducted in arteries cultured with or without leptin (30 ng/mL) and/or fasudil (1 μM) for 8 days. The functional effects of leptin and/or fasudil were conserved in both endothelium intact (Online Resource 2, Figure IVA-C) and endothelium denuded arteries (Online Resource 2, Figure IVA-C) at this time point. Overall KCl-induced vasoconstriction in untreated, control arteries was reduced following 8 days of culture (P < 0.001, Online Resource 2, Figure IVA versus 1B). This reduction in contraction was also observed in response to U46619 (1 μM, P = 0.002), although leptin had no effect on U46619 contractions following chronic, 8 day exposure (P = 0.61, Online Resource 2, Figure I).
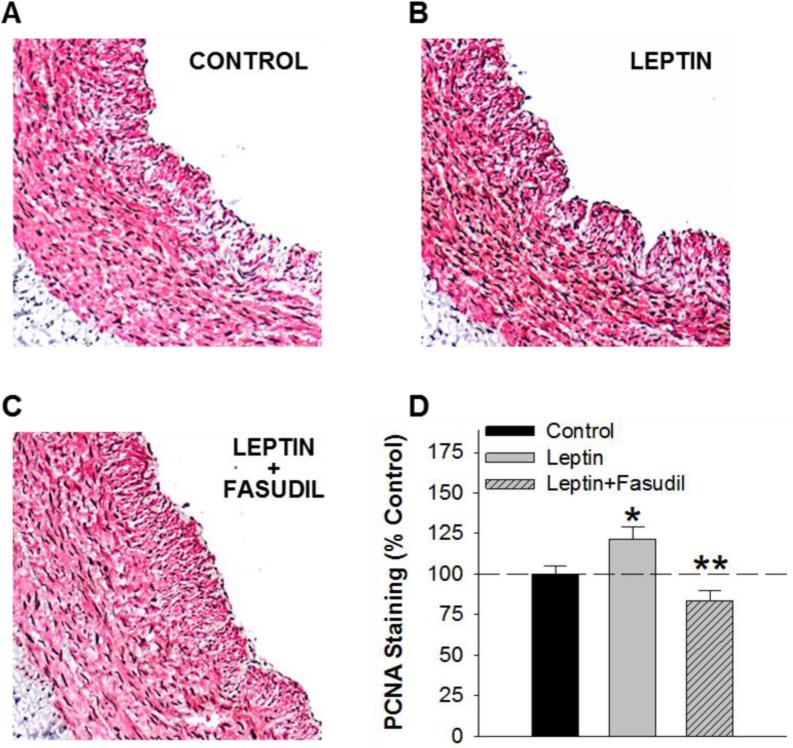
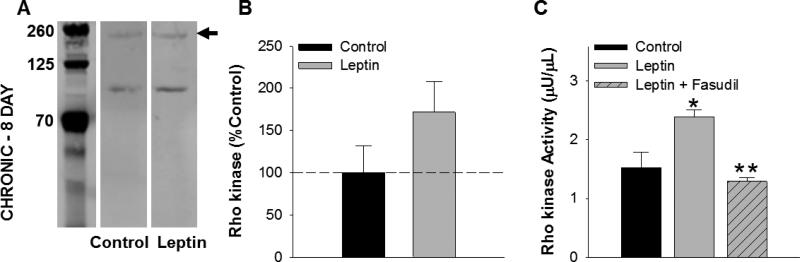
To investigate the role of Rho kinase on leptin-mediated increases in vascular smooth muscle proliferation, arteries were co-immunostained for α-smooth muscle actin and proliferating cell nuclear antigen (PCNA). Systematic quantitation of the PCNA staining pattern within the vascular smooth muscle layer revealed a ~22% increase in PCNA-positive nuclei in leptin treated (Figure 4B) relative to untreated, control arteries (P = 0.04, Figure 4A). Treatment with fasudil alone had no effect on proliferation relative to untreated control arteries (P = 0.50). Co-treatment with leptin and fasudil (Figure 4C) significantly reduced the effect of leptin on vascular smooth muscle proliferation (P = 0.002, Figure 4D). Increases in proliferation with leptin treatment (8 day, serum-containing culture) were associated with a modest increase in Rho kinase protein abundance (P = 0.18, Figure 5A and 5B) and a significant increase in Rho kinase activity, averaging 1.52 ± μU/μL in untreated and 2.40 ± 0.12 μU/μL in leptin treated arteries (P = 0.03, Figure 5C). The effect of leptin on Rho kinase activity was significantly reduced by co-incubation with fasudil (P = 0.002, Figure 5C).
Fig. 4.
Leptin augments coronary vascular smooth muscle proliferation via effects on Rho kinase. Representative images of α-smooth muscle actin (red) and proliferating cell nuclear antigen (brown) co-immunostaining of untreated, control (A), leptin treated (B), and leptin and fasudil co-treated arteries (C) following chronic, 8 day culture in serum-containing media. The increase in PCNA-positive nuclei in leptin treated, relative to untreated arteries was significantly reduced by inhibition of Rho kinase with fasudil (D). All groups n = 5. *P<0.05, leptin versus control. **P<0.05 leptin versus leptin+fasudil
Fig. 5.
Effects of leptin-induced vascular smooth muscle proliferation on Rho kinase. Representative blot of Rho kinase protein abundance in control versus leptin treated arteries following 8 days of culture in serum-containing media [A]. Leptin administration significantly increased Rho kinase activity (C), while only a modest increase in Rho kinase protein abundance was detected (B). The effect of leptin on Rho kinase activity was abolished by co-incubation with fasudil (C) All groups n=5. *P<0.05, leptin versus control. **P<0.05 leptin versus leptin+fasudil
DISCUSSION
The purpose of this investigation was to test the hypothesis that leptin modifies the coronary proteomic expression profile and promotes increases in coronary smooth muscle contraction and proliferation via influences on Rho kinase signaling. The major new findings are: (1) chronic exposure to obese concentrations of leptin (30 ng/mL, 3 day culture) induces marked alterations in the coronary artery proteome (68 proteins) with a significant influence on calcium signaling/contraction and cellular growth and proliferation; (2) acute (30 min) and chronic (3 day, serum-free media) exposure to leptin potentiates voltage-dependent contraction of isolated coronary arteries; (3) acute and chronic leptin-mediated increases in coronary vasoconstriction occur concomitantly with increases in activity and protein abundance of Rho kinase, respectively; (4) chronic leptin administration (8 day, serum-containing media) augments vascular smooth muscle proliferation via increases in Rho kinase activity; and (5) the contractile and proliferative effects of leptin exposure were abolished by the Rho kinase inhibitor fasudil. Taken together, these findings provide novel evidence in support of the paradigm of leptin as an upstream mediator that contributes to marked phenotypic alterations in coronary smooth muscle in the setting of obesity.
Leptin as an upstream mediator of coronary vascular disease
Although recent findings have implicated leptin as an upstream paracrine mediator of a key network of pathways associated with obesity-induced coronary vascular disease, no study has systematically investigated the potential factors and/or pathways involved. Accordingly, we conducted a global proteomic assessment of coronary arteries with and without chronic (3 day) exposure to “obese” concentrations of leptin, i.e., plasma concentrations typically reported in obese subjects (30 ng/ml) [33;46]. This non-biased discovery approach revealed that leptin administration markedly influences the abundance of numerous proteins (Table 1). These data support that leptin signaling alters the expression of a large number of factors which have the potential to mediate additional paracrine effects within the vascular wall. Indeed, Ingenuity Pathway Analysis (IPA) identified alterations within several key cellular processes in leptin treated arteries. Specifically, IPA revealed that the detected changes in the proteomic expression profile corresponded to pathways associated with calcium signaling (e.g., calreticulin, cAMP-dependent protein kinase type II, tropomyosin) and cellular growth and proliferation (e.g., myotrophin, myoferlin, fibrillin-1). These findings are consistent with a previous proteomics assessment from our laboratory which documented an altered secretion profile of coronary PVAT in obese swine which corresponded with pathways associated with cellular growth and proliferation and cellular movement [30]. Furthermore, these alterations in the secretion profile were also associated with increases in vascular smooth muscle contraction [30]. Together with reports of elevated expression of leptin in obese coronary PVAT [36], the current proteomic findings support a role for leptin in coronary vascular contraction and proliferation in the setting of obesity.
These findings have significant (patho)physiologic implications. Recent studies from our laboratory and others indicate that obesity augments coronary smooth muscle CaV1.2 current and voltage dependent increases in intracellular Ca2+ concentration [4;23;30]. Changes in intracellular Ca2+ handling are known to influence both contraction and phenotypic modulation of coronary smooth muscle (i.e. development of a proliferative and/or osteogenic phenotype) [31;50;52]. Recent data also indicate that leptin increases proliferation of isolated smooth muscle cells [19;28] and that perivascular leptin overexpression promotes neointimal formation in peripheral (non-coronary) arteries [44]. Taken together, the present findings and those of others support the growing paradigm regarding a mechanistic role for heightened levels of leptin in obesity-induced coronary disease.
Leptin and coronary smooth muscle contraction
Current understanding of the effects of leptin on vasoconstriction is rather limited and conflicting. Acute leptin administration has been shown to inhibit angiotensin (Ang) II-induced contractions of isolated rat aorta by diminishing the increase in cytosolic [Ca2+] in smooth muscle cells [14]. In contrast, other studies fail to demonstrate an effect of leptin on contractile responses to Ang II, noradrenaline, or endothelin-1 [14]. Chronic systemic leptin administration in normal rats has been shown to produce modest increases in phenlyephrine-induced contractions of aortic rings [32]. These disparate findings are likely related to differences in the concentration of leptin used (0.01 – 100 nmol/L), duration of treatment, vascular bed being studied, and/or the overall health status of the animal model. Results from this study demonstrate that “obese” concentrations of leptin (30 ng/mL) potentiate depolarization-induced contraction of coronary arteries following acute (30 min, Figure 1A) and chronic administration (3 day culture, Figure 1B). These distinct coronary vascular effects of leptin are consistent with recent studies documenting a unique contractile effect of coronary PVAT on vascular smooth muscle compared to other artery/adipose tissue depots that is further augmented in the setting of obesity [30]. Results also demonstrate that leptin has little to no effect on thromboxane A2 receptor-mediated contractions (Online Resource 2, Figure II), suggesting that the functional effects of leptin observed in this study are specifically related to depolarization-induced contraction. This effect of leptin is consistent with reports of augmented coronary vasoconstriction in the setting of obesity [5] and, together with both elevated plasma leptin concentration [15;46] and increased local (coronary PVAT) leptin production [11;36] in the setting of obesity, support the potential for leptin (plasma and/or local PVAT-derived) to contribute to the development of coronary vascular smooth muscle dysfunction.
Rho kinase and coronary smooth muscle contraction
The mechanisms by which leptin influences coronary vascular smooth muscle function remain ill defined. Recent studies suggest a connection between leptin and RhoA/Rho kinase signaling [54-56], which is a well-known major regulator of smooth muscle contraction and vascular tone [25]. However, whether RhoA/Rho kinase contributes to the coronary vascular effects of leptin has not been determined. Data from this investigation support a role for Rho kinase in leptin-mediated increases in depolarization-induced coronary artery contraction. These findings are consistent with previous studies from our laboratory which found marked increases in RhoA expression in obese coronary PVAT and that inhibition of Rho kinase attenuates PVAT-mediated increases in coronary vascular smooth muscle contraction [30]. We propose the effects of leptin occur independent of influences on coronary endothelium as studies in endothelium denuded arteries (Online Resource 2, Figure II) were directionally consistent with studies in endothelium intact arteries.
The present findings also indicate that the effects of leptin on the Rho kinase pathway are time-dependent. Acutely, inhibition of Rho kinase significantly inhibits coronary artery contractions both in the presence (Figure 2C) and absence (Figure 2A) of leptin, whereas following chronic culture for 3 days, the inhibition of Rho kinase significantly inhibits coronary artery contractions only in the presence of leptin (Figure 2D). It is important to note that, in the absence of leptin, the contribution of Rho kinase to KCl-induced contractions was reduced following organ culture (Figure 2B versus 2A) and that the effects of the Rho kinase inhibitor, fasudil, on contractile responses following chronic (Figure 2D) compared to acute (Figure 2C) leptin exposure were relatively modest. Interestingly, these findings are consistent with previous reports of a reduced contribution of Rho kinase to maximal KCl contractions in obese compared to lean arteries [30].
The time-dependent effects of leptin on Rho kinase are further highlighted data indicating that acute (30 min) leptin treatment increased Rho kinase activity (Figure 3C), independent of changes in Rho kinase expression (Figure 3A and 3B), while chronic exposure to leptin (3 day culture) was associated with increased Rho kinase protein abundance (Figure 3D and 3E) with little/no change in overall Rho kinase activity (Figure 3F). It should be noted, however, that overall Rho kinase activity of cultured arteries was significantly elevated relative to untreated, acute controls (Figure 3F versus Figure 3C). Altogether, these findings indicate that the coronary vascular actions of leptin are mediated, at least in part, via time-dependent influences on Rho kinase activity (acute) and expression (chronic) and are consistent with phenotypic changes observed in coronary artery disease.
Although the proteomic analyses in this study indicated alterations in pathways involved in the regulation of vascular tone, no significant alterations in proteins involved in the regulation of Rho kinase activation (e.g., RhoA, rho GDP-dissociation inhibitor, rho GTPase activating protein) or in Rho kinase substrates involved in contraction (e.g., MYPT-1, CPI-17, myosin light chain MLC) were detected by Ingenuity Pathway Analysis following chronic, 3 day leptin treatment (Online Resource 1). However, it is important to consider that a proteomics approach excludes the examination of the phosphorylation status of key regulatory proteins in this cascade as well as the examination of potential lipid mediators. Despite the detection of a direct connection, the proteomic analyses provide valuable insights into the complex cellular processes and pathways influenced by chronic leptin exposure.
Leptin and coronary vascular smooth muscle proliferation
Based on the current and prior evidence supporting that leptin is associated with cellular growth and proliferation pathways, additional experiments were performed to directly investigate the effects of leptin on coronary proliferation. In these studies, coronary arteries were cultured in serum-containing media for 8 days. Quantitation of co-immunostaining for PCNA and α-smooth muscle actin revealed a significant increase in PCNA-positive vascular smooth muscle cells in arteries exposed to leptin (30 ng/ml) that was inhibited by co-incubation with fasudil (Figure 4). The leptin-induced increase in coronary proliferation reported in this study was associated with a modest increase in Rho kinase protein abundance (Figure 5A and 5B) and a significant increase in Rho kinase activity (Figure 5C) relative to untreated, controls. These data further support that the effects of leptin on Rho kinase protein abundance and activity are dependent, at least in part, on the time-course of administration (e.g., acute, 3 day, 8 day) and culture condition (e.g., serum-free versus serum-containing). Although the precise molecular mechanisms responsible for this dynamic effect of leptin on Rho kinase signaling are presently unknown, we postulate that these effects occur independently of influences on coronary endothelium, as endothelial denudation had little to no effect on functional responses following chronic, 8 day treatment with leptin and/or fasudil (Online Resource 2, Figure IV). However, the possibility that the observed effects of leptin on proliferation are mediated by factors released from endothelial cells cannot be ruled out. Our findings are consistent with other studies which have documented leptin-induced vascular smooth muscle hypertrophy [49;56], smooth muscle proliferation [19;28;44], and neointimal formation [42;44] in rodent models, although contrasting evidence in rat aortic smooth muscle cells exposed to Ang II [40] and in a human smooth muscle cell line [6] have also been reported. However, to our knowledge, the present data are the first to demonstrate that patho-physiologically relevant (“obese”) concentrations of leptin are capable of promoting vascular smooth muscle proliferation and to provide evidence in support of a mechanistic linkage between Rho kinase and leptin-mediated increases in proliferation in the coronary circulation.
Limitations
Although this investigation utilizes arteries from the translationally relevant porcine coronary circulation, relevant obese concentrations of leptin, and a time course (i.e., acute and chronic) of leptin administration, it is presently unclear to what extent these findings translate to the human condition. However, the present study is among the first to systematically investigate the factors and pathways involved in leptin-induced phenotypic alterations of coronary arteries (i.e., increased contraction and proliferation). While functional data support that the vascular smooth muscle layer is responsible for the observed effects of leptin, the precise factors and cell types involved remain to be definitively determined. Additionally, a pharmacologic approach was utilized to interrogate the Rho kinase pathway in this study. Based on reported IC50 values for fasudil [1;12;37], a concentration of 1 μM was chosen to potently inhibit Rho kinase and minimize off target effects. Although the possibility of non-specific effects cannot be ruled out, data in which fasudil diminishes Rho kinase activity in this study (Figure 5C) support the specificity and efficacy of this pharmacologic approach. However, future genetic knockdown (e.g. antisense oligonucleotides) studies to confirm the present findings are warranted.
Conclusions & Implications
The present data provide novel evidence that relevant obese concentrations of leptin promote increases in coronary vasoconstriction and smooth muscle proliferation and that these phenotypic effects are directly associated with alterations in the coronary artery proteome and dynamic effects on the Rho kinase pathway. Although the current studies were conducted using (patho)physiologically relevant (“obese”) concentrations of leptin, a critical question remains as to the extent to which locally produced versus circulating leptin contributes to the initiation and progression of coronary disease in vivo. Future studies to directly investigate the mechanisms responsible for the time-dependent effects of leptin on Rho kinase protein abundance and activity are also warranted. Regardless, this investigation provides novel evidence in support of leptin as an upstream mediator of the hypercontractile and proliferative coronary smooth muscle phenotype reported in obesity-induced coronary disease.
Supplementary Material
ACKNOWLEDGEMENTS
This publication was made possible in part by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative (CECARE); HL117620 (Tune-Mather); TL1 TR001107 and UL1 TR001108 (Noblet, Sassoon). Ingenuity Pathway Analyses were made possible by a collaboration with WV-INBRE (supported by NIH grant P20GM103434). The authors also thank Arpad Somogyi and the Proteomics Core at The Ohio State University for performing mass spectrometry.
Footnotes
DISCLOSURES
The authors declare that they have no conflict of interest.
Reference List
- 1.Bain J, Plater L, Elliott M, Shpiro N, Hastie C, McLauchlan H, Klevernic I, Arthur J, Alessi D, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. doi:10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Beltowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. 2012;39:168–178. doi: 10.1111/j.1440-1681.2011.05623.x. doi:10.1111/j.1440-681.2011.05623.x. [DOI] [PubMed] [Google Scholar]
- 4.Berwick ZC, Dick GM, O'Leary HA, Bender SB, Goodwill AG, Moberly SP, Owen MK, Miller SJ, Obukhov AG, Tune JD. Contribution of electromechanical coupling between KV and CaV1.2 channels to coronary dysfunction in obesity. Basic Res Cardiol. 2013;08:370. doi: 10.1007/s00395-013-0370-0. doi:10.1007/s00395-013-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. doi:10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohlen F, Kratzsh J, Mueller M, Seidel B, Friedman-Einat M, Witzigmann H, Teupser D, Koerner A, Storck M, Thiery J. Leptin inhibits cell growth of human vascular smooth muscle cells. Vascul Pharmacol. 2007;46:67–71. doi: 10.1016/j.vph.2006.06.014. doi:10.1016/j.vph.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 8.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. doi:10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MGJ, Weintraub DS, Rudiche SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. doi:10.1152/physiolgenomics.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. doi:10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. doi:10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 12.Davies S, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drolet R, Belanger C, Fortier M, Huot C, Mailloux J, Legare D, Tchernof A. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring) 2009;17:424–430. doi: 10.1038/oby.2008.555. doi:10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
- 14.Fortuno A, Rodriguez A, Gomez-Ambrosi J, Muniz P, Salvador J, Diez J, Fruhbeck G. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology. 2002;143:3555–3560. doi: 10.1210/en.2002-220075. doi:10.1210/en.2002-220075. [DOI] [PubMed] [Google Scholar]
- 15.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. doi:10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 16.Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;65:633–642. doi: 10.1111/j.1476-5381.2011.01430.x. doi:10.1111/j.1476-5381.2011.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der GY, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. doi:10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Gruen M, Hao M, Piston D, Hasty A. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxsis. Am J Physiol Cell Physiol. 2007;293:C1481–C1488. doi: 10.1152/ajpcell.00062.2007. 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Xiong X, Wang H, You S, Zneg H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kB. Acta Biochim Biophys Sin. 2010;42:325–331. doi: 10.1093/abbs/gmq025. doi:10.1093/abbs/gmq025. [DOI] [PubMed] [Google Scholar]
- 20.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Leptin Receptors are Expressed in Coronary Arteries and Hyperleptinemia Causes Significant Coronary Endothelial Dysfunction. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.01159.2004. doi:10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 21.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295:H1514–H1521. doi: 10.1152/ajpheart.00479.2008. doi:10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. doi:10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lin YC, Huang J, Kan H, Castranova V, Frisbee JC, Yu HG. Defective calcium inactivation causes long QT in obese insulin-resistant rat. Am J Physiol Heart Circ Physiol. 2012;302:H1013–H1022. doi: 10.1152/ajpheart.00837.2011. doi:10.1152/ajpheart.00837.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Itoh H, Louie O, Kubota K, Kent K. The signaling protein Rho is necesary for vascular smooth muscle migration and survival but not for proliferation. Surgery. 2002;32:317–325. doi: 10.1067/msy.2002.125786. doi:10.1067/msy.2002.125786. [DOI] [PubMed] [Google Scholar]
- 25.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. doi:10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 26.Mazurek T, Zhang L, Zalewski A, Mannion D, Diehl J, Arafat H, Sarov-Blat L, O'Brien S, Keiper E, Johnson A, Martin J, Goldstein B, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. doi:10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 27.Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD. Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle K+ channels. Arterioscler Thromb Vasc Biol. 2015;35:1393–1400. doi: 10.1161/ATVBAHA.115.305500. doi:10.1161/ATVBAHA.115.305500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- 29.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. doi:10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular Adipose Tissue Potentiates Contraction of Coronary Vascular Smooth Muscle: Influence of Obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. doi:10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. doi:10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ozer C, Gulen S, Dilekoz E, Babul A, Ercan ZS. The effect of systemic leptin administration on aorta smooth muscle responses in diabetic rats. Mol Cell Biochem. 2006;282:187–191. doi: 10.1007/s11010-006-1927-0. doi:10.1007/s11010-006-1927-0. [DOI] [PubMed] [Google Scholar]
- 33.Pardina E, Ferrer R, Baena-Fustegueras J, Lecube A, Fort J, Vargas V, Catalan R, Peinado-Onsurbe J. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg. 2010;40:623–632. doi: 10.1007/s11695-010-0103-5. doi:10.1007/s11695-010-0103-5. [DOI] [PubMed] [Google Scholar]
- 34.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. doi:10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, Tune JD. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. doi:10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. doi:10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikitake Y, Kim H, Huang Z, Seto M, Yano K, Asano T, Moskowitz M, Liao J. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;48:324–331. doi: 10.1210/en.2006-0940. doi:10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez A, Fruhbeck G, Gomez-Ambrosi J, Catalan V, Sainz N, Zalba G, Fortuno A. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction is blunted in spontaneously hypertensive rats. J Hypertens. 2006;24:1589–1597. doi: 10.1097/01.hjh.0000239295.17636.6e. doi:10.1097/01.hjh.0000239295.17636.6e. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez A, Gomez-Ambrosi J, Catalan V, Fortuno A, Fruhbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediator Inflamm. 2010;2010 doi: 10.1155/2010/105489. doi:10.1155/2010/105489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samora JB, Goodwill AG, Frisbee JC, Boegehold MA. Growth-dependent changes in the contribution of carbon monoxide to arteriolar function. J Vasc Res. 2010;47:23–24. doi: 10.1159/000231718. doi:10.1159/000231718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer K, Halle M, Goeschen C, Dellas C, Pynn M, Loskutoff D, Konstantinides S. Leptin promtoes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004;24:112–117. doi: 10.1161/01.ATV.0000105904.02142.e7. doi:10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 43.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White D, Hartenstein V, Eliceiri K, Tomanack P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. doi:10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheif-Nestler M, Czepluch FS, Chalikias G, Konstantinides S, Shafer K. Leptin-dependent and leptin-independent paracrine effects of perivascualr adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol. 2013;33:980–987. doi: 10.1161/ATVBAHA.113.301393. doi:10.1161/ATVBAHA.113.301393. [DOI] [PubMed] [Google Scholar]
- 45.Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci USA. 2008;105:19006–19011. doi: 10.1073/pnas.0809743105. doi:10.1073/pnas.0809743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. doi:10.1161/01.HYP.31.1.409. [DOI] [PubMed] [Google Scholar]
- 47.Shibasaki I, Nishikimi T, Mochizuki Y, Yamada Y, Yoshitatsu M, Inoue Y, Kuwata T, Ogawa H, Tsuchiya G, Ishimitsu T, Fukuda H. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept. 2010;165:210–217. doi: 10.1016/j.regpep.2010.07.169. doi:10.1016/j.regpep.2010.07.169. [DOI] [PubMed] [Google Scholar]
- 48.Shibata R, Kai H, Seki Y, Kato S, Morimatsu M, Kaibuchi K, Imaizumi T. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–289. doi: 10.1161/01.cir.103.2.284. doi:10.1161/01.CIR.103.2.284. [DOI] [PubMed] [Google Scholar]
- 49.Shin HJ, Oh J, Kang SM, Lee JH, Shin MJ, Hwang KC, Jang Y, Chung JH. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;329:18–24. doi: 10.1016/j.bbrc.2004.12.195. doi:10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- 50.Sturek M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J Appl Physiol. 2011;111:573–586. doi: 10.1152/japplphysiol.00373.2011. doi:10.1152/japplphysiol.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterscler Thromb Vasc Biol. 2014;34:1827–1830. doi: 10.1161/ATVBAHA.114.303032. doi:10.1161/ATVBAHA.114.303032. [DOI] [PubMed] [Google Scholar]
- 52.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. doi:10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 53.Withers SB, Bussey CE, Saxton SN, Melrose HM, Watkins AE, Heagerty AM. Mechanisms of adiponectin-associated perivascular function in vascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1637–1642. doi: 10.1161/ATVBAHA.114.303031. doi:10.1161/ATVBAHA.114.303031. [DOI] [PubMed] [Google Scholar]
- 54.Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res. 2008;77:64–72. doi: 10.1093/cvr/cvm020. doi:10.1093/cvr/cvm020. [DOI] [PubMed] [Google Scholar]
- 55.Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res. 2006;72:101–111. doi: 10.1016/j.cardiores.2006.06.024. doi:10.1016/j.cardiores.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 56.Zeidan A, Paylor B, Steinhoff K, Javadov S, Rajapurohitam V, Chakrabarti S, Karmazyn M. Actin cytoskeleton dynamics promotes leptin-induced vascular smooth muscle hypertrophy via RhoA/ROCK- and phosphatidylinositol 3-kinase/protein kinase B-dependent pathways. J Pharmacol Exp Ther. 2007;322:1110–1116. doi: 10.1124/jpet.107.122440. doi:10.1124/jpet.107.122440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.