Abstract
OBJECTIVE:
To investigate the impact of prenatal maternal iron deficiency (ID) on cord blood serum ferritin (CBSF) concentration and infant cognitive and motor development.
METHODS:
Our prospective cohort study included 636 mother-singleton child pairs from 828 eligible pregnant women who were enrolled during their first antenatal care (ANC) visit in Allada, Benin, into a clinical trial comparing the efficacy of mefloquine and sulfadoxine-pyrimethamine. Venous blood samples of women were assessed for ferritin and hemoglobin concentrations at the first and second ANC visits (occurring at least 1-month apart) and at delivery. Women were prescribed daily iron and folic acid supplements throughout pregnancy. Hematologic examinations were repeated for cord blood at birth. At age 1 year, cognitive and motor functions of children were assessed by using the Mullen Scales of Early Learning.
RESULTS:
The prevalence of prenatal ID at first and second ANC visits, and at delivery was 30.5%, 34.0%, and 28.4%, respectively. CBSF concentrations were similar between ID and non-ID pregnant women. Neither prenatal ID nor CBSF concentration was associated with poor cognitive or gross motor function of children at age 1 year. CBSF concentrations were lower among mothers who had ID anemia (IDA) at delivery compared with non-IDA pregnant women (adjusted mean difference: –0.2 [95% confidence interval: –0.4 to –0.0]).
CONCLUSIONS:
In a malaria-endemic region, ID in pregnancy in the context of iron supplementation is neither associated with CBSF concentration nor with infant cognitive and motor development. Prenatal IDA around the time of delivery is associated with lower CBSF concentrations.
What’s Known on This Subject:
Iron deficiency (ID) in children is associated with poor cognitive development. However, the literature on the impact of prenatal ID on ferritin levels at birth and infant cognitive and motor functions are limited.
What This Study Adds:
ID in pregnancy in the context of iron supplementation is not associated with cord blood serum ferritin concentration and infant cognitive and motor development. ID anemia at delivery is, however, associated with lower cord blood serum ferritin concentrations.
Iron deficiency (ID) remains the world’s most prevalent micronutrient deficiency affecting ∼2 billion people worldwide.1 Pregnant women and children <5 years especially, in less developed countries, are most at risk for ID.2 During pregnancy, there is increased demand for iron to accommodate the needs of the fetal-placental unit. By the second trimester of pregnancy, the daily requirements increase to 6.8 mg, which is 3 times that of a nonpregnant woman.3 This increased physiologic demand for iron renders pregnant women highly vulnerable to ID in the absence of iron supplementation or proper nutrition. ID is assumed to account for more than half of the burden of anemia during pregnancy.4
One reason why iron is particularly important during pregnancy is the indispensable role it plays in fetal development and infant cognition, via biochemical processes involved in brain formation and function. Specifically, iron is involved in the appropriate myelination of the white matter of cerebellar folds, hippocampus development, and neurotransmitter synthesis, which are essential in fetal and child brain function.5,6
ID during pregnancy could therefore have deleterious consequences for fetal development via deprived iron availability for fetal neurodevelopment. The precariousness of the fetus during this phase renders it vulnerable to insults including that caused by prenatal maternal micronutrient deficiencies, which could have adverse consequences on fetal brain and cognitive function even after birth.7 Factors associated with delayed or suboptimal infant cognitive functions have been shown to be indicative of child nonreadiness for school.8
Evidence from some studies reveals early postnatal exposure to ID in children to be a risk factor for poor cognitive and psychomotor development and in some cases, has been linked to long-term behavioral consequences.8,9 In children, several studies have shown the importance of iron sufficiency for cognitive development, appropriate behavioral development, and gross motor (GM) function.9,10 However, low maternal prenatal iron levels measured as serum ferritin have not been consistently linked with low cord blood serum ferritin (CBSF) concentrations.11,12
Despite the known detrimental consequences of early ID on cognition, studies on the impact of prenatal ID on neurocognitive outcomes in human subjects remain scanty. Hence, we set out to investigate the impact of ID at 3 periods of pregnancy and in early postnatal stages of infant development on the cognitive development of 1-year-old children.
Methods
Study Design and Population
The prospective cohort study (called TOVI) that was principally set to investigate the relationship between hemoglobin (Hb) concentration and infant cognitive function included 636 mother-singleton child pairs from 828 eligible pregnant women and their offspring.13 This article is therefore a secondary analysis of that data. The mothers had all been enrolled earlier in the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) clinical trial (NCT00811421) that compared the efficacy of mefloquine and sulfadoxine pyrimethamine as intermittent preventive treatment of pregnant women (IPTp).
HIV negative women of, at most, 28 weeks of gestation who had no previous intake of IPTp, anthelmintics, or iron and folic acid supplements were recruited into the MiPPAD clinical trial during their first antenatal care (ANC) visit to any of 3 maternity clinics in 3 subdistricts of Allada, Benin. Detailed inclusion criteria into the MiPPAD trial are explained elsewhere.14 The women were followed throughout pregnancy, at a second ANC of at least 1-month after the first, and at delivery. At the baseline and during the periods of follow-up, anthropometric, socioeconomic, and biological data were obtained from pregnant women. At delivery, infant birth weight and gestational age were determined and recorded. Infants were followed at age 1 year during which the quality of the home environment (using the Home Observatory Measurement of the Environment [HOME] inventory),15 maternal postnatal depression (using the Edinburgh Postnatal Depression Scale),16 maternal nonverbal intelligent quotient (using the Raven’s Progressive Matrices test),17 and infant anthropometric measurements were assessed.
Prenatal Hematologic and Other Biological Assessments
At the first ANC visit, 4-mL venous blood samples of pregnant women were collected into an iron-free dry tube, and stool samples were collected into a clean tube for biological assessments after which the pregnant women were administered IPTp, anthelmintics (if they were in their second trimester), and oral ferrous sulfate (200 mg daily), and folic acid (5 mg daily) as part of the ANC package in Benin. We provided daily iron and folic acid supplements, to be taken at home, throughout pregnancy. Blood withdrawal and biological assessments were repeated at the second ANC visit and at delivery.
Serum ferritin concentrations were measured from 500 µL of serum with an AxSym Immuno-Assay Analyzer (Abbot Laboratories, Abbot Park, IL). To correct for high levels of ferritin concentration resulting from inflammations, a rapid slide test was performed to determine the concentration of C-reactive protein (CRP) in the blood (CRP Latex; Cypress Diagnostics, Inc, Campbellsville, Ontario, Canada). Inflammation was defined as CRP levels higher than 5 mg/L. ID was defined as serum ferritin concentration less than 12 µg/L or serum ferritin concentrations between 12 µg/L and 70 µg/L if CRP concentration in the presence of inflammation.
The Hemo-Control photometer (EKF Diagnostics, Barleben/Magdeburg, Germany) device was used to determine the concentration of Hb. Hb concentration was recorded in g/L. Hb concentration less than 110 g/L was considered anemia. A pregnant woman was deemed to have ID anemia (IDA) if she was iron deficient and anemic at the time of assessment. Malaria infection was determined by using the Lambaréné technique (detection threshold of 5 parasites/µL).18
Hematologic and biological assessments were repeated for cord blood samples taken at delivery for a subset of 582 children, of whom 432 had cord blood ferritin assessments. Anemia at birth was defined as cord blood Hb < 140 g/L.19
Neurocognitive Assessment
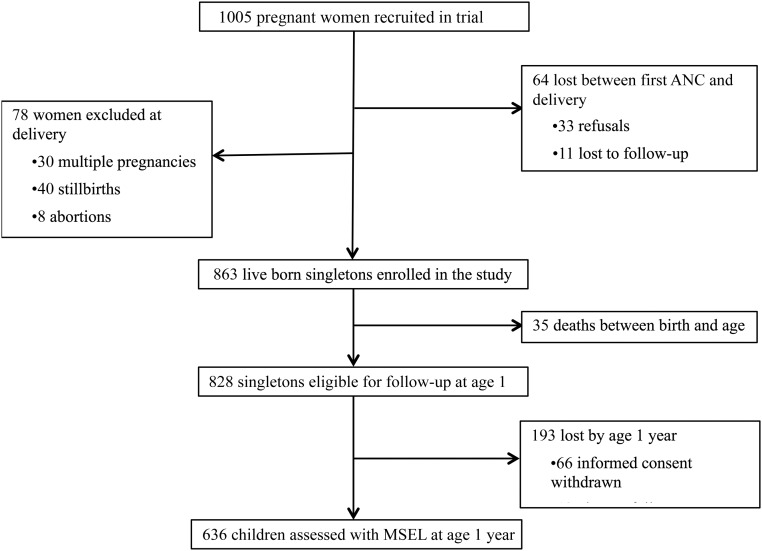
Of the 828 eligible singletons, 76.8% were evaluated for cognitive development at 1 year of age (from April 2011 to November 2012) as shown in Fig 1.
FIGURE 1.
Flowchart of the follow-up of pregnant women and children.
Cognitive and motor developments of children were evaluated by using the American Guidance Service edition of the Mullen Scales of Early Learning (MSEL).20 The MSEL comprises 5 scales: GM, fine motor, expressive language, receptive language, and visual perception. The GM scale assessed central motor control and the ability of movement in several positions of the body. The sum of test scores obtained by the children in each MSEL scale was transformed into standardized age-specific (monthly) scores called the T scores, which are normally distributed. The standardized T scores of the fine motor, expressive language, receptive language, and visual perception scales were combined to produce the Early Learning Composite (ELC) score. The ELC is a measure of the general cognitive factor, which is indicative of early cognitive performance.20
The MSEL were translated and adapted for this setting.21 Detailed quality assurance and reliability of assessments have been published.21 Assessors were blinded to prenatal maternal and neonatal iron levels and birth outcomes.
The sample size of 636 mother–infant pairs was enough to detect small differences of ≥0.16 SD in ELC or GM scores between children of ID mothers and those of non-ID mothers.
Statistical Analysis
First, we compared maternal and infant characteristics between those who had CBSF assessments performed and those who did not. We also compared the maternal characteristics of children who were assessed for cognitive function and those who were not. Then we described separately, maternal and infant hematologic characteristics among the children who were assessed for cognitive and motor functions. We also conducted a univariate analysis between maternal and infant characteristics and CBSF concentration and ELC and GM scores.
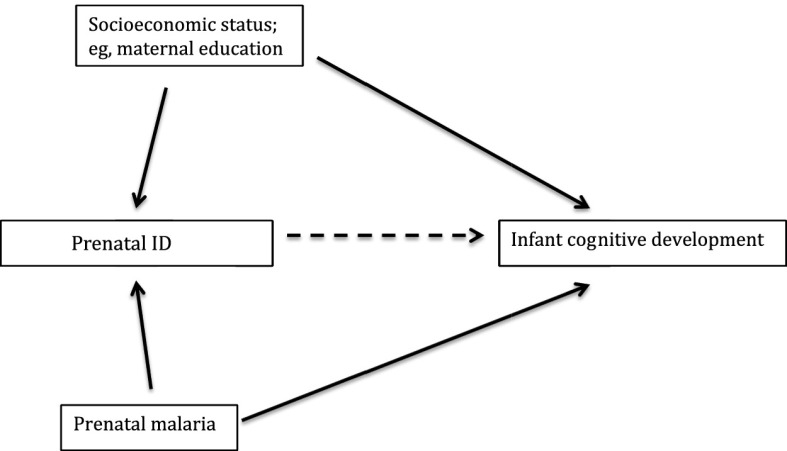
Due to the nonnormal distribution of CBSF concentration and the lack of homoscedasticity, we log transformed the CBSF concentration variable. The transformed variable was normally distributed from a visual inspection from the histogram. Using a multiple linear regression analysis, we compared the mean log CBSF concentration, ELC, and GM scores between iron deficient pregnant women and noniron deficient pregnant women. Directed acyclic graphs, as shown in Fig 2, were used in selecting confounders in the multiple linear regression analysis. All models were adjusted for maternal education and malaria infection at the time of iron assessment irrespective of significance except malaria at birth, as there were only 2 cord blood samples positive with plasmodium parasites. To simplify models, stepwise removal of other covariates was performed until the P values of covariates in the model were less than .05 with the exception of the HOME score, which is an indicator for infant development.
FIGURE 2.
Directed acyclic graph of the relationship between prenatal ID and infant cognitive development.
Statistical analyses were performed by using Stata IC/14.1 for MAC (Stata Corp, College Station, TX). Statistical significance was defined as P < .05 from 2-tailed tests.
Ethical Considerations
The institutional review boards of the University of Abomey-Calavi, Benin; New York University and Michigan State University; and the Research Institute for Development’s Consultative Ethics Committee, France approved the study. At recruitment, we obtained informed consent from all pregnant women and guardians of children who participated in this study.
Results
Maternal and infant characteristics of those who had CBSF assessed were comparable on all covariates to those who did not have CBSF assessed with the exception of gestational age at first ANC visit (small difference of 1 week), maternal occupation, pre-pregnancy BMI, and gestational age at birth. The prevalence of ID at first ANC and at delivery among pregnant women whose children were assessed for cognitive function was significantly less than the prevalence among those whose children were not assessed for cognitive function at age 1 year (Supplemental Table 5). Women who had ID or IDA at baseline had a higher median gestational age compared with those who did not have ID or IDA, respectively. ID was common among primigravida women compared with multigravida women as shown in Table 1. The median age (interquartile range [IQR]) of pregnant women at their first ANC visit was 25 (21–30) years. At baseline, the median gestational age (IQR) was 23 (19–26) weeks. The prevalence of ID among pregnant women at first and second ANC visits, and at delivery was 30.5%, 34.0%, and 28.4%, respectively (Table 2).
TABLE 1.
Comparison of Maternal and Infants Characteristics at Baseline
| Characteristics | ID at First ANC | IDA at First ANC | ||||
|---|---|---|---|---|---|---|
| ID | No ID | P | IDA | No IDA | P | |
| Maternal | ||||||
| Maternal age, ya | 25 (20 to 30) | 25.0 (21 to 30) | .62 | 25 (20 to 30) | 25 (21 to 30) | .79 |
| GA at first ANC, wka | 23 (20 to 26) | 22 (19 to 25) | <.01 | 24 (20 to 26) | 22 (19 to 25) | <10−3 |
| Possessions scorea | 5 (3 to 8) | 5 (3 to 9) | .36 | 5 (3 to 8) | 5 (3 to 9) | .53 |
| Education, no. (%) | ||||||
| Never schooled | 129 (66.5) | 292 (66.1) | .92 | 93 (66.0) | 328 (66.3) | .95 |
| Primary or more | 65 (33.5) | 150 (33.9) | 48 (34.0) | 167 (33.7) | ||
| Gravidity, no. (%) | ||||||
| Primigravida | 24 (16.9) | 93 (21.0) | <.01 | 19 (13.5) | 98 (19.8) | .09 |
| Multigravida | 170 (83.1) | 349 (79.0) | 122 (86.5) | 397 (80.2) | ||
| Occupation, no. (%) | ||||||
| Housewives | 103 (53.1) | 212 (43.5) | .23 | 75 (53.9) | 240 (48.5) | .32 |
| Employed | 91 (46.9) | 230 (56.5) | 66 (46.8) | 255 (51.5) | ||
| BMI, no. (%) | ||||||
| Underweight, <18.5 | 37 (19.1) | 74 (16.7) | .69 | 30 (21.3) | 81 (16.4) | .26 |
| Normal, 18.5 to 24.9 | 139 (71.6) | 320 (72.4) | 100 (70.9) | 359 (72.5) | ||
| Overweight, ≥25.0 | 18 (9.3) | 48 (10.9) | 11 (7.8) | 55 (11.1) | ||
| Infant | ||||||
| Birth weight, g, no. (%) | ||||||
| LBW, <2500 | 13 (9.2) | 48 (12.8) | .11 | 12 (8.7) | 49 (10.0) | .66 |
| Normal, ≥2500 | 178 (90.8) | 391 (87.2) | 126 (91.3) | 443 (90.0) | ||
| GA at birth, wk, no. (%) | ||||||
| Preterm, <37 | 27 (5.3) | 14 (7.4) | .77 | 10 (7.3) | 33 (8.1) | .86 |
| Not preterm, ≥37 | 485 (94.7) | 175 (92.6) | 127 (92.7) | 448 (91.9) | ||
| Sex, no. (%) | ||||||
| Boys | 95 (49.0) | 216 (48.9) | .54 | 71 (50.3) | 240 (48.5) | .70 |
| Girls | 99 (51.0) | 226 (51.1) | 70 (49.7) | 255 (51.5) | ||
| HOME scorea | 27 (25 to 28) | 27 (25 to 28) | .69 | 27 (25 to 28) | 27 (25 to 28) | .85 |
Unless otherwise stated, all numbers represent number (percentage). GA, gestational age; LBW, low birth weight.
Represented as median (IQR).
TABLE 2.
Maternal and Infant Hematologic and Clinical Characteristics of Children Assessed for Cognitive Function Using MSEL
| Number (%) or Mean ± SD | |
|---|---|
| Maternal characteristics | |
| At first ANC visit (N = 636) | |
| Anemia | 426 (67.0) |
| ID | 191 (30.5) |
| IDA | 141 (22.2) |
| Soil-transmitted helminth infection (N = 627) | 73 (11.6) |
| Malaria | 102 (16.0) |
| At second ANC visit (N = 627) | |
| Anemia (N = 626) | 403 (64.4) |
| ID | 213 (34.0) |
| IDA (N = 626) | 151 (24.1) |
| Soil-transmitted helminth infection (N = 619) | 54 (8.7) |
| Malaria | 23 (3.7) |
| At delivery | |
| Anemia (N = 609) | 234 (38.4) |
| ID (N = 606) | 172 (28.4) |
| IDA (N = 605) | 69 (11.4) |
| Soil-transmitted helminth infection (N = 538) | 14 (2.6) |
| Malaria (N = 610) | 62 (10.2) |
| Infant characteristics | |
| At birth | |
| Hb concentration (N = 582), g/L | 140.2 ± 23.3 |
| Anemia (Hb <140 g/L) | 259 (44.5) |
| Ferritin concentration (N = 432), μg/L | 176 ± 171.56 |
CBSF concentration was significantly higher among women who were never schooled (P = .04). Prenatal ID at any of the follow-up periods was not associated with CBSF concentration in the univariate analysis as shown in Table 3. Absent maternal education, lack of employment, and fewer family possessions were all associated with lower ELC and GM scores.
TABLE 3.
Relationship Between Maternal and Infant Characteristics and Cord Blood Ferritin Concentration and Infant Cognitive Development
| Characteristics | Cord Blood Ferritin Concentration | ELC Score | GM Score | |||
|---|---|---|---|---|---|---|
| Median (IQR) | P | Mean ± SD | P | Mean ± SD | P | |
| Maternal | ||||||
| Maternal age, ya | −0.07 | .14 | −0.03 | .41 | 0.05 | .19 |
| GA at first ANC, wka | 0.03 | .48 | 0.01 | .85 | −0.00 | .95 |
| Possessions scorea | −0.03 | .54 | 0.12 | <.01 | 0.14 | <10−3 |
| Education | ||||||
| Never schooled | 145.0 (96.6 to 202.6) | .04 | 96.2 ± 14.8 | <10−3 | 49.4 ± 13.9 | <10−3 |
| Primary or more | 136.7 (63.4 to 203.8) | 102.9 ± 12.0 | 54.1 ± 15.1 | |||
| Gravidity | ||||||
| Primigravida | 159.9 (69.6 to 228.5) | .48 | 99.8 ± 13.4 | .28 | 48.2 ± 15.6 | .02 |
| Multigravida | 140.2 (86.3 to 201.8) | 98.2 ± 14.5 | 51.6 ± 14.1 | |||
| Occupation | ||||||
| Housewives | 138.6 (74.2 to 200.2) | .09 | 96.1 ± 15.1 | <10−3 | 49.2 ± 13.7 | <.01 |
| Employed | 145.3 (98.9 to 204.3) | 100.8 ± 13.1 | 52.8 ± 15.0 | |||
| BMI | ||||||
| Underweight | 153.5 (90.8 to 218.5) | .59 | 96.4 ± 15.1 | .07 | 48.9 ± 14.3 | .03 |
| Normal | 140.5 (81.7 to 201.0) | 98.6 ± 14.4 | 50.9 ± 14.5 | |||
| Overweight | 135.3 (82.4 to 179.5) | 101.5 ± 11.6 | 54.8 ± 14.1 | |||
| ID at first ANC visit | ||||||
| Yes | 132.0 (78.0 to 210.2) | .30 | 98.6 ± 14.1 | .94 | 50.2 ± 14.2 | .40 |
| No | 148.7 (93.1 to 202.9) | 98.5 ± 14.4 | 51.3 ± 14.6 | |||
| ID at second ANC visit | ||||||
| Yes | 135.8 (90.1 to 201.2) | .85 | 99.5 ± 14.3 | .21 | 51.4 ± 14.9 | .72 |
| No | 146.6 (81.8 to 202.6) | 98.0 ± 14.1 | 50.9 ± 14.1 | |||
| ID at delivery | ||||||
| Yes | 127.7 (70.9 to 203.4) | .26 | 98.2 ± 14.2 | .80 | 51.2 ± 13.3 | .90 |
| No | 146.3 (90.7 to 202.7) | 98.5 ± 14.4 | 51.0 ± 14.8 | |||
| Infant | ||||||
| Birth weight, g | ||||||
| LBW, <2500 | 154.1 (100.1 to 243.7) | .27 | 97.8 ± 14.9 | .71 | 45.6 ± 15.0 | <.01 |
| Normal, ≥2500 | 140.3 (81.8 to 201.8) | 98.6 ± 14.3 | 51.6 ± 14.3 | |||
| GA at birth, wk | ||||||
| Preterm, <37 | 138.1 (87.0 to 232.5) | .93 | 95.7 ± 15.5 | .14 | 49.3 ± 14.11 | .35 |
| Not preterm, ≥37 | 143.5 (81.7 to 202.9) | 99.0 ± 14.1 | 51.4 ± 14.4 | |||
| Sex | ||||||
| Boys | 147.2 (90.5 to 205.1) | .59 | 97.8 ± 13.6 | .21 | 51.8 ± 14.8 | .14 |
| Girls | 136.3 (78.5 to 201.0) | 99.2 ± 14.9 | 50.2 ± 14.1 | |||
| HOME scorea | −0.10 | .04 | 0.19 | <10−3 | 0.19 | <10−3 |
| Cord blood ferritin concentrationa | NA | NA | 0.01 | .80 | 0.01 | .89 |
GA, gestational age; LBW, low birth weight; NA, not applicable.
Spearman’s rank correlation coefficients.
As shown in Table 4, there were no statistically significant differences between ELC and GM scores for children whose mothers had prenatal ID or IDA at any of the follow-up visits and those whose mothers did not have ID or IDA at any of the follow-up visits. Likewise, anemia and ferritin levels at birth were not associated with ELC and GM scores at age 1 year. In the adjusted model, there was no difference in CBSF concentration between iron deficient and noniron deficient pregnant women. However, compared with pregnant women with no IDA at delivery, women with IDA at delivery had significantly lower CBSF concentration (adjusted mean difference [AMD] [95% confidence interval (CI)]: –0.2 [–4.0 to –0.0]) but as noted above this did not impact on the child’s ELC or GM scores.
TABLE 4.
Relationship Between Maternal ID, IDA, Cord Blood Ferritin Levels, and Infant Cognitive Development
| Log Cord blood Ferritin Concentration | ELC Score | GM Score | |
|---|---|---|---|
| AMD (95% CI) | AMD (95% CI)a | AMD (95% CI)a | |
| At first ANC visit | |||
| ID | −0.1 (−0.2 to 0.1)b | 0.4 (−1.9 to 2.8)b | −1.1 (−3.5 to 1.3)c |
| IDA | −0.0 (−0.2 to 0.1)b | 0.7 (−1.9 to 3.3)b | −0.1 (−2.8 to 2.5)c |
| At second ANC visit | |||
| ID | 0.0 (−0.1 to 0.2) NS | 1.5 (−0.7 to 3.8)b | −0.0 (−2.4 to 2.3)d |
| IDA | −0.1 (−0.2 to 0.1) NS | 1.3 (−1.2 to 3.8)b | 1.5 (−1.0 to 4.1)d |
| At delivery | |||
| ID | −0.1 (−0.2 to 0.1) | −0.7 (−3.1 to 1.8) | −0.2 (−2.8 to 2.3)d |
| IDA | −0.2 (−0.4 to −0.0)* | −0.8 (−4.3 to 2.7) | 1.3 (−2.3 to 4.8)d |
| At birth | |||
| Log ferritin concentration | NA | 0.8 (−1.0 to 2.5)e | 0.8 (−0.9 to 2.5)f |
| Anemia (Hb <140 g/L) | NA | 0.0 (−2.3 to 2.3) | 0.4 (−1.9 to 2.7)d |
All models were adjusted for maternal education and malaria at iron assessment. NA, not applicable; NS, not significant model.
Models further adjusted for the HOME score
Model adjusted for helminth infection at the time of hematologic assessment.
Model adjusted for helminth infection at the time of hematological assessment, family possession, and gravidity.
Model adjusted for family possession and gravidity.
Model adjusted for prepregnancy BMI, and sex.
Model adjusted for prepregnancy BMI, gravidity, and Edinburgh Postnatal Depression Scale score.
P < .05.
Discussion
In this current study, we found no relationship between prenatal ID or IDA or CBSF concentration and cognitive and motor functions of 1-year-old children. Our study also revealed that ID during pregnancy is not associated with lower CBSF concentrations in the context of iron supplementation. We, however, found that IDA around delivery was associated with low CBSF concentrations. To our knowledge, this is the first study to investigate the impact of prenatal ID on infant cognition in Benin in the context of prescribed iron supplementation and prophylaxis against malaria and helminthes.
Our findings are similar to a recent randomized clinical trial in Hebei, China, that revealed no difference in GM function of children whose mothers were in the iron supplementation arm and those whose mothers were in the placebo arm during pregnancy.22 Our results, however, contradict the findings of another study conducted among a rural Chinese population, in which Hb concentration was used as a marker for IDA in pregnancy. In the Chinese study, the authors reported that children of mothers with IDA had poorer mental development compared with those of non-IDA mothers.23 The contradictory findings may arise from the accurate marker of maternal iron stores, serum ferritin used in our studies, and the account for inflammation that may result in high serum ferritin levels. In our study population, the etiology anemia is multifactorial (helminth infections, malaria, other micronutrient deficiencies) and as such using Hb concentration as a marker for ID may have been misleading. The relationship between prenatal Hb concentration and infant cognitive and motor development in our study population has already been reported in an earlier publication.13
Considering the vulnerability of the fetus to prenatal insults and the essential role of iron in neurodevelopment, it is surprising that we found no association between prenatal ID and infant cognitive development. However, during pregnancy, the fetus has priority to iron supply over the pregnant woman’s needs to ensure adequate iron stores within the first 6 months of life and thus leaving the mother deficient of iron in the absence of adequate supplementation.24,25 This could explain the absence of difference in CBSF between iron deficient and noniron deficient pregnant women although IDA at delivery was associated with lower CBSF concentrations in our study. Other studies among pregnant women have revealed similar findings of fetal impassivity (in reference to ferritin supply) to the ID status of the mother.26,27
In our study population, after the first ANC, all pregnant women were given daily oral iron and folic acid supplementations in addition to the IPTp they were randomized to, in the MiPPAD clinical trial. Pregnant women who remained iron deficient at the second ANC visit and at delivery may have had very low iron stores before pregnancy and as a result remained deficient after fetal demands even after supplementation or may not have taken the supplements, as the intake was not directly observed. The lack of association between prenatal ID and infant cognitive development may result because the fetal iron supply may have been adequate in iron deficient pregnant women who were offered supplementation as in noniron deficient pregnant women.
In the recent past, a substantial number of researchers have shown interest in investigating the impact of ID in infancy on cognitive functions and behavior of children in human subjects as well as in animal models.28,29 However, few studies have been published assessing the impact of prenatal or fetal ID on early child cognitive development. Evidence emanating from randomized controlled trials comparing the cognitive function or behavior of children between pregnant women who had iron (iron and folic acid) supplementation to a placebo or iron and folic acid, to folic acid only treatments are irreconcilable. In a trial among the 4-year-old children of 430 women randomly assigned to daily supplementation of iron or placebo, regardless of previous anemia or ID, from the 20th week of gestation to delivery in Adelaide, Australia, the authors found no difference in the cognitive function.30 A follow-up of these children when they were 6 to 8 years old further confirmed the previous findings of no difference in child behavior among children of iron supplemented and placebo mothers.31 Instead, the authors reported a higher proportion of abnormal behavior among children of iron-supplemented group. However, another trial in Nepal that later assessed cognitive function of 7- to 9-year-old children revealed benefits of iron supplementation in pregnancy for better cognitive function compared with only vitamin A supplementation.10
Although malaria and ID are both important risk factors of anemia in sub-Saharan Africa, the antagonistic role of iron metabolism and malaria remains a controversy among researchers. In a study in Kenya among children of 8 months to 8 years, the authors found a lower incidence of malaria among iron deficient children.32 A similar association has also been reported among pregnant women by other researchers.33 It is hypothesized that the demand for iron by the Plasmodium parasite for growth contributes the disadvantage of iron sufficiency in the presence of malaria. It is for this reason that we adjusted for malaria at the time of ferritin assessment in our analysis in Table 4.
One limitation of this current study is that the intake of the daily iron supplementation among pregnant women was not monitored. Also, because it is unethical to not administer iron supplements to pregnant women, we cannot clearly report the impact of ID in pregnancy in the absence of iron supplementation, on the cognitive development of children. The inability to obtain hematologic data from all children in the study was another limitation to this study, but there was little or no selection bias as maternal and infant characteristics were generally similar among children who had hematologic assessments and those who did not receive hematologic assessments and among those who had MSEL assessments and those who did not. Further, our study also provides an extensive analysis on the impact of ID from the prenatal phase to age 1 year on the cognitive development of children adjusting for all the necessary confounders at each stage of follow-up.
Conclusions
Our study revealed that in a malaria-endemic region, ID in pregnancy, in the context of iron supplementation, is not associated with CBSF concentration, and infant cognitive and motor development. Prenatal IDA around delivery is associated with lower CBSF concentrations. These findings are within a population that implemented current recommendations and we cannot report what could have occurred in the absence of iron supplementation. Hence, antenatal iron supplementation, especially to iron deficient pregnant women as per international guidelines, is encouraged as our findings are reported. Because active brain development continues even after age 1 year, follow-up studies are required to determine the long-term impact of perinatal IDA on infant cognitive development.
Acknowledgments
We are indebted to Drs Ghislain K. Koura, Smaïla Ouédraogo, and Manfred M. K. Accrombessi.
Glossary
- AMD
adjusted mean difference
- ANC
antenatal care
- CBSF
cord blood serum ferritin
- CI
confidence interval
- CRP
C-reactive protein
- ELC
Early Learning Composite
- GM
gross motor
- Hb
hemoglobin
- HOME
Home Observatory Measurement of the Environment
- ID
iron deficiency
- IDA
iron deficiency anemia
- IPTp
intermittent preventive treatment of malaria in pregnancy
- IQR
interquartile range
- MiPPAD
Malaria in Pregnancy Preventive Alternative Drugs
- MSEL
Mullen Scales of Early Learning
Footnotes
The opinions represented here are the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Dr Mireku coordinated and supervised data collection of the TOVI study from the 3 study sites, performed and interpreted data analyses, drafted the initial manuscript, and revised the manuscript; Dr Boivin trained research nurses to use the Mullen Scale of Early Learning cognitive assessment and critically reviewed and revised the manuscript; Dr Davidson designed the TOVI study and critically reviewed and revised the manuscript; Mr Zoumenou supervised initial data collection of the TOVI study and critically reviewed and revised the manuscript; Drs Massougbodji and Cot acquired funding for the Malaria in Pregnancy Preventive Alternative Drugs study, conceptualized, designed and supervised the Malaria in Pregnancy Preventive Alternative Drugs trial, and reviewed and revised the initial manuscript; Dr Bodeau-Livinec conceptualized and designed the TOVI study, acquired funding for the TOVI study, trained research nurses to use the Mullen Scale of Early Learning cognitive assessment, supervised initial data collection, and reviewed and revised the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded The TOVI study, grant R21-HD060524. The Malaria in Pregnancy Preventive Alternative Drugs trial (NCT00811421) was co-funded by the European and Developing Countries Clinical Trials Partnership (EDCTP- IP.07.31080.002). The Fondation pour la Recherche Médicale in France provided final year doctoral funds for Dr Mireku. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454 [DOI] [PubMed] [Google Scholar]
- 2.Pasricha S-R, Drakesmith H, Black J, Hipgrave D, Biggs B-A. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121(14):2607–2617 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29 [PubMed] [Google Scholar]
- 4.World Health Organization Iron deficiency anaemia: assessment, prevention and control. Available at: www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/. Accessed January 30, 2014
- 5.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23(1):41–58 [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165 [DOI] [PubMed] [Google Scholar]
- 7.Garner AS, Shonkoff JP, Siegel BS, et al. ; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics . Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e224 [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Corapci F, Burden MJ, et al. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J Nutr. 2007;137(3):683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency in infancy on patterns of motor development over time. Hum Mov Sci. 2006;25(6):821–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian P, Murray-Kolb LE, Khatry SK, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304(24):2716–2723 [DOI] [PubMed] [Google Scholar]
- 11.Hussain MA, Gaafar TH, Laulicht M, Hoffebrand AV. Relation of maternal and cord blood serum ferritin. Arch Dis Child. 1977;52(10):782–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Rai AK, Basu S, Dash D, Singh JS. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121(3). Available at: www.pediatrics.org/cgi/content/full/121/3/e673 [DOI] [PubMed] [Google Scholar]
- 13.Mireku MO, Davidson LL, Koura GK, et al. Prenatal hemoglobin levels and early cognitive and motor functions of one-year-old children. Pediatrics. 2015;136(1). Available at: www.pediatrics.org/cgi/content/full/121/3/e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González R, Desai M, Macete E, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLoS Med. 2014;11(9):e1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell BM, Bradley RH. Home Inventory Administration Manual. Little Rock, AR: University of Arkansas for Medical Sciences; 2001 [Google Scholar]
- 16.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786 [DOI] [PubMed] [Google Scholar]
- 17.Raven J. The Raven’s progressive matrices: change and stability over culture and time. Cognit Psychol. 2000;41(1):1–48 [DOI] [PubMed] [Google Scholar]
- 18.Planche T, Krishna S, Kombila M, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001;65(5):599–602 [DOI] [PubMed] [Google Scholar]
- 19.Beard J, deRegnier R-A, Shaw MD, Rao R, Georgieff M. Diagnosis of iron deficiency in infants. Lab Med. 2007;38(2):103–108 [Google Scholar]
- 20.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995 [Google Scholar]
- 21.Koura KG, Boivin MJ, Davidson LL, et al. Usefulness of child development assessments for low-resource settings in francophone Africa. J Dev Behav Pediatr. 2013; 34(7):486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angulo-Barroso RM, Li M, Santos DCC, et al. Iron supplementation in pregnancy or infancy and motor development: a randomized controlled trial. Pediatrics. 2016;137(4):e20153547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e755 [DOI] [PubMed] [Google Scholar]
- 24.Shao J, Lou J, Rao R, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sizer F, Whitney E. Nutrition: Concepts and Controversies, MyPlate Update, 12th ed. Belmont, CA: Cengage Learning; 2011 [Google Scholar]
- 26.Harthoorn-Lasthuizen EJ, Lindemans J, Langenhuijsen MM. Does iron-deficient erythropoiesis in pregnancy influence fetal iron supply? Acta Obstet Gynecol Scand. 2001;80(5):392–396 [PubMed] [Google Scholar]
- 27.Sangaré L, van Eijk AM, Ter Kuile FO, Walson J, Stergachis A. The association between malaria and iron status or supplementation in pregnancy: a systematic review and meta-analysis. PLoS One. 2014;9(2):e87743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48(2):169–176 [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152(5):696–702, 31–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr. 2006;83(5):1112–1117 [DOI] [PubMed] [Google Scholar]
- 31.Parsons AG, Zhou SJ, Spurrier NJ, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long-term follow-up of a randomised controlled trial. Br J Nutr. 2008;99(5):1133–1139 [DOI] [PubMed] [Google Scholar]
- 32.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190(3):439–447 [DOI] [PubMed] [Google Scholar]
- 33.Moya-Alvarez V, Cottrell G, Ouédraogo S, Accrombessi M, Massougbodgi A, Cot M. Does iron increase the risk of malaria in pregnancy?. Open Forum Infect Dis. 2015;2(2):ofv038. [DOI] [PMC free article] [PubMed] [Google Scholar]