Abstract
OBJECTIVE:
To identify pathways through which pre- and postnatal factors directly or indirectly affect infant neurodevelopment at 12 months of age among Filipino infants.
METHODS:
The Bayley Scales of Infant Development, third edition was used to assess the development of 314 infants of mothers enrolled in a trial examining the safety and efficacy of praziquantel during pregnancy. Maternal covariates included socioeconomic status, iron and nutritional status, cognitive performance, and alcohol intake. Infant covariates included birth weight and feeding practices, longitudinal growth and nutritional status, hemoglobin and iron status captured at birth, and 6 and 12 months of age. Multivariable regression and structural equation modeling were used to identify significant factors associated with infant development.
RESULTS:
In regression models, maternal education, cognition, and iron status as well as infant weight-for-age z-score (WAZ), weight-for-length z-score, and WAZ gains were significantly associated with infant development at 12 months of age. Structural equation modeling demonstrated a direct effect of maternal cognition on most subscales of infant development and indirect effects on expressive language through effects on infant WAZ. Maternal iron status was a stronger predictor of infant cognition subscale scores than was infant iron status. Exclusive breastfeeding had a direct influence on expressive language rather than acting through improved infant iron or nutritional status.
CONCLUSIONS:
We identified key modifiable risk factors for impaired neurodevelopment, including prenatal risk factors such as maternal iron status. Integrated nutritional interventions that impact both maternal and infant nutritional status are likely to positively affect infant neurodevelopment through identified pathways.
What’s Known on This Subject:
Studies have identified independent risk factors for impaired infant neurodevelopment, including birth weight, nutritional status, and feeding practices, yet few examined the pathways through which these factors directly or indirectly influence infant neurodevelopment.
What This Study Adds:
A longitudinal examination of risk factors from early gestation to 12 months of age identified mechanistic pathways through which known (maternal cognition, infant nutrition) and newly identified (maternal iron status) risk factors impact neurodevelopment.
By 2005, the World Bank had financed $1.7 billion in loans to 52 low- and middle-income countries (LMICs) to design early child development programs.1 Nevertheless, more than 200 million children <5 years of age in LMICs, representing approximately one-third of children living in this context, fail to achieve their developmental potential.2 In separate studies that included a broader age range, developmental disabilities among children aged 2 to 9 years were identified in 3% to 48% (median, 23%) of children in 18 LMICs assessed.3 Cognitive and behavioral development during infancy and childhood affects general and mental health and well-being in adulthood.4,5 Therefore, identifying risk factors, which may begin in utero, and understanding pathways through which they contribute to neurodevelopmental deficits are key steps to inform the timing and content of interventions.
Cognitive, language, and motor development of children at different stages can be affected by numerous and varying factors.6 As these factors are often complexly interdependent, the use of independent regression models is cumbersome. In addition, these models may not elucidate mechanisms through which early factors influence other more proximate risk factors, which may underestimate their contribution. This has important implications for the development of interventions, as more distal causes and their contribution to both proximate causes and neurodevelopmental outcomes may otherwise be underestimated, thus missing potential opportunities to intervene. Structural equation modeling (SEM) is a powerful statistical approach to address interdependent relationships among multiple variables simultaneously, and thus may be useful to investigate causal pathways associated with infant development beginning in early pregnancy.
Although studies have investigated postnatal factors influencing infant development, few have captured key prenatal factors and used SEM to understand the mechanistic pathways through which these factors affect development. Further, no studies using SEM have been conducted in the LMIC context, where iron deficiency, undernutrition, decreased educational opportunity, and poverty are likely to have a profound effect on neurodevelopment. The objective of this study was to identify risk or protective factors that directly or indirectly influence infant neurodevelopment as captured by the Bayley Scales of Infant Development, third edition (BSID-III). We addressed this objective by constructing SEM models to examine potential mechanisms through which measured predictors influence BSID-III subscale and composite scores.
Methods
Study Participants
Study subjects were infants of pregnant women living in Leyte, the Philippines, who were enrolled in a phase 2 randomized placebo-controlled trial to examine the effects of praziquantel given at 12 to 16 weeks of gestation on pregnancy outcomes. Details of the trial are described elsewhere.7 Briefly, 370 pregnant women were recruited in their villages or municipal health centers by trained midwives. To be eligible to participate, women had to be infected with Schistosoma japonicum; be otherwise healthy as determined by history, physical examination, and laboratory studies; and have a viable, singleton gestation. Women were enrolled and randomly assigned to the praziquantel or placebo groups. The trial captured birth weight and health status of 361 newborns. Of these, 314 infants had BSID-III administered between 332 and 430 days after birth, and are included in this study. Of note, treatment with praziquantel did not affect the primary outcome, birth weight.
Primary Determinants
Demographic Covariates
Household socioeconomic status (SES) was captured in 4 domains determined a priori as previously developed and validated in this study area: educational status, social class position, material wealth, and a composite SES score by using all questionnaire items.8 Scores for each of these 4 domains reflect numeric weights assigned to SES questionnaire items, as described by Filmer and Pritchett,9 by using the FACTOR procedure in SAS 9.3 (SAS Institute Inc, Cary, NC). The primary determinant used was the derived summary SES variable, a weighted combination of all items, which was dichotomized. Maternal education was also evaluated separately and dichotomized as having completed high school or not.
Nutritional Covariates
At 12 to 16 weeks of gestation, maternal height and weight (model BD-585 portable scale; Tanita, Arlington Heights, MD) were measured to 0.1 cm and 0.1 kg, respectively. At 32 weeks’ gestation, maternal complete blood count was captured as well as iron status by using a multiplex bead-based platform (Bio-Rad, Hercules, CA) as described previously.7 Iron status was determined by quantifying serum Transferrin Receptor (sTfR), a receptor predominantly expressed by red blood cells when they are iron thirsty, and ferritin, indicative of stored iron. The sTfR:log10(ferritin) ratio (sTfR-F index) at 32 weeks’ gestation was used as the primary measure of maternal iron status, as it is thought to be more robust in the setting of inflammation and has been validated against a gold standard of bone marrow iron in a setting with high rates of infection and inflammation.10
Newborn birth weight was measured to within 0.01 kg within 48 hours of delivery by using a portable Tanita digital infant scale. Small-for-gestational age (SGA), used as an indicator for possible intrauterine growth restriction, was defined based on gestational age determined at the 12- to 16-week ultrasound. SGA was defined as a birth weight below the 10th percentile for gestational age based on the sex-specific curves generated for close to 1 million healthy Chinese newborns, to attenuate genetic contribution to SGA.11 Prematurity was defined as gestation <37 weeks. Low birth weight (LBW) was defined as birth weight <2.5 kg. Length was measured in a recumbent position by using a length board stadiometer (Ellard Instrumentation Ltd, Monroe, WA). Weight-for-age (WAZ), length-for-age (LAZ), weight-for-length (WLZ) z-scores were calculated by using the 2006 World Health Organization Anthro Growth Standards (www.who.int/childgrowth/software/en/).
At 1, 6, and 12 months of age, the infant was again weighed and measured and WAZ, LAZ, and WLZ were derived. In addition, sTfR, ferritin levels, and sTfR-F index were quantified at birth, and 6 and 12 months of age as described. To assess change in nutritional status and linear growth, we additionally calculated WAZ, LAZ, and WLZ gains per month between birth and 12 months.
Maternal Cognitive Function.
Maternal cognition was captured with the Philippine Nonverbal Intelligence Test (PNIT), which measures concept recognition and abstract thinking.12 In our previous studies in the same study area, interrater and test-retest reliability for this test were found to be excellent (0.99 and 0.89, respectively).9 Participants are asked to choose which 1 of 5 drawings is conceptually different from the other 4. The score (range, 0–100) was the total number of correct responses.
Primary Outcome
Infant cognitive function was assessed at 12 months of age with the BSID-III. The BSID-III consists of 3 primary developmental scales: cognitive, language (composed of receptive and expressive communication subscales), and motor (composed of fine and gross motor subscales). The social/adaptive scales were not evaluated, as they were too difficult to adapt to this cultural setting. The 5 raw subscales were converted to scaled scores by age grouping (30-day windows).13 Scaled scores were then used to derive the 3 composite BSID scores.13
Statistical Analysis
sTfR and ferritin levels and sTfR-F index were natural log-transformed before regression modeling. If sTfR or ferritin were below detection, the level of those was recorded as the detection limit. As some ferritin levels produce negative levels in sTfR-F index, integer 1 was added to ferritin values before producing sTfR-F index.
Associations between predictors and BSID-III subscale scores were first evaluated by univariable linear regression models. All variables listed in Table 1, except for BSID-III test age, were analyzed in the statistical modeling. Predictors with a P < .2 in the univariable model were considered for inclusion in multivariable modeling. If >1 predictor capturing a related construct had a P < .2 (eg, ferritin levels and sTfR-F index), we selected a representative predictor that was the most significant. Five multivariable linear regression models were built, 1 for each of the BSID-III subscales, by using forward stepwise selection; predictors that were significant at P < .05 were retained. In multivariable modeling, the expanded model included predictors whose addition significantly decreased residual deviance. Significant differences of model deviance between 2 nested models were evaluated based on the likelihood ratio test (P < .05). With the exception of SEM, all statistical analyses were performed by using SAS 9.4 software.
TABLE 1.
Characteristics of Study Participants
| n | Mean (95% CI) or Proportion | |
|---|---|---|
| Maternal characteristics | ||
| Treatment with praziquantel | 314 | 49.0 |
| Age, y | 314 | 26.0 (25.3 to 26.7) |
| Weight at 12 wk gestation, kg | 314 | 47.7 (46.9 to 48.6) |
| Height, cm | 314 | 147.3 (146.7 to 147.9) |
| BMI at 12 wk gestation, kg/m2 | 314 | 22.0 (21.6 to 22.3) |
| High education (≥high school), % | 312 | 58.7 |
| High SES (≥median), % | 314 | 48.1 |
| Parity, number | 314 | 3.57 (3.33 to 3.81) |
| PNIT score | 241 | 62.5 (60.7 to 64.3) |
| Hemoglobin at 32 wk gestation, g/dL | 312 | 11.0 (10.9 to 11.1) |
| sTfR at 32 wk gestation, mg/La | 312 | 1.27 (1.23 to 1.32) |
| Ferritin at 32 wk gestation, ng/mLa | 312 | 6.2 (5.0 to 7.8) |
| sTfR-F index at 32 wk gestationa | 312 | 1.97 (1.68 to 2.29) |
| Smoker at 12 wk gestation, % | 314 | 0.3 |
| Alcohol drinker at 12 wk gestation, % | 314 | 78.3 |
| Delivery and infant characteristics | ||
| Gestational age, mo | 314 | 38.6 (38.5 to 38.8) |
| Boys, % | 314 | 54.8 |
| BSID-III test age, d | 314 | 369.9 (368.6 to 371.1) |
| Exclusively breastfeeding, % | 297 | 88.6 |
| At birth | ||
| SGA, %b | 314 | 33.8 |
| LBW, %c | 314 | 13.1 |
| WAZ | 314 | −0.98 (−1.08 to −0.87) |
| LAZ | 314 | −1.46 (−1.66 to −1.26) |
| WLZ | 245 | −0.4 (−0.59 to −0.2) |
| sTfR, mg/La | 228 | 2.92 (2.77 to 3.03) |
| Ferritin, ng/mLa | 303 | 129.0 (116.7 to 141.2) |
| sTfR-F indexa | 228 | 1.42 (1.34 to 1.49) |
| At 12 mo | ||
| WAZ | 310 | −1.62 (−1.72 to −1.51) |
| LAZ | 310 | −1.24 (−1.35 to −1.13) |
| WLZ | 310 | −1.38 (−1.49 to −1.27) |
| Hemoglobin, g/dL | 307 | 11.8 (10.7 to 13.0) |
| sTfR, mg/La | 305 | 2.72 (2.61 to 2.83) |
| Ferritin, ng/mLa | 308 | 3.42 (2.69 to 4.35) |
| sTfR-F indexa | 305 | 5.75 (4.81 to 6.82) |
| z-score gain/mo | ||
| WAZ | 310 | −0.05 (−0.06 to −0.04) |
| LAZ | 310 | 0.02 (0.00 to 0.03) |
| WLZ | 241 | −0.07 (−0.09 to −0.06) |
| BSID-III | ||
| Raw scores | ||
| Cognitive | 314 | 41.5 (41.2 to 41.9) |
| Receptive language | 314 | 12.1 (12.0 to 12.3) |
| Expressive language | 314 | 11.0 (10.8 to 11.2) |
| Fine motor | 314 | 28.6 (28.4 to 28.9) |
| Gross motor | 314 | 40.2 (39.8 to 40.7) |
| Scaled scores | ||
| Cognitive | 314 | 10.8 (10.6 to 11.0) |
| Receptive language | 314 | 7.2 (7.0 to 7.4) |
| Expressive language | 314 | 7.0 (6.8 to 7.2) |
| Fine motor | 314 | 9.9 (9.7 to 10.2) |
| Gross motor | 314 | 9.1 (8.8 to 9.4) |
| Composite scaled scores | ||
| Cognitive | 314 | 102.1 (100.4 to 103.8) |
| Language | 314 | 83.2 (82.3 to 84.1) |
| Motor | 314 | 95.6 (94.1 to 97.2) |
Geometric mean (95% confidence interval [CI]).
Defined as birth weight <10th percentile for gestational age.
Defined as birth weight <2.5 kg.
SEM was used to examine explanatory models for BSID-III subscale scores suggested by results of multivariable models by using Stata SEM Builder (Stata 14.0; StataCorp, College Station, TX). SEM provides simultaneous estimation of associations among variables as well as the estimation of individual associations. Therefore, SEM may be useful in confirming or refuting a theoretical framework suggested by multivariable modeling and mechanistic pathways culminating in a specific outcome.
A simple SEM was initially constructed based on the multivariable models for 5 BSID-III subscale scores. The language and motor subscales were evaluated in distinct models as we hypothesized that specific predictors might differently influence these subscales. Each variable in multivariable models was iteratively considered alone and in combination, because many predictors were correlated. Predictors that were not retained in the multivariable models, but were correlated with other predictors in the multivariable models (Supplemental Table 4) were also considered for inclusion in the SEM. Finally, BSID-III subscale scores were connected to 3 composite scores to evaluate the indirect effects of predictors on those 3 summary scores. The model with appropriate measure of fit and theoretical validity was then chosen. A good model was considered to have relative χ2 (χ2/df) <3 with P > .05 of χ2, the root mean square error of approximation <0.07, the comparative fit index >0.95, and the standardized root mean squared residual <0.08.14
Results
Characteristics of 314 mother-infant pairs for whom infant development was captured at 12 months of age is presented in Table 1. Infants who completed the BSID-III (n = 314) were not significantly different from noncompleters (n = 47) with respect to maternal, delivery, and infant characteristics, including maternal treatment status, age, parity, education, SES, and infants’ sex, birth weight, and feeding practices (data not shown). The mean maternal age was 26.0 years and the mean age at which the BSID-III was completed was 369.9 days. A total of 88.6% infants were exclusively fed breast milk at 12 months of age; 33.8% and 13.1% of infants were SGA and LBW, respectively.
In univariable models for 5 subscale scores, maternal education and PNIT scores were the most significant predictors across the subscales (Supplemental Table 5). In multivariable models, higher maternal PNIT score significantly increased all subscale scores with the exception of receptive language (Table 2). The cognitive subscale was additionally associated with maternal sTfR-F index; expressive language with exclusive breastfeeding and WAZ at 12 months; fine motor with maternal randomization to the praziquantel arm; and gross motor with WLZ at 12 months. Higher receptive language scores were associated with maternal education and higher infant WLZ at 12 months of age.
TABLE 2.
Multivariable Regression Models for Each of BSID-III Subscale Scores at 1 Year of Age
| Variable | n | Coefficient (95% CI) | P |
|---|---|---|---|
| Cognitive | |||
| Maternal PNIT score | 239 | 0.022 (0.003 to 0.041) | .020 |
| Maternal Ln (sTfR-F index) | −0.25 (−0.44 to −0.07) | .007 | |
| Receptive language | |||
| Maternal education (≥high school) | 308 | 0.51 (0.13 to 0.90) | .009 |
| WLZ at 12 mo | 0.19 (0.00 to 0.38) | .045 | |
| Expressive language | |||
| Maternal PNIT score | 224 | 0.015 (0.000 to 0.030) | .048 |
| Exclusively breastfeeding | 0.79 (0.06 to 1.53) | .035 | |
| WAZ at 12 mo | 0.33 (0.12 to 0.55) | .003 | |
| Fine motor | |||
| Maternal use of praziquantel | 241 | −0.70 (−1.28 to −0.13) | .017 |
| Maternal PNIT score | 0.036 (0.016 to 0.056) | <.001 | |
| Gross motor | |||
| Maternal PNIT score | 237 | 0.033 (0.009 to 0.058) | .008 |
| WLZ at 12 mo | 0.51 (0.17 to 0.86) | .004 |
CI, confidence interval; Ln, natural log.
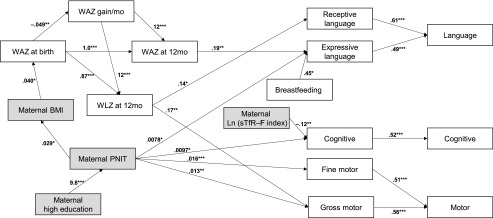
Our SEM model represents a set of theoretical causal pathways based on regression modeling by using observed predictors (Fig 1). Direct, indirect, and total relationships may be inferred from SEM models (Fig 1). For example, a higher maternal PNIT score directly increased the gross motor score (β = 0.013, P = .006), and the higher PNIT score increased the gross motor score through indirect effects on maternal and infant nutritional status. Accordingly, the gross motor subscale score increased by approximately 0.013 for every 1-point increase in maternal PNIT score (β = 0.013, P = .005; Table 3). Of note, in SEM models without the PNIT score, maternal education was significantly and directly related to both language subscales, as well as the cognitive and fine motor subscales (data not shown).
FIGURE 1.
SEM predicting BSID-III subscale scores (n = 222) (relative χ2 [χ2/df], 1.2 [P value of χ2 test, 0.116]; the root mean square error of approximation, 0.027; the comparative fit index, 0.994; the standardized root mean squared residual, 0.065). Arrow indicates significant direct effects with unstandardized coefficients (presented next to arrows). Shaded boxes indicate maternal characteristics. Correlated residuals (ie, error term) were omitted from the diagram. *P < .05, **P < .01, ***P < .001.
TABLE 3.
Total Effect Sizes of Predictors (Unstandardized Coefficient [Standardized Coefficient]) for BSID-III Scores at 1 Year of Age (n = 222)
| Cognitive | Language | Motor | ||||||
|---|---|---|---|---|---|---|---|---|
| Subscale | Composite | Subscale | Composite | Subscale | Composite | |||
| Cognitive | Receptive | Expressive | Fine | Gross | ||||
| Maternal characteristics | ||||||||
| BMI, kg/m2 | — | — | 0.0016 (0.0046)* | 0.0030 (0.0097)* | 0.0024 (0.0075)* | — | 0.0020 (0.0064)* | 0.0011 (0.0033)* |
| High education (≥high school) | 0.095 (0.047)* | 0.050 (0.022) | 0.00046 (0.00021) | 0.077 (0.040) | 0.038 (0.018) | 0.16 (0.075)** | 0.12 (0.062)* | 0.15 (0.069)** |
| PNIT score | 0.0097 (0.14)* | 0.0051 (0.065)* | 0.000047 (0.00060)* | 0.0079 (0.12)* | 0.0039 (0.054)* | 0.016 (0.22)** | 0.013 (0.18)** | 0.016 (0.20)*** |
| Ln (sTfR-F index) | −0.12 (−0.17)** | −0.062 (−0.082)** | — | — | — | — | — | — |
| Infant characteristics | ||||||||
| Exclusively breastfeeding | — | — | — | 0.45 (0.13)* | 0.22 (0.058)* | — | — | — |
| WAZ at birth | — | — | 0.041 (0.032)*** | 0.075 (0.067)*** | 0.061 (0.052)*** | — | 0.051 (0.045)*** | 0.029 (0.023)*** |
| WAZ at 12 mo | — | — | — | 0.19 (0.18)** | 0.091 (0.085)** | — | — | — |
| WLZ at 12 mo | — | — | 0.14 (0.13)* | — | 0.085 (0.085)* | — | 0.17 (0.18)** | 0.097 (0.092)** |
| WAZ gain/mo | — | — | 1.62 (0.12)*** | 2.30 (0.20)*** | 2.12 (0.17)*** | — | 2.02 (0.17)*** | 1.1 (0.087)*** |
| Subscale scores | ||||||||
| Cognitive | — | 0.52 (0.48)*** | — | — | — | — | — | — |
| Receptive language | — | — | — | — | 0.61 (0.66)*** | — | — | — |
| Expressive language | — | — | — | — | 0.49 (0.46)*** | — | — | — |
| Fine motor | — | — | — | — | — | — | — | 0.51 (0.50)*** |
| Gross motor | — | — | — | — | — | — | — | 0.56 (0.51)*** |
Ln, natural log; —, P > .05.
P < .05.
P < .01.
P < .001.
Interestingly, maternal iron status at 32 weeks (sTfR-F index) was more strongly related to the cognitive subscale than was the infant’s own measures of iron status. With respect to exclusive breastfeeding, there was a strong direct effect on expressive language, but not other subscales. Importantly, this was a direct effect rather than an indirect effect through improved infant iron status or nutritional status, as has been hypothesized. The effect of WAZ at birth acted indirectly through WAZ at 12 months of age to affect expressive language, implying that catch-up growth may allow improvement in expressive language that might not occur among smaller newborns who do not experience catch-up growth, as is the case in many resource-poor settings.15–18 This was further supported by the indirect effect of monthly WAZ gains on both language and gross motor subscales.
Discussion
Our study examined the roles of pre- and postnatal maternal and infant determinants of neurodevelopment, including pathways through which these may act in the LMIC context. Importantly, developmental subscales were differentially affected by a variety of pre- and postnatal factors, which one would expect based on the importance of both the timing of specific insults and mechanisms through which they act on different cognitive processes. SEMs allowed us to explain the contributions of those predictors on neurodevelopment, while elucidating potential mechanisms through which pre- and postnatal predictors may affect a range of cognitive domains. Importantly, this allows identification of potentially modifiable pre- and postnatal risk factors that may continue to place infants at risk for cognitive impairment.
Our study identified maternal PNIT score as one of the most important predictors of neurodevelopment, with strong direct effects on 4 of 5 BSID-III subscales. Described as an “intelligence” test, this covariate likely also captures educational experience. This is supported by education’s direct effect on PNIT scores. Also of note, we identified indirect effects of maternal PNIT scores on expressive language and motor development subscales through both maternal and infant nutritional status. A few studies in industrialized nations have attempted to identify both pre- and postnatal factors, including maternal education and IQ, influencing neurodevelopment.19–21 In an Italian birth cohort using SEM, both SES and home environment were key mediators of the effect of maternal IQ on BSID-III cognition and language scores, with the effect of IQ “lost” after considering these covariates.19 In many studies not using SEM, maternal IQ was a strong independent predictor of neurodevelopment.20,21 Some of these studies also note the correlation between maternal IQ and other key predictors of neurodevelopment, such as home environment.19,21,22 Importantly, evaluation of many potential distal and proximal risk factors and the use of SEM allowed us to identify both the direct and indirect pathways through which maternal cognition affects neurodevelopment.
We further found that infant nutritional status most significantly affected BSID-III language and motor subscales. Specifically, there was a direct effect of WAZ at 12 months of age on expressive language and a direct effect of WLZ, a measure of wasting, on gross motor development. This is in agreement with cross-sectional studies that have found an adjusted relationship between WLZ and gross motor development.23–25 In addition, we specifically examined gains in nutritional z-scores given the importance of linear growth and improved WLZ in modifying risk for neurodevelopmental delays.23 Greater gains in WAZ culminating in higher WAZ and WLZ, directly affected the aforementioned subscales, highlighting the importance of infant catch-up growth as a potential buffer. Longitudinal data and SEM allowed us to identify the indirect effect of maternal BMI on these subscales through its impact of WAZ at birth, highlighting the distal, yet persistent, role that maternal nutritional status has on infant neurodevelopment.
Interestingly, our results found that infants who were exclusively breastfed at 12 months demonstrated higher BSID-III score for only the expressive language subscale. Although we evaluated indirect effects through which breastfeeding might affect neurodevelopment, such as infant nutritional status and iron nutriture, the effect was better explained as direct in SEMs. Similarly, in a group of Asian toddlers, increased exposure to breastfeeding was associated with higher scores on tests of both expressive and receptive language,26 and higher BSID-III scores on all subscales except gross motor among Greek toddlers.27 Differences in affected subscales by feeding practices among studies may be due to the differences in defining practices, timing of neurodevelopmental testing, as well as analytic approach. Specifically, many studies have failed to adjust for potential confounders, such as SES, which may overattribute the effect of feeding practices.28 An SEM approach both allows adjustment for confounders, which may explain lack of effect on nonlanguage subscales in this study, as well as capture of mechanistic pathways, which may lead to “dropping” out of covariates. The direct effect of breastfeeding on expressive language may be attributed to the shared network of brain regions controlling speech and swallowing,29 as well as a hypothesized effect of emotional bonding and greater physical proximity during breastfeeding.
In our SEM model, we found a direct effect of maternal iron status at 32 weeks’ gestation on the BSID-III cognition subscale. Interestingly, we did not observe direct or indirect effects of the infant’s own iron status at 6 or 12 months of age on cognition. During human pregnancy, maternal iron deficiency poses a significant threat to fetal iron, which may decrease the iron concentration of the rapidly growing fetal and neonatal brain.30 In rodent models, this culminates in iron-dependent changes in neurometabolism and neuroanatomy during late gestation and the neonatal period, many of which are not reversible.31–33 A recent study conducted in China demonstrated that iron deficiency anemia in the third trimester significantly reduced neurodevelopment at 12, 18, and 24 months of age and this could be corrected by adequate supplementation even if the woman’s anemia was not fully resolved.34
This study has some limitations that should be noted. First, our measure of maternal cognitive function, the PNIT score, likely captures both IQ and aspects of educational opportunity. It is therefore difficult to assess how modifiable this construct is, although the direct effect of educational status on PNIT score and neurodevelopment suggests this may be a modifiable risk factor. Another limitation relates to the selection of primary predictors in SEM; when conducting the same analyses (n = 314) without PNIT score, some maternal characteristics, such as maternal education and SES, were additionally included in the multivariable and SEM models, again suggesting this covariate may be a proxy for the broader context of poverty. With respect to the generalizability of our findings, we would expect that the relationships among risk factors, pathways, and neurodevelopment would be generalizable to other populations, although the attributable risk due to exposures such as maternal iron deficiency that occur at higher frequency in LMICs, may differ from industrialized nations. Finally, the PNIT was originally developed to measure the intelligence of Filipino children; lack of a “ceiling effect” during testing, however, suggests that we were able to capture a full range of cognitive function.12
Conclusions
The longitudinal design of this study beginning in early gestation allowed the evaluation of both distal and proximal risk factors contributing to neurodevelopment. We identified key risk factors and pathways influencing this construct. Importantly, many of these are at least partially modifiable, including maternal cognitive function, which was strongly influenced by educational status, exclusive breastfeeding, infant nutritional status at 12 months of age, and maternal iron status. The influence of key maternal factors, such as iron status, and maternal BMI acting through infant WAZ, highlights the importance of maternal health and nutritional status on the longitudinal development of their infants. The protective effect of infant catch-up growth as well as the direct effect of exclusive breastfeeding provides further support for continued efforts to improve infant nutritional status and promote exclusive breastfeeding.
Acknowledgments
We thank our study staff and participants from the Philippines.
Glossary
- BSID-III
the Bayley Scales of Infant Development, third edition
- LAZ
length-for-age z-score
- LBW
low birth weight
- LMIC
low- and middle-income countries
- PNIT
the Philippine Nonverbal Intelligence Test
- SEM
Structural Equation Model
- SES
socioeconomic status
- SGA
small-for-gestational age
- sTfR
soluble transferrin receptor
- sTfR-F index
sTfR:log10(ferritin) ratio
- WAZ
weight-for-age z-score
- WLZ
weight-for-length z-score
Footnotes
Dr Park conceptualized and designed the study, completed data analysis, drafted the original manuscript, and revised and reviewed the manuscript; Drs Baltazar and Tallo designed the data collection instruments and coordinated and supervised data collection at all sites; Drs Ayaso and Monterde designed the data collection instruments, received training in administration of the Bayley Scales of Infant Development, third edition, and administered these tests, and assessed nutritional status of infants at their follow-up visits; Dr Acosta developed study protocols, submitted institutional review board materials, and coordinated hiring and training of field staff; Dr Olveda co-conceptualized the grants that support this work and worked closely with institutional review boards and Philippines Department of Health to ensure the trial was coordinated with other helminth treatment campaigns; Dr Bellinger provided expertise in the choice of cognitive test and trained the pediatricians in test administration both in Boston and as a refresher in the Philippines; Mr Bennett conducted initial bivariate analyses that informed the Structural Equation Model; Dr Choi provided expertise in statistical analyses and critically reviewed the manuscript; Ms Adamo finalized the data set for analysis and critically reviewed the manuscript; Dr Kurtis developed the laboratory assays and supervised their execution and critically reviewed and revised the manuscript; Dr Friedman conceptualized and designed the study, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00486863).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The randomized controlled trial was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases, “S. japonicum and pregnancy outcomes: An RCT” (grant U01AI066050) with relevant data for this manuscript collected through newborn day of life 28. The Thrasher Research Fund supported the infant follow-up studies, including infant hemoglobin, nutritional status, and neuro-cognitive development. (grant 02826-5). Other support included Alpert Medical School of Brown University Summer Assistantship (Ms Adamo). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Engle PL, Black MM, Behrman JR, et al. ; International Child Development Steering Group . Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369(9557):229–242 [DOI] [PubMed] [Google Scholar]
- 2.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group . Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb CA, Maenner MJ, Cappa C, Durkin MS. Child disability screening, nutrition, and early learning in 18 countries with low and middle incomes: data from the third round of UNICEF’s Multiple Indicator Cluster Survey (2005-06). Lancet. 2009;374(9704):1831–1839 [DOI] [PubMed] [Google Scholar]
- 4.Mensah FK, Hobcraft J. Childhood deprivation, health and development: associations with adult health in the 1958 and 1970 British prospective birth cohort studies. J Epidemiol Community Health. 2008;62(7):599–606 [DOI] [PubMed] [Google Scholar]
- 5.Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Dev. 1986;57(2):251–274 [DOI] [PubMed] [Google Scholar]
- 6.Walker SP, Wachs TD, Gardner JM, et al. ; International Child Development Steering Group . Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157 [DOI] [PubMed] [Google Scholar]
- 7.Olveda RM, Acosta LP, Tallo V, et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16(2):199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezeamama AE, Friedman JF, Acosta LP, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72(5):540–548 [PMC free article] [PubMed] [Google Scholar]
- 9.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132 [DOI] [PubMed] [Google Scholar]
- 10.Phiri KS, Calis JC, Siyasiya A, Bates I, Brabin B, van Hensbroek MB. New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. J Clin Pathol. 2009;62(12):1103–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS One. 2014;9(8):e104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthrie GM, Tayag AH, Jacobs PJ. Philippine Nonverbal Intelligence-Test. J Soc Psychol. 1977;102(1):3–11839743 [Google Scholar]
- 13.Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: Harcourt Assessment; 2006 [Google Scholar]
- 14.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6(1):53–60 [Google Scholar]
- 15.Kebede A, Larson C. The health consequences of intrauterine growth retardation in southwestern Ethiopia. Trop Doct. 1994;24(2):64–69 [DOI] [PubMed] [Google Scholar]
- 16.Sania A, Spiegelman D, Rich-Edwards J, et al. The contribution of preterm birth and intrauterine growth restriction to childhood undernutrition in Tanzania. Matern Child Nutr. 2015;11(4):618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci JA, Becker S. Risk factors for wasting and stunting among children in Metro Cebu, Philippines. Am J Clin Nutr. 1996;63(6):966–975 [DOI] [PubMed] [Google Scholar]
- 18.Adair LS, Guilkey DK. Age-specific determinants of stunting in Filipino children. J Nutr. 1997;127(2):314–320 [DOI] [PubMed] [Google Scholar]
- 19.Ronfani L, Vecchi Brumatti L, Mariuz M, et al. The complex interaction between home environment, socioeconomic status, maternal IQ and early child neurocognitive development: a multivariate analysis of data collected in a newborn cohort study. PLoS One. 2015;10(5):e0127052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong S, Baghurst P, Vimpani G, McMichael A. Socioeconomic position, maternal IQ, home environment, and cognitive development. J Pediatr. 2007;151(3):284–288, 288.e1 [DOI] [PubMed] [Google Scholar]
- 21.Bacharach VR, Baumeister AA. Effects of maternal intelligence, marital status, income, and home environment on cognitive development of low birthweight infants. J Pediatr Psychol. 1998;23(3):197–205 [DOI] [PubMed] [Google Scholar]
- 22.Madigan S, Wade M, Plamondon A, Browne D, Jenkins JM. Birth weight variability and language development: risk, resilience, and responsive parenting. J Pediatr Psychol. 2015;40(9):869–877 [DOI] [PubMed] [Google Scholar]
- 23.Cheung YB, Yip PS, Karlberg JP. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int J Epidemiol. 2001;30(1):66–72 [DOI] [PubMed] [Google Scholar]
- 24.Siegel EH, Stoltzfus RJ, Kariger PK, et al. Growth indices, anemia, and diet independently predict motor milestone acquisition of infants in south central Nepal. J Nutr. 2005;135(12):2840–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudfeld CR, McCoy DC, Fink G, et al. Malnutrition and its determinants are associated with suboptimal cognitive, communication, and motor development in Tanzanian children. J Nutr. 2015;145(12):2705–2714 [DOI] [PubMed] [Google Scholar]
- 26.Cai S, Pang WW, Low YL, et al. ; GUSTO Study Group . Infant feeding effects on early neurocognitive development in Asian children. Am J Clin Nutr. 2015;101(2):326–336 [DOI] [PubMed] [Google Scholar]
- 27.Leventakou V, Roumeliotaki T, Koutra K, et al. Breastfeeding duration and cognitive, language and motor development at 18 months of age: Rhea mother-child cohort in Crete, Greece. J Epidemiol Community Health. 2015;69(3):232–239 [DOI] [PubMed] [Google Scholar]
- 28.Walfisch A, Sermer C, Cressman A, Koren G. Breast milk and cognitive development—the role of confounders: a systematic review. BMJ Open. 2013;3(8):e003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Semin Speech Lang. 2006;27(4):300–309 [DOI] [PubMed] [Google Scholar]
- 30.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165 [DOI] [PubMed] [Google Scholar]
- 31.Clardy SL, Wang X, Zhao W, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;(71):173–196 [DOI] [PubMed] [Google Scholar]
- 32.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126(3):693–701 [DOI] [PubMed] [Google Scholar]
- 33.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48(2):169–176 [DOI] [PubMed] [Google Scholar]
- 34.Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e755 [DOI] [PubMed] [Google Scholar]