Abstract
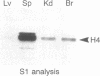
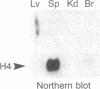
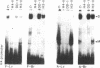
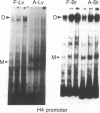
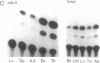
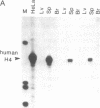
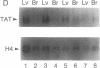
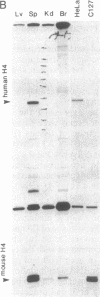
Regulation of the cell cycle-controlled histone gene promoter factor HiNF-D was examined in vivo. Proliferative activity was measured by DNA replication-dependent histone mRNA levels, and HiNF-D binding activity was found to correlate with cell proliferation in most tissues. Furthermore, HiNF-D is down-regulated during hepatic development, reflecting the onset of differentiation and quiescence. The contribution of transcription to histone gene expression was directly addressed in transgenic mice by using a set of fusion constructs containing a human H4 histone gene promoter linked to three different genes. Transgene expression in both fetal and adult mice paralleled endogenous mouse histone mRNA levels in most tissues, consistent with this promoter conferring developmental, cell growth-related transcriptional regulation. Our results suggest that HiNF-D is stringently regulated in vivo in relation to cell growth and support a primary role for HiNF-D in the proliferation-specific expression of H4 histone genes in the intact animal. Further, the data presented here provide an example in which apparent tissue specificity of gene expression reflects the proliferative state of various tissues and demonstrate that multiple levels of histone gene regulation are operative in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artishevsky A., Wooden S., Sharma A., Resendez E., Jr, Lee A. S. Cell-cycle regulatory sequences in a hamster histone promoter and their interactions with cellular factors. 1987 Aug 27-Sep 2Nature. 328(6133):823–827. doi: 10.1038/328823a0. [DOI] [PubMed] [Google Scholar]
- Cheng G. H., Nandi A., Clerk S., Skoultchi A. I. Different 3'-end processing produces two independently regulated mRNAs from a single H1 histone gene. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7002–7006. doi: 10.1073/pnas.86.18.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart D., Ramsey-Ewing A., Bortell R., Lian J., Stein J., Stein G. Isolation and characterization of a cDNA from a human histone H2B gene which is reciprocally expressed in relation to replication-dependent H2B histone genes during HL60 cell differentiation. Biochemistry. 1991 Feb 12;30(6):1610–1617. doi: 10.1021/bi00220a024. [DOI] [PubMed] [Google Scholar]
- Dailey L., Roberts S. B., Heintz N. Purification of the human histone H4 gene-specific transcription factors H4TF-1 and H4TF-2. Genes Dev. 1988 Dec;2(12B):1700–1712. doi: 10.1101/gad.2.12b.1700. [DOI] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. A gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase. EMBO J. 1988 Jan;7(1):49–56. doi: 10.1002/j.1460-2075.1988.tb02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. Maximal binding levels of an H1 histone gene-specific factor in S-phase correlate with maximal H1 gene transcription. Mol Cell Biol. 1988 Oct;8(10):4576–4578. doi: 10.1128/mcb.8.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinari P., La Bella F., Heintz N. Characterization and purification of H1TF2, a novel CCAAT-binding protein that interacts with a histone H1 subtype-specific consensus element. Mol Cell Biol. 1989 Apr;9(4):1566–1575. doi: 10.1128/mcb.9.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes S., Weisz-Carrington P., Daum H., 3rd, Smith J., Green L., Wright K., Stein G., Stein J. A rat histone H4 gene closely associated with the testis-specific H1t gene. Exp Cell Res. 1987 Dec;173(2):534–545. doi: 10.1016/0014-4827(87)90293-x. [DOI] [PubMed] [Google Scholar]
- Hatch C. L., Bonner W. M. The human histone H2A.Z gene. Sequence and regulation. J Biol Chem. 1990 Sep 5;265(25):15211–15218. [PubMed] [Google Scholar]
- Holthuis J., Owen T. A., van Wijnen A. J., Wright K. L., Ramsey-Ewing A., Kennedy M. B., Carter R., Cosenza S. C., Soprano K. J., Lian J. B. Tumor cells exhibit deregulation of the cell cycle histone gene promoter factor HiNF-D. Science. 1990 Mar 23;247(4949 Pt 1):1454–1457. doi: 10.1126/science.247.4949.1454. [DOI] [PubMed] [Google Scholar]
- Hwang I., Chae C. B. S-phase-specific transcription regulatory elements are present in a replication-independent testis-specific H2B histone gene. Mol Cell Biol. 1989 Mar;9(3):1005–1013. doi: 10.1128/mcb.9.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Sharma A., Lee A. S., Maxson R. Cell cycle regulation of H2b histone octamer DNA-binding activity in Chinese hamster lung fibroblasts. Mol Cell Biol. 1989 Feb;9(2):869–873. doi: 10.1128/mcb.9.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger P., Stewart C., Schaap T., van Wijnen A., Hirshman J., Helms S., Stein G., Stein J. Proximal and distal regulatory elements that influence in vivo expression of a cell cycle-dependent human H4 histone gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3982–3986. doi: 10.1073/pnas.84.12.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Gallinari P., McKinney J., Heintz N. Histone H1 subtype-specific consensus elements mediate cell cycle-regulated transcription in vitro. Genes Dev. 1989 Dec;3(12A):1982–1990. doi: 10.1101/gad.3.12a.1982. [DOI] [PubMed] [Google Scholar]
- Liu T. J., Levine B. J., Skoultchi A. I., Marzluff W. F. The efficiency of 3'-end formation contributes to the relative levels of different histone mRNAs. Mol Cell Biol. 1989 Aug;9(8):3499–3508. doi: 10.1128/mcb.9.8.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Pandey N. B. Multiple regulatory steps control histone mRNA concentrations. Trends Biochem Sci. 1988 Feb;13(2):49–52. doi: 10.1016/0968-0004(88)90027-8. [DOI] [PubMed] [Google Scholar]
- Morris T. D., Weber L. A., Hickey E., Stein G. S., Stein J. L. Changes in the stability of a human H3 histone mRNA during the HeLa cell cycle. Mol Cell Biol. 1991 Jan;11(1):544–553. doi: 10.1128/mcb.11.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Aronow M., Shalhoub V., Barone L. M., Wilming L., Tassinari M. S., Kennedy M. B., Pockwinse S., Lian J. B., Stein G. S. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990 Jun;143(3):420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Owen T. A., Holthuis J., Markose E., van Wijnen A. J., Wolfe S. A., Grimes S. R., Lian J. B., Stein G. S. Modifications of protein-DNA interactions in the proximal promoter of a cell-growth-regulated histone gene during onset and progression of osteoblast differentiation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5129–5133. doi: 10.1073/pnas.87.13.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli U., Chrysogelos S., Stein G., Stein J., Nick H. Protein-DNA interactions in vivo upstream of a cell cycle-regulated human H4 histone gene. Science. 1987 Jun 5;236(4806):1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- Peltz S. W., Ross J. Autogenous regulation of histone mRNA decay by histone proteins in a cell-free system. Mol Cell Biol. 1987 Dec;7(12):4345–4356. doi: 10.1128/mcb.7.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Peltz S. W., Kobs G., Brewer G. Histone mRNA degradation in vivo: the first detectable step occurs at or near the 3' terminus. Mol Cell Biol. 1986 Dec;6(12):4362–4371. doi: 10.1128/mcb.6.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D. Multilevel regulation of replication-dependent histone genes. Trends Genet. 1988 Jul;4(7):187–191. doi: 10.1016/0168-9525(88)90074-1. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Paterson B. M. Cell cycle regulation of a mouse histone H4 gene requires the H4 promoter. Mol Cell Biol. 1987 Mar;7(3):1048–1054. doi: 10.1128/mcb.7.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub V., Gerstenfeld L. C., Collart D., Lian J. B., Stein G. S. Downregulation of cell growth and cell cycle regulated genes during chick osteoblast differentiation with the reciprocal expression of histone gene variants. Biochemistry. 1989 Jun 27;28(13):5318–5322. doi: 10.1021/bi00439a002. [DOI] [PubMed] [Google Scholar]
- Sharma A., Bos T. J., Pekkala-Flagan A., Vogt P. K., Lee A. S. Interaction of cellular factors related to the Jun oncoprotein with the promoter of a replication-dependent hamster histone H3.2 gene. Proc Natl Acad Sci U S A. 1989 Jan;86(2):491–495. doi: 10.1073/pnas.86.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber C., Schümperli D. 3' processing of pre-mRNA plays a major role in proliferation-dependent regulation of histone gene expression. Nucleic Acids Res. 1988 Oct 25;16(20):9399–9414. doi: 10.1093/nar/16.20.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., Lian J. B., Van Wijnen A. J., Wright K. L., Pauli U. Modifications in molecular mechanisms associated with control of cell cycle regulated human histone gene expression during differentiation. Cell Biophys. 1989 Dec;15(3):201–223. doi: 10.1007/BF02989684. [DOI] [PubMed] [Google Scholar]
- Stein G., Lian J., Stein J., Briggs R., Shalhoub V., Wright K., Pauli U., van Wijnen A. Altered binding of human histone gene transcription factors during the shutdown of proliferation and onset of differentiation in HL-60 cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1865–1869. doi: 10.1073/pnas.86.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasserot A. P., Schaufele F. J., Birnstiel M. L. Conserved terminal hairpin sequences of histone mRNA precursors are not involved in duplex formation with the U7 RNA but act as a target site for a distinct processing factor. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4345–4349. doi: 10.1073/pnas.86.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D., Hoffman D., Kedes L. Unusual structure, evolutionary conservation of non-coding sequences and numerous pseudogenes characterize the human H3.3 histone multigene family. Nucleic Acids Res. 1987 Apr 10;15(7):2871–2889. doi: 10.1093/nar/15.7.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti G., Stein J., Stein G. Role of messenger RNA subcellular localization in the posttranscriptional regulation of human histone gene expression. J Cell Physiol. 1990 Jul;144(1):175–182. doi: 10.1002/jcp.1041440123. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Stein J., Stein G. Targeting of a chimeric human histone fusion mRNA to membrane-bound polysomes in HeLa cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2683–2687. doi: 10.1073/pnas.84.9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Massung R. F., Stein J. L., Stein G. S. Human H1 histone gene promoter CCAAT box binding protein HiNF-B is a mosaic factor. Biochemistry. 1988 Aug 23;27(17):6534–6541. doi: 10.1021/bi00417a051. [DOI] [PubMed] [Google Scholar]
- van Wijnen A. J., Stein J. L., Stein G. S. A nuclear protein with affinity for the 5' flanking region of a cell cycle dependent human H4 histone gene in vitro. Nucleic Acids Res. 1987 Feb 25;15(4):1679–1698. doi: 10.1093/nar/15.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Wright K. L., Lian J. B., Stein J. L., Stein G. S. Human H4 histone gene transcription requires the proliferation-specific nuclear factor HiNF-D. Auxiliary roles for HiNF-C (Sp1-like) and HiNF-A (high mobility group-like). J Biol Chem. 1989 Sep 5;264(25):15034–15042. [PubMed] [Google Scholar]