Abstract
Cryptococcus neoformans is a fungal pathogen with worldwide distribution. Serological studies of human populations show a high prevalence of human infection, which rarely progresses to disease in immunocompetent hosts. However, decreased host immunity places individuals at high risk for cryptococcal disease. The disease can result from acute infection or reactivation of latent infection, in which yeasts within granulomas and host macrophages emerge to cause disease. In this review, we summarize what is known about the cellular recognition, ingestion, and killing of C. neoformans and discuss the unique and remarkable features of its intracellular life, including the proposed mechanisms for fungal persistence and killing in phagocytic cells.
Keywords: granuloma, Trojan horse, disease tolerance, fungal immunity, intracellular pathogen, C. neoformans killing
Introduction to The Biology of Cryptococcus Neoformans
Environmental Organism and Treatment of Disease
Cryptococcus neoformans was first described in 1894 by Otto Busse, when the organism was recovered from a lesion in a woman's tibia (1). The pathogenic yeast can be found worldwide in several environmental niches and has been isolated from soil, trees, and animals, in particular from avian guano (1, 2). Exposure to C. neoformans does not usually lead to overt disease, and epidemiological data led to the accepted view that establishment of an asymptomatic latent state may be the most common outcome of infection (3–5). Even from the early clinical cases described, an association between cryptococcosis and immunosuppression was already inferred (39, 40). In fact, in immunosuppressed patients, reactivation of infection is frequently fatal. Patients develop pneumonia and meningoencephalitis, and brain involvement predicts high mortality and morbidity, even with aggressive antifungal drug therapy (6).
Immunity to Cryptococcosis
Serological studies show that 80% of children in urban environments have been infected with C. neoformans, without any discernible clinical manifestations (4, 7). Primary infection most likely occurs via inhalation of spores or desiccated yeast cells from environmental sources. The physical characteristics of these infectious particles, such as size and capacity to become airborne, allow deposition in the lungs (8). There, the yeast particles encounter an alveolar macrophage or dendritic cell and trigger an immune response, culminating in sterilization or, most likely, restriction of infection within a granuloma. The resulting granulomas are usually well circumscribed, self-limited, and benign (Figure 1) and are composed mainly of mature mononuclear phagocytes, histiocytes, and giant multinucleated cells enveloping the yeast cells (9).
Figure 1.

Histopathology of Cryptococcus neoformans lung infection. Photomicrographs of lung tissue from Balb/c mice infected with C. neoformans (blue arrowheads), stained with hematoxylin and eosin. (a) Initial infection, showing diffuse pneumonitis and infiltration of immune cells and yeast into the alveolar space (200×). (b) Typical granuloma formation 5 days postinfection (200×). (c) Typical granuloma formation 15 days postinfection (100×). (d) Magnification of panel c, showing the presence of histiocytes (red arrowheads) (400×). (e) At later stages of infection, giant cells (yellow arrowhead) contain C. neoformans (400×). (f) C. neoformans replicating within the alveolar space, visualized by periodic acid–Schiff stain (400×).
Efficient control of C. neoformans requires a delicate balance of both Th1- and Th2-type responses (10–12). Depletion of cytokines by genetic disruption or antibody neutralization has confirmed that a Th1-type response is essential to control infection; these studies are summarized in Table 1. In fact, mouse strains show differential susceptibilities that correlate with a stronger Th1 versus Th2 skewing (13) and with the presence of complement cascade member C5 (14). Depletion of Th1-type cytokines, such as interferon-γ (IFN-γ) and interleukin (IL)-12, consistently results in decreased mouse survival (15, 16), whereas loss of hallmark Th2-type cytokines increases mouse survival (17). In these models, Th1 or Th2 cytokine bias is reflected in both granuloma composition and control of fungal burden (18). Although a predominantly Th1-type response results in mouse survival, too strong of a Th1-type polarization cannot prevent brain dissemination (19–22) and associated mortality, and the Th2 component is required for the most efficient immune response. Although an impressive body of work has been carried out to characterize cytokine dependence, an understanding of immunity to cryptococcosis is still incomplete. For example, lack of the Th1 major cytokine tumor necrosis factor α (TNF-α) did not influence mouse survival, but administration of TNF-α was beneficial (23). As another example, Th17 immunity was crucial for Candida albicans mucosal immunity (24) but appears to play a lesser role in cryptococcal disease: In models of cryptococcosis, deletion of Th17-type responses did not influence the outcome of primary infection or the efficiency of vaccination (25).
Table 1. Role of immune components in mouse model of cryptococcosis.
| Type | Immune polarization | Immune component | Infection route | Outcome when removeda | Reference(s) |
|---|---|---|---|---|---|
| Recognition/binding | — | CD36 | i.v. | Decreased survival | 53 |
| Mannose receptor | i.n. | Decreased survival | 59 | ||
| C5 | i.v. | Decreased survivalb | 14 | ||
| C3 | i.v. | Decreased survival | 52 | ||
| TLR2 | i.p. | Decreased survival | 58 | ||
| TLR4 | i.p. | No or limited effect | 58, 123 | ||
| TLR9 | i.n. | Decreased survival | 61 | ||
| Dectin-1 | i.v. | No effect | 56 | ||
| Immune cells | — | Neutrophils | i.t. | Increased survival | 30 |
| Eosinophils | i.t., i.n. | Increased survivalc | 29, 31 | ||
| Macrophages | i.v. | Decreased survival | 26 | ||
| Macrophages/dendritic cells | i.t, i.v. | Decreased survival | 27 | ||
| B cells | i.v. | No effectd | 124 | ||
| CD4+ T cells | i.t. | Decreased survivalc | 11, 125 | ||
| CD8+ T cells | i.v. | Decreased survival | 11, 126 | ||
| Cytokines | Th1 | IL-12 | i.v. | Decreased survival | 17 |
| Th1 | IFN-γ | i.v., i.t | Decreased survival | 15, 126 | |
| Th1 | IL-18 | i.n., i.t. | Decreased survival | 61, 127 | |
| Th1 | TNF-α | i.t. | No effect | 23 | |
| Th2 | IL-13 | i.n. | Increased survival | 128 | |
| Th2 | IL-4 | i.v. | Increased survival | 17 | |
| Th1/Th17 | IL-23 | i.p, i.v | Decreased survival | 129 | |
| Th17 | IL-17 | i.n. | Important for early response | 25 | |
| Th2 | uPA | i.t. | Decreased survival | 130 | |
| Antiinflammatory | IL-10 | i.v. | Decreased survival | 131 | |
| Inflammatory | IL-6 | i.v. | Earlier death | 131 | |
| Antiinflammatory | TGF-β | i.n. | Mixed resultsc | 132 | |
| Effector molecules | — | Nitric oxide synthase | i.t | Decreased survival rate | 86 |
| NADPH oxidase | i.n. | Increased survivalc | 82 | ||
| Myeloperoxidase | i.n., i.v. | Decreased survival | 92 | ||
| Signaling molecules | — | MyD88 | i.p. | Earlier death | 58 |
Abbreviations: IFN, interferon; IL, interleukin; i.n., intranasal; i.p., intraperitoneal; i.t., intratracheal; i.v., intravenous; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumor necrosis factor; uPA, urokinase-type plasminogen activator.
Compared with wild-type mice.
Inferred from correlating mouse strain susceptibility with presence of C5.
No survival study performed, but conclusion is supported by pathology and cytokine profile.
According to Reference 133, B cells play a role in regulating immunity and establishing protection.
Macrophages are crucial for control of cryptococcosis, as evidenced by the observation that depletion of host macrophages and dendritic cells results in dramatically reduced survival after C. neoformans challenge (26, 27). Two studies of the effects of macrophage depletion on lung fungal burden produced contradictory results (27, 28); however, both studies demonstrated that mouse macrophages require a particular activation profile to become fungicidal (28). Macrophages with a mixed classical and alternative activation phenotype are seen during experimental models of cryptococcosis (19). Although they are less studied, other types of innate immune cells are found in granulomas and may play a role in defense against cryptococcosis (29). The presence of either excess eosinophils or excess neutrophils is associated with poor control of infection in mice (30, 31), whereas eosinophils might have a beneficial role in rats (32).
An extensive body of literature shows that induced or passively administered antibodies can mediate significant protection from cryptococcosis (33). However, the role of humoral immunity in the cryptococcosis model is not adequately explained by classical mechanisms of antibody-mediated immunity, which has led to the discovery of novel immunoregulatory functions of antibodies (33).
Various investigators have addressed the immunological mechanism for effective immunization against C. neoformans challenge (3, 27, 34, 35). For example, immunization with capsular mannoproteins was able to prolong mouse survival (34). An alternative approach was to design an IFN-γ-producing C. neoformans (IFN-γ is a strong Th1-type cytokine) (25, 36–38). This strategy resulted in complete protection from a posterior challenge, accompanied by a Th1-biased lung cytokine pattern, classical activation of macrophages, and increased production of nitric oxide (NO) (37), and demonstrated how appropriate manipulation of the host immune system, in particular macrophage activation, can be an effective therapeutic option. At this time, there is a reasonable consensus that defense against cryptococcosis depends on an appropriate collaboration of Th1 cells with macrophages.
Evidence That Intracellular Residence Contributes to Virulence and Immune Escape
Evidence from Pathological Studies
C. neoformans lesions in autopsies (9, 39–41) and experimental models (42) show fungal cells inside granulomas, known as cryptococcomas (Figure 1b,c). Cryptococcal granulomas are less inflammatory than Mycobacterium tuberculosis granulomas, suggesting a dormant and controlled infection. In well-organized granulomas the yeast is localized within the cytosol of giant cells or macrophages, but in the absence of granulomas yeasts are both intracellular and extracellular (Figure 1f) (41). Neutrophilic infiltrates are not common in human cryptococcal lesions, whereas CD4+ T cells are found in immunocompetent patients.
In rats (42), mice (3, 43), and rabbits (44), C. neoformans can be found associated with lung macrophages, in some cases for months, without obvious clinical manifestations. In mice, C. neoformans is rapidly ingested by phagocytes, and in one model of experimental infection, there was a fluctuation in intracellular and extracellular residence during the first 24 h (43). At day 7, a shift occurred toward the intracellular lifestyle, coincident with formation of granulomas. At day 28, most yeast cells were found within multinucleated giant cells, as illustrated in Figure 1e. In this model, the budding index was higher for intracellular than for extracellular C. neoformans, sparking the hypothesis that intracellular residency is favorable for C. neoformans growth. Hence, both early infection and long-term persistence find C. neoformans cells associated with host macrophages, supporting the importance of intracellular residence within them (43).
Evidence from Animal Models
Animal models have found evidence consistent with the view that fungal residency within macrophages contains the infection while allowing the fungus to persist in tissue. Rats are more resistant than mice are to C. neoformans infection, but the two rodent systems have provided complementary information. Rats' superior resistance to cryptococcosis is associated with a more effective macrophage fungicidal capacity, an effect attributed to increased production by macrophages of lysozyme and reactive oxygen species (ROS) (28, 42). Similar to the situation in mice, C. neoformans resistance in rats is associated with a strong Th1 response balanced with an adequate Th2 component (18). Rats that control infection develop mature granulomas containing eosinophils, whereas rats with an excessive Th1 response develop more inflammatory granulomas with central necrosis and caseation. Early in the course of rat infection, extracellular C. neoformans is prominent, but after granuloma formation the percentage of intracellular fungi increases, with a concomitant reduction in fungal burden (45).
Further evidence that macrophages are required for both control and persistence of disease came from the observation that macrophage depletion can prevent yeast dissemination into the mouse brain (46, 47). This result is consistent with the notion that fungal dissemination to the brain involves the transport of viable yeast cells inside host macrophages. The idea that C. neoformans has a favorable niche within murine macrophages was directly investigated by constructing a yeast strain that could survive only within acidic environments. During the course of infection, an acidic environment is found solely in the phagosome. This strain, although confined to the phagocytic compartment, was still virulent in natural killer– and T cell–depleted mice, indicating that yeast virulence occurs from the intracellular compartment (47). In the same immunosuppressed mice, depletion of alveolar macrophages delayed mouse death, supporting the concept that the macrophages are a niche for intracellular survival of C. neoformans (47).
The Intracellular Life Cycle of Cryptococcus Neoformans
Fungal Entry and Recognition
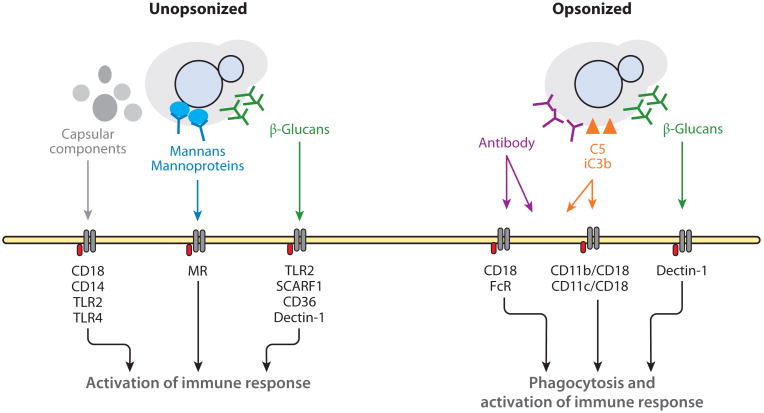
Fungal cell wall components, such as α-glucans, β-glucans, and chitin, are recognized by pattern recognition receptors (PRRs) present in immune cells, triggering cellular activation and, in the case of phagocytic receptors, ingestion of the fungal particle. However, the capsule is highly antiphagocytic, and without opsonins there is no significant ingestion of yeast cells in vitro. Because acapsular C. neoformans is readily ingested through complement receptors and/or β-glucan receptors (48), it has been hypothesized that the large polysaccharide capsule conceals most fungal PRR ligands, thereby decreasing phagocytosis by host cells (Figure 2) (49). In fact, for efficient phagocytosis in vitro (Figure 3), opsonization with antibody or complement is necessary, after which phagocytosis proceeds through a complex interplay of Fc receptors, complement receptors (50), and Dectin-1 (51). Despite the capsule's antiphagocytic properties in vitro, C. neoformans ingestion occurs readily in vivo. The opsonin or the receptor responsible for in vivo ingestion has not been definitively identified. The complement system is the most likely candidate because complement-deficient animals have greater susceptibility to cryptococcosis (14, 52). C. neoformans spores are acapsular, and thus their surfaces expose more β-glucans than do the surfaces of the yeasts; therefore, when spores are the infectious particles, Dectin-1 and other β-glucan PRRs might be readily activated (8) and mediate rapid ingestion of C. neoformans.
Figure 2.
Schematic of recognition of Cryptococcus neoformans by immune cells. Recognition of C. neoformans by immune cells depends on several receptors and extensive cross talk between those receptors. Recognition of capsular components was determined in isolation and likely also occurs for the whole capsule. Most of these receptors are not opsonic, meaning they cannot mediate ingestion. The in vivo opsonins are thought to be serum components iC3b and C5, such that the yeast is ingested via cooperation between complement receptors, FcRs, and possibly Dectin-1. Abbreviations: FcR, Fc receptor; MR, mannose receptor; TLR, Toll-like receptor.
Figure 3.
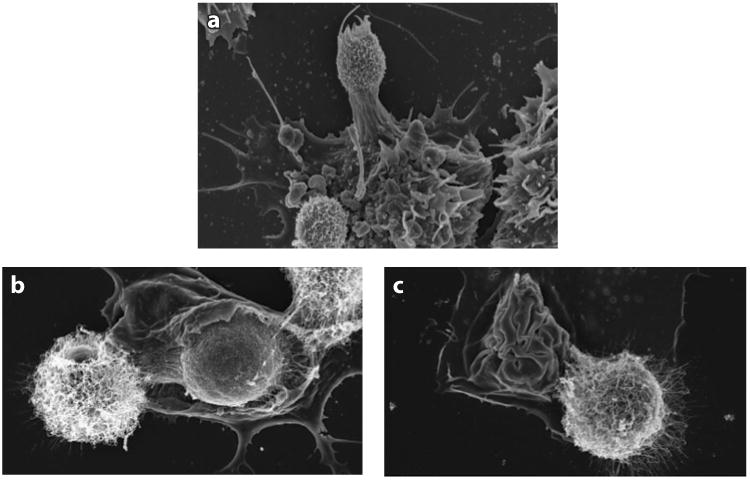
Scanning electron micrographs showing Cryptococcus neoformans and macrophage interaction in vitro. Bone marrow–derived macrophages were infected with antibody-opsonized C. neoformans, and macrophage membranes are shown interacting with yeast cells. (a) Yeast cells are recognized when macrophage membranes probe the extracellular environment around them. (b) Capsulated yeast cells are ingested as the macrophage membrane engulfs them. (c) Ingestion is finalized when the membrane closes upon the yeast cell; a neighboring extracellular yeast is also shown. Panel a courtesy of Sabriya Stukes; panels b and c acquired with the help of Julie M. Wolf.
Cell wall β-glucans can be recognized by Dectin-1, Toll-like receptor 2 (TLR2), Nodlike receptors, and several scavenger receptors. In addition, CD36 and scavenger receptor F1 (SCARF1) are responsible for immune cell binding of C. neoformans in the mouse lung (53). Recognition of the yeast particle is not limited to the immune cell extracellular membrane but continues within the phagolysosome, and even the host cytosol is monitored for the presence of fungal components. In C. albicans infection, Dectin-1 and complement receptors accumulated at sites of phagocytosis but dissociated from the phagosome shortly after internalization, while mannose receptors fused into nascent phagosomes, displaying a coordinated cooperation (54). In contrast, in Aspergillus fumigatus, Dectin-1 remained within the phagosome and was capable of interacting with β-glucans within the acidic compartment (55). Activation of Dectin-1 by β-glucans in vitro led to enhanced macrophage fungicidal activity, presumably because Dectin-1 mediated inflammasome activation and proinflammatory cytokine production (Figure 4a), which can trigger a more effective antifungal response. Therefore, disguise of β-glucans by the C. neoformans capsule may impair maximal macrophage activation. Thus, defects in recognition of C. neoformans by Dectin-1 might explain why mice deficient in Dectin-1 do not have increased susceptibility to C. neoformans infection (56). This hypothesis has been proven in C. albicans, where Dectin-1 dependency is fungal strain dependent due to differences in cell wall composition (57). Other receptors have been shown to be crucial for C. neoformans recognition. Both TLR2- (58) and mannose-deficient mice (59) have decreased immunity to cryptococcal challenge, and the TLR9 receptor is important because of cytosolic detection of fungal DNA (60, 61). In summary, mannose receptor, complement receptors, CD36, SCARF1, TLR2, and TLR9 are all crucial receptors for C. neoformans recognition in the lung, and cross talk between multiple PRRs is necessary for maximal immune response.
Figure 4.
Schematic of immune signaling cascades triggered by Cryptococcus neoformans recognition. (a) Dectin-1 signaling pathway. Dectin-1 can induce both Syk-dependent and Raf (Syk-independent) pathways. Dectin-1 can activate macrophages through the Syk pathway, triggering phagocytosis; following phagocytosis, Dectin-1 activation, coupled to ROS production, contributes to inflammasome activation or fungal killing and activates the transcription factor NF-κB through CARD9, triggering inflammatory cytokine production. The Raf-1 (Syk-independent) pathway enhances NF-κB and inflammatory cytokines. (b) Inflammasome pathway. The Syk-dependent pathway requires combination of two signals. The first signal, which can be mediated by TLR activation, together with a second signal, such as ROS production and/or lysosomal damage, induces the oligomerization of the NLRP3 complex, activation of caspase 1, and production of IL-1β. Abbreviations: ASC, apoptosis-associated speck-like protein containing a C-terminal CARD; Bcl10, B cell leukemia/lymphoma 10; CARD9, caspase recruitment domain–containing protein 9; CLR, C-type lectin receptor; IL, interleukin; MALT-1, mucosa-associated lymphoid tissue 1; NF-κB, nuclear factor κ-light-chain enhancer of activated B cells; NLRP3, Nod-like receptor family, pyrin domain–containing 3; PLCγ2, phospholipase Cγ2; ROS, reactive oxygen species; Syk, spleen tyrosine kinase; TLR, Toll-like receptor; TNF-α, tumor necrosis factor α
Cells other than immune cells might also recognize the presence of C. neoformans, and IL-8 secretion by epithelial cells has been detected (62). Within the lungs, despite extensive adhesion to the epithelium, very little invasion of epithelial cells by C. neoformans occurs (63). However, the yeast is commonly found within lung capillaries and can cross the blood-brain and endothelial barriers, which leads to the conclusions that the yeast is able to cross host tissues (64) and that epithelial cells play a role in the pathogenesis of C. neoformans.
Phagosome Maturation
C. neoformans has not been shown to interfere with phagosomal maturation. A phagosome containing C. neoformans is able to acidify (65), and this acidification is beneficial for fungal replication (65–67). The existing characterization of the C. neoformans phagosome shows that lysosomal fusion occurs (Figure 5) and phagosomes quickly acquire an array of phagosomal markers (Figure 6) (65, 66, 68). Autophagic markers colocalize to the C. neoformans phagosome (65), but the yeast has not been found within an autophagic compartment. Autophagy mediators may perform functions in this phagosome distinct from their canonical functions; such hypothetical activities would explain why depletion of Atg2, Atg5, or Atg9 decreases uptake and/or replication of C. neoformans (65, 69) and why depletion of Atg5 affects survival after C. albicans but not C. neoformans challenge.
Figure 5.
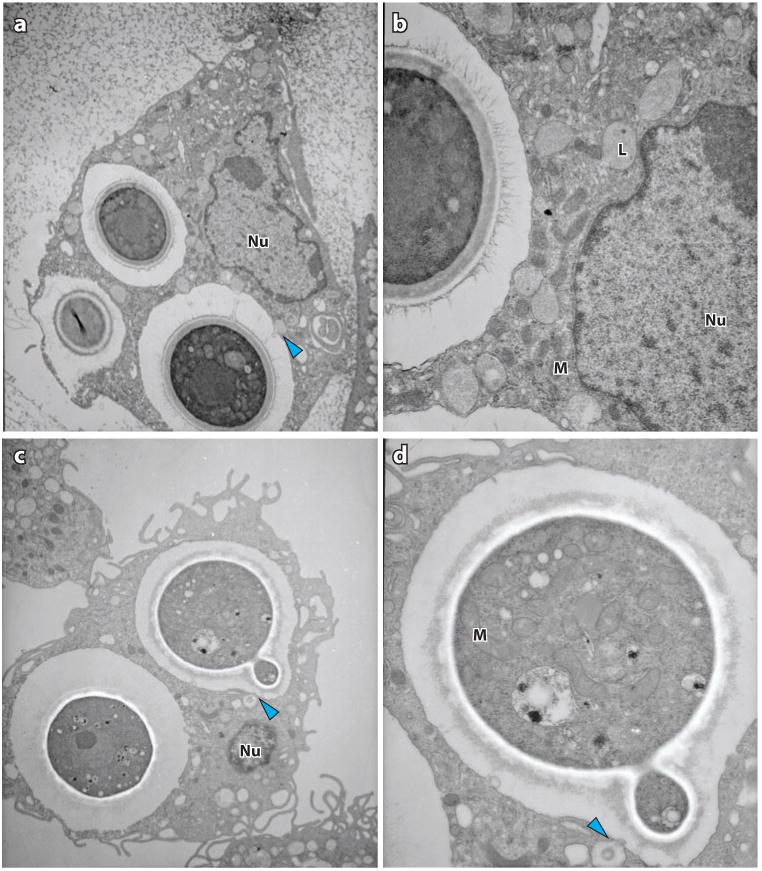
Transmission electron micrographs showing Cryptococcus neoformans and macrophage interaction in vitro. Blue arrowheads indicate possible lysosomal fusion events. (a) Macrophage with ingested C. neoformans. (b) Magnification of panel a, highlighting macrophage organelles, particularly lysosomes, in proximity with the phagosome. (c) C. neoformans budding within a phagosome. (d) Magnification of panel c, displaying C. neoformans organelles. Abbreviations: L, lysosome; M, mitochondrion; Nu, nucleus.
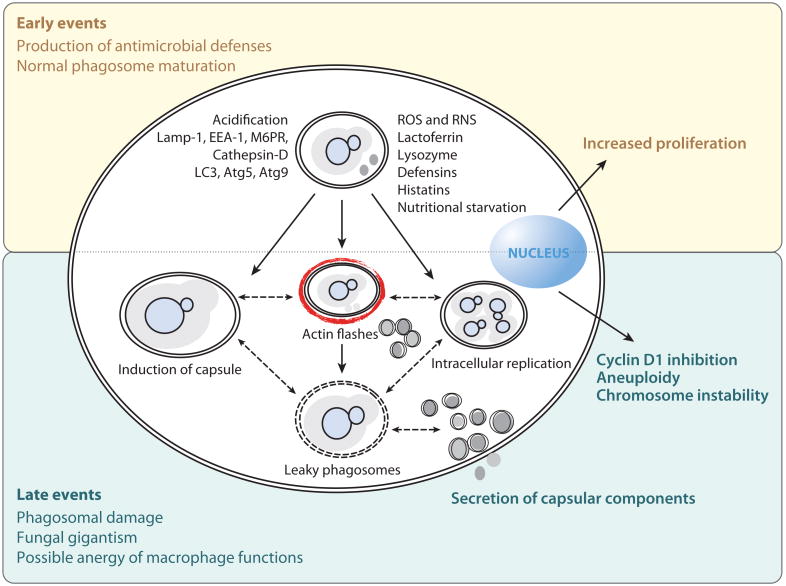
Figure 6.
Phagocytic events upon Cryptococcus neoformans ingestion. To date, no manipulation of the phagocytic compartment by C. neoformans has been described. The interplay between macrophage fungicidal mechanisms and C. neoformans results in host damage, mainly to the phagosomal compartment and to the regulation of the host cell cycle. Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species.
Evidence for Host Cytotoxicity
Despite normal phagosome maturation, macrophage phagosomes become leaky after C. neoformans infection, as measured by light and electron microscopy (70). Leakiness of the phagosome would have a myriad of consequences: loss of acidity, leakage of macrophage-damaging phagosomal enzymes, easy fungal access to cytoplasmic nutrients, and release of strong immunomodulatory capsular components into the cytosol (Figure 4b). At this time, it is not clear whether the leakiness of phagosomes reflects a loss of phagosomal integrity due to macrophage damage, a direct effect of the fungus, or a combination of both. It is also hard to reconcile the fact that the yeast prefers an acidic phagosome with the notion of fungi residency within a leaky, nonacidic phagosome.
Reports of fungal damage to host macrophages are scarce. Lipid peroxidation was observed in rat alveolar macrophages exposed to C. neoformans, which presumably occurs as a result of excessive ROS production by the macrophage (71) and not due to direct fungal toxicity. In vivo, cells that have ingested C. neoformans display features of affected lysosomes; they are known as hueco cells, after the Spanish word for hole, given their perforated appearance in electron microscopy preparations (43). Capsulated, but not acapsular, C. neoformans can trigger apoptosis in macrophages (72), and this observation has been replicated for isolated capsular components (73). Phagocytosis can stimulate proliferation of macrophage cells (74, 75), yet in prolonged C. neoformans infection, ingestion of yeast cells specifically inhibited cyclin D1 expression (75) and decreased macrophage mitosis, indicating cell cycle arrest (76). Similarly, the presence of extracellular yeast triggered aneuploidy and cell cycle impairment in macrophages (72). The realization that fungi, like bacteria such as Mycobacterium tuberculosis (77), can manipulate the host cell cycle to their advantage is an exciting development in fungal pathogenesis. However, the type of macrophage adaptations necessary to support the observed long-term residence of fungal pathogens has not been elucidated.
Killing of Cryptococcus neoformans
Human macrophages restrict C. neoformans growth for up to 24 h after infection (78), a finding indicative of damage to the fungus. Within the phagosome, the yeast is exposed simultaneously to low pH, ROS, reactive nitrogen species, and nutrient starvation (79). These challenges are counteracted by equally powerful mechanisms on the yeast side. Upon ingestion, the yeast upregulates gene expression of oxidative stress enzymes (80), starvation responses, and the autophagic machinery (81). These collaborate with the antioxidant properties of fungal melanin and the capsule to efficiently protect the fungus from host attack. In a model of NADPH oxidase–null mice, cryptococcal infection is contained and the fungal load in both brain and lung is decreased (82), suggesting that inflammatory ROS are prejudicial to the host rather than to the fungus. One antimicrobial molecule proven to be inhibitory to C. neoformans in acidic conditions is NO (83). The enzyme that produces NO, iNOS (inducible nitric oxide synthase), is present in C. neoformans granulomas in the lung (37, 42, 84), and NO has a protective role in the cryptococcosis mouse model (85, 86). The understanding of these complex effects is hampered by the difficulty in separating the direct fungicidal and indirect immunoregulatory effects of NO, but because C. neoformans with defective nitrosative defenses is only slightly less virulent than is wild-type C. neoformans (87), immunoregulation seems to be the predominant effect of NO.
T cells and natural killer cells exert direct antifungal activity, at least in vitro (88, 89), through an unknown mechanism. Neutrophils and dendritic cells can kill opsonized fungi through oxidative and nonoxidative mechanisms (90, 91), and the myeloperoxidase system contributes significantly to antifungal activity against C. neoformans, given that myeloperoxidase-knockout mice have dramatically decreased survival after cryptococcal infection (92). Nonoxidative mechanisms include Cathepsin-B-induced structural changes and rupture of the fungal cell wall (93) in dendritic cells, whereas neutrophils have been reported to use both oxidative burst and nonoxidative molecules such as calprotectin and defensins (91).
In macrophages, microbicidal activity depends on macrophage activation, in which Th1-type responses result in the upregulation of ROS, reactive nitrogen species, proteases, and lipid mediators (94), all of which would render macrophages more effective in pathogen killing. Such Th1 stimulation can also decrease phagosomal hydrolase activity to increase major histocompatibility complex presentation and stimulation of adaptive immunity (95). However, in the case of C. neoformans infection, even IFN-γ stimulation of macrophages failed to elicit efficient killing in vitro (78). Therefore, the contribution of macrophages' oxidative and nonoxidative defenses to fungal control remains unknown.
Nonlytic Exocytosis
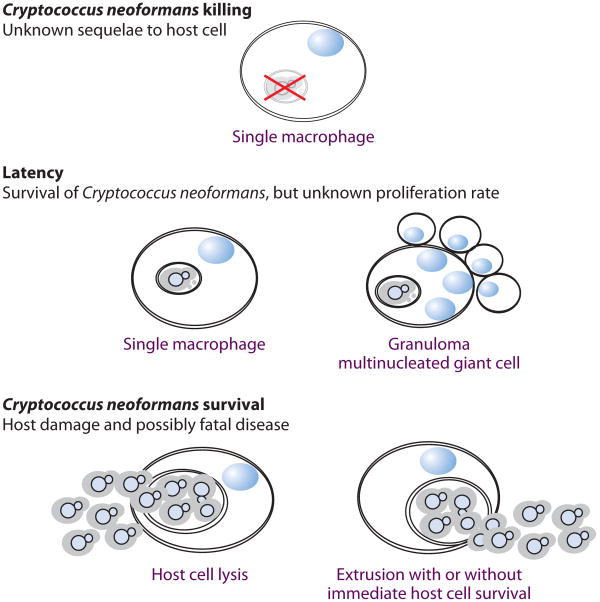
Upon phagocytosis, C. neoformans can undergo morphological changes, such as capsular enlargement, that aid its survival within, and even its escape from, host phagocytes (96). Some of these changes include fungal giant cell (titan cell) formation (97, 98), cell-to-cell spread (99), and nonlytic exocytosis (NLE) (100, 101). The presence of mechanisms to flee from phagosomes or traverse to an adjacent cell is compelling evidence of the yeast's adaptation to an intracellular lifestyle. NLE occurs after phagosomal maturation and requires fungal viability (100–102). Curiously, phagosomal permeability always precedes NLE, whereas actin flashes around the phagosome seem to counteract fungal escape (Figure 7) (103). Interference with host cytoskeletal machinery decreases NLE (104), and yeast cells have been found to interact with host cytoskeletal Rac1, a small GTP-binding Rho family protein, to penetrate the blood-brain barrier (105), indicating that the host cytoskeleton can be subverted to promote fungal escape. The most surprising feature of NLE is how little macrophage damage ensues immediately afterward, with the exception of giant vacuole formation in the cytoplasm of the host cell (100).
Figure 7.
Possible outcomes for Cryptococcus neoformans infection of murine macrophages. The interaction between C. neoformans and host macrophages can result in different outcomes, and the frequency with which they occur influences the course of infection.
NLE appears to be tightly modulated by macrophage permissiveness. Macrophages activated by Th2 cytokines in vitro showed an increase in intracellular proliferation and a decrease in extrusion rate when compared with nonstimulated macrophages (106). Th2 cytokines enhance iron uptake and storage by macrophages (107), which may transform the phagosome into a more hospitable environment for the yeast. As mentioned above, acidification of the phagosome is beneficial for C. neoformans (65), and blockage of acidification increases NLE rates (102, 104). These results could be interpreted to suggest the curious hypothesis that a less favorable intracellular niche leads to increased fungal escape via NLE.
Trojan Horse Hypothesis for Extrapulmonary Dissemination
The Trojan horse hypothesis posits that a pathogen gains entry into the blood-brain barrier through dissemination within immune cells (108). In this scenario, the macrophage functions as a Trojan horse, carrying the fungus throughout the body and contributing to dissemination and the breaching of epithelial and endothelial barriers. For C. neoformans, a Trojan horse mechanism for dissemination is supported by the observation that depletion of alveolar macrophages prevents brain dissemination (47). Similarly, injection of ex vivo infected macrophages into mice resulted in increased brain fungal burden (46). However, alternative mechanisms of penetration into the brain are possible, such as active penetration of endothelial cells, by either a transcellular or paracellular mechanism (64, 109), and C. neoformans proteins that contribute to differential lung/brain infection ratios have been identified in a mutant screen (110, 111). For example, phospholipase B mutants have reduced virulence and invasion of the brain (112). Phospholipase B was found to interact with host cytoskeletal Rac1 to promote brain invasion (105), supporting the idea that C. neoformans may use transcellular mechanisms in addition to the Trojan-horse mechanism.
Cryptococcus Neoformans is An Intracellular Pathogen
Establishment of a latent intracellular residency is a very common outcome after phagocyte– fungal cell interactions (see sidebar, The Amoeba-Macrophage Connection). Although C. neoformans is not an obligate intracellular pathogen, intracellular residency is an environment where C. neoformans can persist and even travel, if we attribute brain invasion to migratory infected macrophages. However, it remains unclear why latency, and not eradication of infection, is such a common outcome. One explanation postulated the damage-response framework (113), which was further developed with the tolerance hypothesis (114). According to the tolerance hypothesis, resistance mechanisms minimize pathogen burden, whereas tolerance mechanisms maximize host function without affecting microbe burden. Consequently, brain, lungs, and heart are the most susceptible organs to immune damage (114). Two of these organs are major targets of C. neoformans. In chronic infection models, the yeast spreads to spleen and liver early in infection but is later cleared (42), consistent with a lower risk to these organs of immune damage (due in part to their greater regenerative capacities). Thus, control of infection by intracellular latency, but not clearance, might be a tolerance mechanism to minimize brain and lung damage. Within this postulate, intracellular residency is a tolerance mechanism that would minimize both direct fungal damage to the host and exposure of fungi to the immune response (which would trigger immunopathology), allowing maximal host function (114). These considerations raise the question of why C. neoformans has particular tropism for the lung and brain, but not the heart, for which we cannot formulate a credible explanation. When cryptococcal pathogenesis is viewed in the context of the tolerance hypothesis, it appears that fungal intracellular residence is an outcome that presents advantages to both organisms.
Conclusions and Unresolved Questions
There is still much to be discovered regarding the survival of C. neoformans within macrophages and its capacity for lung intracellular residence in pathogenesis. Most cases of cryptococcosis are initiated by lung pathology, a finding that provides evidence consistent with a pulmonary reservoir for latency. However, animal studies show that dissemination to the brain occurs shortly after pulmonary infection, which suggests that the brain could also be a reservoir for the yeast. If so, how does the yeast establish latency within the immunoprivileged brain, and are there particular mechanisms of fungal control within the brain?
Within the lung, yeast control is achieved through the formation of specialized granulomas. Granulomas originate from immune cell cooperation, including macrophages and granulomas generated in vitro that have already been used as a C. albicans infection model (115). That macrophage granulomas and giant cells possess cellular and molecular characteristics distinct from those of macrophages (116) could explain the observed reduced fungicidal capacity of macrophages in vitro. An alternative explanation could be that immune cells must cooperate, meaning that macrophages would have to acquire a microbicidal molecule from other immune cells.
Microbial ligands can activate innate immunity in the absence of adequate adaptive immunity (117). Our results (51) have shown an increase in fungicidal activity due to β-glucan stimulation. Given that protection could be elicited with proper innate cell stimulation, without the need for CD4+ T cells, we suggest that microbial ligands might have therapeutic value, in particular for immunocompromised patients in whom proper T cell stimulation is not possible.
In conclusion, C. neoformans is capable of surviving within mammalian hosts, contained within the intracellular environment of macrophages. The intracellular residency might reflect the most advantageous equilibrium for the host and the pathogen duo and seems to have evolved serendipitously from an ancient relationship with amoebae. Understanding the features of intracellular life can help to prevent C. neoformans–associated deaths.
The Amoeba-Macrophage Connection.
Cryptococcus neoformans is a soil organism that has no requirement for mammalian pathogenesis in its life cycle. Why would C. neoformans develop such a sophisticated intracellular pathogenic strategy? Studies of the interaction of C. neoformans with amoebae suggested how this strategy might have evolved. Amoebae are predators on C. neoformans in soil, which can be replicated in a laboratory setting (118, 119). Analysis of the interactions of C. neoformans with Acanthamoeba castellanii revealed remarkable similarities to the response elicited by interaction with mammalian macrophages; similar virulence factors are required for pathogenesis in both hosts (120). Subsequent studies have established that other phenomena associated with the interaction of macrophages, such as capsule growth and NLE, can be replicated in C. neoformans–amoeba interactions (121, 122). On the basis of these observations, the capacity of C. neoformans to survive in macrophages and cause disease in mammals was proposed to be the result of selection by such biotic factors as amoebae in the environment (113). According to this synthesis, environmental pressures selected for traits that were needed to survive phagocytic predators and that incidentally also conferred the capacity for mammalian virulence (113).
Future Issues.
What is the mechanism of control of the primary C. neoformans infection?
What immune effector mechanism, lost during immunosuppression or loss of CD4+ T cells, is responsible for control of latent C. neoformans infection?
Are the mechanisms responsible for control of a primary infection the same as those that will control an established disseminated infection?
Is it possible to prevent C. neoformans from crossing the blood-brain barrier?
Acknowledgments
The authors acknowledge Julie M. Wolf for help in obtaining the TEM images and for invaluable critical reading of the manuscript. We also acknowledge all the personnel at the Analytical Imaging Facility, National Cancer Institute support grant P30CA013330, for their technical assistance on the electron microscopy images. This work was supported by NIH grants HL059842-3, A1033774, A1052733, and AI033142 to A.C. and PhD grant SFRH/BD/33471/2008 by Fundação Ciência e Tecnologia to C.C.
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Barnett JA. A history of research on yeasts 14: medical yeasts part 2, Cryptococcus neoformans. Yeast. 2010;27(11):875–904. doi: 10.1002/yea.1786. [DOI] [PubMed] [Google Scholar]
- 2.Lin X, Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 3.Lindell DM, Ballinger MN, McDonald RA, Toews GB, Huffnagle GB. Immunologic homeostasis during infection: coexistence of strong pulmonary cell–mediated immunity to secondary Cryptococcus neoformans infection while the primary infection still persists at low levels in the lungs. J Immunol. 2006;177(7):4652–61. doi: 10.4049/jimmunol.177.7.4652. [DOI] [PubMed] [Google Scholar]
- 4.Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski La, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107(5):e66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37(10):3204–9. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's Principles of Internal Medicine. 18th 1, 2. New York: McGraw-Hill; 2011. [Google Scholar]
- 7.Chen LC, Goldman DL, Doering TL, Pirofski La, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67(5):2218–24. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles SS, Dagenais TRT, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun. 2009;77(8):3491–500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibuya K, Hirata A, Omuta J, Sugamata M, Katori S, et al. Granuloma and cryptococcosis. J Infect Chemother. 2005;11(3):115–22. doi: 10.1007/s10156-005-0387-x. [DOI] [PubMed] [Google Scholar]
- 10.Lindell DM, Ballinger MN, McDonald RA, Toews GB, Huffnagle GB. Diversity of the T-cell response to pulmonary Cryptococcus neoformans infection. Infect Immun. 2006;74(8):4538–48. doi: 10.1128/IAI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173(4):793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim TS, Murphy JW. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun. 2007;75(6):2729–39. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes JC. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia. 1985;23(3):225–34. doi: 10.1080/00362178585380331. [DOI] [PubMed] [Google Scholar]
- 15.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17(6):733–39. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 16.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, Toews GB. The γinterferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73(3):1788–96. doi: 10.1128/IAI.73.3.1788-1796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66(10):4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi M, Ito M, Sano K, Koyama M. Granulomatous and cytokine responses to pulmonary Cryptococcus neoformans in two strains of rats. Mycopathologia. 2001;151(3):121–30. doi: 10.1023/a:1017900604050. [DOI] [PubMed] [Google Scholar]
- 19.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 2011;79(5):1915–26. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, et al. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun. 2009;77(12):5389–99. doi: 10.1128/IAI.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175(6):2489–500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenzel W, Müller U, Köhler G, Heppner FL, Blessing M, et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol. 2009;174(2):486–96. doi: 10.2353/ajpath.2009.080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K, Qifeng X, Tohyama M, Qureshi MH, Saito A. Contribution of tumour necrosis factor-alpha (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106(3):468–74. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12(7):518–27. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wozniak KL, Young ML, Wormley FL. Protective immunity against experimental pulmonary cryptococcosis in T cell–depleted mice. Clin Vaccine Immunol. 2011;18(5):717–23. doi: 10.1128/CVI.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monga DP. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. 1981;32(3):975–78. doi: 10.1128/iai.32.3.975-978.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osterholzer JJ, Milam JE, Chen GH, Toews GB, Huffnagle GB, Olszewski MA. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infect Immun. 2009;77(9):3749–58. doi: 10.1128/IAI.00454-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005;175(5):3244–51. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 29.Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil–Cryptococcus neoformans interactions in vivo and in vitro. Infect Immun. 1997;65(5):1899–907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mednick AJ, Feldmesser M, Rivera J, Casadevall A. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol. 2003;33(6):1744–53. doi: 10.1002/eji.200323626. [DOI] [PubMed] [Google Scholar]
- 31.Piehler D, Stenzel W, Grahnert A, Held J, Richter L, et al. Eosinophils contribute to IL-4 production and shape the T-helper cytokine profile and inflammatory response in pulmonary cryptococcosis. Am J Pathol. 2011;179(2):733–44. doi: 10.1016/j.ajpath.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garro AP, Chiapello LS, Baronetti JL, Masih DT. Rat eosinophils stimulate the expansion of Cryptococcus neoformans-specific CD4+ and CD8+ T cells with a T-helper 1 profile. Immunology. 2010;132(2):174–87. doi: 10.1111/j.1365-2567.2010.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casadevall A. Amoeba provide insight into the origin of virulence in pathogenic fungi. Adv Exp Med Biol. 2012;710:1–10. doi: 10.1007/978-1-4419-5638-5_1. [DOI] [PubMed] [Google Scholar]
- 34.Mansour MK, Yauch LE, Rottman JB, Levitz SM. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infect Immun. 2004;72(3):1746–54. doi: 10.1128/IAI.72.3.1746-1754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami K. Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans. Jpn J Infect Dis. 2004;57(4):137–45. [PubMed] [Google Scholar]
- 36.Hardison SE, Wozniak KL, Kolls JK, Wormley FL. Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun. 2010;78(12):5341–51. doi: 10.1128/IAI.00845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J Immunol. 2012;189(8):4060–68. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL. Pulmonary infection with an interferon-γ-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol. 2010;176(2):774–85. doi: 10.2353/ajpath.2010.090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker RD, Haugen RK. Tissue changes and tissue diagnosis in cryptococcosis; a study of 26 cases. Am J Clin Pathol. 1955;25(1):14–24. doi: 10.1093/ajcp/25.1.14. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz DA. Characterization of the biological activity of Cryptococcus infections in surgical pathology. The Budding Index and Carminophilic Index. Ann Clin Lab Sci. 1988;18(5):388–97. [PubMed] [Google Scholar]
- 41.Shibuya K, Coulson WE, Wollman JS, Wakayama M, Ando T, et al. Histopathology of cryptococcosis and other fungal infections in patients with acquired immunodeficiency syndrome. Int J Infect Dis. 2001;5(2):78–85. doi: 10.1016/s1201-9712(01)90030-x. [DOI] [PubMed] [Google Scholar]
- 42.Goldman DL, Lee SC, Mednick AJ, Montella L, Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68(2):832–38. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68(7):4225–37. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nessa K, Gross NT, Jarstrand C, Johansson A, Camner P. In vivo interaction between alveolar macrophages and Cryptococcus neoformans. Mycopathologia. 1997;139(1):1–7. doi: 10.1023/a:1006843202124. [DOI] [PubMed] [Google Scholar]
- 45.Goldman D, Lee SC, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62(11):4755–61. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlier C, Nielsen K, Daou S, Brigitte M, Chrétien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–27. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–98. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cross CE, Bancroft GJ. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and β-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect Immun. 1995;63(7):2604–11. doi: 10.1128/iai.63.7.2604-2611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giles SS, Zaas AK, Reidy MF, Perfect JR, Wright JR. Cryptococcus neoformans is resistant to surfactant protein A mediated host defense mechanisms. PLoS ONE. 2007;2(12):e1370. doi: 10.1371/journal.pone.0001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16(6):791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 51.Bocca AL, Coelho C, Casadevall A. Dectin-1 pathway activation is important to Cryptococcus neoformans killing. J Immunol. 2012;188(Meet. Abstr. Suppl):67.10. http://www.jimmunol.org/cgi/content/meeting_abstract/188/1_MeetingAbstracts/67.10. [Google Scholar]
- 52.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, et al. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun. 2002;70(5):2598–604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206(3):637–53. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinsbroek SEM, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 2008;4(11):e1000218. doi: 10.1371/journal.ppat.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faro-Trindade I, Willment JA, Kerrigan AM, Redelinghuys P, Hadebe S, et al. Characterisation of innate fungal recognition in the lung. PLoS ONE. 2012;7(4):e35675. doi: 10.1371/journal.pone.0035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura K, Kinjo T, Saijo S, Miyazato A, Adachi Y, et al. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol Immunol. 2007;51(11):1115–19. doi: 10.1111/j.1348-0421.2007.tb04007.x. [DOI] [PubMed] [Google Scholar]
- 57.Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by Dectin-1. PLoS Pathog. 2013;9(4):e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biondo C, Midiri A, Messina L, Tomasello F, Garufi G, et al. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur J Immunol. 2005;35(3):870–78. doi: 10.1002/eji.200425799. [DOI] [PubMed] [Google Scholar]
- 59.Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect Immun. 2008;76(6):2362–67. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura K, Miyazato A, Xiao G, Hatta M, Inden K, et al. Deoxynucleic acids from Cryptococcus neoformans activate myeloid dendritic cells via a TLR9-dependent pathway. J Immunol. 2008;180(6):4067–74. doi: 10.4049/jimmunol.180.6.4067. [DOI] [PubMed] [Google Scholar]
- 61.Wang JP, Lee CK, Akalin A, Finberg RW, Levitz SM. Contributions of the MyD88-dependent receptors IL-18R, IL-1R, and TLR9 to host defenses following pulmonary challenge with Cryptococcus neoformans. PLoS ONE. 2011;6(10):e26232. doi: 10.1371/journal.pone.0026232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guillot L, Carroll SF, Badawy M, Qureshi ST. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir Res. 2008;9(1):9. doi: 10.1186/1465-9921-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganendren R, Carter E, Sorrell T, Widmer F, Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006;8(4):1006–15. doi: 10.1016/j.micinf.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Sabiiti W, May RC. Capsule independent uptake of the fungal pathogen Cryptococcus neoformans into brain microvascular endothelial cells. PLoS ONE. 2012;7(4):e35455. doi: 10.1371/journal.pone.0035455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin QM, Luo J, Lin X, Pei J, Li L, et al. Functional analysis of host factors that mediate the intracellular lifestyle of Cryptococcus neoformans. PLoS Pathog. 2011;7(6):e1002078. doi: 10.1371/journal.ppat.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Artavanis-Tsakonas K, Love JC, Ploegh HL, Vyas JM. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci USA. 2006;103(43):15945–50. doi: 10.1073/pnas.0607528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levitz SM, Harrison TS, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100(6):1640–46. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wozniak KL, Levitz SM. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun. 2008;76(10):4764–71. doi: 10.1128/IAI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, et al. Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans. Infect Immun. 2012;80(9):3065–76. doi: 10.1128/IAI.00358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci USA. 2002;99(5):3165–70. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gross NT, Hultenby K, Mengarelli S, Camner P, Jarstrand C. Lipid peroxidation by alveolar macrophages challenged with Cryptococcus neoformans, Candida albicans or Aspergillus fumigatus. Med Mycol. 2000;38(6):443–49. doi: 10.1080/mmy.38.6.443.449. [DOI] [PubMed] [Google Scholar]
- 72.Ben-Abdallah M, Sturny-Leclère A, Avé P, Louise A, Moyrand F, et al. Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB. PLoS Pathog. 2012;8(3):e1002555. doi: 10.1371/journal.ppat.1002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villena SN, Pinheiro RO, Pinheiro CS, Nunes MP, Takiya CM, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008;10(6):1274–85. doi: 10.1111/j.1462-5822.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 74.Luo Y, Tucker SC, Casadevall A. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J Immunol. 2005;174(11):7226–33. doi: 10.4049/jimmunol.174.11.7226. [DOI] [PubMed] [Google Scholar]
- 75.Luo Y, Casadevall A. Intracellular cryptococci suppress Fc-mediated cyclin D1 elevation. Commun Integr Biol. 2010;3(4):390–91. doi: 10.4161/cib.3.4.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coelho C, Tesfa L, Zhang J, Rivera J, Gonçalves T, Casadevall A. Analysis of cell cycle and replication of mouse macrophages after in vivo and in vitro Cryptococcus neoformans infection using laser scanning cytometry. Infect Immun. 2012;80(4):1467–78. doi: 10.1128/IAI.06332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thi EP, Lambertz U, Reiner NE. Sleeping with the enemy: how intracellular pathogens cope with a macrophage lifestyle. PLoS Pathog. 2012;8(3):e1002551. doi: 10.1371/journal.ppat.1002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levitz SM, Farrell TP. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990;58(5):1201–9. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129(5):569–82. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 80.Derengowski LS, Paes HC, Albuquerque P, Tavares AHFP, Fernandes L, et al. The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation into the mammalian host. Eukaryot Cell. 2013;12(5):761–74. doi: 10.1128/EC.00073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan W, Kraus PR, Boily MJ, Heitman J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot Cell. 2005;4(8):1420–33. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snelgrove RJ, Edwards L, Williams AE, Rae AJ, Hussell T. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. J Immunol. 2006;177(8):5509–16. doi: 10.4049/jimmunol.177.8.5509. [DOI] [PubMed] [Google Scholar]
- 83.Alspaugh JA, Granger DL. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59(7):2291–96. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldman D, Cho Y, Zhao M, Casadevall A, Lee SC. Expression of inducible nitric oxide synthase in rat pulmonary Cryptococcus neoformans granulomas. Am J Pathol. 1996;148(4):1275–82. [PMC free article] [PubMed] [Google Scholar]
- 85.Rivera J, Mukherjee J, Weiss LM, Casadevall A. Antibody efficacy in murine pulmonary Cryptococcus neoformans infection: a role for nitric oxide. J Immunol. 2002;168(7):3419–27. doi: 10.4049/jimmunol.168.7.3419. [DOI] [PubMed] [Google Scholar]
- 86.Aguirre KM, Gibson GW. Differing requirement for inducible nitric oxide synthase activity in clearance of primary and secondary Cryptococcus neoformans infection. Med Mycol. 2000;38(5):343–53. doi: 10.1080/mmy.38.5.343.353. [DOI] [PubMed] [Google Scholar]
- 87.de Jesús-Berríos M, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J. Enzymes that counteract nitrosative stress promote fungal virulence. Curr Biol. 2003;13(22):1963–68. doi: 10.1016/j.cub.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 88.Murphy JW, Hidore MR, Wong SC. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Investig. 1993;91(4):1553–66. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levitz SM, Dupont MP, Smail EH. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun. 1994;62(1):194–202. doi: 10.1128/iai.62.1.194-202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelly RM, Chen J, Yauch LE, Levitz SM. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect Immun. 2005;73(1):592–98. doi: 10.1128/IAI.73.1.592-598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect Immun. 2000;68(11):6257–64. doi: 10.1128/iai.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aratani Y. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol. 2006;55(9):1291–99. doi: 10.1099/jmm.0.46620-0. [DOI] [PubMed] [Google Scholar]
- 93.Hole CR, Bui H, Wormley FL, Wozniak KL. Mechanisms of dendritic cell lysosomal killing of Cryptococcus. Sci Rep. 2012;2:739. doi: 10.1038/srep00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-γ-activated macrophages. Immunity. 2009;30(1):143–54. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Bliska JB, Casadevall A. Intracellular pathogenic bacteria and fungi—a case of convergent evolution? Nat Rev Microbiol. 2009;7:165–71. doi: 10.1038/nrmicro2049. [DOI] [PubMed] [Google Scholar]
- 97.Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6(6):e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6(6):e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alvarez M, Burn T, Luo Y, Pirofski La, Casadevall A. The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol. 2009;9:51. doi: 10.1186/1471-2180-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16(21):2161–65. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 101.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–60. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 102.Nicola AM, Robertson EJ, Albuquerque P, Derengowski LDS, Casadevall A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. mBio. 2011;2(4):e00167–11. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex–mediated actin polymerisation. PLoS Pathog. 2010;6(8):e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carnell M, Zech T, Calaminus SD, Ura S, Hagedorn M, et al. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J Cell Biol. 2011;193(5):831–39. doi: 10.1083/jcb.201009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maruvada R, Zhu L, Pearce D, Zheng Y, Perfect J, et al. Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell Microbiol. 2012;14(10):1544–53. doi: 10.1111/j.1462-5822.2012.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77(8):3450–57. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weiss G, Bogdan C, Hentze MW. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol. 1997;158(1):420–25. [PubMed] [Google Scholar]
- 108.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74(5):650–56. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 109.Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72(9):4985–95. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He X, Lyons DM, Toffaletti DL, Wang F, Qiu Y, et al. Virulence factors identified by Cryptococcus neoformans mutant screen differentially modulate lung immune responses and brain dissemination. Am J Pathol. 2012;181(4):1356–66. doi: 10.1016/j.ajpath.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rao PK, Singh CR, Jagannath C, Li Q. A systems biology approach to study the phagosomal proteome modulated by mycobacterial infections. Int J Clin Exp Med. 2009;2(3):233–47. [PMC free article] [PubMed] [Google Scholar]
- 112.Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39(1):166–75. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 113.Casadevall A, Pirofski La. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1(1):17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–41. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alvarez-Rueda N, Albassier M, Allain S, Deknuydt F, Altare F, Le Pape P. First human model of in vitro Candida albicans persistence within granuloma for the reliable study of host-fungi interactions. PLoS ONE. 2012;7(6):e40185. doi: 10.1371/journal.pone.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vignery A. Macrophage fusion: molecular mechanisms. Methods Mol Biol. 2008;475:149–61. doi: 10.1007/978-1-59745-250-2_9. [DOI] [PubMed] [Google Scholar]
- 117.Evans SE, Tuvim MJ, Fox CJ, Sachdev N, Gibiansky L, Dickey BF. Inhaled innate immune ligands to prevent pneumonia. Br J Pharmacol. 2011;163(1):195–206. doi: 10.1111/j.1476-5381.2011.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bunting LA, Neilson JB, Bulmer GS. Cryptococcus neoformans: gastronomic delight of a soil ameba. Sabouraudia. 1979;17(3):225–32. doi: 10.1080/00362177985380341. [DOI] [PubMed] [Google Scholar]
- 119.Ruiz A, Neilson JB, Bulmer GS. Control of Cryptococcus neoformans in nature by biotic factors. Sabouraudia. 1982;20(1):21–29. [PubMed] [Google Scholar]
- 120.Steenbergen JN, Casadevall A. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 2003;5(7):667–75. doi: 10.1016/s1286-4579(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 121.Chrisman CJ, Alvarez M, Casadevall A. Phagocytosis of Cryptococcus neoformans by, and nonlytic exocytosis from, Acanthamoeba castellanii. Appl Environ Microbiol. 2010;76(18):6056–62. doi: 10.1128/AEM.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chrisman CJ, Albuquerque P, Guimaräes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011;7(5):e1002047. doi: 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura K, Miyagi K, Koguchi Y, Kinjo Y, Uezu K, et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol. 2006;47(1):148–54. doi: 10.1111/j.1574-695X.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 124.Monga DP, Kumar R, Mohapatra LN. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect Immun. 1979;23(1):1–3. doi: 10.1128/iai.26.1.1-3.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hill JO, Aguirre KM. CD4+ T cell–dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152(5):2344–50. [PubMed] [Google Scholar]
- 126.Yuan RR, Casadevall A, Oh J, Scharff MD. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94(6):2483–88. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawakami K, Koguchi Y, Qureshi MH, Kinjo Y, Yara S, et al. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol Lett. 2000;186(1):121–26. doi: 10.1111/j.1574-6968.2000.tb09092.x. [DOI] [PubMed] [Google Scholar]
- 128.Müller U, Stenzel W, Köhler G, Werner C, Polte T, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179(8):5367–77. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 129.Kleinschek MA, Müller U, Brodie SJ, Stenzel W, Köhler G, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176(2):1098–106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 130.Gyetko MR, Chen GH, McDonald RA, Goodman R, Huffnagle GB, et al. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans. A murine transgenic model. J Clin Investig. 1996;97(8):1818–26. doi: 10.1172/JCI118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beenhouwer DO, Shapiro S, Feldmesser M, Casadevall A, Scharff MD. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect Immun. 2001;69(10):6445–55. doi: 10.1128/IAI.69.10.6445-6455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams AE, Humphreys IR, Cornere M, Edwards L, Rae A, Hussell T. TGF-β prevents eosinophilic lung disease but impairs pathogen clearance. Microbes Infect. 2005;7(3):365–74. doi: 10.1016/j.micinf.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 133.Casadevall A, Pirofski La. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012;11(5):447–56. doi: 10.1016/j.chom.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]