Abstract
OBJECTIVE
Continuing to smoke after a cancer diagnosis can adversely influence the prognosis for cancer patients. However, remarkably few studies have carefully examined the use of first-line FDA-approved medications for nicotine dependence in cancer patients. This study evaluated the feasibility, safety, and effect on cessation of varenicline for smoking cessation in cancer patients.
METHODS
Data from 132 treatment-seeking smokers who received 12 weeks of open-label varenicline and 5 brief behavioral counseling sessions were used to evaluate the feasibility, safety, and impact on cessation of varenicline. The effects of abstinence on cognitive function and affect were also explored.
RESULTS
Of 459 patients screened, 306 were eligible for the study (66.7%) and 132 entered treatment (43.1%). Retention was 84.1% over 12 weeks. The rate of biochemically verified abstinence at Week 12 was 40.2%. Expected side effects were reported (e.g., sleep problems, nausea) but there were no reports of elevated depressed mood, suicidal thoughts, or cardiovascular events. Abstinence was associated with improved cognitive function and reduced negative affect over time (p’s < .05).
CONCLUSIONS
Although many cancer patients who smoke did not enroll in treatment, the side effect profile of varenicline and its effect on short-term cessation converge with what is seen in the general population. Further, as with the general population, abstinence while taking varenicline may lead to improved cognitive function and reduced negative affect. The present data support the use of varenicline to help cancer patients to quit smoking.
Keywords: varenicline, smoking cessation, cancer, cognition, affect, oncology
BACKGROUND
In 2014, the Surgeon General’s Report concluded that there is sufficient evidence to infer a causal relationship between cigarette smoking and adverse health outcomes in cancer patients and that quitting smoking improves cancer patient prognosis [1]. Although this evidence points to the importance of smoking cessation among cancer patients, remarkably few studies have examined evidence-based treatments for smoking in this population. Population-based estimates of smoking prevalence across cancer type suggest that smoking rates remain high among individuals who have been diagnosed with cancer (16.1% vs 18.6% among those without a history of cancer) [2]. The paucity of studies in this area may underlie reluctance among patients and physicians to use best-in-class treatment options for nicotine dependence [3–5]. With medical advances in cancer care yielding a growing constituency of individuals diagnosed with cancer, numbering close to 14 million today [6], demonstrating the safety and efficacy of treatments for nicotine dependence in this population is a priority.
Varenicline, a nicotinic acetylcholine receptor (nAChRs) partial agonist, could be particularly well-suited for cancer patients who smoke. Varenicline is currently the most efficacious FDA-approved medication for nicotine dependence, yielding quit rates that significantly exceed those produced by bupropion [7] and nicotine patch [8]. Varenicline also mitigates the adverse psychological effects of quitting smoking and can reduce withdrawal-related cognitive impairment associated with quitting smoking through direct medication effects on the β2 acetylcholine receptors [9–10]. While varenicline is efficacious and safe for treating nicotine dependence among various clinical populations [11–12], there have been reports of adverse psychiatric events following varenicline use, leading the FDA to mandate a black-box warning for varenicline, likely decreasing willingness to use this medication in the oncologic context. Further, varenicline may not be well-tolerated by cancer patients due to the similarity of its side effect profile to cancer treatment side effects (e.g., nausea). A preliminary study by Park and colleagues [13] provided some encouraging data, reporting significantly higher end of treatment quit rates for varenicline, compared to matched controls, and a side effect profile that mirrored the general population. However, this study used a small sample of only thoracic cancer patients.
In this study, we attempted to examine the feasibility of recruiting cancer patients into a smoking cessation treatment program involving varenicline and behavioral counseling and examined the safety and effects of varenicline on short-term abstinence. Further, we examined if abstinence was associated with changes in perceived cognitive function and affect in order to explore if the mechanism of effect of varenicline on abstinence could be similar to that documented in the general population. With the recognition of the importance of treating nicotine dependence among cancer patients, this study sought to provide data that could help support the broader acceptance of varenicline as a first-line option for cancer patients who continue to smoke.
METHODS
This study used data collected for an ongoing NCI-funded clinical trial that involved randomization to either 12 weeks of varenicline followed by 12 weeks of placebo, or a full 24 weeks of varenicline (clinicaltrials.gov Identifier: NCT01756885). All participants received 5 standardized, manual-based brief smoking cessation counseling sessions based on established guidelines [14]. For this analysis, only data to 12 weeks were used to standardize treatment across participants (i.e., all participants received 12 weeks of varenicline treatment and 5 counseling sessions). Potential participants were identified from electronic medical records at two academic cancer centers. These patients were screened for tobacco use and contacted by telephone to assess trial eligibility and interest. Interested patients attended an in-person eligibility visit and those eligible were registered for the trial.
Participants
Eligible participants were ≥18 years of age, had a diagnosis of cancer, reported smoking ≥5 cigarettes per week, and were interested in quitting smoking. All participants enrolled in the study were either receiving active treatment for their cancer or were being monitored post-treatment following a diagnosis within the past 5 years. Participants were required to have a Karnofsky score ≥50 or an ECOG Performance status ≤2 to enroll. Exclusion criteria included use of other tobacco products or smoking cessation treatments, current diagnosis of substance abuse disorder, having a current medical problem for which varenicline use is contraindicated (e.g., allergy), a lifetime DSM-IV diagnosis of psychotic or bipolar disorder or current unstable or untreated major depression, current suicidality or a past attempt as identified by the Mini International Neuropsychiatric Interview (MINI)[15] or the Columbia-Suicide Severity Rating Scale[16], or being unable to communicate in English. Female participants were excluded if they were pregnant, planning a pregnancy, or lactating.
Procedure
This study was performed after approval by the university’s Institutional Review Boards and participants provided informed consent. Participants received 12 weeks of standard open-label varenicline treatment in accordance with U.S. Food and Drug Administration approved labeling: Day 1–Day 3 (0.5 mg once daily); Day 4–7 (0.5 mg twice daily); and Day 8–Day 84 (1.0 mg twice daily). Participants also received behavioral counseling based on social cognitive theory described in more detail in previous studies [17–18]. Counseling included one 60 minute in-person pre-quit counseling visit at Week 0, which focused on preparing for cessation and providing the participant with their first 4 weeks of medication, which they began taking at Week 0. At Weeks 1, 4, 8 and 12, participants received 20 minute in-person counseling sessions focusing on managing smoking-related urges and triggers and developing strategies to avoid relapse. Assessments were conducted Weeks 0, 1, 4, 8, and 12. Self-reports of smoking cessation at Week 12 (for the 7 days preceding the assessment) were biochemically confirmed with breath carbon monoxide (CO).
Measures
Covariates
Participants self-reported demographics (e.g., age, race) and smoking behavior [19].
Affective Variables
The Positive and Negative Affect Schedule [20], a 20-item self-report measure, assesses positive (PA; 10 items, e.g., enthusiastic) and negative (NA; 10 items, e.g. distressed) affect.
Cognitive Variables
The FACT-Cog-Version 2 is a 42-item measure of perceived cognitive function designed for cancer patients. The FACT-Cog Version 2 consists of 3 sub-domains: Cognitive Deficits (measuring mental acuity, concentration, memory, and verbal fluency), Consequences of Cognitive Deficits (measuring functional interference, noticeability, and changes in cognitive function), and Cognitive Capabilities (including all positively-worded items reflecting higher cognitive functioning). [21].
Side Effects
As in our past varenicline trials [9, 18], a checklist and open-ended items assessed the incidence and severity of varenicline-related side effects, including potential psychiatric [18] and cardiovascular side effects. Each symptom was rated from 1 (none) to 4 (severe). Side effects assessments were completed in person or over the phone by trained staff at each session. Blood pressure was assessed at each in-person visit. Participants were instructed to contact study personnel if they experienced any serious problems between assessments. Adverse events were considered serious if the participant considered them debilitating or if they required hospitalization.
Treatment Adherence
Medication adherence was assessed by self-report using a time-line follow-back method and by pill count and collection of used blister packs as in past trials [7, 22]. An adherence measure represented the proportion of total dose taken (adherence = >80% of dose taken) [18]. Completion of counseling sessions was also tracked.
Smoking Cessation
Smoking status was assessed using the timeline follow-back method [23] as done previously [24, 25] and by using breath CO to biochemically verify self-reports. Participants were considered abstinent at Week 12 if they self-reported abstinence for 7 days prior to the assessment and provided a breath sample with CO ≤10ppm. Participants who withdrew from the study, failed to provide a sample, or provided a CO breath sample >10ppm were considered smokers.
Data Analysis
The sample was characterized in terms of demographic and smoking-related data (e.g., age, nicotine dependence). Feasibility was assessed by rates of study eligibility, recruitment, and retention. We calculated rate of eligibility by totaling the number of participants who were considered eligible, vs. all screened. The recruitment rate was calculated by comparing the number of patients enrolled in the study to the number of patients who were eligible. The retention rate was calculated by determining the number of patients remaining in the study by Week 12 versus those who initiated treatment. Lastly, we examined variability in side effects, affect, cognitive function, and cessation across primary demographic characteristics (i.e., sex, race, education, age, time since diagnosis). The intent to treat (ITT) sample included all participants who completed their Week 0 visit. We calculated the rate of biochemically-confirmed abstinence by obtaining the ratio of participants providing a CO sample ≤10ppm over the total number of participants reaching Week 12. The rate of false reporting was calculated by identifying the number of participants self-reporting abstinence with CO levels >10ppm. Adherence was assessed by determining the proportion of patients who completed counseling sessions and used ≥80% of medication. Side effect data were reported as the frequency of moderate-to-severe reports and the overall mean side effect for each was assessed over time using repeated measures ANOVA. We used Generalized Estimating Equations (GEE) to determine whether changes in cognitive function and positive and negative affect from baseline to Week 12 were associated with smoking abstinence at Week 12. Main and interaction effects for the repeated measures independent variable (i.e., time; Week 0 to Week 12) and the between-group independent variable (i.e., smoking status at Week 12: smoking or abstinent) were assessed separately for each subscale.
RESULTS
Feasibility and Sample Characteristics
Of the 459 current smokers assessed for eligibility, 153 (33.3%) were ineligible (rate of eligibility = 67.7%). The most common reasons for ineligibility were low smoking rate (n=20), followed by no cancer diagnosis in the past 5 years (n=17), a diagnosis of bipolar disorder or current use of bipolar disorder medications (n=14), and a history of suicidal ideation or attempt (n=14). Of the 306 eligible patients, 174 refused enrollment or withdrew after intake, leading to an enrollment rate of 43.1%. The most common reasons for refusal or withdrawal were not being interested in the program, a general term to include lack of participant response despite repeated attempts to contact as well as the participant failing to specify a reason for refusal (n=80), not having time or adequate transportation to participate (n=27), preferring to quit on one’s own (n=26), concerns about the study medication (n=11), and disease progression or death (n=9). Of the 155 participants completing the intake session, 132 enrolled in the trial (85.2%) and completed the first treatment session. Table 1 displays the characteristics of the study sample. Of note, over 30% of the sample is African American, which exceeds the rate in the overall population but reflects the increased incidence of cancer in this community. Lastly, Caucasian participants reported more side effects (F [1, 80] = 8.71, p = .004), women had higher negative affect scores (F[1,129] = 12.18, p = .001), individuals with some college education reported increased cognitive capabilities (F[1,129] = 5.01, p = .03), and older participants reported greater cognitive deficits (p’s < 0.05). Time since diagnosis was not associated with side effects, affect, cognitive function, or cessation.
TABLE 1.
Characteristics of Sample
| Characteristic |
ITT (N=132) |
Reached Week 12 (N=117) |
Completed Week 12 (N=82) |
|---|---|---|---|
| Sex | |||
| Female | 66(50.0%) | 58(49.6%) | 41(50.0%) |
| Male | 66(50.0%) | 59(50.4%) | 41(50.0%) |
| Race | |||
| Black/African American | 41(31.1%) | 32(27.4%) | 24(29.3%) |
| White | 89(67.4%) | 83(70.9%) | 57(69.5%) |
| Unknown/Not Reported | 2(1.5%) | 2(1.7%) | 1(1.2%) |
| Ethnicity | |||
| Hispanic/Latino | 5 (3.8%) | 4 (3.4%) | 3 (3.7%) |
| Not Hispanic/Latino | 127 (96.2%) | 113 (96.6%) | 79 (96.3%) |
| Marital Status | |||
| Married | 63(47.7%) | 56(47.9%) | 37(45.1%) |
| Not Married | 69(52.3%) | 61(52.1%) | 45(54.9%) |
| Education | |||
| GED or less | 33(25.0%) | 28(23.9%) | 17(20.7%) |
| Some College or More | 99(75.0%) | 89(76.1%) | 65(79.3%) |
| Income | |||
| <50,000/year | 70(53.0%) | 61(52.1%) | 42(51.2%) |
| <50,000/year | 61(46.2%) | 55(47.0%) | 40(48.8%) |
| Unknown/Not reported | 1(0.8%) | 1(0.9%) | 0(0.0%) |
| Employment | |||
| Employed | 55(41.7%) | 54(46.2%) | 37(45.1%) |
| Unemployed/Retired | 77(58.3%) | 63(53.8%) | 45(54.9%) |
| Tumor Type | |||
| Genitourinary | 32(24.2%) | 29(24.8%) | 19(23.2%) |
| Breast | 27(20.5%) | 23(19.7%) | 15(18.3%) |
| Lung | 20(15.2%) | 16(13.7%) | 12(14.6%) |
| Skin | 15(11.4%) | 15(12.8%) | 10(12.2%) |
| Hematological | 14(10.6%) | 14(12.0%) | 9(11.0%) |
| Head and Neck | 9(6.8%) | 9(7.7%) | 8(9.8%) |
| Gastrointestinal | 8(6.1%) | 6(5.1%) | 5(6.1%) |
| Kidney, Pancreas, and Liver | 7(5.3%) | 5(4.3%) | 4(4.9%) |
| Cancer Stage | |||
| Stage 0 | 3(2.3%) | 3(2.6%) | 2(2.4%) |
| Stage I | 9(6.8%) | 9(7.7%) | 7(8.5%) |
| Stage II | 11(8.3%) | 10(8.5%) | 8(9.8%) |
| Stage III | 12(9.1%) | 10(8.5%) | 7(8.5%) |
| Stage IV | 19(14.4%) | 16(13.7%) | 10(12.2%) |
| Remission | 34(25.8%) | 33(28.2%) | 22(26.8%) |
| Not specified | 44(33.3%) | 36(30.8%) | 26(31.7%) |
| Age (Mean, SD) | 58.7(8.9) | 58.6(9.1) | 58.9(8.7) |
| Cigarettes per Day (Mean, SD, range) | 14.1(9.9), 1–40 | 14.5(8.2), 1–40 | 13.3(8.1), 1–40 |
| FTND* (Mean, SD) | 4.6(2.1) | 4.6(2.1) | 4.4(2.2) |
| CO at Intake (Mean, SD) | 16.8(5.6) | 16.6(9.6) | 15.2(8.4) |
| Karnofsky Score (Mean, SD) | 90.7(11.2) | 90.8(10.9) | 91.0(9.9) |
| ECOG score (Mean, SD) | .28(.45) | .30(.46) | .30(.47) |
| Age started smoking (Mean, SD) | 16.8(5.6) | 16.6(5.7) | 16.8(5.6) |
| Years smoked (Mean, SD) | 40.5(11.3) | 40.4(11.6) | 39.9(11.2) |
Note. Since this is an ongoing trial, we present sample characteristics for sub-groups within the study. There were no significant differences across the sub-groups (p>.05).
Fagerstrom Test of Nicotine Dependence.
Treatment Effects: Cessation Rate, Side Effects, and Adherence
These analyses used participants who reached Week 12 at the time of analyses (n=117). Of these participants, 53 (45.3%) self-reported abstinence, but only 47 (40.2%) of these 53 had CO readings <10ppm, confirming abstinence. Of the 117 participants reaching the Week 12 session, 113 provided valid pill count data. Of those 113, 62 (54.9%) took 80% or more of the study medication. Of the 82 participants who completed their Week 12 session, 61 (74.4%) were in the ≥80% adherence group and 21 (25.6%) were in the < 80% adherence group. Medication adherence was significantly associated with CO-confirmed abstinence at Week 12 (p ≤0.05).
As shown in Table 2, the most frequently reported moderate-to-severe side effects were fatigue (12.2%–14.4%), sleep problems (8.4%–14.4%), nausea (2.3%–9.6%), and constipation (1.6%–8.4%). Side effects that increased in reported severity between Week 0 and Week 12 were nausea, constipation, abnormal dreams, flatulence, and irritability (p’s ≤0.05). Weakness on one or both sides of the body decreased in reported severity between Week 0 and Week 12 (p <0.05). There were no reports of suicidal ideation or behavior, and there was no difference across time with respect to depressed mood, anxiety, hostility, or agitation (p’s > .05). There were 10 moderate-to-severe side effects reported by participants in addition to the checklist, but these were not considered related to study participation. There were 28 reported serious adverse events, one of which (anxiety) was considered possibly related to study participation. Serious adverse events were neurological (n=1), infectious (n=3), psychiatric (n=1), cardiovascular (n=2), fall/accident (n=4), dehydration/electrolyte imbalance (n=4), respiratory (n=6), death attributable to disease progression (n=4), and gastrointestinal (n=3) in nature.
TABLE 2.
Frequency of Moderate-Severe Side Effects by Time-point
| Variable | Pre-Quit (N=132) |
Week 4 (N=112) |
Week 8 (N=101) |
Week 12 (N= 83) |
|---|---|---|---|---|
| Irritability | 4(3.1) | 4(3.6) | 1(1.0) | 4(4.8) |
| Irregular Heartbeat | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| Depressed Mood | 0(0.0) | 6(5.4) | 2(2.0) | 1(1.2) |
| Increased Heart Rate or Palpitations | 0(0.0) | 0(0.0) | 0(0.0) | 1(1.2) |
| Suicidal Thoughts | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| Nausea | 3(2.3) | 10(8.9) | 2(2.0) | 8(9.6) |
| Sleep Problems | 11(8.4) | 11(9.8) | 9(8.9) | 12(14.4) |
| Constipation | 2(1.6) | 5(4.5) | 2(2.0) | 7(8.4) |
| Vomiting | 3(2.3) | 5(4.5) | 1(1.0) | 2(2.4) |
| Dry Mouth | 8(6.1) | 6(5.4) | 3(3.0) | 6(7.2) |
| Gas | 2(1.5) | 8(7.1) | 2(2.0) | 4(4.8) |
| Anxiety | 3(2.3) | 1(.9) | 3(3.0) | 2(2.4) |
| Skin swelling or rash | 2(1.5) | 3(2.7) | 2(2.0) | 1(1.2) |
| Indigestion | 1(.8) | 2(1.8) | 1(1.0) | 3(3.6) |
| Agitation | 1(.8) | 1(.9) | 0(0.0) | 2(2.4) |
| Insomnia | 9(6.9) | 4(3.6) | 4(4.0) | 5(6.0) |
| Headache | 3(2.3) | 4(3.6) | 3(3.0) | 1(1.2) |
| Abnormal Dreams | 0(0.0) | 5(4.5) | 3(3.0) | 3(3.6) |
| Disturbance in attention | 0(0.0) | 1(.9) | 4(4.0) | 1(1.2) |
| Diarrhea | 4(3.1) | 2(1.8) | 3(3.0) | 1(1.2) |
| Abdominal pain | 1(.8) | 3(2.7) | 1(1.0) | 4(4.8) |
| Flatulence | 1(.8) | 4(3.6) | 1(1.0) | 3(3.6) |
| Dizziness | 1(.8) | 3(2.7) | 0(0.0) | 1(1.2) |
| Fatigue | 16(12.2) | 11(9.8) | 10(9.9) | 13(15.7) |
| Feeling of weakness | 8(6.1) | 6(5.4)) | 5(5.0) | 5(6.0) |
| Skin redness | 2(1.5) | 0(0.0) | 2(2.0) | 0(0.0) |
| Hostility | 0(0.0) | 1(.9) | 0(0.0) | 1(1.2) |
| Chest pain | 1(.8) | 0(0.0) | 0(0.0) | 1(1.2) |
| Weakness (one/both sides of body) | 5(3.8) | 1(.9) | 2(2.0) | 2(2.4) |
Note: Number listed indicates the number of participants at time-point reporting moderate or severe side effect; the number in parentheses represents proportion of patients at that time-point.
Of the 132 participants who completed Week 0, 128 (97.7%) completed Week 1; of the 129 participants who reached Week 4, 112 (86.8%) completed the session; of the 124 participants who reached Week 8, 101 (81.5%) completed the session; and of the 117 participants who reached Week 12, 82 (70.1%) completed the session. Overall study retention for the 132 ITT participants was 111 (84.1%).
Abstinence, Cognition, and Affect
The GEE analysis showed that change in cognitive deficits from Week 0 to Week 12 was significantly related to Week 12 abstinence (χ2[3] = 9.12, p = .03; Table 3). Both the main effect of time and smoking status were not significant (p’s > .05). But, the interaction between time and smoking status was significant (β = −3.22, 95% CI: −6.07–−0.37, p = .03), indicating that, while cognitive deficits remained stable from Week 0 to Week 12 for participants who were smoking at Week 12 (Ms = 9.9 and 9.9), there was a significant decrease in the cognitive deficit measure for participants who were abstinent at Week 12 (Ms = 10.0 and 7.1), indicating improved cognitive function. Change in consequences of cognitive deficits from Week 0 to Week 12 was also associated with Week 12 abstinence (χ2[3] = 8.81, p = .03; Table 3). Both the main effect of time and smoking status were not significant (p’s > .05). But, the interaction between time and smoking status was significant (β = −2.61, 95% CI: −4.74–−.50, p = .02), indicating that, while consequences of cognitive deficits remained stable from Week 0 to Week 12 for participants who were smoking at Week 12 (Ms = 6.7 and 7.2), there was a significant decrease in the cognitive deficit measure for participants who were abstinent at Week 12 (Ms = 7.0 and 4.9), indicating improved cognitive function. Changes in the cognitive capabilities sub-scale were not associated with Week 12 abstinence (Figures 1 and 2).
Table 3.
Means and Standard Errors for Measures of Cognitive Function and Affect
| Measure | Smokers | Abstainers | ||
|---|---|---|---|---|
| Pre-Quit (Mean, SE) | Week 12 (Mean, SE) | Pre-Quit (Mean, SE) | Week 12 (Mean, SE) | |
| Cognitive Deficits | 9.9 (1.6) | 9.9 (1.4) | 10.0 (1.4) | 7.1 (1.2) |
| Consequences of Cognitive Deficits | 6.7 (1.2) | 7.2 (1.0) | 7.0 (1.1) | 4.9 (.91) |
| Cognitive Capabilities | 32.3 (1.2) | 33.8 (1.0) | 31.3 (1.1) | 32.5 (.88) |
| Negative Affect | 13.8 (.56) | 13.2 (.49) | 15.2 (.69) | 12.5 (.60) |
| Positive Affect | 36.7 (1.4) | 36.4 (1.2) | 36.1 (1.2) | 37.7 (1.1) |
Note. Interaction effect for changes in cognitive deficits was significant (p = .03); interaction effect for changes in the consequences of cognitive deficits was significant (p = .02); the interaction effect for changes in cognitive capabilities was not significant (p = .68); the interaction for negative affect was significant (p = .04) and the interaction for positive affect was not significant (p=.29).
Figure 1. CONSORT Diagram.

Note. *A list of the reasons for participant ineligibility can be provided by the authors upon request
FIGURE 2.
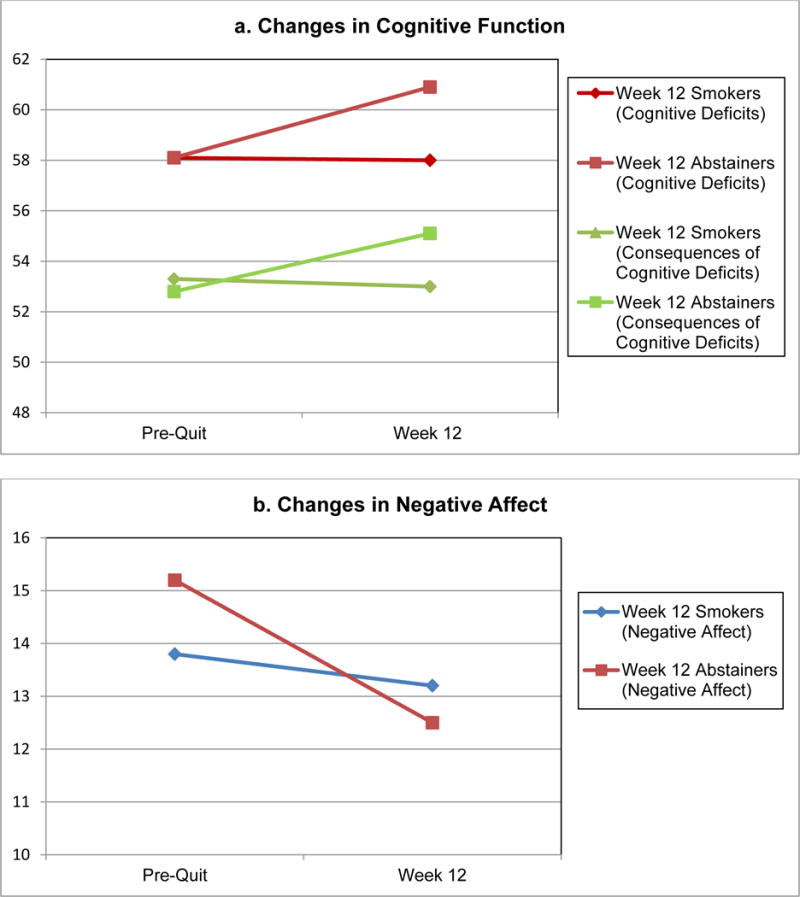
Changes in Cognitive Function and Negative Affect Over 12 Weeks for Smokers and Abstainers
The GEE analysis also showed that change in negative affect from Week 0 to Week 12 was significantly associated with week 12 abstinence (χ2[3] = 10.56, p = .01; Table 3). Both the main effect of time and smoking status were not significant (p’s > .05). But, the interaction between time and smoking status was significant (β = −1.99, 95% CI: −3.88–−.093, p = .04), indicating that, while negative affect remained stable from Week 0 to Week 12 for participants who were smoking at Week 12 (Ms = 13.8 and 13.2), there was a significant decrease in negative affect for participants who were abstinent at Week 12 (Ms = 15.2 and 12.5). Changes in positive affect were not associated with Week 12 abstinence.
CONCLUSIONS
This study provides support for the safety, feasibility, and efficacy of varenicline to treat nicotine dependence in cancer patients in the form of abstinence rates, treatment adherence, and side effect profile. Furthermore, this study demonstrated that abstainers showed a significant decrease in cognitive deficits and negative affect across 12 weeks of treatment, suggesting potential mechanisms by which varenicline may promote smoking cessation in cancer patients.
These findings converge well with those reported previously in terms of side effect profile, enrollment rate, cessation rate, and adherence [13], but offer enhanced generalizability of the safety of varenicline for cancer patients. Compared with this past study, the present sample was more reflective of the United States cancer population in terms of cancer site and race [6]. Because the present study included a wide variety of cancers, the findings provide additional evidence that the varenicline side effect profile for cancer patients is similar to that of the general population. Further, African Americans comprised close to a third of our sample, providing more generalizability of varenicline’s efficacy and safety for racial minority patients.
This is the largest study of its kind to combine behavioral and varenicline treatments for a cancer patient population. Despite concerns that cancer patients might not tolerate varenicline due to side effects similar to cancer treatment side effects (e.g. nausea)[26], participant adherence rates to varenicline (with 54.9% of the ITT population taking 80% or more of the total varenicline dose) were similar to rates of medication adherence found among cancer patients and non-cancer patients[27–28]. These adherence rates suggest that varenicline was tolerated among patients in our trial. Overall study retention (84%) suggests that studies of this kind can be implemented successfully. Similar to observations in the general population [28], sleep problems, abnormal dreams, and gastrointestinal distress (nausea, constipation, and flatulence) were among the most frequently reported side effects. Fatigue and dry mouth were also commonly reported, although the role of varenicline in these cases is unclear. Fatigue and dry mouth, with or without varenicline, are also common side effects of cancer treatments. The role of varenicline in the case of increased irritability is also not clear, because irritability, with or without varenicline, is associated with nicotine withdrawal symptoms [29].
The 40.2% rate of biochemically verified abstinence is also promising and mirrors abstinence rates for non-cancer populations [7,8]. Further, the finding that abstainers at Week 12 showed a significant decrease in cognitive deficits and negative affect, compared to Week 12 smokers, lends further support for recent calls for the use of varenicline in cancer patients [30] and the potential importance of addressing psychological distress and cognitive impairment when developing smoking cessation interventions for this population. These results are also consistent with previous research that showed that reduced negative affect and improved cognition are potential mechanisms by which varenicline may be effective for cancer patients.
The results have clinical implications for improving knowledge of the safety and feasibility of varenicline as a smoking cessation intervention for cancer patients. Given that side effects, abstinence rates, and adherence rates are similar to the general population, and improvements in cognitive function and negative affect are associated with short-term abstinence, the present study produced promising initial feasibility and potential effectiveness results that could broaden the acceptability of varenicline by both patients and clinicians.
This study must be considered within the context of its limitations. First, this study did not include long-term assessment outcomes. Similarly, this study counts those unable to attend the Week 12 session in person as smokers, therefore counting potential Week 12 abstainers as smokers on the basis of session attendance and availability of biochemical abstinence verification. Second, this study examined 12-weeks of standard, open-label varenicline treatment in cancer patients, but it lacked a placebo control, therefore assessment of side effects is limited to a comparison across time-points rather than between varenicline and placebo groups. The evaluation of treatment efficacy is similarly limited by this open-label design without a comparison group. Additionally, though the results suggest that patients who were able to quit smoking using varenicline exhibited improved affect and cognitive function, the absence of a control group and the presence of behavioral counseling make it impossible to discern whether these were the mechanisms of varenicline’s effect on cessation. Lastly, this study utilized a non-biological measurement of medication adherence, instead relying on pill count and participant self-report. Use of pill count data is practical, however. A study by Buchanan et al. found that pill count correctly classified non-adherence 80% of the time when verified by plasma varenicline concentration [31]. Our use of expired breath carbon monoxide rather than cotinine as a measure of self-report verification also limited the ability to capture the behavior of the subset of our population who were light smokers at baseline.
Nevertheless, the present findings advance understanding of the use of varenicline for smoking cessation among cancer patients. This study provides important feasibility, safety, and effectiveness data in a larger and more diverse sample of treatment-seeking smokers with cancer and provides support for the use of varenicline to treat nicotine dependence in this important clinical population. Varenicline may be particularly well-suited for this population due to its potential therapeutic effect on negative affect and cognitive function. Future studies should assess longer-term benefits of varenicline in this population.
Acknowledgments
Funding: This research was supported by National Cancer Institute grants R01 CA165001; P30 CA16520.
Footnotes
Financial Disclosures: Dr. Schnoll and Dr. Hitsman receive medication and placebo free from Pfizer and Dr. Schnoll has provided consultation to Pfizer and GlaxoSmithKline. These companies had no involvement in this study.
References
- 1.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Mowls DS, Brame LS, Martinez SA, et al. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2016 doi: 10.1007/s11764-016-0515-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Ostroff JS, Copeland A, Borderud SP, et al. Readiness of Lung Cancer Screening Sites to Deliver Smoking Cessation Treatment: Current Practices, Organizational Priority, and Perceived Barriers. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv177. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farley A, Aveyard P, Kerr A, et al. Surgical lung cancer patients’ views about smoking and support to quit after diagnosis: a qualitative study. J Cancer Surviv. 2015 doi: 10.1007/s11764-015-0477-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Balmford J, Leifert JA, Jaehne A. “Tobacco dependence treatment makes no sense because”…: rebuttal of commonly-heard arguments against providing tobacco dependence treatment in the hospital setting. BMC Public Health. 2014;14:1182. doi: 10.1186/1471-2458-14-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: 2015. [Google Scholar]
- 7.Jorenby DE, Hays JT, Rigotti N, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: Results form a randomized, open-label trial. Thorax. 2008;63:717–24. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson F, Jepson C, Strasser AA, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65(2):144–9. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollema H, Hajós M, Seymour PA, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78(7):813–24. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthenelli RM, Morris C, Ramey TS, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: A randomized trial. Ann Intern Med. 2013;159:390–400. doi: 10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- 13.Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot clinical trial. J Thorac Oncol. 2011;6(6):1059–65. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: US Public Health Service; 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. (Suppl 20) [PubMed] [Google Scholar]
- 16.Posner K, Brown GK, Stanley B, et al. The Columbia–Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168:12,1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015 Apr;175(4):504–11. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lerman C, Schnoll RA, Hawk LW, Jr, et al. for PGRN-PNAT Research Group Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomized, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–8. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heatherton TF, Kozlowski L, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 21.Lai JS, Butt Z, Wagner L, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage. 2009;37(6):982–95. doi: 10.1016/j.jpainsymman.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebbert JO, Croghan IT, Sood A, et al. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11(3):234–9. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- 23.Brown R, Burgess E, Sales S, et al. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 24.Lerman C, Kaufmann V, Rukstalis M, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med. 2004;140:426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- 25.Schnoll RA, Patterson F, Wileyto P, et al. Efficacy of extended duration transdermal nicotine therapy: A randomized trial. Ann Intern Med. 2010b;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balmford J, Borland R, Hammond D, et al. Adherence to and reasons for premature discontinuation from stop-smoking medications: Data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13(2):94–102. doi: 10.1093/ntr/ntq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnoll RA, Martinez E, Tatum KL, et al. A buproprion smoking cessation clinical trial for cancer patients. Cancer Causes Control. 2010a;21(6):811–20. doi: 10.1007/s10552-010-9507-8. [DOI] [PubMed] [Google Scholar]
- 28.Williams KE, Reeves KR, Billing CB, Jr, et al. A double-blind study evaluation the long-term safety of varenicline for smoking cessation. Curr med Res Opin. 2007;23(4):793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- 29.Foulds J, Russ C, Yu C, et al. Effect of varenicline on individual nicotine withdrawal symptoms: A combined analysis of eight randomized, placebo-controlled trials. Nicotine Tob Res. 2013;15(11):1849–1857. doi: 10.1093/ntr/ntt066. [DOI] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines in Oncology: Smoking Cessation, Version I.2015. J Natl Compr Canc Netw. 2015;13(4):xliv. [Google Scholar]
- 31.Buchanan TS, Berg CJ, Cox LS, et al. Adherence to varenicline among African American smokers: An exploratory analysis comparing plasma concentration, pill count, and self-report. Nicotine Tob Res. 2012;14(9):1083–1091. doi: 10.1093/ntr/ntr333. [DOI] [PMC free article] [PubMed] [Google Scholar]


