Abstract
Most Gram-negative plant pathogenic bacteria translocate effector proteins (T3Es) directly into plant cells via a conserved type III secretion system, which is essential for pathogenicity in susceptible plants. In resistant plants, recognition of some T3Es is mediated by corresponding resistance (R) genes or R proteins and induces effector triggered immunity (ETI) that often results in programmed cell death reactions. The identification of R genes and understanding their evolution/distribution bears great potential for the generation of resistant crop plants. We focus on T3Es from Xanthomonas campestris pv. vesicatoria (Xcv), the causal agent of bacterial spot disease on pepper and tomato plants. Here, 86 Solanaceae lines mainly of the genus Nicotiana were screened for phenotypical reactions after Agrobacterium tumefaciens-mediated transient expression of 21 different Xcv effectors to (i) identify new plant lines for T3E characterization, (ii) analyze conservation/evolution of putative R genes and (iii) identify promising plant lines as repertoire for R gene isolation. The effectors provoked different reactions on closely related plant lines indicative of a high variability and evolution rate of potential R genes. In some cases, putative R genes were conserved within a plant species but not within superordinate phylogenetical units. Interestingly, the effector XopQ was recognized by several Nicotiana spp. lines, and Xcv infection assays revealed that XopQ is a host range determinant in many Nicotiana species. Non-host resistance against Xcv and XopQ recognition in N. benthamiana required EDS1, strongly suggesting the presence of a TIR domain-containing XopQ-specific R protein in these plant lines. XopQ is a conserved effector among most xanthomonads, pointing out the XopQ-recognizing RxopQ as candidate for targeted crop improvement.
Keywords: Non-host resistance, Solanaceae, Nicotiana benthamiana, Xanthomonas, XopQ, XopC, EDS1, ETI
Introduction
Plants have evolved different defense mechanisms for protection against potentially pathogenic microbes. As a first line of defense, surface-localized plant receptors recognize pathogen-associated molecular patterns (PAMPs) such as flagellin or lipopolysaccharide and initiate PAMP-triggered immunity, PTI (Jones and Dangl, 2006; Schwessinger and Ronald, 2012). Most Gram-negative plant-pathogenic bacteria express a conserved type III secretion system (T3SS) and translocate type III effector (T3E) proteins directly into the plant cell cytosol (Büttner and He, 2009). Here, T3Es manipulate plant cellular processes in various ways for the benefit of the bacteria, e.g., to suppress PTI (Büttner, 2016). On the other hand, plants can recognize T3Es via resistance (R) genes or proteins that in return initiate effector-triggered immunity, ETI (Khan et al., 2016). PTI and ETI are characterized by different cellular defense mechanisms, i.e., induction of mitogen-activated protein kinases, transcriptional reprogramming, formation of reactive oxygen species and a Ca2+-burst (Meng and Zhang, 2013; Buscaill and Rivas, 2014; Cui et al., 2015; Kadota et al., 2015). Most plant R proteins belong to the nucleotide-binding leucine-rich repeat receptors (NLRs), usually harboring an N-terminal coiled-coil domain (CNLs) or a Toll interleukin-1 receptor domain (TNLs) (Li et al., 2015). Typically, NLRs are bound to adenosine diphosphate (ADP) in an inactive state. Presence of a corresponding effector most likely induces a conformational change, leading to the exchange of ADP to adenosine triphosphate (ATP) and ultimately the exposure of the N-terminal domain, which is believed to initiate downstream signaling processes (Takken and Goverse, 2012; Sukarta et al., 2016). NLR specificity is usually conferred by the highly diverse C-terminal LRR domain, and direct effector-binding has been shown in some cases (Li et al., 2015). Alternatively, effectors can be sensed indirectly by NLRs guarding effector targets (guardee) or mimics thereof (decoy) (Khan et al., 2016), and decoys were recently found to also persist integrated into NLRs (Cesari et al., 2014). In some cases, ETI is induced without NLRs. This was shown for transcription activator-like effectors (TALEs), which activate transcription of non-NLR encoding R genes (Boch et al., 2014). ETI often results in the hypersensitive response (HR), a rapid programmed cell death limiting bacterial multiplication (Klement and Goodman, 1967).
In contrast to host plant-specific resistance, plant non-host resistance (NHR) is defined as the resistance of all genotypes of an entire plant species to all genotypes of a pathogen species (Gill et al., 2015). NHR is the most common form of plant resistance, directed against a multitude of pathogens (Heath, 2000; Niks and Marcel, 2009; Fan and Doerner, 2012). NHR is complex and includes physical barriers (e.g., the plant cuticle), plant species-specific secondary metabolites which are sufficient to defend poorly adapted pathogens and might include PTI and even ETI mechanisms (Thordal-Christensen, 2003; Maekawa et al., 2011). Plant NHR reactions vary from symptomless reactions to HR (Uma et al., 2011). Non-host plants represent an excellent repertoire of R genes and potentially novel resistance mechanisms, which can be employed to generate resistant crop plants (Bent, 2016; Lee et al., 2016).
We study the γ-proteobacterium Xanthomonas campestris pv. vesicatoria (Xcv), the causal agent of bacterial spot disease on pepper and tomato plants which causes enormous yield losses in regions with a warm and humid climate (Stall, 1995). Xcv translocates approximately 35 different T3Es into the host cell cytosol (Thieme et al., 2005; Teper et al., 2016). Here, T3Es interfere with plant cellular processes, e.g., via transcriptional reprogramming (Kay et al., 2007; Römer et al., 2007), ubiquitination (Singer et al., 2013), desumoylation (Kim et al., 2013), or modulation of proteasome activity (Üstün et al., 2013), and often suppress PTI (Popov et al., 2016). A helpful tool for T3E characterization is the Agrobacterium tumefaciens-mediated transient expression of individual T3Es in model plants of the genus Nicotiana, particularly N. benthamiana and N. tabacum both non-host plants for Xcv. Several Xcv T3Es induce cell death reactions in Nicotiana spp., presumably as a result of ETI upon T3E recognition. For example, transient expression of XopJ (Thieme et al., 2007), XopE1 (Thieme et al., 2007), XopL (Singer et al., 2013), XopX (Metz et al., 2005; Stork et al., 2015), AvrRxv, and AvrBsT (Schulze et al., 2012) induces severe cell death reactions in N. benthamiana, whereas expression of XopG induces cell death in N. tabacum (Schulze et al., 2012).
To obtain a larger picture on the recognition of Xcv effectors in Solanaceae spp., we used in this study a set of 21 T3Es, which were transiently expressed in a large panel of plant lines. Our results indicate that T3E families or homologies do not correlate with recognition in different plant lines. Furthermore, assumed R genes for recognition of T3Es are highly divergent at all phylogenetic levels. One particular Xcv effector, XopQ, was identified as a host range-limiting factor in several Nicotiana species, and is most likely recognized by a TIR-type NLR at least in N. benthamiana.
Materials and methods
Bacterial strains and growth conditions
Escherichia coli TOP10 (Thermo Fisher Scientific), DH5α λpir (Ménard et al., 1993) and derivatives were cultivated in LB (lysogeny broth) medium at 37°C. A. tumefaciens GV3101(pMP90) (Koncz and Schell, 1986) and derivatives were grown at 30°C in YEB (yeast extract broth) medium, and Xcv 85-10 (Thieme et al., 2005), Xcv 85-10ΔxopQ, Xcv 85-10ΔxopC, and Xcv 85-10ΔhrcN (Lorenz and Büttner, 2009) at 30°C in nutrient yeast glycerol (Daniels et al., 1984). Plasmids were introduced into E. coli and A. tumefaciens by chemical transformation and electroporation, respectively, and into Xcv by conjugation, using pRK2013 as helper plasmid in triparental matings (Figurski and Helinski, 1979). Plasmids used in this study are listed in Table S1.
Plant material and inoculations
Plants were grown at day and night temperatures of 23° and 19°C, respectively, with 60/40% relative humidity and 16 h light. Plant lines used for the T3E screen are listed in Table S2. For detailed analysis of NHR of N. tabacum against Xanthomonas, the plant line Nicotiana tabacum L. cv. Petit Havana was used. Generation of the Nbeds1 mutant N. benthamiana line was described previously (Ordon et al., 2016).
Two to four most expanded leaves of 5- to 9-week-old plants were used for inoculations. Xcv bacteria were hand-inoculated at an optical density (OD600) of 0.4 in 10 mM MgCl2 using a needleless syringe. For transient expression studies in planta, A. tumefaciens strains were resuspended in inoculation medium (10 mM MgCl2, 5 mM MES, pH 5.3, 150 μM acetosyringone) and hand-inoculated at OD600 = 0.8. For in planta growth curves, Xcv strains were inoculated at OD600 = 0.0004, and bacterial growth was determined as described (Bonas et al., 1991).
Generation of expression constructs
For Golden Gate cloning, coding sequences of xopC, xopG, xopO, xopP, and xopQ were PCR-amplified from genomic DNA of Xcv 85-10 using oligonucleotides with BsaI restriction sites (Table S3). Fragments were cloned into pUC57 or pJET1.2/blunt (Thermo Fisher Scientific), respectively, and then by BsaI cut-ligation (Engler et al., 2008) into the expression vectors pBRM (Szczesny et al., 2010b) or pGGX1 for Xcv, and pGGA1 (Schulze et al., 2012), pGGA2 (Schreiber et al., 2015) and pGGA7, respectively, for Agrobacterium-mediated expression in planta. The binary vector pGGA7 contains the backbone of pBGWFS7 (Karimi et al., 2002), the chloramphenicol resistance-ccdB selection cassette from pGWB2 (Nakagawa et al., 2007), and allows in planta expression of genes 3′-translationally fused to 4 × c-Myc under the control of the cauliflower mosaic virus 35S promoter. The Xcv expression vector pGGX1 contains the backbone of pBBR1MCS-5 (Kovach et al., 1995), the chloramphenicol resistance-ccdB selection cassette from pGWB2 (Nakagawa et al., 2007), and allows expression of genes 3′-translationally fused to a FLAG epitope under the control of the lac promoter. Cloning details are available upon request.
A DNA-fragment corresponding to the NbEDS1a cDNA and flanked by BpiI restriction sites was synthesized as gBlocks fragment by Integrated DNA Technologies (IDT, Germany). The synthesized fragment did not contain internal BsaI or BpiI restriction sites, and codon usage was additionally altered to eliminate target sites of Cas9 nucleases used for generation of eds1 mutant plants (Ordon et al., 2016). The fragment was cloned into pAGM1287 yielding pJOG285, and subsequently assembled together with pICH51277, pICH50010, and pICH41432 in pICH47732 to yield pJOG296 (Engler et al., 2014).
For Gateway cloning, coding sequences of avrBsT, avrRxv, xopC, and xopH were PCR-amplified from genomic DNA of Xcv 85-10 or Xcv 75-3 using oligonucleotides listed in Table S3. Fragments were cloned into pENTR/D-TOPO (Thermo Fisher Scientific) and subsequently recombined into the binary vectors pGWB5 (Nakagawa et al., 2007), pGWB6 (Nakagawa et al., 2007), or pK7FWG2 (Karimi et al., 2002) using Gateway® technology (Thermo Fisher Scientific).
Construction of xopQ and xopC deletion strains
To generate Xcv 85-10ΔxopQ, 1-kb fragments upstream and downstream of xopQ were amplified from genomic DNA of Xcv 85-10 by PCR using oligonucleotides incorporating BsaI restriction sites (Table S3). Because xopC is flanked by IS elements, xopC was only partial deleted. A 5′ fragment (298 bp upstream of xopC and the first 452 bp of xopC) and a 3′ fragment (last 327 bp of xopC and 121 bp downstream of xopC) were PCR-amplified from genomic DNA of Xcv 85-10 using oligonucleotides incorporating BsaI restriction sites (Table S3). Corresponding 5′ and 3′ fragments were cloned into SmaI-digested pUC57 (Thermo Fisher Scientific) and subsequently into the suicide vector pOGG2 (Schulze et al., 2012). The resulting plasmids pOGG2:xopC and pOGG2:xopQ were conjugated into Xcv 85-10, and mutants were selected by PCR.
Immunoblot analysis
For Agrobacterium-mediated expression studies, two 0.785 cm2 leaf discs per inoculated strain were ground in liquid nitrogen, resuspend in 130 μl 2 × Laemmli buffer and boiled. For analysis of protein synthesis in Xcv, bacteria were resuspended in 10 mM MgCl2 to OD600 = 0.4, 500 μl were pelleted, resuspended in 40 μl 2 × Laemmli and boiled. Proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting. Strep Tag II Antibody HRP Conjugate (Merck Chemicals GmbH), anti-c-Myc (Roche Diagnostics) anti-GFP (Thermo Fisher Scientific) primary antibodies and horseradish peroxidase-labeled α-rabbit and α-mouse antibodies (GE Healthcare) were used.
Results
T3ES from Xcv induce necrosis or chlorosis on non-host Solanaceae
To identify T3Es that induce a macroscopic reaction in non-host plants, 21 T3Es from different Xcv strains (Table 1) were synthesized via Agrobacterium-mediated transient expression in leaves of 86 non-host Solanaceae lines, mostly Nicotiana species (Table S2). Plant reactions were scored over 8 days and categorized into six classes as exemplified in Figure 1. Protein synthesis was probed by immunoblot analysis. Plant reactions and expression data are summarized in Tables 2, 3 and Table S4. Expression of GFP did not trigger visible reactions, indicating that Agrobacterium itself was not recognized by any plant line. Upon effector expression, plants showed a range of macroscopic responses, from no reaction to chlorosis and to more or less severe cell death. AvrBs2, AvrBsT, AvrRxv, XopE1, XopG, XopL, XopM, and XopQ caused reactions, often fast cell death, on the majority of the plant lines analyzed (Tables 2, 3). XopC, XopK, AvrBs3, XopJ, and XopV triggered reactions in a few lines tested, whereas only one plant line reacted to XopH (Nnud) and XopO (Nvel), respectively. Intriguingly, XopE2, XopI, and XopP never caused any visible reactions although they were mostly well expressed. We often observed no plant reaction in the infected tissue. Even in these cases, the majority of effectors was detectable by immunoblot, indicating that a lack of phenotype is not due to transformation efficiency.
Table 1.
T3Es from Xcv analyzed in this study.
| Effectora | Comment(s)b | References |
|---|---|---|
| AvrBs1 | Unknown function | Ronald and Staskawicz, 1988; Escolar et al., 2001 |
| AvrBs2 | Putative glycerophosphoryl-diester phosphodiesterase | Kearney and Staskawicz, 1990; Zhao et al., 2011 |
| AvrBs3 | TAL effector family, transcriptional activator | Bonas et al., 1989; Kay et al., 2007 |
| AvrBsT | YopJ/AvrRxv family, acetyltransferase | Escolar et al., 2001; Kim et al., 2010; Szczesny et al., 2010a; Cheong et al., 2014 |
| AvrRxv | YopJ/AvrRxv family, putative cysteine protease and/or acetyltransferase | Whalen et al., 1993, 2008 |
| XopB | HopD1 family, unknown function | Noël et al., 2001; Schulze et al., 2012 |
| XopC | Putative haloacid dehalogenase-like hydrolase | Noël et al., 2003; Salomon et al., 2011 |
| XopE1 | HopX family, putative transglutaminase, N-myristoylation motif | Thieme et al., 2007 |
| XopE2 | HopX family, putative transglutaminase, N-myristoylation motif | Thieme et al., 2007 |
| XopG | HopH family, putative zinc metalloprotease | Potnis et al., 2011; Schulze et al., 2012 |
| XopH (AvrBs1.1) | Protein tyrosine phosphatase | Potnis et al., 2012 |
| XopI | F-box motif | Schulze et al., 2012 |
| XopJ | YopJ/AvrRxv family, putative cysteine protease and/or acetyltransferase | Noël et al., 2003; Üstün et al., 2013 |
| XopK | Unknown function | Schulze et al., 2012 |
| XopL | E3 ubiquitin ligase | Singer et al., 2013 |
| XopM | Unknown function | Schulze et al., 2012 |
| XopO | Homology to HopK1 and AvrRps4 (P. syringae) | Roden et al., 2004; Sohn et al., 2009 |
| XopP | Unknown function | Roden et al., 2004 |
| XopQ | HopQ1-1 family, putative inosine-uridine nucleoside N-ribohydrolase | Roden et al., 2004; Teper et al., 2014 |
| XopS | Unknown function | Schulze et al., 2012 |
| XopV | Unknown function | Schulze et al., 2012 |
T3Es isolated from Xcv strain 85-10 with the exception of AvrBs3 (from Xcv 82-8) and AvrBsT (from Xcv 75-3).
Putative molecular function, conserved motifs and/or homology to known T3Es from Pseudomonas and other Xanthomonas spp. For Pseudomonas effectors, the unified nomenclature was used (Lindeberg et al., 2005).
Figure 1.
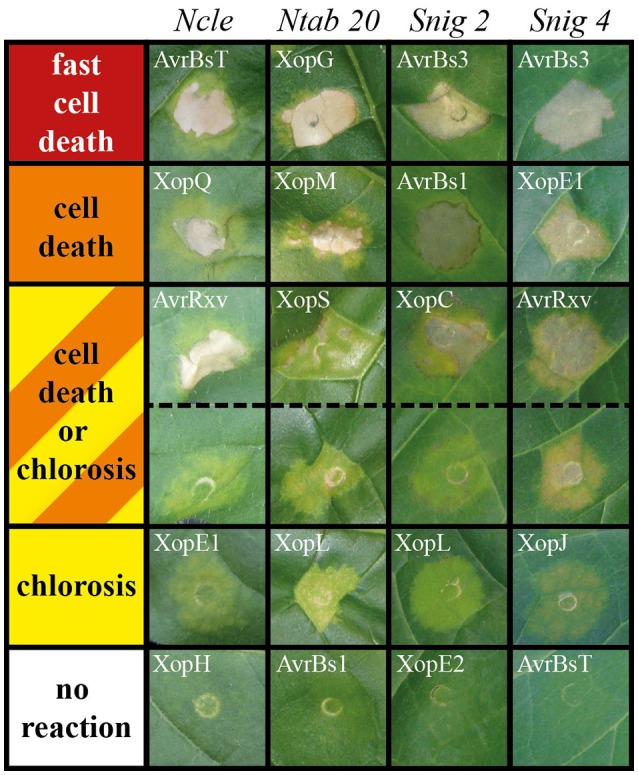
Plant phenotypes resulting from Agrobacterium-mediated effector expression. T3Es from Xcv were transiently expressed in 86 Solanaceae lines via Agrobacterium-mediated T-DNA transfer (see Table 2 for details). Plant reactions were classified into five groups, each represented by a color: red, fast cell death (3 dpi); orange, cell death (8 dpi); yellow, chlorosis (8 dpi); orange/yellow striped, chlorosis or cell death (8 dpi); white, no visible reaction (8 dpi). As examples, phenotypes of four plant lines after expression of different T3Es are shown. Plant lines were abbreviated according to Table S2. The Xcv effector causing the respective reaction is indicated. Photographs were taken 8 dpi.
Table 2.
Reactions of 40 solanaceous plants to Agrobacterium-mediated expression of Xcv T3Es.
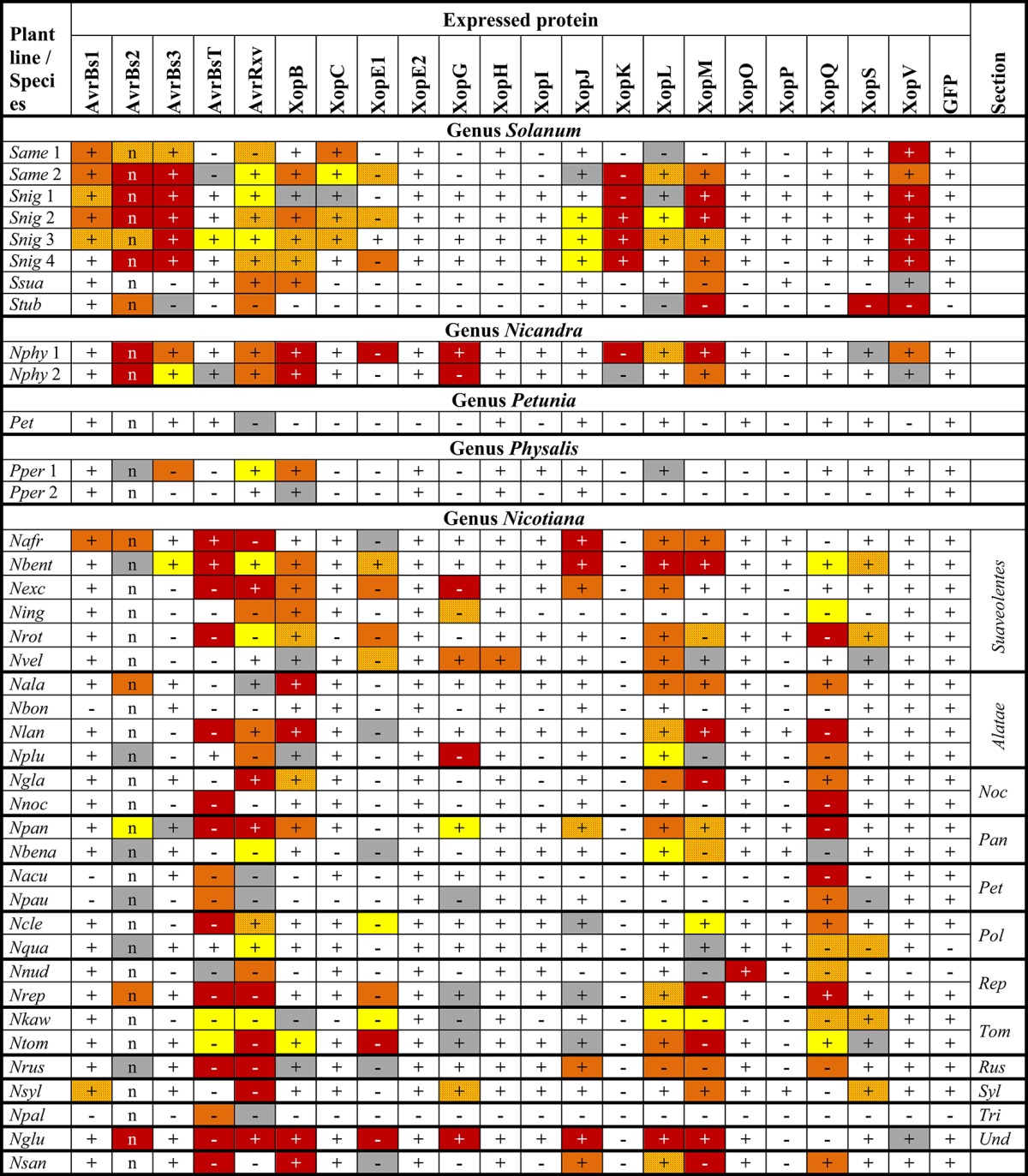
Five plants per line, two leaves each, were inoculated with A. tumefaciens mediating the expression of the T3Es indicated or of GFP as control. Plant reactions were scored over 8 dpi and are represented by the color code as exemplified in Figure 1: red, strong necrosis on ≥7/10 spots (3 dpi); orange, weak necrosis on ≥7/10 spots (8 dpi); yellow, chlorosis on ≥7/10 spots (8 dpi); yellow/orange striped: chlorosis or cell death on ≥7/10 spots (8 dpi); white, no visible reaction on ≥7/10 spots (8 dpi); gray: data inconsistent (reactions on 4–6 spots). T3E expression analyzed by immunoblot is indicated: +, expression detectable; −, no expression detectable; n, expression not analyzed. Noc, Noctiflorae; Pan, Paniculatae; Pet, Petunioides; Pol, Polydicliae; Rep, Repandae; Tom, Tomentosae; Rus, Rusticae; Syl, Sylvestres; Tri, Trigonophyllae; Und, Undulatae.
Table 3.
Reactions of 46 Nicotiana tabacum plant lines to Agrobacterium-mediated expression of Xcv T3Es.
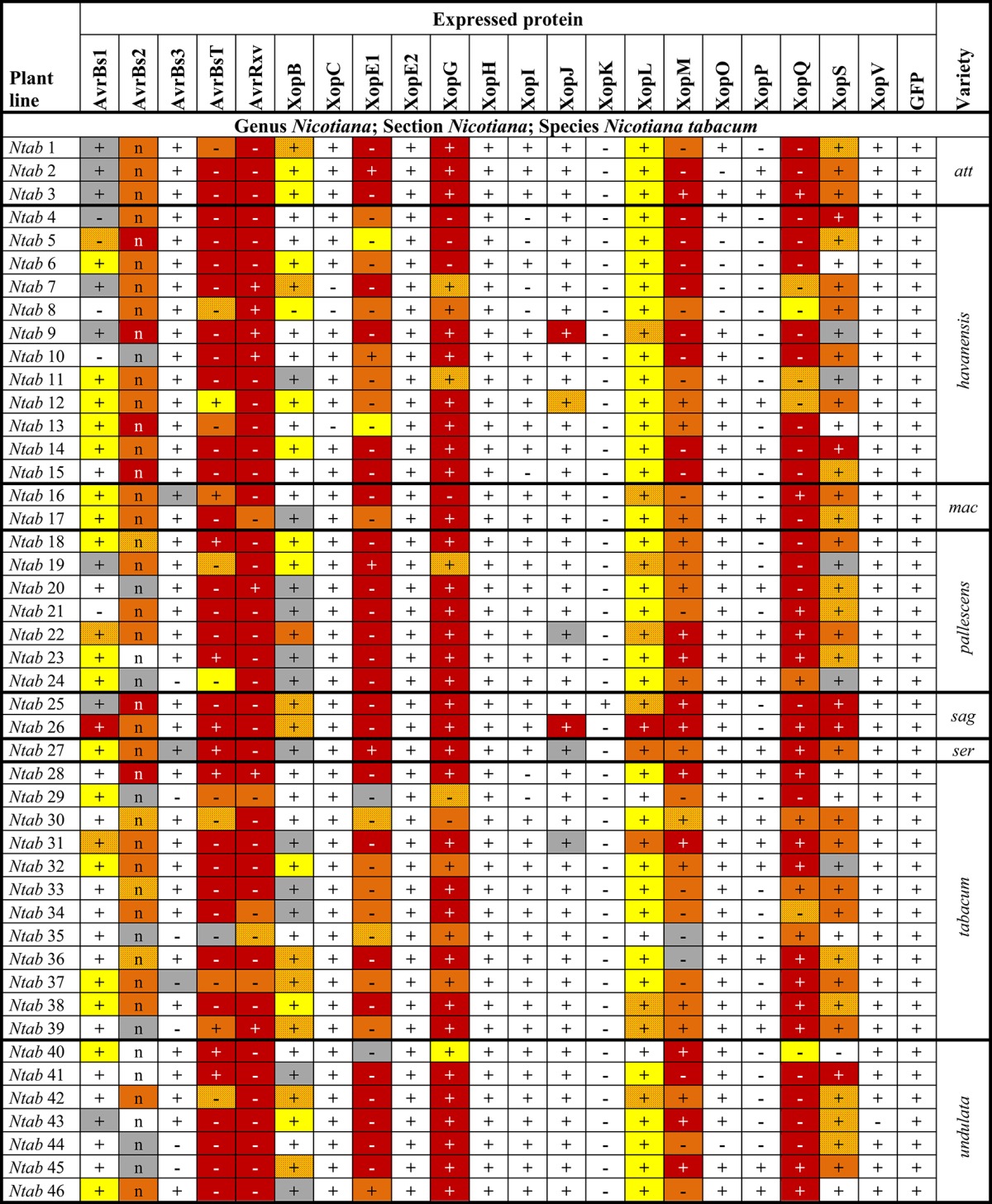
The same experimental procedure and color code as described in Table 2 was used. att, attenuata; mac, macrophylla; sag, sagittata; ser, serotina.
After the first survey, a subset of 18 plant accessions encompassing most phylogenetic groups was tested again in at least two additional independent experiments which generally confirmed the first results (Table S5).
Members of T3E families trigger diverse plant-reaction patterns
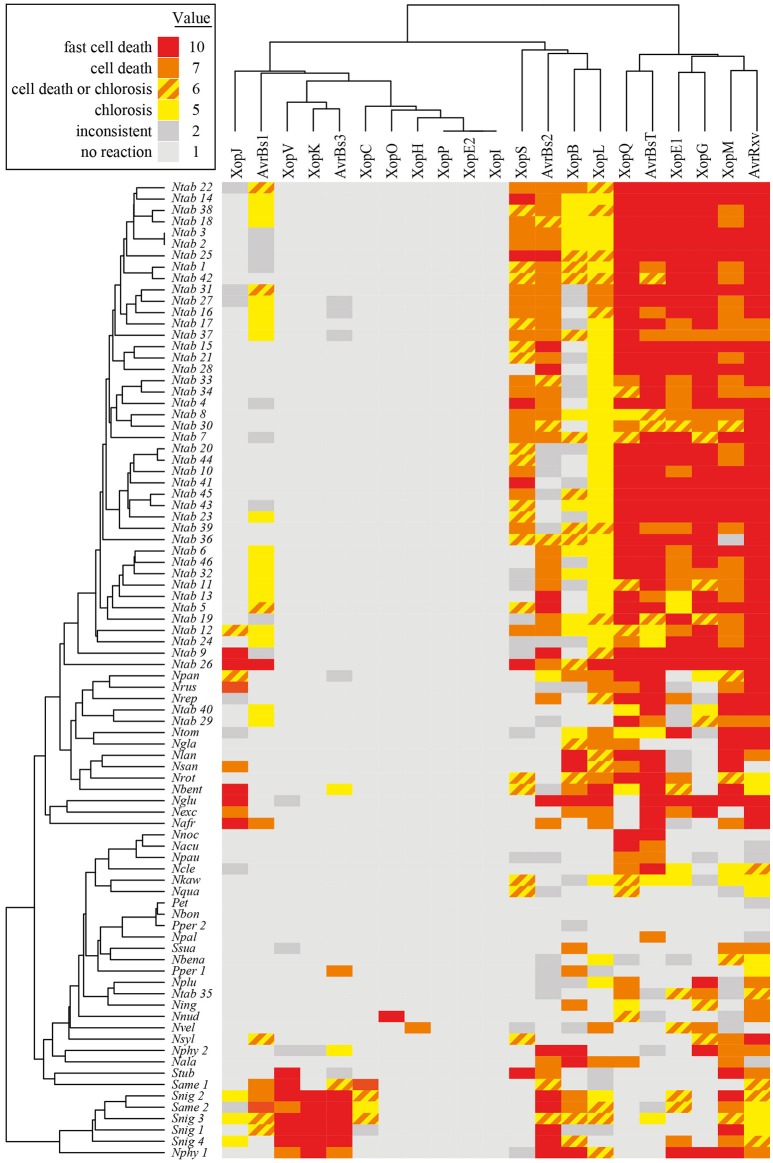
Hierarchical cluster analysis was performed to identify potential commonalities between T3Es with respect to the induced plant reactions. Since we aimed at the identification of T3Es that are recognized in solanaceous non-host plants, special emphasis was laid on fast cell death, i.e., HR-like reactions, by scoring of the observed reactions on a numerical scale from 1 (no reaction) to 10 (fast cell death). Hierarchical cluster analysis of effectors and plant accessions revealed two branches of T3Es (Figure 2): T3Es, which triggered reactions on most lines of the genus Nicotiana (AvrBsT, AvrRxv, XopE1, XopG, XopM, XopQ) and T3Es, which induced reactions less frequently (all other T3Es). Only a few T3Es showed similar reaction patterns: AvrBs3, XopK, and XopV, which induced cell death in most Solanum species cluster together, as well as T3Es that triggered visible reactions in only few lines (XopC, XopE2, XopH, XopI, XopO, and XopP). All other T3Es triggered rather unique reaction patterns (Figure 2). Considering the overrepresentation of N. tabacum lines (Table S2), one line of each N. tabacum variety was randomly selected and hierarchical cluster analysis repeated (Figure S1). This led to only minor changes in T3E clustering (compare Figure 2 and Figure S1). The tested T3E set contained three members of the YopJ/AvrRxv T3E family (AvrBsT, AvrRxv, and XopJ) and two members of the HopX T3E family (XopE1 and XopE2). Interestingly, members of a given T3E family did not group together in hierarchical cluster analysis. Thus, the classification into a “family” does not allow conclusions or the predictions about a T3E's capacity to induce plant reactions or about their putative recognition via corresponding R genes/R proteins.
Figure 2.
Plant reactions to Agrobacterium-mediated transient expression of Xcv T3Es. Heatmap representation of effector responses in 86 different non-host Solanaceae plant lines (for abbreviations see Table S2). Five plants per line, two leaves per plant, resulting in 10 spots per Agrobacterium strain, were inoculated with Agrobacterium strains mediating expression of the T3Es indicated on top. Plant reactions observed on at least 7/10 spots were classified as follows: fast cell death (3 dpi); cell death (6 dpi); chlorosis (6 dpi); chlorosis or cell death (6 dpi); no visible reaction (6 dpi). Reactions on only 4-6/10 spots were judged to be inconsistent. Plant reactions were visualized in a heatmap using the color code indicated. Each reaction type was assigned a value serving as the basis for clustering. The dendrogram shows the results of hierarchical clustering using average linkage and euklidean distance measures for T3Es and plant genotypes, respectively.
Conservation of putative R genes
The observed T3E-induced plant reactions in different species might rely on the presence of corresponding R genes. Among our set of plant lines, in particular the Nicotiana phylogeny has been extensively studied. Based on phylogenetic studies, e.g., sequence analyses of plastid- and nuclear-encoded genes and genomic in situ hybridization experiments (Chase et al., 2003; Clarkson et al., 2010; Kelly et al., 2013), the genus Nicotiana has been divided into 13 sections (Knapp et al., 2004). To study conservation of putative R genes in this genus in more detail, representative lines of all sections were tested. No T3E triggered a reaction in all tested Nicotiana lines (Table 2 and Table S2). We furthermore included six species of section Suaveolentes and four species of section Alatae to test for conservation of putative R genes within a given section. Since none of the tested T3Es triggered reactions in all representatives of the two sections (Table 2), putative corresponding R genes within Suaveolentes and Alatae appear not to be conserved.
Finally, 46 members of the species N. tabacum (sect. Nicotiana) were analyzed. AvrBsT, AvrRxv, XopE1, XopG, XopL, XopM, and XopQ triggered consistent reactions in all or most lines of the species N. tabacum (at least 43 out of 46 lines), suggesting a high conservation of putative corresponding R genes (Table 3). Four T3Es triggered consistent reactions in 21–34 N. tabacum lines tested: AvrBs1 (21/46), AvrBs2 (34/46), XopB (21/46), and XopS (34/46). Putative R genes recognizing these T3Es appear less conserved, but retain a high persistence among N. tabacum lines.
Taken together, some putative R genes are conserved within the species N. tabacum, whereas no conservation was observed within the superordinate phylogenetic units section and genus.
XopQ is a host range determinant in a number of Nicotiana species
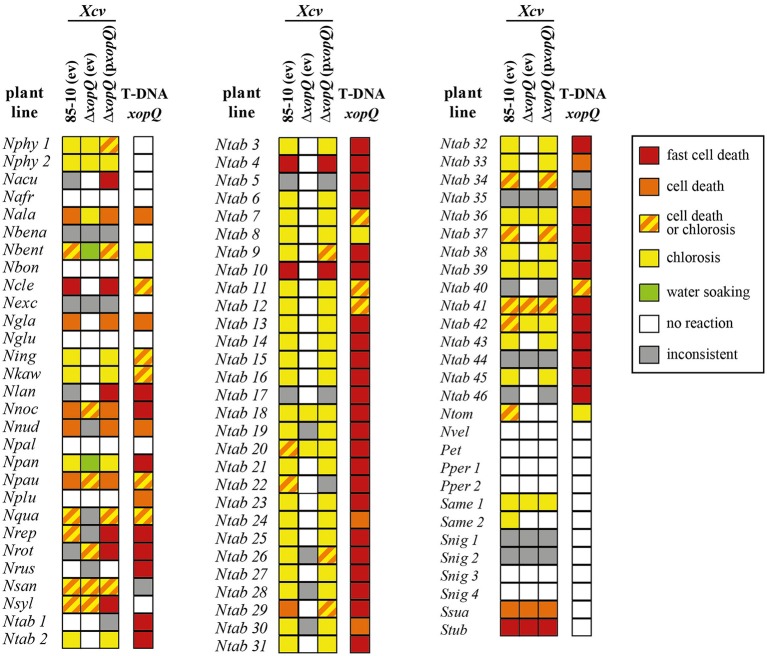
Strikingly, XopQ expression induced necrotic or chlorotic reactions exclusively in Nicotiana species (Figure 2, Table 2), suggesting the presence of a XopQ-specific R gene (RxopQ) in most members of this genus. We speculated that XopQ is also recognized during infection of Nicotiana spp. with Xcv and therefore contributes to Xcv-induced NHR. To test the influence of XopQ on NHR, all 86 Solanaceae lines were infected with the wild-type strain Xcv 85-10, the Xcv 85-10ΔxopQ deletion mutant and an Xcv 85-10ΔxopQ strain ectopically expressing xopQ. Xcv 85-10ΔxopQ caused weaker or no reactions compared to the wild-type strain on approximately two-thirds of the accessions tested (Figure 3). The plant phenotypes after Xcv infection correlated well with reactions observed after Agrobacterium-mediated XopQ expression: If T-DNA delivery of xopQ induced a cell death or chlorosis, Xcv-induced reactions also were xopQ-dependent (Figure 3). Intriguingly, two plant lines, N. benthamiana (Nbent) and N. paniculata (Npan), showed water-soaked lesions after infection with Xcv 85-10ΔxopQ, whereas infection with the wild-type and the complemented ΔxopQ mutant triggered chlorotic or cell death reactions (Figures 3, 4A).
Figure 3.
Avirulence activity of XopQ is restricted to Nicotiana species. Eighty-six different non-host Solanaceae plant lines (for abbreviations see Table S2) were inoculated with Xcv strains 85-10 and Xcv 85-10ΔxopQ, harboring empty vector (ev) or pBRM:xopQ (pxopQ), at OD600 = 0.4; reactions were scored for 6 days. Five plants per line with two leaves per plant were inoculated resulting in 10 spots per analyzed Xcv strain. Plant reactions are indicated according to the following color code: red, fast cell death (3 dpi); orange, cell death (6 dpi); yellow, chlorosis (6 dpi); orange/yellow striped, chlorosis or cell death (6 dpi); green, water-soaked lesions (6 dpi); white, no visible reaction (6 dpi). Colors were assigned if the same type of reaction was observed on ≥7/10 spots, reactions on only 4-6/10 spots were judged inconsistent, indicated in gray. Plant phenotypes 8 dpi of Agrobacterium mediating xopQ expression are indicated on the right-hand side of each column.
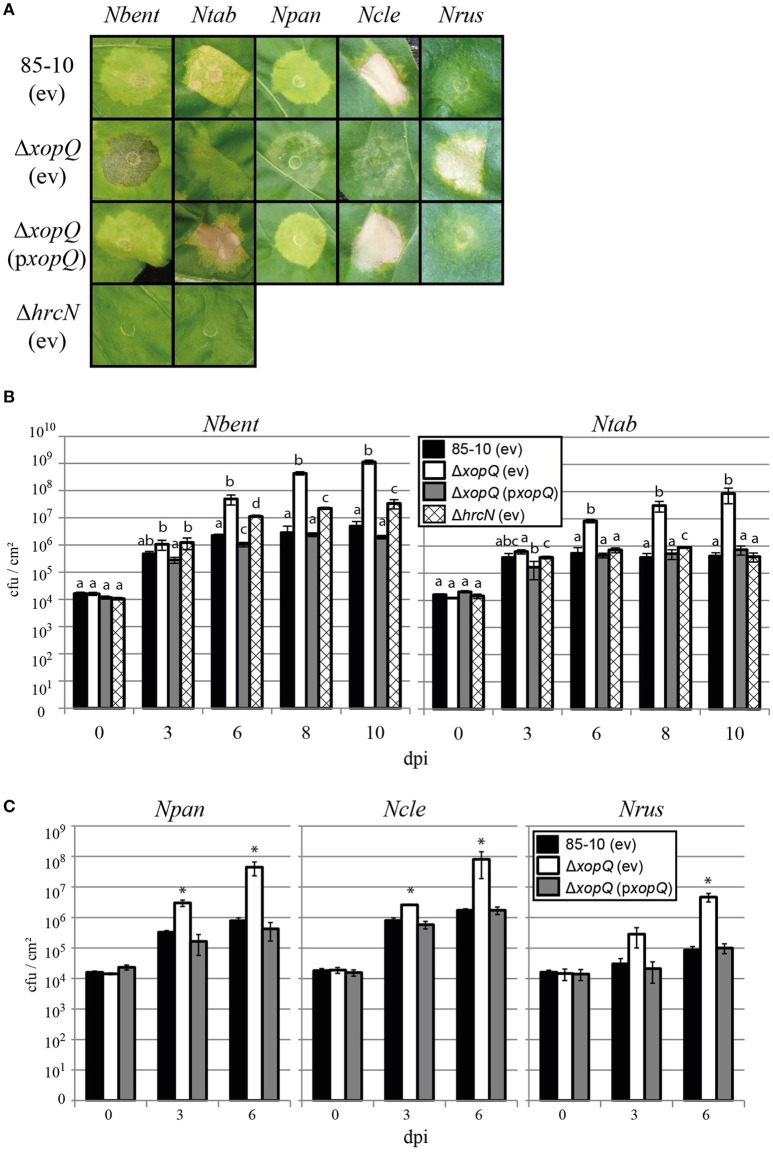
Figure 4.
XopQ shows avirulence activity in Nicotiana spp. Five non-host lines were infected with Xcv: N. benthamiana (Nbent), N. tabacum (Ntab), N. paniculata (Npan), N. clevelandii (Ncle), and N. rustica (Nrus). (A) Leaves were inoculated with Xcv strains 85-10, 85-10ΔxopQ, and 85-10ΔhrcN, harboring empty vector (ev) or pBRM:xopQ (pxopQ) at OD600 = 0.4. Photographs were taken 6 dpi (Nbent, Ntab), 7 dpi (Npan, Ncle) and 12 dpi (Nrus), respectively. (B,C) Bacterial growth of Xcv strains in leaves was tested. The same Xcv strains as above were inoculated and bacterial multiplication was monitored over a period of 10 days. Values represent the mean of three samples from three different plants. Error bars indicate standard deviation. Different letters represent statistically significant differences; asterisks indicate statistically significant differences when compared to the wild-type strain (two sided t-test, P < 0.05). Experiments were repeated at least twice with similar results.
Similarly to the transient expression via Agrobacterium, a subset of 18 plant accessions encompassing most phylogenetic groups was analyzed in at least two additional independent experiments. Results largely confirmed the reactions shown in Figure 3 (Table S5). Inoculation of Xcv 85-10ΔhrcN (Lorenz and Büttner, 2009), a T3SS-deficient and non-pathogenic mutant, never resulted in visible reactions (Table S5). Thus, macroscopic NHR reactions depend on T3E translocation, whereas T3SS-independent recognition of Xcv, i.e., during PTI, failed to induce visible NHR reactions.
Next, we determined whether XopQ contributes to bacterial multiplication in leaves of N. benthamiana (Nbent), N. tabacum (Ntab), N. paniculata (Npan), N. clevelandii (Ncle), and N. rustica (Nrus). In these lines, xopQ differentially determines the Xcv-induced NHR reaction: Xcv 85-10 induces a xopQ-dependent chlorotic reaction in Nbent, Ntab, and Npan and a HR-like reaction in Ncle (Figure 4A). Xcv 85-10ΔxopQ triggered water soaking on Nbent and Npan and nearly no visible reactions on Ntab and Ncle. Nrus was the only plant line in which Xcv 85-10ΔxopQ triggered cell death, whereas Xcv 85-10 caused no visible reactions (Figures 3, 4A). As shown in Figures 4B,C, Xcv 85-10 moderately multiplied in all plant lines, whereas Xcv 85-10ΔxopQ grew significantly better. We also analyzed in planta growth of the T3S-deficient strain Xcv 85-10ΔhrcN in Ntab and Nbent. Interestingly, Xcv 85-10ΔhrcN multiplied significantly better in Nbent than Xcv 85-10 (Figure 4B) indicating a strong impact of ETI on NHR of Nbent. Taken together, in all Nicotiana species analyzed, XopQ displays an avirulence activity triggering plant defenses and restricting the growth of Xcv in the leaf tissue.
XopC determines the Xcv-induced phenotype in S. americanum
As described above, XopQ affected Xcv-mediated NHR reactions in all Nicotiana plant lines in which Agrobacterium-mediated expression of XopQ triggered a reaction. We wondered if this is also true for other T3Es. In contrast to XopQ, transient expression of XopC exclusively induced plant reactions in lines of the genus Solanum (Table 2). We speculated that XopC contributes to Xcv-induced reactions in these plant lines and generated a xopC deletion mutant. As shown in Figure 5, Xcv 85-10ΔxopC induced weaker reactions than Xcv 85-10 in S. americanum (Same 1), which could be complemented by ectopic expression of xopC. Deletion of xopC did not affect visible reactions in N. benthamiana and N. tabacum to infection with Xcv (data not shown). Thus, similarly to recognition of XopQ, also XopC contributes to Xcv-induced phenotypes in certain non-host plants.
Figure 5.
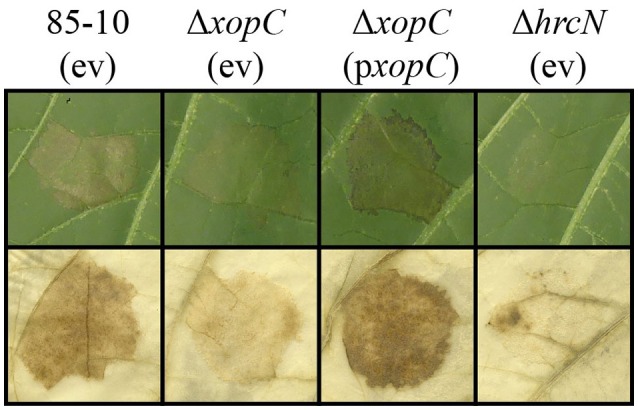
XopC influences Xcv-mediated non-host resistance in Solanum americanum. Solanum americanum (Same 1) leaves were inoculated with Xcv strains 85-10, 85-10ΔxopC, and 85-10ΔhrcN, harboring an empty vector (ev) or pBRM:xopC (pxopC), at OD600 = 0.4. Plant reactions were documented 5 dpi (upper panel) and 3 dpi (lower panel). For better visualization of cell death reactions at 3 dpi, the leaf was bleached in EtOH (lower panel). The experiment was repeated twice with similar results.
XopQ-mediated recognition in N. benthamiana depends on EDS1
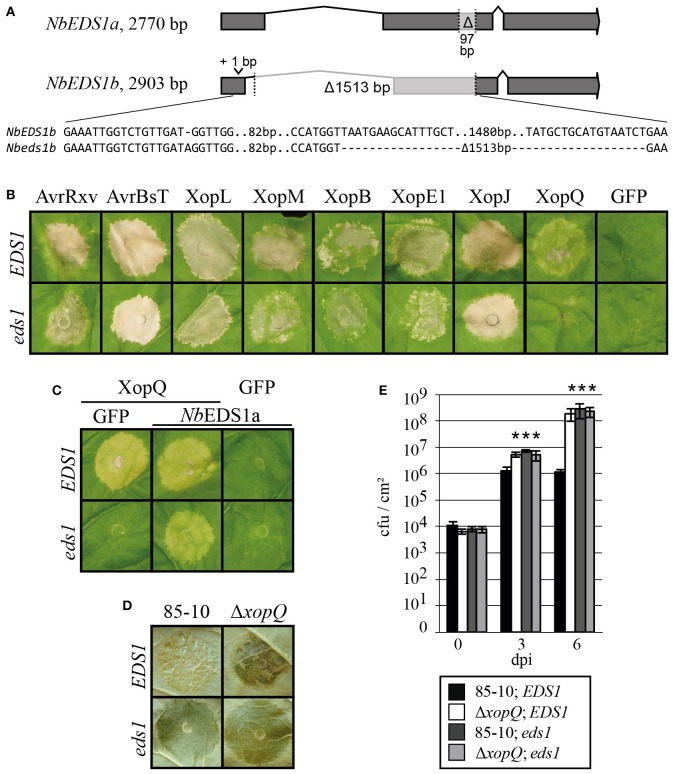
In most cases, T3E recognition takes place within the plant cell via corresponding NLR-type R proteins (Khan et al., 2016). TIR domain-containing TNLs represent one large NLR subgroup, and TNL-mediated immunity required the lipase-like protein EDS1 in N. benthamiana (Peart et al., 2002), tomato (Hu et al., 2005) and Arabidopsis thaliana (Aarts et al., 1998; Wirthmueller et al., 2007). We employed a recently reported Nbeds1a-1 line to test EDS1 dependency of T3E-induced plant reactions in N. benthamiana, which encodes two EDS1 orthologs, NbEDS1a and NbEDS1b (Ordon et al., 2016). Nbeds1a-1 was reported to contain a 97-bp deletion in exon 2 of NbEDS1a, which was generated using Cas9-based nucleases. Since employed guide RNAs also targeted NbEDS1b, this locus was sequence-verified. Indeed, the Nbeds1a-1 line additionally contained both a point mutation and a large deletion at the NbEDS1b locus (Figure 6A). However, this line will be further referred to as Nbeds1a-1, since NbEDS1b is most likely a pseudogene (Figure S2). When T3Es were transiently expressed in Nbeds1a-1 leaf tissues, plant reactions induced by AvrBsT, AvrRxv, XopE1, XopJ, XopL, and XopM were unaltered, indicating EDS1-independent recognition of these effectors (Figure 6B). In contrast, XopQ-induced chlorosis was abolished on eds1 mutant plants, suggesting activation of an EDS1-dependent resistance pathway (Figure 6B). A transient complementation assay was used to unequivocally show EDS1-dependent recognition of XopQ in N. benthamiana. XopQ or GFP were transiently co-expressed with NbEDS1a in wild-type or eds1 mutant leaf tissues (Figure 6C). XopQ expression induced chlorosis on wild type, but not eds1 plants, and chlorosis was restored upon co-expression of NbEDS1b (Figure 6C). Thus, the putative RxopQ for recognition of XopQ most likely encodes a TIR-type NLR protein.
Figure 6.
Recognition of XopQ and Xcv in N. benthamiana depends on EDS1. (A) Schematic representation of EDS1 loci in N. benthamiana. The genomic mutations harbored in the Nbeds1a-1 line are indicated. (B–E) Leaves of wild-type N. benthamiana (EDS1) and Nbeds1a-1 (eds1) mutant plants were inoculated. (B) A. tumefaciens strains mediating expression of the indicated T3Es and GFP with OD600 = 0.8 were inoculated. Photographs were taken 7 dpi. (C) A. tumefaciens strains mediating expression of XopQ, GFP or NbEDS1a with OD600 = 0.8 were mixed in a 1:1 ratio and inoculated. Photographs were taken 10 dpi. (D) Inoculation of Xcv 85-10 and 85-10ΔxopQ at OD600 = 0.4. Phenotypes were documented 7 dpi. (E) Bacterial multiplication was monitored over a period of 6 days after inoculation of Xcv 85-10 and 85-10ΔxopQ at OD600 = 0.0004. Values represent the mean of three samples from three different plants. Error bars indicate standard deviations. Asterisks indicate significant differences compared to Xcv 85-10 in EDS1 plants (two-sided t-test, P < 0.05). Experiments were repeated at least twice with similar results.
To analyze the role of EDS1 in the NHR of N. benthamiana against Xcv, we inoculated Xcv 85-10 and Xcv 85-10ΔxopQ into N. benthamiana wild-type (EDS1) and Nbeds1a-1 (eds1) plants. Xcv 85-10 triggered no disease symptoms and showed a moderate growth, whereas Xcv 85-10ΔxopQ multiplied significantly better and caused disease symptoms in wild-type N. benthamiana (Figures 6D,E). In eds1 plants, both Xcv strains caused disease and multiplied equally well (Figures 6D,E). Thus, EDS1 is essential for the NHR of N. benthamiana against Xcv 85-10, most likely due to its essential role in XopQ recognition via a corresponding TIR-type NLR.
Discussion
Different Solanaceae encode a diverse set of putative R genes
Our work is the first larger study on reactions caused by Xanthomonas T3Es in non-host plants. Plant phenotypes upon T3E expression reached from fast, HR-like cell death over chlorotic reactions to no visible reaction. T3E-induced cell death reactions are a hallmark of ETI (Henry et al., 2013) and, therefore, suggest the presence of one or several corresponding R genes/R proteins. Chlorotic reactions might also result from ETI, as shown for recognition of the Pseudomonas syringae T3E AvrB by the TNL TAO1 in A. thaliana (Eitas et al., 2008). In some cases, however, the observed phenotypes might result from a virulence-associated activity of the respective strongly expressed effector and occur independently of an R gene/R protein. Transient expression of T3Es in different Solanaceae species led to diverse reaction patterns (Tables 2, 3), suggesting variable sets of putative R genes among Solanaceae or different sensitivities of plant lines to virulence activities of T3Es. A genetic variation of R genes has often been described, whereas a genetic variation of plant susceptibility against T3Es virulence activities is rarely reported. Therefore, we basically interpret our data according to the presence or absence of putative R genes. However, this simplification requires further analysis, i.e., the isolation of corresponding R genes. The number of plant R genes varies strongly in different Solanaceae species, e.g., 2042 NLRs were annotated in pepper (Chiltepin), whereas tomato (Heinz1706) only encodes 478 NLRs (Wei et al., 2016). Furthermore, a high evolution rate of R genes and R gene clusters was shown, e.g., in various Solanaceae plants (Jupe et al., 2012; Quirin et al., 2012; Andolfo et al., 2013), Fabaceae (Zheng et al., 2016), Arabidopsis lyrata (Buckley et al., 2016) and grasses (Yang et al., 2008, 2013; Luo et al., 2012; Zhang et al., 2014). We observed variable plant responses between members of the section Nicotiana and even between closely related members of the species N. tabacum, indicating dynamic acquisition and loss of R genes.
Conservation of putative R genes in N. tabacum lines
N. tabacum is an allotetraploid species which originated approximately 200,000 years ago from an interspecific cross of N. sylvestris (2n = 24, maternal progenitor) with N. tomentosiformis (2n = 24, paternal progenitor) (Leitch et al., 2008; Sierro et al., 2013, 2014). Interestingly, two sets of T3Es triggered consistent reactions in N. tomentosiformis (AvrBsT, AvrRxv, XopB, XopE1, XopL, XopM, and XopQ) and N. sylvestris (AvrBs1, AvrRxv, XopG, XopM, and XopS), respectively, with AvrRxv and XopM being recognized in both species. Taken together, one can speculate that R genes recognizing these 10 T3Es were combined upon genome fusion in N. tabacum (Figure 7). Indeed, putative R genes recognizing seven of the 10 T3Es (AvrBsT, AvrRxv, XopE1, XopG, XopL, XopM, and XopQ) appear to be conserved in N. tabacum until today. By contrast, putative R genes recognizing the T3Es AvrBs1, XopB and XopS got (functionally) lost in a number of cultivated N. tabacum lines, e.g., Ntab 8, 36, 39 (no AvrBs1-mediated reaction) and Ntab 5, 16 (no XopB-mediated reaction). Interestingly, AvrBs2 also triggered reactions in most N. tabacum lines, but not in lines of the progenitor species N. tomentosiformis or N. sylvestris tested here, suggesting loss of the putative corresponding R genes in N. tomentosiformis and N. sylvestris or gain in N. tabacum in the course of evolution (Table 3, Figure 7). The T3E AvrBs2 is recognized in pepper ECW-20R plants (Minsavage et al., 1990) and is a virulence factor across xanthomonads (Kearney and Staskawicz, 1990).
Figure 7.
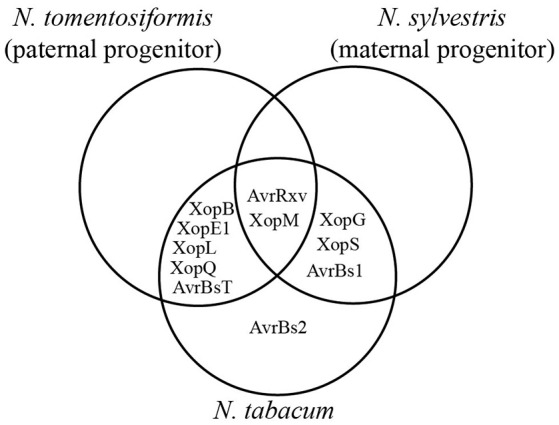
N. tabacum recognizes T3Es similarly to its progenitors. T3Es which trigger consistent plant reactions in N. tomentiformis, N. sylvestris and at least 21 of 46 tested N. tabacum lines were compared. For details see Table 3.
Out of the 46 tested N. tabacum lines, only Ntab 9, Ntab 12 and Ntab 26 showed consistent reactions to XopJ (Table 3). A plausible explanation could be that these lines acquired XopJ-specific R genes only recently. The same might be true for N. velutina (Nvel) and N. nudicaulis (Nnud), which were the only lines recognizing XopH and XopO, respectively (Table 2). As mentioned above, the observed reaction patterns might also rely on a genetically determined variation of plant susceptibility against the virulence activity of a given T3E.
ETI contributes to the Xcv-induced NHR
Up to now it was largely unknown whether Xcv translocates T3Es into non-host plants and whether ETI is induced during NHR. We identified XopQ as avirulence determinant within several non-host plant lines and found that XopC contributes to Xcv-induced plant reactions during infection of S. americanum. These results indicate that Xcv translocates T3Es into the plant cells of non-host species. In contrast to Xcv 85-10, the T3SS-deficient strain Xcv 85-10ΔhrcN did not induce phenotypic reactions on non-host plants (Table S5). We, therefore, assume that ETI significantly contributes to NHR against Xcv. Similarly, ETI also contributes to the NHR of diverse plant lines during interaction with Pseudomonas syringae (Lindeberg et al., 2009, 2012; Senthil-Kumar and Mysore, 2013).
Since Xcv 85-10ΔhrcN multiplied significantly better than Xcv 85-10 in N. benthamiana (Figure 4B), PTI appears to restrict Xcv growth in N. benthamiana less efficiently than the combination of PTI and ETI. This is reminiscent of a recent model by Cui et al. (2015) which describes PTI as a balance of positive and negative immunity signals to prevent plants from overreactions to harmless microbes. Initiation of ETI, signaling the presence of a serious pathogen threat, dampens negative regulation of PTI, resulting in an efficient plant immunity to halt the infection (Cui et al., 2015). This, however, cannot be generalized as in N. tabacum the hrcN deletion strain affected NHR phenotypes but not in planta growth of Xcv. Future studies on the interaction of Xcv with N. benthamiana and N. tabacum might help to understand quantitative differences in plant immunity responses.
XopQ is probably recognized by a TIR-type NLR in Nicotiana spp.
Here, we identified XopQ as a key host range factor in Xcv for the interaction with Nicotiana species. A recent study performed at the same time as ours also identified XopQ as a host range factor in N. benthamiana and proposed a XopQ-specific R protein, RXopQ (Schwartz et al., 2015). In most cases, Xcv 85-10ΔxopQ induced weaker NHR reactions on Nicotiana spp. compared to Xcv 85-10 and only caused disease on N. benthamiana and N. paniculata. This might be due to the recognition of at least one additional T3E or due to the inability to modulate virulence targets in most non-host plants.
The finding that N. benthamiana EDS1 is essential for the XopQ-mediated NHR suggests that RxopQ encodes a TIR-type NLR. To our knowledge, this is the first report on the role of EDS1 in NHR against a bacterial pathogen in N. benthamiana. It is worth to note that several Xcv T3Es (Figure 6B) can induce HR-like reactions when expressed in N. benthamiana, but the deletion of xopQ in Xcv 85-10 is sufficient to abolish NHR, and allows full plant colonization and disease symptom formation (Figure 6E; Schwartz et al., 2015). Thus, remaining T3Es are either translocated at low levels, below a threshold for avirulence activity, or avirulence activities might be suppressed by simultaneously translocated other T3Es. XopQ was identified as the only effector recognized in an EDS1-dependent manner, and Xcv 85-10 and Xcv 85-10ΔxopQ strains grew equally well on eds1 mutant plants. These observations suggest that XopQ is most likely the only Xcv T3E recognized in an EDS1-dependent manner in N. benthamiana, and resistance defects in eds1 mutant lines do not extend beyond abolished TNL signaling.
Interestingly, there are several parallels between recognition of XopQ from Xcv and recognition of the XopQ homolog from Pseudomonas syringae, HopQ1. As XopQ, HopQ1 from P. syringae DC3000 induces chlorosis in N. benthamiana (Wroblewski et al., 2009) and a fast cell death in N. tabacum (Li et al., 2013a) after transient expression. Additionally, HopQ1 restricts host range of P. syringae strains in N. benthamiana dependent on SGT1, indicating the presence of a HopQ1-specific R protein (Wei et al., 2007; Ferrante et al., 2009). In case of HopQ1, its virulence activity can be clearly separated from its avirulence activity because the nucleoside hydrolase-like domain of HopQ1 and the interaction of HopQ1 with host 14-3-3 proteins contribute to virulence but are dispensable for recognition in N. tabacum (Li et al., 2013a,b). It could very well be that recognition of XopQ and HopQ1 is mediated by a single TIR-type NLR. Identification of the representative R gene might represent a promising avenue for generation of more resistant crop plants.
Author contributions
NA together with UB designed experiments and interpreted results. NA, DB, PJ, ON, HP, and SS performed the screen on Solanaceae spp. and NA performed all other experiments. AB, JG, PJ, ON, HP, JS, and SS provided strains and expression constructs. JG and JS provided the eds1 mutant line. CD performed cluster analysis. NA, ST, and UB prepared the manuscript with contribution from JS and all authors reviewed the manuscript.
Funding
This work was funded by grants to UB from the Deutsche Forschungsgemeinschaft (CRC 648 “Molecular mechanisms of information processing in plants”) and the Bundesministerium für Bildung und Forschung (“tools, targets & therapeutics–ProNet-T3”).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank B. Rosinsky, K. Pflüger, C. Kretschmer, and M. Jordan for excellent technical assistance. We are grateful to R. Szczesny, E. Herzfeld, and A. Schonsky for providing unpublished material.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01796/full#supplementary-material
References
- Aarts N., Metz M., Holub E., Staskawicz B. J., Daniels M. J., Parker J. E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 95, 10306–10311. 10.1073/pnas.95.17.10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo G., Sanseverino W., Rombauts S., Van de Peer Y., Bradeen J. M., Carputo D., et al. (2013). Overview of tomato (Solanum lycopersicum) candidate pathogen recognition genes reveals important Solanum R locus dynamics. New Phytol. 197, 223–237. 10.1111/j.1469-8137.2012.04380.x [DOI] [PubMed] [Google Scholar]
- Bent A. (2016). Resistance from relatives. Nat. Biotechnol. 34, 620–621. 10.1038/nbt.3591 [DOI] [PubMed] [Google Scholar]
- Boch J., Bonas U., Lahaye T. (2014). TAL effectors–pathogen strategies and plant resistance engineering. New Phytol. 204, 823–832. 10.1111/nph.13015 [DOI] [PubMed] [Google Scholar]
- Bonas U., Schulte R., Fenselau S., Minsavage G. V., Staskawicz B. J., Stall R. E. (1991). Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol. Plant Microbe Interact. 4, 88. [Google Scholar]
- Bonas U., Stall R. E., Staskawicz B. (1989). Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol. Gen. Genet. 218, 127–136. [DOI] [PubMed] [Google Scholar]
- Buckley J., Kilbride E., Cevik V., Vicente J. G., Holub E. B., Mable B. K. (2016). R-gene variation across Arabidopsis lyrata subspecies: effects of population structure, selection and mating system. BMC Evol. Biol. 16:93. 10.1186/s12862-016-0665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaill P., Rivas S. (2014). Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 20, 35–46. 10.1016/j.pbi.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Büttner D. (2016). Behind the lines–actions of bacterial type III effector proteins in plant cells. FEMS Microbiol. Rev. 40, 894–937. 10.1093/femsre/fuw026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D., He S. Y. (2009). Type III protein secretion in plant pathogenic bacteria. Plant Physiol. 150, 1656–1664. 10.1104/pp.109.139089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S., Bernoux M., Moncuquet P., Kroj T., Dodds P. N. (2014). A novel conserved mechanism for plant NLR protein pairs: the “integrated decoy” hypothesis. Front. Plant Sci. 5:606. 10.3389/fpls.2014.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M. W., Knapp S., Cox A. V., Clarkson J. J., Butsko Y., Joseph J., et al. (2003). Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann. Bot. 92, 107–127. 10.1093/aob/mcg087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong M. S., Kirik A., Kim J.-G., Frame K., Kirik V., Mudgett M. B. (2014). AvrBsT acetylates Arabidopsis ACIP1, a protein that associates with microtubules and is required for immunity. PLoS Pathog. 10:e1003952. 10.1371/journal.ppat.1003952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. J., Kelly L. J., Leitch A. R., Knapp S., Chase M. W. (2010). Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids. Mol. Phylogenet. Evol. 55, 99–112. 10.1016/j.ympev.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Cui H., Tsuda K., Parker J. E. (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 66, 487–511. 10.1146/annurev-arplant-050213-040012 [DOI] [PubMed] [Google Scholar]
- Daniels M. J., Barber C. E., Turner P. C., Sawczyc M. K., Byrde R. J., Fielding A. H. (1984). Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 3, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitas T. K., Nimchuk Z. L., Dangl J. L. (2008). Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. U.S.A. 105, 6475–6480. 10.1073/pnas.0802157105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C., Kandzia R., Marillonnet S. (2008). A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3:e3647. 10.1371/journal.pone.0003647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C., Youles M., Gruetzner R., Ehnert T.-M., Werner S., Jones J. D., et al. (2014). A golden gate modular cloning toolbox for plants. ASC Synth. Biol. 3, 839–843. 10.1021/sb4001504 [DOI] [PubMed] [Google Scholar]
- Escolar L., Van Den Ackerveken G., Pieplow S., Rossier O., Bonas U. (2001). Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol. Plant Pathol. 2, 287–296. 10.1046/j.1464-6722.2001.00077.x [DOI] [PubMed] [Google Scholar]
- Fan J., Doerner P. (2012). Genetic and molecular basis of nonhost disease resistance: complex, yes; silver bullet, no. Curr. Opin. Plant Biol. 15, 400–406. 10.1016/j.pbi.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Ferrante P., Clarke C. R., Cavanaugh K. A., Michelmore R. W., Buonaurio R., Vinatzer B. A. (2009). Contributions of the effector gene hopQ1-1 to differences in host range between Pseudomonas syringae pv. phaseolicola and P. syringae pv. tabaci. Mol. Plant Pathol. 10, 837–842. 10.1111/J.1364-3703.2009.00577.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. (1979). Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U.S.A. 76, 1648–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill U. S., Lee S., Mysore K. S. (2015). Host versus nonhost resistance: distinct wars with similar arsenals. Phytopathology 105, 580–587. 10.1094/PHYTO-11-14-0298-RVW [DOI] [PubMed] [Google Scholar]
- Heath M. C. (2000). Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 3, 315–319. 10.1016/S1369-5266(00)00087-X [DOI] [PubMed] [Google Scholar]
- Henry E., Yadeta K. A., Coaker G. (2013). Recognition of bacterial plant pathogens: local, systemic and transgenerational immunity. New Phytol. 199, 908–915. 10.1111/nph.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., deHart A. K., Li Y., Ustach C., Handley V., Navarre R., et al. (2005). EDS1 in tomato is required for resistance mediated by TIR-class R genes and the receptor-like R gene Ve. Plant J. 42, 376–391. 10.1111/j.1365-313X.2005.02380.x [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dangl J. L. (2006). The plant immune system. Nature 444, 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Jupe F., Pritchard L., Etherington G. J., Mackenzie K., Cock P. J., Wright F., et al. (2012). Identification and localisation of the NB-LRR gene family within the potato genome. BMC Genomics 13:75. 10.1186/1471-2164-13-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y., Shirasu K., Zipfel C. (2015). Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 56, 1472–1480. 10.1093/pcp/pcv063 [DOI] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195. 10.1016/S1360-1385(02)02251-3 [DOI] [PubMed] [Google Scholar]
- Kay S., Hahn S., Marois E., Hause G., Bonas U. (2007). A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318, 648–651. 10.1126/science.1144956 [DOI] [PubMed] [Google Scholar]
- Kearney B., Staskawicz B. J. (1990). Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene avrBs2. Nature 346, 385–386. [DOI] [PubMed] [Google Scholar]
- Kelly L. J., Leitch A. R., Clarkson J. J., Knapp S., Chase M. W. (2013). Reconstructing the complex evolutionary origin of wild allopolyploid tobaccos (Nicotiana section Suaveolentes). Evolution 67, 80–94. 10.1111/j.1558-5646.2012.01748.x [DOI] [PubMed] [Google Scholar]
- Khan M., Subramaniam R., Desveaux D. (2016). Of guards, decoys, baits and traps: pathogen perception in plants by type III effector sensors. Curr. Opin. Microbiol. 29, 49–55. 10.1016/j.mib.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Kim J.-G., Stork W., Mudgett M. B. (2013). Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host Microbe 13, 143–154. 10.1016/j.chom.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. H., Choi H. W., Hwang B. K. (2010). Xanthomonas campestris pv. vesicatoria effector AvrBsT induces cell death in pepper, but suppresses defense responses in tomato. Mol. Plant Microbe Interact. 23, 1069–1082. 10.1094/MPMI-23-8-1069 [DOI] [PubMed] [Google Scholar]
- Klement Z., Goodman R. (1967). The hypersensitive reaction to infection by bacterial plant pathogens. Annu. Rev. Phytopathol. 5, 17–44. [Google Scholar]
- Knapp S., Chase M. W., Clarkson J. J. (2004). Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 53, 73–82. 10.2307/4135490 [DOI] [Google Scholar]
- Koncz C., Schell J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204, 383–396. [Google Scholar]
- Kovach M. E., Elzer P. H., Hill D. S., Robertson G. T., Farris M. A., Roop R. M., et al. (1995). Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176. [DOI] [PubMed] [Google Scholar]
- Lee S., Whitaker V. M., Hutton S. F. (2016). Mini review: potential applications of non-host resistance for crop improvement. Front. Plant Sci. 7:997. 10.3389/fpls.2016.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch I. J., Hanson L., Lim K. Y., Kovarik A., Chase M. W., Clarkson J. J., et al. (2008). The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Ann. Bot. 101, 805–814. 10.1093/aob/mcm326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Chiang Y. H., Coaker G. (2013a). The HopQ1 effector's nucleoside hydrolase-like domain is required for bacterial virulence in arabidopsis and tomato, but not host recognition in tobacco. PLoS ONE 8:e59684 10.1371/journal.pone.0059684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yadeta K. A., Elmore J. M., Coaker G. (2013b). The Pseudomonas syringae effector HopQ1 promotes bacterial virulence and interacts with tomato 14-3-3 proteins in a phosphorylation-dependent manner. Plant Physiol. 161, 2062–2074. 10.1104/pp.112.211748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kapos P., Zhang Y. (2015). NLRs in plants. Curr. Opin. Immunol. 32, 114–121. 10.1016/j.coi.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Lindeberg M., Cunnac S., Collmer A. (2009). The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol. Plant Pathol. 10, 767–775. 10.1111/J.1364-3703.2009.00587.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg M., Cunnac S., Collmer A. (2012). Pseudomonas syringae type III effector repertoires: last words in endless arguments. Trends Microbiol. 20, 199–208. 10.1016/j.tim.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Lindeberg M., Stavrinides J., Chang J. H., Alfano J. R., Collmer A., Dangl J. L., et al. (2005). Proposed guidelines for a unified nomenclature and phylogenetic analysis of type III Hop effector proteins in the plant pathogen Pseudomonas syringae. Mol. Plant Microbe Interact. 18, 275–282. 10.1094/MPMI-18-0275 [DOI] [PubMed] [Google Scholar]
- Lorenz C., Büttner D. (2009). Functional characterization of the type III secretion ATPase HrcN from the plant pathogen Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 191, 1414–1428. 10.1128/JB.01446-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Zhang Y., Hu Q., Chen J., Li K., Lu C., et al. (2012). Dynamic nucleotide-binding site and leucine-rich repeat-encoding genes in the grass family. Plant Physiol. 159, 197–210. 10.1104/pp.111.192062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Kufer T. A., Schulze-Lefert P. (2011). NLR functions in plant and animal immune systems: so far and yet so close. Nat. Immunol. 12, 817–826. 10.1038/ni.2083 [DOI] [PubMed] [Google Scholar]
- Ménard R., Sansonetti P. J., Parsot C. (1993). Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175, 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Zhang S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266. 10.1146/annurev-phyto-082712-102314 [DOI] [PubMed] [Google Scholar]
- Metz M., Dahlbeck D., Morales C. Q., Al Sady B., Clark E. T., Staskawicz B. J. (2005). The conserved Xanthomonas campestris pv. vesicatoria effector protein XopX is a virulence factor and suppresses host defense in Nicotiana benthamiana. Plant J. 41, 801–814. 10.1111/j.1365-313X.2005.02338.x [DOI] [PubMed] [Google Scholar]
- Minsavage G., Dahlbeck D., Whalen M., Kearney B., Bonas U., Staskawicz B., et al. (1990). Gene-for-gene relationships specifying disease resistance in Xanthomonas campestris pv. vesicatoria - pepper interactions. Mol. Plant Microbe Interact. 3, 41–47. [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., et al. (2007). Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41. 10.1263/jbb.104.34 [DOI] [PubMed] [Google Scholar]
- Niks R. E., Marcel T. C. (2009). Nonhost and basal resistance: how to explain specificity? New Phytol. 182, 817–828. 10.1111/j.1469-8137.2009.02849.x [DOI] [PubMed] [Google Scholar]
- Noël L., Thieme F., Gäbler J., Büttner D., Bonas U. (2003). XopC and XopJ, two novel type III effector proteins from Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 185, 7092–7102. 10.1128/JB.185.24.7092-7102.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël L., Thieme F., Nennstiel D., Bonas U. (2001). cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 41, 1271–1281. 10.1046/j.1365-2958.2001.02567.x [DOI] [PubMed] [Google Scholar]
- Ordon J., Gantner J., Kemna J., Schwalgun L., Reschke M., Streubel J., et al. (2016). Generation of chromosomal deletions in dicotyledonous plants employing a user-friendly genome editing toolkit. Plant J. [Epub ahead of print]. 10.1111/tpj.13319 [DOI] [PubMed] [Google Scholar]
- Peart J. R., Cook G., Feys B. J., Parker J. E., Baulcombe D. C. (2002). An EDS1 orthologue is required for N-mediated resistance against tobacco mosaic virus. Plant J. 29, 569–579. 10.1046/j.1365-313X.2002.029005569.x [DOI] [PubMed] [Google Scholar]
- Popov G., Fraiture M., Brunner F., Sessa G. (2016). Multiple Xanthomonas euvesicatoria Type III Effectors Inhibit flg22-Triggered Immunity. Mol. Plant Microbe Interact. 29, 651–660. 10.1094/MPMI-07-16-0137-R [DOI] [PubMed] [Google Scholar]
- Potnis N., Krasileva K., Chow V., Almeida N. F., Patil P. B., Ryan R. P., et al. (2011). Comparative genomics reveals diversity among xanthomonads infecting tomato and pepper. BMC Genomics 12:146. 10.1186/1471-2164-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potnis N., Minsavage G., Smith J. K., Hurlbert J. C., Norman D., Rodrigues R., et al. (2012). Avirulence proteins AvrBs7 from Xanthomonas gardneri and AvrBs1. 1 from Xanthomonas euvesicatoria contribute to a novel gene-for-gene interaction in pepper. Mol. Plant Microbe Interact. 25, 307–320. 10.1094/MPMI-08-11-0205 [DOI] [PubMed] [Google Scholar]
- Quirin E. A., Mann H., Meyer R. S., Traini A., Chiusano M. L., Litt A., et al. (2012). Evolutionary meta-analysis of Solanaceous resistance gene and Solanum resistance gene analog sequences and a practical framework for cross-species comparisons. Mol. Plant Microbe Interact. 25, 603–612. 10.1094/MPMI-12-11-0318-R [DOI] [PubMed] [Google Scholar]
- Roden J. A., Belt B., Ross J. B., Tachibana T., Vargas J., Mudgett M. B. (2004). A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc. Natl. Acad. Sci. U.S.A. 101, 16624–16629. 10.1073/pnas.0407383101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer P., Hahn S., Jordan T., Strauss T., Bonas U., Lahaye T. (2007). Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318, 645–648. 10.1126/science.1144958 [DOI] [PubMed] [Google Scholar]
- Ronald P. C., Staskawicz B. J. (1988). The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50-kD protein. Mol. Plant Microbe Interact. 1, 191–198. [PubMed] [Google Scholar]
- Salomon D., Dar D., Sreeramulu S., Sessa G. (2011). Expression of Xanthomonas campestris pv. vesicatoria type III effectors in yeast affects cell growth and viability. Mol. Plant Microbe Interact. 24, 305–314. 10.1094/MPMI-09-10-0196 [DOI] [PubMed] [Google Scholar]
- Schreiber T., Sorgatz A., List F., Blüher D., Thieme S., Wilmanns M., et al. (2015). Refined requirements for protein regions important for activity of the TALE AvrBs3. PLoS ONE 10:e0120214. 10.1371/journal.pone.0120214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze S., Kay S., Büttner D., Egler M., Eschen-Lippold L., Hause G., et al. (2012). Analysis of new type III effectors from Xanthomonas uncovers XopB and XopS as suppressors of plant immunity. New Phytol. 195, 894–911. 10.1111/j.1469-8137.2012.04210.x [DOI] [PubMed] [Google Scholar]
- Schwartz A. R., Potnis N., Timilsina S., Wilson M., Patané J., Martins J., Jr., et al. (2015). Phylogenomics of Xanthomonas field strains infecting pepper and tomato reveals diversity in effector repertoires and identifies determinants of host specificity. Front. Microbiol. 6:535. 10.3389/fmicb.2015.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Ronald P. C. (2012). Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 63, 451–482. 10.1146/annurev-arplant-042811-105518 [DOI] [PubMed] [Google Scholar]
- Senthil-Kumar M., Mysore K. S. (2013). Nonhost resistance against bacterial pathogens: retrospectives and prospects. Annu. Rev. Phytopathol. 51, 407–427. 10.1146/annurev-phyto-082712-102319 [DOI] [PubMed] [Google Scholar]
- Sierro N., Battey J. N. D., Ouadi S., Bakaher N., Bovet L., Willig A., et al. (2014). The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 5:3833. 10.1038/ncomms4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro N., Battey J. N., Ouadi S., Bovet L., Goepfert S., Bakaher N., et al. (2013). Reference genomes and transcriptomes of Nicotiana sylvestris and Nicotiana tomentosiformis. Genome Biol. 14:R60. 10.1186/gb-2013-14-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A. U., Schulze S., Skarina T., Xu X., Cui H., Eschen-Lippold L., et al. (2013). A pathogen type III effector with a novel E3 ubiquitin ligase architecture. PLoS Pathog. 9:e1003121. 10.1371/journal.ppat.1003121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K. H., Zhang Y., Jones J. D. (2009). The Pseudomonas syringae effector protein, AvrRPS4, requires in planta processing and the KRVY domain to function. Plant J. 57, 1079–1091. 10.1111/j.1365-313X.2008.03751.x [DOI] [PubMed] [Google Scholar]
- Stall R. E. (1995). Xanthomonas campestris pv. vesicatoria, in Pathogenesis and Host-Parasite Specificity in Plant Diseases, eds Singh R. P. S. U. S., Kohmoto K. (Tarrytown, NY: Pergamon, Elsevier Science Inc.), 167–184. [Google Scholar]
- Stork W., Kim J.-G., Mudgett M. B. (2015). Functional analysis of plant defense suppression and activation by the Xanthomonas core type III effector XopX. Mol. Plant Microbe Interact. 28, 180–194. 10.1094/MPMI-09-14-0263-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukarta O. C., Slootweg E. J., Goverse A. (2016). Structure-informed insights for NLR functioning in plant immunity. Semin. Cell Dev. Biol. 56, 134–149. 10.1016/j.semcdb.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Szczesny R., Büttner D., Escolar L., Schulze S., Seiferth A., Bonas U. (2010a). Suppression of the AvrBs1-specific hypersensitive response by the YopJ effector homolog AvrBsT from Xanthomonas depends on a SNF1-related kinase. New Phytol. 187, 1058–1074. 10.1111/j.1469-8137.2010.03346.x [DOI] [PubMed] [Google Scholar]
- Szczesny R., Jordan M., Schramm C., Schulz S., Cogez V., Bonas U., et al. (2010b). Functional characterization of the Xcs and Xps type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria. New Phytol. 187, 983–1002. 10.1111/j.1469-8137.2010.03312.x [DOI] [PubMed] [Google Scholar]
- Takken F. L., Goverse A. (2012). How to build a pathogen detector: structural basis of NB-LRR function. Curr. Opin. Plant Biol. 15, 375–384. 10.1016/j.pbi.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Teper D., Burstein D., Salomon D., Gershovitz M., Pupko T., Sessa G. (2016). Identification of novel Xanthomonas euvesicatoria type III effector proteins by a machine-learning approach. Mol. Plant Pathol. 17, 398–411. 10.1111/mpp.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper D., Salomon D., Sunitha S., Kim J. G., Mudgett M. B., Sessa G. (2014). Xanthomonas euvesicatoria type III effector XopQ interacts with tomato and pepper 14-3-3 isoforms to suppress effector-triggered immunity. Plant J. 77, 297–309. 10.1111/tpj.12391 [DOI] [PubMed] [Google Scholar]
- Thieme F., Koebnik R., Bekel T., Berger C., Boch J., Büttner D., et al. (2005). Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 187, 7254–7266. 10.1128/JB.187.21.7254-7266.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme F., Szczesny R., Urban A., Kirchner O., Hause G., Bonas U. (2007). New type III effectors from Xanthomonas campestris pv. vesicatoria trigger plant reactions dependent on a conserved N-myristoylation motif. Mol. Plant Microbe Interact. 20, 1250–1261. 10.1094/MPMI-20-10-1250 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H. (2003). Fresh insights into processes of nonhost resistance. Curr. Opin. Plant Biol. 6, 351–357. 10.1016/S1369-5266(03)00063-3 [DOI] [PubMed] [Google Scholar]
- Uma B., Rani T. S., Podile A. R. (2011). Warriors at the gate that never sleep: non-host resistance in plants. J. Plant Physiol. 168, 2141–2152. 10.1016/j.jplph.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Üstün S., Bartetzko V., Börnke F. (2013). The Xanthomonas campestris type III effector XopJ targets the host cell proteasome to suppress salicylic-acid mediated plant defence. PLoS Pathog. 9:e1003427. 10.1371/journal.ppat.1003427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Chen J., Kuang H. (2016). Dramatic Number Variation of R Genes in Solanaceae Species Accounted for by a Few R Gene Subfamilies. PLoS ONE 11:e0148708. 10.1371/journal.pone.0148708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. F., Kvitko B. H., Shimizu R., Crabill E., Alfano J. R., Lin N. C., et al. (2007). A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1-1 is able to cause disease in the model plant Nicotiana benthamiana. Plant J. 51, 32–46. 10.1111/j.1365-313X.2007.03126.x [DOI] [PubMed] [Google Scholar]
- Whalen M. C., Wang J. F., Carland F. M., Heiskell M. E., Dahlbeck D., Minsavage G. V., et al. (1993). Avirulence gene avrRxv from Xanthomonas campestris pv. vesicatoria specifies resistance on tomato line Hawaii 7998. Mol. Plant Microbe Interact. 6, 616–627. [DOI] [PubMed] [Google Scholar]
- Whalen M., Richter T., Zakhareyvich K., Yoshikawa M., Al-Azzeh D., Adefioye A., et al. (2008). Identification of a host 14-3-3 Protein that Interacts with Xanthomonas effector AvrRxv. Physiol. Mol. Plant Pathol. 72, 46–55. 10.1016/j.pmpp.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirthmueller L., Zhang Y., Jones J. D., Parker J. E. (2007). Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr. Biol. 17, 2023–2029. 10.1016/j.cub.2007.10.042 [DOI] [PubMed] [Google Scholar]
- Wroblewski T., Caldwell K. S., Piskurewicz U., Cavanaugh K. A., Xu H., Kozik A., et al. (2009). Comparative large-scale analysis of interactions between several crop species and the effector repertoires from multiple pathovars of Pseudomonas and Ralstonia. Plant Physiol. 150, 1733–1749. 10.1104/pp.109.140251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Gu T., Pan C., Feng Z., Ding J., Hang Y., et al. (2008). Genetic variation of NBS-LRR class resistance genes in rice lines. Theor. Appl. Genet. 116, 165–177. 10.1007/s00122-007-0656-4 [DOI] [PubMed] [Google Scholar]
- Yang S., Li J., Zhang X., Zhang Q., Huang J., Chen J.-Q., et al. (2013). Rapidly evolving R genes in diverse grass species confer resistance to rice blast disease. Proc. Natl. Acad. Sci. U.S.A. 110, 18572–18577. 10.1007/s00122-007-0656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Murat F., Pont C., Langin T., Salse J. (2014). Paleo-evolutionary plasticity of plant disease resistance genes. BMC Genomics 15:187. 10.1186/1471-2164-15-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Dahlbeck D., Krasileva K. V., Fong R. W., Staskawicz B. J. (2011). Computational and biochemical analysis of the Xanthomonas effector AvrBs2 and its role in the modulation of Xanthomonas type three effector delivery. PLoS Pathog. 7:e1002408. 10.1371/journal.ppat.1002408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Wu H., Zhang R., Li S., He W., Wong F.-L., et al. (2016). Molecular phylogeny and dynamic evolution of disease resistance genes in the legume family. BMC Genomics 17:402. 10.1186/s12864-016-2736-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.