Abstract
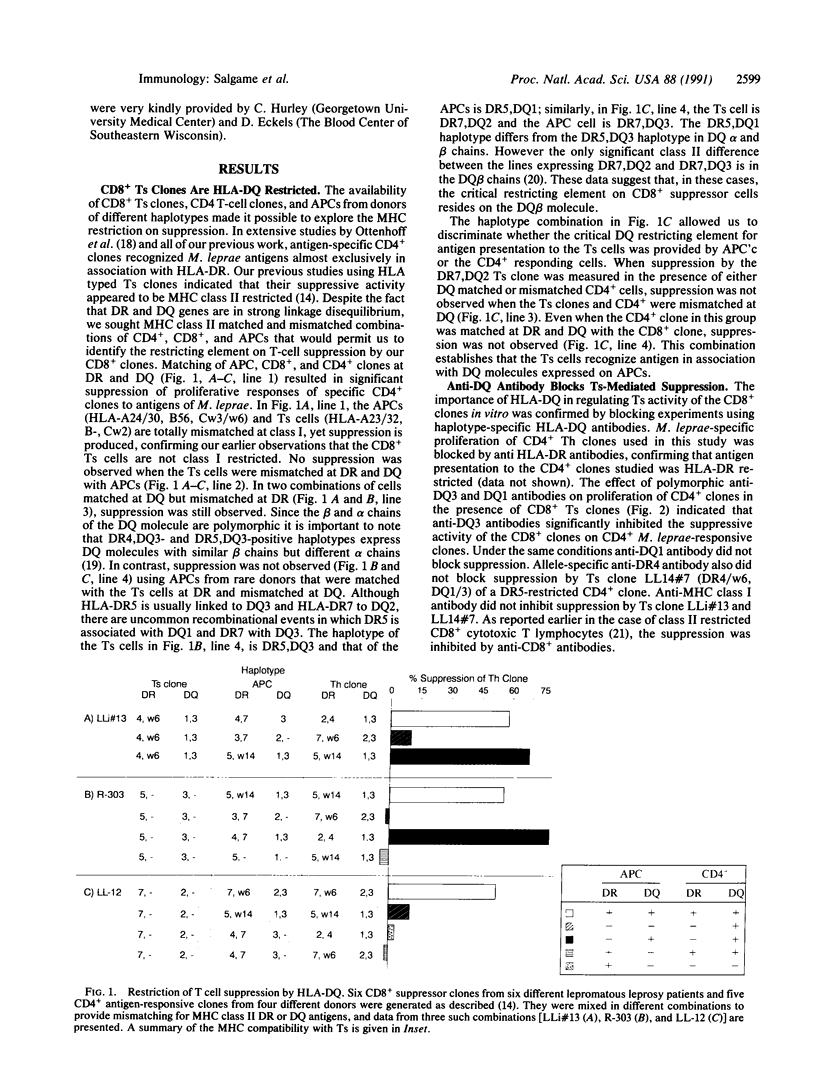
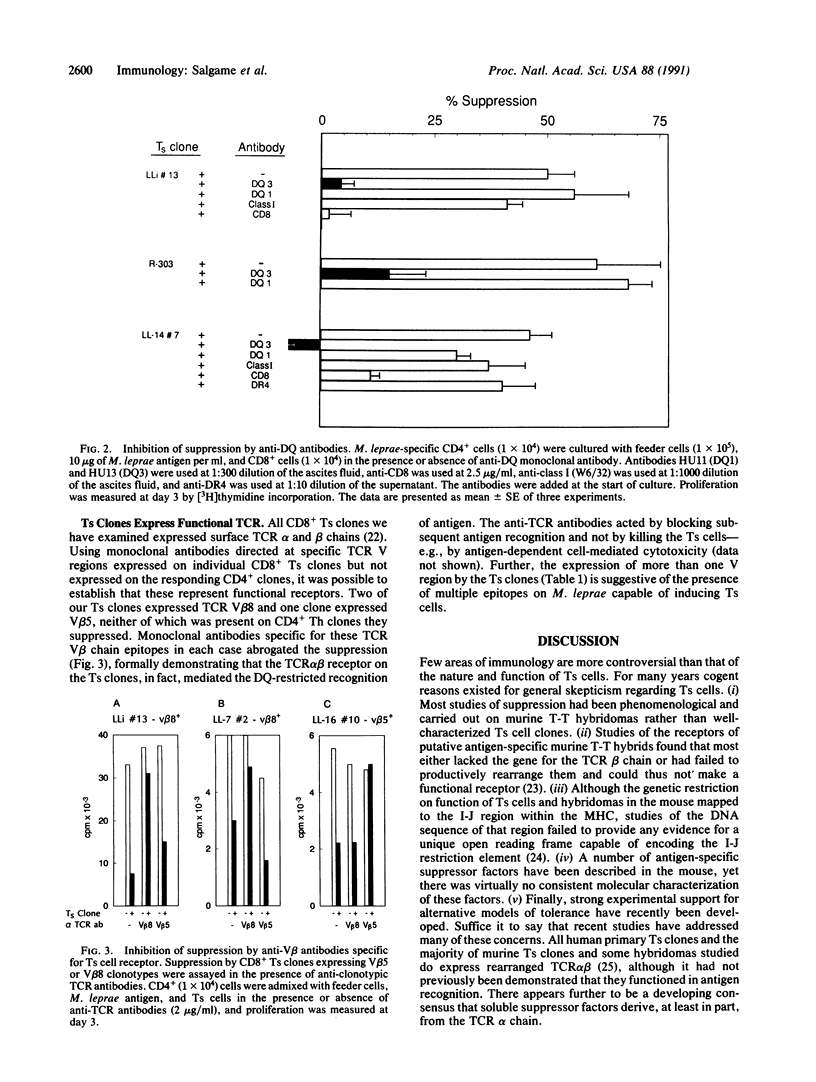
Mechanisms of specific immunologic unresponsiveness or tolerance and their regulation by the major histocompatibility complex remain central issues in immunology. Recent findings that potentially reactive anti-self T cells are not completely clonally deleted in the thymus and that specific immunological unresponsiveness can be acquired in certain infectious diseases, such as leprosy, suggest that peripheral unresponsiveness can be developed and maintained in adults. Human antigen-specific T suppressor cells represent one mechanism of peripheral tolerance. Clones of CD8+ T suppressor cells have been derived from blood or lesions of patients with lepromatous leprosy who are selectively unable to mount cellular immunity to Mycobacterium leprae. Using a panel of M. leprae-specific CD4+ and CD8+ T-cell clones of differing major histocompatibility complex class II haplotypes, suppression in vitro was found to be restricted by HLA-DQ and not by HLA-DR and inhibited by antibodies to HLA-DQ. In addition, antigen-induced suppression could be inhibited by antibodies specific to appropriate polymorphic T-cell receptor beta chains of the CD8+ clones. The results establish that activation of specific T suppressor cells is dependent on their polymorphic T-cell receptors and suggest that HLA-DQ serves as the preferred restricting element for suppression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxevanis C. N., Ishii N., Nagy Z. A., Klein J. Role of the Ek molecule in the generation of suppressor T cells in response to LDHB. Scand J Immunol. 1982 Jul;16(1):25–31. doi: 10.1111/j.1365-3083.1982.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Flavell R. A. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science. 1990 Jun 15;248(4961):1364–1368. doi: 10.1126/science.1694042. [DOI] [PubMed] [Google Scholar]
- Böhme J., Schuhbaur B., Kanagawa O., Benoist C., Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990 Jul 20;249(4966):293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S., Fotedar A., Talwar G. P. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):159–168. [PubMed] [Google Scholar]
- Collins M., Kuchroo V. K., Whitters M. J., O'Hara R. M., Jr, Kelleher K., Kubo R. T., Dorf M. E. Expression of functional alpha beta T cell receptor gene rearrangements in suppressor T cell hybridomas correlates with antigen binding, but not with suppressor cell function. J Immunol. 1990 Nov 1;145(9):2809–2819. [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Ulrich M., Pinardi M. E., Reyes O., Alvarado J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negative contacts. Int J Lepr Other Mycobact Dis. 1982 Dec;50(4):415–424. [PubMed] [Google Scholar]
- Deo M. G., Bapat C. V., Bhalerao V., Chaturvedi R. M., Bhatki W. S., Chulawala R. G. Antileprosy potentials of ICRC vaccine. A study in patients and healthy volunteers. Int J Lepr Other Mycobact Dis. 1983 Dec;51(4):540–549. [PubMed] [Google Scholar]
- Fink P. J., Shimonkevitz R. P., Bevan M. J. Veto cells. Annu Rev Immunol. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [DOI] [PubMed] [Google Scholar]
- Gao E. K., Kanagawa O., Sprent J. Capacity of unprimed CD4+ and CD8+ T cells expressing V beta 11 receptors to respond to I-E alloantigens in vivo. J Exp Med. 1989 Dec 1;170(6):1947–1957. doi: 10.1084/jem.170.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Germain R. N., Bevan M. J., Dorf M., Engel I., Fink P., Gascoigne N., Heber-Katz E., Kapp J., Kaufmann Y. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985 Jan;82(2):531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K., Matsushita S., Kikuchi I., Iuchi M., Ohta N., Sasazuki T. HLA-DQ is epistatic to HLA-DR in controlling the immune response to schistosomal antigen in humans. Nature. 1987 Jun 4;327(6121):426–430. doi: 10.1038/327426a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Karr R. W., Gregersen P. K., Obata F., Goldberg D., Maccari J., Alber C., Silver J. Analysis of DR beta and DQ beta chain cDNA clones from a DR7 haplotype. J Immunol. 1986 Nov 1;137(9):2886–2890. [PubMed] [Google Scholar]
- Kikuchi I., Ozawa T., Hirayama K., Sasazuki T. An HLA-linked gene controls susceptibility to lepromatous leprosy through T cell regulation. Lepr Rev. 1986 Dec;57 (Suppl 2):139–142. doi: 10.5935/0305-7518.19860064. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Steinmetz M., Kobori J., Kraig E., Kapp J. A., Pierce C. W., Sorensen C. M., Suzuki G., Tada T., Hood L. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5704–5708. doi: 10.1073/pnas.80.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Brenner M. B., Krangel M. S., Duby A. D., Bloom B. R. T-cell receptors of human suppressor cells. Nature. 1987 Oct 8;329(6139):541–545. doi: 10.1038/329541a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Kato H., Mehra V., Nelson E. E., Fan X. D., Rea T. H., Pattengale P. K., Bloom B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. 1986 Jul 31-Aug 6Nature. 322(6078):459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Wong L., Fujimiya Y., Chang W. C., Horwitz D. A., Bloom B. R., Rea T. H., Pattengale P. K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986 Nov 1;137(9):2831–2834. [PubMed] [Google Scholar]
- Morahan G., Allison J., Miller J. F. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989 Jun 22;339(6226):622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nagy Z. A., Baxevanis C. N., Klein J. Cross-reactivity of suppressor T cells specific for lactate dehydrogenase B and IgG2a myeloma protein. J Immunol. 1983 Apr;130(4):1498–1499. [PubMed] [Google Scholar]
- Oliveira D. B., Blackwell N., Virchis A. E., Axelrod R. A. T helper and T suppressor cells are restricted by the A and E molecules, respectively, in the F antigen system. Immunogenetics. 1985;22(2):169–175. doi: 10.1007/BF00563514. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Klatser P. R., de Vries R. R. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. 1986 Jul 31-Aug 6Nature. 322(6078):462–464. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Neuteboom S., Elferink D. G., de Vries R. R. Molecular localization and polymorphism of HLA class II restriction determinants defined by Mycobacterium leprae-reactive helper T cell clones from leprosy patients. J Exp Med. 1986 Dec 1;164(6):1923–1939. doi: 10.1084/jem.164.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. Autoimmunity. A diversity of diabetes. Nature. 1990 Jun 21;345(6277):662–664. doi: 10.1038/345662a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Salgame P., Modlin R., Bloom B. R. On the mechanism of human T cell suppression. Int Immunol. 1989;1(2):121–129. doi: 10.1093/intimm/1.2.121. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Freidmann A., Steinman L., Brautbar C., Erlich H. A. Specific HLA-DQB and HLA-DRB1 alleles confer susceptibility to pemphigus vulgaris. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6215–6219. doi: 10.1073/pnas.86.16.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Watanabe M., Sachs D. H., Hozumi N. Killing of antigen-reactive B cells by class II-restricted, soluble antigen-specific CD8+ cytolytic T lymphocytes. Nature. 1988 Dec 1;336(6198):481–484. doi: 10.1038/336481a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- de Vries R. R., van Eden W., van Rood J. J. HLA-linked control of the course of M. leprae infections. Lepr Rev. 1981 Dec;52 (Suppl 1):109–119. [PubMed] [Google Scholar]
- van Eden W., Elferink B. G., Hermans J., de Vries R. R., van Rood J. J. Role of HLA class II products in proliferative T-lymphocyte responses to PPD. Evidence of a regulatory influence associated with MB1. Scand J Immunol. 1984 Dec;20(6):503–510. doi: 10.1111/j.1365-3083.1984.tb01032.x. [DOI] [PubMed] [Google Scholar]



