Abstract
We present here the integration of brain-computer interfacing (BCI) technology with functional electrical stimulation therapy to restore voluntary function. The system was tested with a single man with chronic (6 years) severe left hemiplegia resulting from a stroke. The BCI, implemented as a simple “brain-switch” activated by power decreases in the 18 Hz – 28 Hz frequency range of the participant’s electroencephalograpic signals, triggered a neuroprosthesis designed to facilitate forward reaching, reaching to the mouth, and lateral reaching movements. After 40 90-minute sessions in which the participant attempted the reaching tasks repeatedly, with the movements assisted by the BCI-triggered neuroprosthesis, the participant’s arm function showed a clinically significant six point increase in the Fugl-Meyer Asessment Upper Extermity Sub-Score. These initial results suggest that the combined use of BCI and functional electrical stimulation therapy may restore voluntary reaching function in individuals with chronic severe hemiplegia for whom the rehabilitation alternatives are very limited.
Key Words: stroke, severe hemiplegia, rehabilitation, functional electrical stimulation therapy, brain-computer interface
Stroke is the most common cause of long-term disability.1,2 It is the result of interrupted blood supply following a blocked or ruptured vessel, and it often affects one cerebral hemisphere leading to paralysis of one side of the body referred to as hemiplegia. Despite significant therapeutic and technological advances to restore motor function after stroke, individuals with severe hemiplegia continue to face a significant lack of rehabilitation alternatives. Severe hemiplegia, characterized by a total or almost complete lack of the ability to move, is often incompatible with current best-practice rehabilitation interventions, which typically require that patients retain residual movement.1,2 One important exception is functional electrical stimulation therapy (FEST),3 which has been used successfully to restore voluntary motor function after stroke4-6 and spinal cord injury.7,8 In this therapeutic intervention patients are asked to repeatedly perform a series of functional movements and, after a few seconds of trying, a therapist triggers a train of electrical pulses applied non-invasively to specific muscles in a synergy that produces the intended movement. The assistance provided by the electrical stimulation is reduced gradually as recovery takes place until it is discontinued completely at the end of the intervention. Brain-computer interfaces (BCI) translate brain signals into control commands for electronic devices. The technology can be operated in complete absence of voluntary movement making it an important access method for individuals who are completely paralyzed. Although originally conceived as assistive devices, BCIs have been actively studied in the last decade as a potential tool to promote recovery of voluntary motor function after paralysis. One possible way to use a BCI to restore voluntary function involves triggering an external device designed to facilitate movement of a paralyzed limb upon detecting the intention to move through analysis of a person’s electroencephalographic (EEG) activity.9 This can be done by close monitoring of reductions in power in the alpha (8 Hz - 12 Hz) or beta (13 Hz - 30 Hz) frequency ranges. The power decrease, frequently called event-related desynchronization (ERD), is a typical response observed during preparation, execution, and imagination of voluntary movement,10 and it is often used to implement BCI systems. Applications of ERD-based BCIs have included the control of orthotic11 and functional electrical stimulation devices,12-14 but exploration of the therapeutic effects of this technological synergy has only started recently with most of the work focused on BCI-control of rehabilitation robots.15 To the authors’ knowledge, only one report describes the use of a BCI to deliver FEST.16 In that work, the researchers tested the BCI-triggered FEST for restoring voluntary finger movement 10 months after a stroke resulting in impaired hand function. The participant’s finger movements improved after only nine sessions. We present here a BCI-triggered FEST to restore arm reaching function. The participant of this study had chronic severe left hemiplegia, representing a population that does not benefit from current best-practice rehabilitation interventions.1
Materials and Methods
Participant
The single participant of this study was a man with left severe hemiplegia resulting from a stroke six years (72 months) earlier. His arm and hand were at stage 1 on Chedoke-MacMaster Stages of Movement Recovery17 with no voluntary movement. He was 64 years old and every other rehabilitation intervention in which he participated, including FEST (without BCI activation), had failed to produce any change in his ability to move his affected upper limb.
Neuroprosthesis for Reaching
We created a neuroprosthesis for reaching using a four-channel stimulator (Compex, Switzerland),18 which was programmed to perform
forward reaching (making it possible to reach to the right knee as well) – for which the anterior deltoid and triceps brachii were stimulated, with arm retreival produced by stimulating the posterior deltoid and biceps brachii.
reaching to the mouth (which also allowed reaching to the right shoulder) – achieved by aplying stimulation to the anterior deltoid and biceps brachii, while retrieval was produced by stimulation the posterior deltoid and triceps brachii.
lateral reaching – produced by stimulating biceps brachii followed by anterior and posterior deltoid and finally by the triceps brachii, while retrieving was achieved by stimulating the biceps brachii muscle, discontinuing deltoid stimulation, and stimulating the triceps brachii to produce arm extension while lowering the arm.
Transitioning between movement phases (i.e., reach and retrieve) could be triggered using a TTL-level signal provided by an external switch or a BCI.
Brain-Computer Interface
The BCI was implemented as a simple “brain-switch”, which was activated whenever the power within the beta range (13 Hz- 30 Hz) of the participant’s EEG decreased below a threshold, indicating the participant’s intention to move his arm. This frequency range was selected by identifying spectral components displaying ERD19 using data collected when the participant attempted repeatedly to perform hand (palmar and lateral grasps, precision pinch, and hand opening) movements following a Ready-Go-Stop experimental cue. The analysis was performed using a total of 104 attempted movement repetitions and applied to six EEG electrode positions (F3, Fz, F4, C3, Cz, C4 of the 10-20 electrode placement system). The frequency band between 18 Hz and 28 Hz at the Fz location were found to be the most suitable for implementing the BCI. EEG recordings for this process were performed using a SynAmpRT EEG amplifier (Compumedics, U.S.A.) at a sampling rate of 1 KHz and filtered between 0.05 Hz and 40 Hz. ERD estimation was performed with custom-made software (Matlab, Mathworks, U.S.A.). To create the BCI, acquisition of EEG activity was performed using a desktop biopotential amplifier (QP511, Grass Telefunken, Germany) and a data acquisition system (USB-6363, National Instruments, U.S.A.) using a sampling frequency of 300 Hz. The EEG signal from electrode Fz was band-pass filtered (10-Hz - 100 Hz) and amplified (20,000x) prior to its digital acquisition. All processing was implemented using custom-made software (LabView, National Instruments, U.S.A.). The BCI estimated a moving average (over a 500 ms period) of the EEG RMS value measured every 125 ms. For an activation to take place, the moving average had to decrease below a threshold for a pre-specified duration (500 ms to 1,200 ms). The experimenters could adjust both parameters at any moment during the operation of the system.
Brain-Computer Interface-Triggered Neuroprosthesis for Reaching
The BCI produced a TTL-level pulse whenever it was activated. This pulse was delivered to the neuroprosthesis for reaching where it triggered a transition in the state of the electrical stimulation (e.g., from active reaching to retrieving the arm). In addition, an external manual switch could also be used by the experimenters bypassing the BCI altogether in cases in which the brain interface failed to identify the intention to move.
Intervention
Two experimenters delivered the BCI-triggered FEST. One of them guided the arm in motion (assisted by the electrical stimulation) and could trigger the stimulation manually bypassing the BCI completely. The second experimenter demonstrated the movement to perform at every repetition and could also adjust the BCI activation parameters (i.e., activation threshold and latency). The intervention consisted of 40 90-minute sessions (30-minute setup and 60-minute active therapy) delivered three times a week. During each session the participant attempted to reach to different targets (listed above in the neuroprosthesis implementation) repeatedly following the experimenters’ instructions. Each movement was performed with a brief pause between phases (i.e., the participant would reach, hold the reaching position for a few seconds, and then return his arm to a starting position followed by a few moments of relaxation with no stimulation).
Outcome Measures
We measured the effects of the intervention using five assessments, measured at beginning, midpoint and end of the study. The assessments were the Toronto Rehabilitation Institute - Hand Function Test (TRIHFT),20 the Action Research Arm Test (ARAT),21 Functional Independence Measure (FIM),22 Self-Care Component of the Functional Independence Measure,22 and Fugl-Meyer Assessment Upper Extremity Sub-Score.23 The recorded values are displayed in Table 1.
Table 1.
Performed Assessments
| Baseline | Mid-Point | Discharge | |
|---|---|---|---|
| TRI Hand Function Test Object Manipulation Sub-Score |
0 | 0 | 0 |
| Action Research Arm Test |
0 | 0 | 0 |
| FIM Self-Care Sub-Score | 28 | 35 | 35 |
| FIM Total | 104 | 118 | 118 |
| Fugl-Meyer Assessment Upper Extremity Sub-Score |
13 | 18 | 19 |
Results and Discussion
The Fugl-Meyer Assessment Upper Extremity sub-score had a clinically significant change (6 points; 13 points at baseline and 19 points at the end of the intervention). The FIM Self-Care subscores registered non-significant improvements (28 and 35 at baseline and end, respectively). All the other assessments remained unchanged (Table 1), likely due to the fact that the TRIHFT and ARAT require hand function beyond the functional gains observed. In addition, although the FMA-UE sub-score and FIM self-care subscores were very similar (or the same) between the midpoint and discharges assessments, it was evident that the quality of the movement (e.g., speed and smootheness) improved between these two measurements. Unfortunately, these aspects are not covered by the applied tests. Qualitatively, the BCI was able to detect the participant’s intention to move and consequently trigger the neuroprosthesis when required. Our preliminary results suggests that the BCI was able to trigger the neuroprosthesis for the majority of reaching movement repetitions (sometimes with accuracies as high as 90%), although a full data analysis is yet to be completed. The most common source of interference that affected the BCI was electrical interference originating from activation of facial muscles (i.e., reflective of the participants physical effort). In conclusion, the results of this study suggest that integration of a simple single-channel BCI with FEST may help individuals with severe hemiplegia resulting from stroke regain voluntary arm reaching function, even 6 years (72 months) after having a stroke. Further testing is required to verify the findings presented here including a large number of participants.
Fig 1.
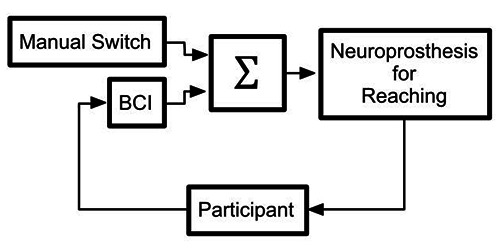
Block diagram of the integrated BCI and FEST sytem. The neuroprosthesis for reaching was designed to faciliate forward and lateral reaching, as well as reaching to the mouth. The stimulation could be triggered using a BCI implemented with a single EEG electrode (Fz) activated with decreases in power in the 18 Hz-28 Hz frequency range. The manual switch could also trigger the electrical stimulation when the BCI failed to identify the intention to move.
Acknowledgment
The authors thank Mr. Chaim Katz and Mr. Gary Evans for their invaluable assistance in integrating our brain-computer interfacing and neuroprosthetic systems. This work received funding from the Natural Sciences and Engineering Research Council: Discovery Grant (#249669) and Toronto Rehab Foundation.
Contributor Information
Aaron Marquis, Email: Aaron.Marquis@uhn.ca.
Milos R. Popovic, Email: milos.popovic@utoronto.ca.
References
- 1.Dobkin BH. Strategies for stroke rehabilitation. The Lancet Neurology. 2004;3:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group CCW, Cheeran B, Cohen L, et al. The Future of Restorative Neurosciences in Stroke: Driving the Translational Research Pipeline From Basic Science to Rehabilitation of People After Stroke. Neurorehabil Neural Repair 2009;23:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popovic MB, Popovic DB, Sinkjaer T, et al. Restitution of reaching and grasping promoted by functional electrical therapy. Artif Organs 2002;26:271–5. [DOI] [PubMed] [Google Scholar]
- 4.Popovic MR, Thrasher TA, Zivanovic V, et al. Neuroprosthesis for retraining reaching and grasping functions in severe hemiplegic patients. Neuromodulation 2005;8:58–72. [DOI] [PubMed] [Google Scholar]
- 5.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of Reaching and Grasping Function in Severe Hemiplegic Patients Using Functional Electrical Stimulation Therapy. Neurorehabil Neural Repair 2008;22:706–14. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia NM, Nagai MK, Zivanovic V, et al. Functional Electrical Stimulation Therapy for Recovery of Reaching and Grasping in Severe Chronic Pediatric Stroke Patients. J Child Neurol 2014;29:493–9. [DOI] [PubMed] [Google Scholar]
- 7.Popovic MR, Thrasher TA, Adams ME, et al. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006;44:143–51. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia N, Masani K, Catharine Craven, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;37:511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly JJ, Wolpaw JR. Brain–computer interfaces in neurological rehabilitation. The Lancet Neurology. 2008;7:1032–43. [DOI] [PubMed] [Google Scholar]
- 10.Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr Clin Neurophysiol 1979;46:138–46. [DOI] [PubMed] [Google Scholar]
- 11.Pfurtscheller G, Guger C, Müller G, et al. Brain oscillations control hand orthosis in a tetraplegic. Neuroscience Letters 2000. 13;292:211–4. [DOI] [PubMed] [Google Scholar]
- 12.Márquez-Chin C, Popovic MR, Cameron T, et al. Control of a neuroprosthesis for grasping using offline classification of electrocorticographic signals: case study. Spinal Cord 2009;47:802–8. [DOI] [PubMed] [Google Scholar]
- 13.Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ. “Thought” – control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neuroscience 2003;351:33–6. [DOI] [PubMed] [Google Scholar]
- 14.Müller-Putz GR, Scherer R, Pfurtscheller G, Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neuroscience Letters 2005;382:169–74. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK, et al. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clin EEG Neurosci 2014; 46:310-320. [DOI] [PubMed] [Google Scholar]
- 16.Daly JJ, Cheng R, Rogers J, et al. Feasibility of a New Application of Noninvasive Brain Computer Interface (BCI): A Case Study of Training for Recovery of Volitional Motor Control After Stroke. J Neurol Phys Ther 2009;33:203–11. [DOI] [PubMed] [Google Scholar]
- 17.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993;24:58–63. [DOI] [PubMed] [Google Scholar]
- 18.Popovic MR, Keller T. Modular transcutaneous functional electrical stimulation system. Med Eng Phys 2005;27:81–92. [DOI] [PubMed] [Google Scholar]
- 19.Graimann B, Huggins JE, Levine SP, Pfurtscheller G. Visualization of significant ERD/ERS patterns in multichannel EEG and ECoG data. Clin Neurophysiol 2002;113:43–7. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia N, Zivanovic V, Verrier M, Popovic MR. Toronto Rehabilitation Institute–Hand Function Test: Assessment of Gross Motor Function in Individuals With Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2012;18:167–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 1981;4:483. [DOI] [PubMed] [Google Scholar]
- 22.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993;74:531–6. [DOI] [PubMed] [Google Scholar]
- 23.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]


