Abstract
Objective
To identify nailfold videocapillaroscopic features and other clinical risk factors for new digital ulcers (DUs) during a 6‐month period in patients with systemic sclerosis (SSc).
Methods
In this multicenter, prospective, observational cohort study, the videoCAPillaroscopy (CAP) study, we evaluated 623 patients with SSc from 59 centers (14 countries). Patients were stratified into 2 groups: a DU history group and a no DU history group. At enrollment, patients underwent detailed nailfold videocapillaroscopic evaluation and assessment of demographic characteristics, DU status, and clinical and SSc characteristics. Risk factors for developing new DUs were assessed using univariable and multivariable logistic regression (MLR) analyses.
Results
Of the 468 patients in the DU history group (mean ± SD age 54.0 ± 13.7 years), 79.5% were female, 59.8% had limited cutaneous SSc, and 22% developed a new DU during follow‐up. The strongest risk factors for new DUs identified by MLR in the DU history group included the mean number of capillaries per millimeter in the middle finger of the dominant hand, the number of DUs (categorized as 0, 1, 2, or ≥3), and the presence of critical digital ischemia. The receiver operating characteristic (ROC) of the area under the curve (AUC) of the final MLR model was 0.738 (95% confidence interval [95% CI] 0.681–0.795). Internal validation through bootstrap generated a ROC AUC of 0.633 (95% CI 0.510–0.756).
Conclusion
This international prospective study, which included detailed nailfold videocapillaroscopic evaluation and extensive clinical characterization of patients with SSc, identified the mean number of capillaries per millimeter in the middle finger of the dominant hand, the number of DUs at enrollment, and the presence of critical digital ischemia at enrollment as risk factors for the development of new DUs.
Systemic sclerosis (SSc) is a rare multisystem connective tissue disease characterized by microvascular damage, fibrosis of the skin and internal organs, and specific immunologic abnormalities. Digital ulceration, which represents a visible manifestation of peripheral vasculopathy, is a frequent complication of SSc, with an estimated lifetime prevalence of as much as 50% 1, 2.
Digital ulcers (DUs) often occur relatively early in the course of the disease, causing severe pain and functional impairment, and have a great impact on patients’ quality of life 3, 4, 5, 6, 7, 8, 9, 10, 11. DUs can also result in significant disfigurement and infection and may lead to gangrene, osteomyelitis, and eventually, amputation 4. Furthermore, DUs are often persistent, recurrent, and slow to heal, requiring considerable resources for wound management and nursing care 2, 12. Given the clinical and financial burden, as well as the availability of therapies to prevent DUs in patients with SSc 13, there is a need to identify risk factors for the development of new DUs. In addition to the established role of capillaroscopy in the diagnosis of SSc 14, 15, 16 and the evaluation of its possible role in monitoring SSc, some studies have reported that abnormalities noted on capillaroscopy are associated with DUs 17, 18, 19, 20, 21, 22, 23, 24, 25.
The aim of this study was to identify potential risk factors for the occurrence of new DUs during a 6‐month period in patients with SSc, based on nailfold videocapillaroscopy (NVC) findings and other clinical characteristics.
PATIENTS AND METHODS
Study design
The videoCAPillaroscopy (CAP) study was a multicenter, prospective, observational cohort study with stratified enrollment into DU history and no DU history groups. Potential risk factors for the development of DUs were evaluated in the DU history group. The no DU history group was included for exploratory purposes only, as the incidence of new DUs was expected to be low. Enrollment occurred over a 1‐year period to minimize seasonal effects. Patients were monitored from the time of enrollment until the occurrence of a new DU or a maximum of 6 months, whichever came first. At enrollment, patients were provided with an educational leaflet on the identification of DUs, and staff at each center telephoned patients monthly to inquire about the occurrence of new DUs. If a DU was reported, a patient visit was organized so that the physician could confirm or exclude the presence of a DU.
Data management was performed centrally, and data quality was rigorously monitored. Consistency of the source data with the clinical database was verified for critical variables for 3 randomly selected patients per site (or fewer, if fewer patients had been enrolled). Data were reviewed regularly.
The CAP study was led by an independent steering committee (see the Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). The complete list of CAP study investigators is also given in the Supplementary Materials. This study was conducted in accordance with the Declaration of Helsinki and its amendments, followed the Guidelines for Good Clinical Practice of the International Conference on Harmonisation, and was approved by the local institutional review boards and ethics committees. All patients provided written informed consent.
Study population
Fifty‐nine SSc centers in 14 countries (12 European countries and Turkey and Israel) participated in the study between January 31, 2011 and July 26, 2012. Patients ages ≥18 years with a diagnosis of SSc according to the American College of Rheumatology (ACR) 26 and/or the LeRoy and Medsger 14 criteria were eligible for inclusion. The inclusion criteria were broad to permit generalizability to a wider SSc population. To enrich for the occurrence of new DUs in the study population, the patients had to meet 1 of the following 2 criteria: 1) a history of DUs or a DU at enrollment (DU history group), or 2) a disease duration of ≤2 years (no DU history group), defined as the time since the first physician‐documented non–Raynaud's phenomenon clinical feature of SSc 3.
As the study was conducted to allow for extrapolation of the results to the real‐world setting, patients were permitted to continue their ongoing treatments. Patients unable to undergo NVC assessment were not eligible for study inclusion. Patients with SSc sine scleroderma were excluded as they were not expected to develop DUs frequently during the 6‐month observation period. Furthermore, patients who had undergone stem cell transplantation or had participated in an interventional clinical trial within 3 months prior to enrollment were excluded since these interventions may have unknown effects on the occurrence of new DUs.
Data collection
Covariables of demographic features, SSc clinical characteristics, DUs, and other clinical characteristics, as well as findings of the NVC were collected at enrollment and are summarized in Table 1 and Supplementary Tables 1–4 (non‐NVC covariables) and in Table 2 and Supplementary Tables 5 and 6 (NVC covariables) (Supplementary Materials are available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). Covariables of DU characteristics included a history of DUs (assessed at the investigator's discretion), the presence of DUs (number and location), and previous and current DU‐associated complications/interventions, including critical digital ischemia (defined as a prolonged, severe, persistent reduction in digital tissue perfusion without rewarming [see Supplementary Table 7 and Supplementary Figure 1]).
Table 1.
Non–nailfold videocapillaroscopy covariables selected at the ULR analysis stage and carried forward to the MLR within‐bundle analysis in the DU history groupa
| Variable | Summary statistics | ULR | |||||
|---|---|---|---|---|---|---|---|
| Cases (n = 103) | Noncases (n = 365) | Parameter | Coefficient estimate (SE) | OR (95% CI) | P b | ROC AUC (95% CI) | |
| Demographic features (bundle 1) | |||||||
| Age at enrollment, mean ± SD yearsc | 51.5 ± 13.9 | 54.8 ± 13.6 | Intercept | −0.3343 (0.4434) | 0.983 (0.967–0.999) | 0.033 | 0.573 (0.509–0.636) |
| Linear | −0.0175 (0.0082) | ||||||
| Currently smoking, n/N (%) | 21/103 (20.4) | 49/365 (13.4) | Intercept | −1.3490 (0.1239) | 1.652 (0.938–2.909) | 0.082 | 0.535 (0.492–0.578) |
| Factor | 0.5017 (0.2888) | ||||||
| Comprehensive smoking index, mean ± SD; nd | 0.1 ± 0.2; 99 | 0.1 ± 0.3; 351 | Intercept | −1.3697 (0.1332) | e | 0.032 | 0.535 (0.480–0.590) |
| Linear | 4.5275 (1.9283) | ||||||
| Quadratic | −6.2329 (2.9057) | ||||||
| SSc clinical characteristics (bundle 2) | |||||||
| Age at first physician‐documented non‐RP clinical feature, mean ± SD years; nc | 43.1 ± 14.8; 103 | 45.6 ± 13.6; 363 | Intercept | −0.6748 (0.3734) | 0.987 (0.971–1.003) | 0.106 | 0.561 (0.496–0.626) |
| Linear | −0.0132 (0.0082) | ||||||
| SSc subtype, n/N (%) | |||||||
| Diffuse cutaneous | 48/103 (46.6) | 140/365 (38.4) | Intercept | −1.0704 (0.1673) | 0.713 (0.459–1.108)f | 0.133f | 0.541 (0.487–0.596) |
| Limited cutaneous | 55/103 (53.4) | 225/365 (61.6) | Factor | −0.3383 (0.2249) | |||
| MRSS, mean ± SD; n | 13.0 ± 9.0; 100 | 11.3 ± 8.4; 354 | Intercept | −1.5363 (0.1955) | 1.023 (0.998–1.048) | 0.076 | 0.562 (0.496–0.627) |
| Linear | 0.0224 (0.0127) | ||||||
| Kidney involvement: SSc renal crisis, kidney failure, n/N (%)c | 2/103 (1.9) | 22/365 (6.0) | Intercept | −1.2226 (0.1132) | 0.309 (0.071–1.336) | 0.116 | 0.520 (0.502–0.539) |
| Factor | −1.1746 (0.7470) | ||||||
| Heart involvement, n/N (%)c | 22/103 (21.4) | 55/365 (15.1) | Intercept | −1.3421 (0.1248) | 1.531 (0.882–2.658) | 0.130 | 0.532 (0.488–0.575) |
| Factor | 0.4261 (0.2814) | ||||||
| Joint involvement, n/N (%) | 45/103 (43.7) | 127/365 (34.8) | Intercept | −1.4118 (0.1464) | 1.454 (0.932–2.269) | 0.099 | 0.545 (0.491–0.599) |
| Factor | 0.3743 (0.2270) | ||||||
| DU characteristics (bundle 3) | |||||||
| No. of DUs, n/N (%)c | |||||||
| 0 DUs | 41/103 (39.8) | 262/365 (71.8) | |||||
| 1 DU | 24/103 (23.3) | 57/365 (15.6) | Intercept | −1.8548 (0.1679) | 2.691 (1.507–4.803)g | <0.001g | 0.678 (0.622–0.734) |
| Factor | 0.9898 (0.2957) | ||||||
| 2 DUs | 16/103 (15.5) | 27/365 (7.4) | Factor | 1.3315 (0.3574) | 3.787 (1.879–7.630)h | <0.001h | |
| ≥3 DUs | 22/103 (21.4) | 19/365 (5.2) | Factor | 2.0014 (0.3554) | 7.399 (3.687–14.848)i | <0.001i | |
| Previous complications, n/N (%) | |||||||
| Soft tissue infection requiring antibiotics | 52/103 (50.5) | 142/361 (39.3) | Intercept | −1.4708 (0.1553) | 1.594 (1.027–2.475) | 0.038 | 0.557 (0.503–0.612) |
| Factor | 0.4663 (0.2245) | ||||||
| Autoamputationc | 13/103 (12.6) | 14/365 (3.8) | Intercept | −1.3610 (0.1182) | 3.621 (1.644–7.977) | 0.001 | 0.544 (0.510–0.578) |
| Factor | 1.2869 (0.4029) | ||||||
| Critical digital ischemia | 35/103 (34.0) | 94/362 (26.0) | Intercept | −1.3752 (0.1357) | 1.473 (0.920–2.358) | 0.107 | 0.540 (0.489–0.592) |
| Factor | 0.3873 (0.2401) | ||||||
| Gangrene | 16/103 (15.5) | 37/365 (10.1) | Intercept | −1.3271 (0.1206) | 1.630 (0.866–3.068) | 0.130 | 0.527 (0.489–0.565) |
| Factor | 0.4888 (0.3226) | ||||||
| Complications at enrollment, n/N (%) | |||||||
| Soft tissue infection requiring antibiotics | 23/99 (23.2) | 24/311 (7.7) | Intercept | −1.4499 (0.1242) | 4.085 (2.194–7.606) | <0.001 | 0.579 (0.536–0.621) |
| Factor | 1.4073 (0.3171) | ||||||
| Critical digital ischemiac | 15/99 (15.2) | 12/311 (3.9) | Intercept | −1.3891 (0.1191) | 5.014 (2.266–11.094) | <0.001 | 0.556 (0.521–0.592) |
| Factor | 1.6122 (0.4052) | ||||||
| Previous DU‐associated interventions, n/N (%) | |||||||
| Hospitalization for DU | 55/103 (53.4) | 144/365 (39.5) | Intercept | −1.5268 (0.1592) | 1.759 (1.132–2.732) | 0.012 | 0.570 (0.515–0.624) |
| Factor | 0.5645 (0.2247) | ||||||
| Other clinical characteristics (bundle 4) | |||||||
| Both hands abnormal on Allen test, n/N (%) | 32/95 (33.7) | 85/338 (25.1) | Intercept | −0.9768 (0.2074) | 0.661 (0.405–1.081) | 0.099 | 0.543 (0.490–0.596) |
| Factor | −0.4135 (0.2507) | ||||||
| Presence of paronychia, n/N (%)‡ | 20/103 (19.4) | 38/365 (10.4) | Intercept | −1.3588 (0.1231) | 2.048 (1.132–3.705) | 0.018 | 0.545 (0.503–0.586) |
| Factor | 0.7170 (0.3024) | ||||||
| Presence of pitting scars, n/N (%) | 60/103 (58.3) | 182/365 (49.9) | Intercept | −1.4815 (0.1752) | 1.498 (0.958–2.342) | 0.076 | 0.550 (0.496–0.604) |
| Factor | 0.4043 (0.2279) | ||||||
Data are summary statistics of clinical covariables selected and carried forward to the multivariable logistic regression (MLR) within‐bundle analysis in patients who developed (cases) and those who did not develop (noncases) a digital ulcer (DU) during the observation period. ULR = univariable logistic regression; 95% CI = 95% confidence interval; RP = Raynaud's phenomenon; MRSS = modified Rodnan skin thickness score.
By Wald's chi‐square test.
Variable was also carried forward to the MLR across‐bundles analysis.
Integrates the component variables of smoking intensity, smoking duration, and time since smoking cessation into a single covariate of smoking effect 61.
The odds ratio (OR) is not given since the functional relationship is quadratic. The associated P value and receiver operating characteristic (ROC) area under the curve (AUC) are quadratic terms.
For limited cutaneous systemic sclerosis (SSc) versus diffuse cutaneous SSc.
For 1 DU versus 0 DUs.
For 2 DUs versus 0 DUs.
For ≥3 DUs versus 0 DUs.
Table 2.
NVC covariables selected at the ULR analysis stage and carried forward to the MLR within‐bundle analysis in the DU history groupa
| Variable | Summary statistics | ULR | |||||
|---|---|---|---|---|---|---|---|
| Cases (n = 103) | Noncases (n = 365) | Parameter | Coefficient estimate (SE) | OR (95% CI) | P b | ROC AUC (95% CI) | |
| Quantitative NVC characteristics (bundle 5) | |||||||
| Finger level: dominant hand, 4 individual fingers (sub‐bundle 5.5)c | |||||||
| Index finger, mean ± SD/mm; n | |||||||
| No. of capillaries | 3.9 ± 2.2; 89 | 4.5 ± 2.3; 325 | Intercept | −0.7504 (0.2573) | 0.879 (0.787–0.981) | 0.022 | 0.603 (0.538–0.668) |
| Linear | −0.1294 (0.0564) | ||||||
| No. of microhemorrhagesd | 0.1 ± 0.4; 89 | 0.2 ± 0.5; 325 | Intercept | −1.2157 (0.1270) | 0.592 (0.305–1.150) | 0.122 | 0.552 (0.512–0.593) |
| Linear | −0.5239 (0.3386) | ||||||
| Middle finger, mean ± SD/mm; n | |||||||
| No. of capillariesd | 3.8 ± 1.9; 97 | 4.7 ± 2.2; 339 | Intercept | −0.3115 (0.2790) | 0.801 (0.707–0.907) | <0.001 | 0.614 (0.553–0.674) |
| Linear | −0.2217 (0.0634) | ||||||
| No. of microhemorrhages | 0.2 ± 0.8; 97 | 0.3 ± 0.6; 339 | Intercept | −1.1172 (0.1247) | e | 0.007 | 0.565 (0.524–0.605) |
| Linear | −1.6839 (0.5904) | ||||||
| Quadratic | 0.5243 (0.1933) | ||||||
| No. of neoangiogenesesd | 0.6 ± 0.9; 97 | 0.4 ± 0.8; 339 | Intercept | −1.4030 (0.1390) | 1.326 (1.024–1.718) | 0.032 | 0.558 (0.498–0.617) |
| Linear | 0.2823 (0.1319) | ||||||
| Ring finger, mean ± SD/mm; n | |||||||
| No. of capillaries | 4.2 ± 2.1; 99 | 4.6 ± 2.1; 343 | Intercept | −0.7917 (0.2677) | 0.902 (0.807–1.009) | 0.070 | 0.564 (0.500–0.629) |
| Linear | −0.1031 (0.0570) | ||||||
| Little finger, mean ± SD/mm; n | |||||||
| No. of capillaries | 4.1 ± 1.8; 97 | 5.0 ± 2.1; 347 | Intercept | −0.3062 (0.2920) | 0.808 (0.715–0.913) | <0.001 | 0.620 (0.558–0.681) |
| Linear | −0.2137 (0.0624) | ||||||
| Qualitative NVC characteristics (variable not included in a bundle)f | |||||||
| NVC pattern at enrollment, n/N (%) | |||||||
| Normal | 0/103 (0.0) | 4/363 (1.1) | 0.597 (0.548–0.647) | ||||
| Early | 4/103 (3.9) | 40/363 (11.0) | |||||
| Active | 25/103 (24.3) | 123/363 (33.9) | Intercept | −2.3972 (0.5221) | 2.234 (0.736–6.779)g | 0.156g | |
| Factor | 0.8039 (0.5663) | ||||||
| Late | 74/103 (71.8) | 196/363 (54.0) | Factor | 1.4231 (0.5396) | 4.150 (1.441–11.950)h | 0.008h | |
Data are summary statistics of nailfold videocapillaroscopy (NVC) covariables selected and carried forward to the multivariable logistic regression (MLR) within‐bundle analysis in patients who developed (cases) and those who did not develop (noncases) a digital ulcer (DU) during the observation period. Quantitative NVC characteristics carried forward to the MLR within‐bundle and across‐bundles analyses for sub‐bundles 5.1–5.4 and sub‐bundle 5.6 are shown in Supplementary Table 5 (in section 3 of the Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). ULR = univariable logistic regression; 95% CI = 95% confidence interval.
By Wald's chi‐square test.
The mean value at the finger level is the mean count per measurement from 2 capillaroscopic images.
Variable was also carried forward to the MLR across‐bundles analysis.
The odds ratio (OR) is not given since the functional relationship is quadratic. The associated P value and receiver operating characteristic (ROC) area under the curve (AUC) are quadratic terms.
Used in an alternative final model as a “surrogate” for NVC quantitative variables.
Active versus normal/early pattern.
Late versus normal/early pattern.
Information on medication use within 3 months prior to enrollment, at enrollment, and during the observation period was recorded as predefined classes of medications, including vasoactive medications and immunosuppressants.
Study outcome
A DU was defined clinically as a denuded area located on the fingers and with a defined border and loss of epithelialization and a loss of epidermis and dermis. The definition excluded fissures, paronychia, pitting scars, or ulcers located over the metacarpophalangeal joints or elbows (see Supplementary Table 7, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). DUs distal to the metacarpophalangeal joint and on the volar and dorsal aspects of the hand were included (see Supplementary Figure 1). Calcinosis‐induced ulcers were not specifically excluded.
A patient's DU outcome was recorded as a binary variable: either the occurrence or nonoccurrence of a new DU. Cases were defined as patients who experienced a new DU that was confirmed by the investigator during the 6‐month observation period. Noncases were defined as patients who did not experience a new DU during the 6‐month observation period. For noncases, the nonoccurrence of a new DU was recognized only if the patient had been contacted successfully by staff for at least 3 of the monthly phone calls, including the month 6 phone call, and had reported no new DUs or if the noncases had reported a new DU that had not been confirmed by the investigators. Physicians assessing the presence of DUs were not blinded to the data collected at enrollment.
Collection and assessment of the NVC images
The nailfolds of the second, third, fourth, and fifth fingers of both hands were examined in each patient with the use of a videocapillaroscope equipped with a 200× magnification lens, which is commonly used for NVC 5, 27, 28, and connected to image analysis software. Two adjacent fields extending over 1 mm, in the middle of the nailfold, and corresponding to the distal row of capillaries were studied 27. Images were evaluated using qualitative and quantitative assessment techniques 18, 27, 29.
Qualitative assessment of images (1 covariable) (see Supplementary Table 6) classified the patient as having the normal, early, active, or late scleroderma NVC pattern according to Cutolo et al 29 (see Supplementary Materials, Investigator booklet, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). Quantitative assessment of images (6 covariables in each of the 8 fingers) consisted of counting the following 5 covariables per linear millimeter: capillaries, giant capillaries (hairpin‐shaped or horseshoe‐shaped, homogenously large capillary with a diameter >50 μm), irregularly enlarged capillaries (diameter >20 μm; morphology can be hairpin‐shaped, tortuous, or crossing once), microhemorrhages (dark masses due to hemosiderin deposits, which can be linked to a disappearing capillary), and neoangiogeneses (meandering, ramified, branching, bushy, bizarre capillaries and capillaries with >2 crossings), plus a sixth covariable consisting of measuring the maximal capillary diameter in capillaries with a diameter >50 μm (see Supplementary Materials, Investigator booklet).
To ensure optimal reliability in the assessment, staff at all centers had been trained on the assessment of capillaroscopic images in an interactive workshop with practical use of the capillaroscopy devices and were provided with a booklet containing illustrated definitions of the capillaroscopic features (see Supplementary Materials, Investigator booklet). To reflect real‐world clinical practice in this observational study, the images were analyzed at each participating center. Picture quality was evaluated for the first 2–3 patients at each center by 3 members of the steering committee (MC, ALH, and VS). If the picture quality was not optimal, further training on the correct use of NVC was provided.
Statistical analysis
Sample size
Sample size was based on feasibility considerations, where 350 patients enrolled in the DU history group would provide a reasonable number of cases (n = 150) for model building using a stagewise process to explore risk factor associations and discrimination 30, 31, 32. Exploratory statistical analyses were planned for 150 patients in the no DU history group, where the number of cases was expected to be low. Stratification was used to increase homogeneity within the DU history and no DU history groups, because the factors associated with the occurrence of new DUs in the two groups were expected to be different.
Analysis of risk factors for the occurrence of new DUs
Covariables were described for cases and noncases using summary statistics. Associations between the individual categorical covariables and new DU outcomes were initially explored using chi‐square test or Fisher's exact test, as appropriate. Summary statistics were provided for continuous variables within the case and noncase groups and for the differences between the 2 groups. Logistic regression modeling was the main analytical method for examining the associations and discriminatory ability of potential risk factors for the occurrence of new DUs, including linear and quadratic functional relationships. The strength of association between a risk factor and new DU outcome was given by the odds ratio (OR) with its 95% confidence interval (95% CI); statistical significance was given via Wald's chi‐square test. Model calibration in the multivariable logistic regression (MLR) analysis was assessed via the Hosmer‐Lemeshow chi‐square test. The discriminatory performance of the various risk factors (individually and combined) was given by the receiver operating characteristic (ROC) area under the curve (AUC) and its corresponding 95% CI.
The statistical strategy for selecting the best‐performing risk factors for the final MLR model was a stagewise process in 3 broad stages using “bundles” of covariables 33, 34 (see Supplementary Tables 1–6), where stage 1 is univariable logistic regression (ULR) analysis, stage 2 is MLR within‐bundle analysis, and stage 3 is MLR across‐bundles analysis. The number of covariables was reduced at each stage, and the best‐performing covariables from the bundles were carried forward to the next stage.
The non‐NVC covariables with similar characteristics (according to the domains on the case report forms) were organized into 4 bundles: bundle 1 for demographics, bundle 2 for SSc clinical characteristics, bundle 3 for DU characteristics, and bundle 4 for other clinical characteristics. Six sub‐bundles of NVC covariables (bundles 5.1–5.6) were derived in various ways from the 6 assessed covariables for the 4 fingers on each hand. The NVC sub‐bundles were organized on 3 levels for building competing MLR models: the patient, hand, and finger levels (see Supplementary Table 5). The 1 NVC pattern qualitative covariable was used in an alternative final model as a surrogate for the NVC quantitative covariables. Bundle 6 was formed for statistical investigation of interactions between covariables, which were prespecified by clinical consensus (see Supplementary Table 8, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract).
The pattern of medication use (by class) varied from one country to another, and patients took a wide range of combinations from the different medication classes. An analysis showed that medication use within 3 months prior to and at enrollment was associated with DU disease severity prior to and at enrollment (number of DUs, by Cochran‐Armitage trend test, and previous hospitalizations due to DUs, by chi‐square test) (Table 3). Specifically, DU disease severity and medication use were collinear, meaning that one could be predicted from the other with reasonable accuracy. Therefore, due to the collinearity in the multivariable regression and the complexity of medication use patterns across the countries, it was decided to not include medication use as a potential risk factor, but to instead include DU disease severity in the model‐building process.
Table 3.
Medication use and relationship to DU disease severitya
| Medication class | Summary statistics | P for association with DU disease severity | ||
|---|---|---|---|---|
| No. (%) of cases (n = 103) | No. (%) of noncases (n = 365) | No. of DUs (n = 468)b | Hospitalization due to DUs (n = 468)c | |
| At least 1 medication class | 97 (94.2) | 343 (94.0) | – | – |
| Vasoactive medication | ||||
| Endothelin receptor antagonists | 43 (41.7)d | 113 (31.0) | 0.0251 | 0.0856 |
| Phosphodiesterase 5 inhibitors | 15 (14.6) | 31 (8.5) | 0.0088 | 0.8604 |
| Prostanoids, including intravenous formulation | 54 (52.4)d | 140 (38.4) | 0.3159 | <0.0001 |
| Angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers | 27 (26.2) | 112 (30.7) | 0.3283 | 0.5535 |
| Calcium‐channel blockers | 57 (55.3) | 206 (56.4) | 0.1044 | 0.0553 |
| Selective serotonin reuptake inhibitors | 11 (10.7) | 39 (10.7) | 0.1935 | 0.0192 |
| Nitrates | 3 (2.9) | 15 (4.1) | 0.9737 | 0.7506 |
| Statins | 10 (9.7)d | 69 (18.9) | 0.0568 | 0.1097 |
| Other vasodilators | 10 (9.7) | 53 (14.5) | 0.3635 | 0.1534 |
| Platelet aggregation inhibitors | 39 (37.9) | 163 (44.7) | 0.1265 | 0.2490 |
| Immunosuppressants | 53 (51.5)d | 139 (38.1) | 0.1989 | 0.7551 |
Includes ongoing medication use and medication use within 3 months prior to and at enrollment. DU = digital ulcer.
By Cochran‐Armitage trend test.
By chi‐square test.
P < 0.05 versus noncases, by chi‐square test and Fisher's exact test.
Statistical criteria for selecting good‐performing covariables via forward stepwise selection (FSS) at the ULR stage were the Wald's chi‐square test statistic, P < 0.15 for linear terms, or P < 0.05 for quadratic terms. Categorical variables with a frequency of <20 patients were not moved forward from ULR to MLR (see Supplementary Table 9, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). In addition to FSS, the nominal group technique was used by the steering committee to exclude some covariables based on lack of clinical plausibility and/or feasibility, with particular regard to use in standard practice (see Supplementary Table 10). Statistical criteria for selecting covariables via FSS during MLR stage 2 (within‐bundle) analysis were to enter if P < 0.15 and retain if P < 0.10. At MLR stage 3 (across‐bundles) analysis, statistical criteria for selecting covariables via FSS were to enter if P < 0.15 and retain if P < 0.05. The reduction of covariables at each of the 3 stages resulted in the final MLR model.
Modeling within each of the NVC sub‐bundles resulted in 6 competing MLR models. In order to retain only 1 type of NVC assessment in the final model from among the several assessment options for the NVC (qualitative or quantitative [on the patient, hand, and finger levels]), it was decided to carry forward the sub‐bundle with the highest ROC AUC into the across‐bundles stage.
Model validation
Internal validation of the final MLR model was performed through the bootstrap method 35, with 2,000 re‐samples using the same model‐building and covariable selection procedures as for the final model.
SAS software, version 9.1.3, was used for the statistical analysis and the reporting of clinical data.
RESULTS
Findings in the study population
DU outcome was known for 591 of the 623 enrolled patients. Among those 591 patients, 468 (79.2%) belonged to the DU history group (Figure 1), of whom 103 (22.0%) developed a DU during the observation period. Of the 123 patients in the no DU history group, only 5 (4.1%) developed a new DU (Figure 1). As the incidence of new DUs in the no DU history group was low, this report focuses on the DU history group. The distribution of the total number of patients and the number of patients who developed a new DU (cases) were highly variable across countries and centers (see Supplementary Figure 2, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). The results of the covariable selection process for the best‐performing risk factors for new DU outcome for each bundle at stages 1, 2, and 3 of model development are presented in detail in Supplementary Tables 1–6.
Figure 1.
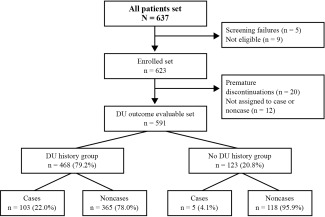
Flow chart showing the distribution of the study patients and stratification of the digital ulcer (DU) outcome set. In total, 637 patients with systemic sclerosis were screened. After exclusion of screening failures, ineligible patients, premature discontinuations, and patients not assigned to case or noncase categories, the DU outcome evaluable set (n = 591) was obtained. Because the variables that influence the occurrence of a new DU were thought to be different in the DU history group (those with a history of DUs or with DUs at enrollment) versus the no DU history group (those without a history of DUs), the data analysis was stratified in the same two study arms.
Demographic features, digital ulcers, and clinical characteristics
Of the DU history group, 79.5% of patients were female, 280 (59.8%) were classified as having limited cutaneous SSc, and 188 (40.2%) as having diffuse cutaneous SSc. The demographic, DU, and clinical characteristics, according to DU outcome, are reported in Table 1. The presence and number of DUs at enrollment were significantly associated with the occurrence of new DUs (P < 0.001), with the ORs increasing with an increasing number of DUs at enrollment. The OR of having a new DU was 2.691 (95% CI 1.507–4.803) in patients with 1 DU at enrollment, 3.787 (95% CI 1.879–7.630) in those with 2 DUs, and 7.399 (95% CI 3.687–14.848) in those with ≥3 DUs.
The ROC AUCs for individual non‐NVC covariables by ULR (stage 1) had a range between 0.520 and 0.678 (Table 1). The highest ROC AUC for the non‐NVC covariables selected by MLR within‐bundle (stage 2) was for the DU characteristics bundle, at 0.694 (95% CI 0.637–0.751) (see Supplementary Tables 11–14, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). Calcinosis was present in 20.4% of cases and 18.4% of noncases. On ULR analysis, calcinosis was not significantly associated with the development of new DUs (OR 1.090 [95% CI 0.637–1.866]).
The vasomodulating and immunosuppressive medications taken during the 3 months prior to or at enrollment and their association with DU disease severity, are described in Table 3. During the observation period, medication use was stable for the drug classes recorded.
NVC covariables, qualitative and quantitative measurements
Descriptive analysis of the NVC covariables selected at the ULR stage and carried forward to the MLR within‐bundle analysis, are reported in Table 2 and in Supplementary Tables 5 and 6. The NVC pattern was significantly associated with the DU outcome (P < 0.002); the proportion of patients with a late SSc NVC pattern was higher in the cases than in the noncases (71.8% versus 54.0%), and the OR for a late versus normal/early SSc NVC pattern was 4.150 (95% CI 1.441–11.950), which is consistent with previous reports 20, 21. The mean number of capillaries per millimeter was significantly reduced in cases versus noncases regardless of the NVC quantitative assessment type used (patient, hand, or finger level).
The NVC covariables selected by MLR within‐bundle analysis (stage 2) are depicted in Supplementary Tables 15 and 16 (available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). The NVC sub‐bundle encompassing analysis at the finger level of the dominant hand in the 4 individual fingers (sub‐bundle 5.5) had the highest ROC AUC among the 6 competing NVC sub‐bundle models (ROC AUC 0.677 [95% CI 0.614–0.740]) and was carried forward to the MLR across‐bundles analysis (stage 3). The complete list of NVC covariables carried forward from MLR within‐bundle to MLR across‐bundles analysis is presented in Supplementary Table 17 (available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract).
Interactions between covariables
Analysis of the interactions bundle 6 showed that none of the 8 interaction terms were statistically significant. Therefore, no interaction terms were carried forward to the MLR across‐bundles analysis.
Non‐NVC and NVC covariables in MLR across‐bundles analysis
MLR across‐bundles analysis (stage 3) resulted in the final model with 3 risk factors: 1) the mean number of capillaries/mm in the middle finger of the dominant hand (evaluated on 2 adjacent fields in the middle of the nailfold), with an OR of 0.838 (95% CI 0.735–0.955), 2) the number of DUs at the enrollment visit (categorized as 0, 1, 2, or ≥3), with an OR for ≥3 DUs versus 0 DUs at enrollment of 6.160 (95% CI 2.999–12.653), and 3) the presence of critical digital ischemia at enrollment, with an OR of 3.194 (95% CI 1.284–7.945) (Table 4). The ROC AUC for the model was 0.738 (95% CI 0.681–0.795), and the trade‐off between sensitivity and specificity can be seen in the crossover curves (see Supplementary Figure 3, available online at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract). The Hosmer‐Lemeshow test indicated that the final model did not show a significant lack of fit (P = 0.751). Internal validation of the final MLR model through bootstrap generated a ROC AUC of 0.633 (95% CI 0.510–0.756).
Table 4.
Final multivariable logistic regression modela
| Variable | Coefficient estimate | MLR | |||
|---|---|---|---|---|---|
| Standard error | Odds ratio (95% CI) | Wald's chi‐square test | P | ||
| Intercept | −1.0864 | 0.3299 | 0.337 (0.177–0.644) | 10.8445 | 0.0010 |
| Mean no. of capillaries/mm in middle finger of dominant hand | −0.1770 | 0.0670 | 0.838 (0.735–0.955) | 6.9801 | 0.0082 |
| No. of DUs at enrollment | |||||
| 1 | 0.7460 | 0.3307 | 2.109 (1.103–4.032) | 5.0878 | 0.0241 |
| 2 | 1.1696 | 0.3889 | 3.221 (1.503–6.902) | 9.0458 | 0.0026 |
| ≥3 | 1.8181 | 0.3672 | 6.160 (2.999–12.653) | 24.5082 | <0.0001 |
| Critical digital ischemia present at enrollment | 1.1613 | 0.4649 | 3.194 (1.284–7.945) | 6.2388 | 0.0125 |
The final prognostic model used 3 variables to predict the occurrence of digital ulcers (DUs) within 6 months: the mean number of capillaries/mm in the middle finger of the dominant hand, the number of DUs at enrollment (categorized as 0, 1, 2, or ≥3), and the presence/absence of critical digital ischemia (defined as prolonged, severe, persistent reduction in digital tissue perfusion without rewarming) at enrollment. The multivariable logistic regression (MLR) coefficient estimates indicate that the risk of developing a DU within 6 months increases in patients with critical digital ischemia at enrollment, in patients with a greater number of DUs, and in patients with a lower number of capillaries/mm in the middle finger of the dominant hand. No variable among the demographic, SSc clinical, or other clinical characteristic bundle was retained. Receiver operating characteristic curve analysis showed an area under the curve of 0.738 (95% confidence interval [95% CI] 0.681–0.795). The Hosmer‐Lemeshow goodness‐of‐fit test yielded the following values: χ2 = 5.0602, 8 df, P = 0.751. The MLR equation, based on the estimates shown in the table, was as follows:
where the linear predictor is −1.0864 (intercept) −0.1770 multiplied by the mean number of capillaries per mm in the middle finger of the dominant hand, plus either 0.7460 for the presence of 1 DU, 1.1696 for 2 DUs, or 1.8181 for ≥3 DUs at enrollment, plus 1.1613 for the presence of critical digital ischemia at enrollment. Thus, a patient with 5 capillaries/mm in the middle finger of the dominant hand, plus 2 DUs at enrollment, plus critical digital ischemia at enrollment has a 59% probability of developing new DUs within 6 months.
A supplementary analysis in which the NVC pattern was used as the NVC covariable in the final model yielded similar model performance, with a ROC AUC of 0.715 (95% CI 0.658–0.771).
DISCUSSION
The CAP study was the first large, prospective, international, multicenter study to evaluate capillaroscopic and other clinical characteristics to determine risk factors for the development of new DUs during a 6‐month period in patients with SSc. A very low number of cases were reported in the no DU history group during the 6‐month observation period; therefore, risk factor analysis was performed only on the DU history group. The strongest performing risk factors for the occurrence of new DUs identified by MLR analysis were the mean number of capillaries per millimeter in the middle finger of the dominant hand, the number of DUs at enrollment, and the presence of critical digital ischemia at enrollment.
The characteristics of our study population were similar to those in the European League Against Rheumatism (EULAR) Scleroderma Trials and Research Group (EUSTAR) cohort of patients with SSc 36. As expected, it appeared that the number of DUs had a large influence on the risk of future DUs, which is consistent with data reported previously 37, 38, and may be linked to increased severity of SSc disease, since patients with DUs are more likely to have an earlier onset of SSc and more extensive skin involvement 3. There are a number of therapies available for the prevention of DUs 32, 39, 40, and it is therefore important to identify patients who are at risk of developing DUs so that they can receive preventive management.
Consistent with previous studies, the CAP study revealed capillary density (number of capillaries/mm) as the most robust NVC risk factor for new DUs. Of note, loss of capillaries has been linked to an increased risk of developing SSc and may therefore predict an early diagnosis, more severe skin involvement, and a poorer prognosis 21, 41, 42, 43, 44, 45, 46. While the current study found that the capillary density on the third digit of the dominant hand was sufficient for predicting the risk of new DUs, it is still necessary to evaluate at least 4 digits per hand for the diagnosis of SSc in patients with Raynaud's phenomenon. The study also indicated that the NVC pattern may play a role in predicting DUs, confirming the results of smaller studies that have shown that the late SSc NVC pattern is associated with an increased risk of DUs 20, 47, 48. Ideally, future studies would encompass NVC‐based indices assessing eventual disease‐modifying characteristics of treatment on the clinical complications of SSc such as DUs 49, 50.
With regard to the individual performance of each of the final model's 3 variables in the ULR analysis, the number of DUs was the strongest risk factor (ROC AUC 0.678), followed by the number of capillaries/mm in the middle finger of the dominant hand (ROC AUC 0.614), and then the presence of critical digital ischemia at enrollment (ROC AUC 0.556). The combination of the 3 variables in the final MLR model improved the model's discriminatory ability (ROC AUC 0.738). Furthermore, the relative weights of the Wald's chi‐square values for the 3 variables (Table 4) demonstrated that both the number of capillaries/mm in the middle finger of the dominant hand (NVC variable) and critical digital ischemia at enrollment make important additional contributions to the number of DUs for determining the risk of new DUs in the final MLR model.
Interestingly, whereas a 50% incidence of new DUs in the DU history group had been assumed in the sample size estimation of our study, in reality, there was only a 22% incidence. The lower‐than‐expected incidence of new DUs may be the result of patients already receiving best practice standard of care for the management of their disease at enrollment. Medication use was not restricted, varied between countries, and was found to be associated with DU disease severity, which is consistent with findings from the Digital Ulcer Outcome Registry showing that patients with chronic and/or recurrent DUs have a shorter time to new DUs as compared with patients with no or episodic DUs 51. Therefore, medication use was not considered to be an independent variable and was not included as a potential risk factor for new DUs.
Because the no DU history subgroup was considered exploratory, it was not possible to identify variables that predicted DUs because of the small number of cases in this subgroup. We had anticipated at least 20% of cases, based on the report that 50% of patients with SSc experience DUs 1 and the fact that the first DU usually occurs early in the disease course 3; however, we observed only 4.1% of cases of new DUs in this subgroup. This may have 2 explanations. First, the SSc population may have changed in the last few years: patients with SSc may be diagnosed earlier than in the past, and preventive and efficient measures are now more widely used. Second, it might have been beneficial to restrict patients in the no DU history group to those with a first non–Raynaud's phenomenon symptom within 1 year (instead of 2 years) in order to be closer to the population described by Hachulla et al 3, in which 43% of patients had their first DU within 1 year of their first clinical sign of SSc.
NVC has the potential to be a useful tool for monitoring the progression of microvascular disease associated with SSc and for measuring the response to treatment 52, 53. It is a well‐established, noninvasive technique that allows for higher‐magnification analysis compared with the older widefield capillaroscopic method 53, 54. The training needed for the device is minimal (∼5 days 55) and the required examination time is short (∼10 minutes including image recording). An NVC apparatus is more costly than handheld devices such as dermatoscopes, but NVC has been shown to permit more‐detailed assessments 56 and the grading of more images 54. During patient follow‐up, detailed assessment and quantification of abnormalities is important, and therefore, handheld tools may not be as useful in this setting 53. Of note, capillaroscopy has been recently introduced as a criterion in the ACR/EULAR classification criteria for SSc 16, thereby increasing their sensitivity and specificity.
Overall, there are several key strengths of this study. First is its generalizability, ensured by the broad distribution of participating centers and patients representing current standard of care. Second is its applicability in real‐world clinical practice, owing to the broad study population, the simplicity and ease of clinical evaluation of the NVC, and the clinical risk factors that built the final model. Third is its value in the management of patients with a history or presence of DUs. The scope of the CAP study was to determine risk factors for developing DUs by using NVC and other clinical characteristics in routine clinical practice and health care environments, including centers with different levels of NVC experience. Thus, the “center” was not regarded as a potential risk factor, which could allow generalizability of the study findings for patients with SSc outside of the study centers. The interrater variability of NVC assessments has been of concern, and the capillary density has been identified as the NVC variable with the best interrater agreement in earlier, smaller studies 28, 57. In the CAP study, interrater variability was addressed by practical training and teaching booklets that were offered to the investigators. Despite these efforts, the ambitious intention to allow extrapolation to the real‐world setting may have introduced a large amount of noise that was detrimental to obtaining a final model with high discriminatory ability.
Limitations of this study include the fact that the diagnosis of DUs and critical digital ischemia is not always unequivocal 58, 59, although definitions were provided in the protocol and/or the case report form. Investigators were not blinded to the NVC and other clinical characteristics assessed at enrollment and may therefore have been biased in favor of a diagnosis of a new DU. However, this was inherent to the real‐world nature of the study, as physicians in clinical practice are not blinded to the results of other assessments. Although the wide geographic spread of centers permits generalizability, it was conducive to introducing heterogeneity in the data. The center effect could not be explored in depth because of the wide distribution of patients across a large number of centers, with a small number of cases per center. Although it was estimated that 150 cases would be a reasonable number for exploring risk factor associations with the development of DUs, only 103 cases were observed in this cohort and, as such, the study was underpowered. Nevertheless, the modeling strategy (ULR, MLR within‐bundle, MLR across‐bundles analyses), together with the reduction in the number of variables entered into the model and the bootstrap validation demonstrated the robustness of the variables in the final model, thereby compensating for the lower‐than‐prespecified number of cases.
Laboratory biomarkers were not included in this study, which could have helped to improve the discriminatory ability of the final model. Biomarkers have previously been shown to be useful for predicting DUs 60 and may be useful to include in future studies. Given the nature of this real‐world observational study, it was not feasible to determine the presence of an association between medication use and development of DUs. Future larger studies could be designed to explore this association by controlling for confounding factors such as DU disease severity and the use of medications (individually and in combination) in different countries.
In conclusion, this longitudinal, multicenter study of almost 500 patients with SSc has shown that the mean number of capillaries/mm on the middle finger of the dominant hand, the number of DUs at enrollment, and the presence of critical digital ischemia at enrollment are risk factors for the development of new DUs. The risk factors identified are simple to evaluate in the clinic, and the real‐world nature of the study allows the results to be generalized to a wider SSc population, thereby providing the physician with useful information when considering a patient's risk of future DUs.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Cutolo and Smith had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Cutolo, Herrick, Distler, Chadha‐Boreham, Cottreel, Pfister, Rosenberg, Smith.
Acquisition of data
Cutolo, Herrick, Distler, Beltran, Carpentier, Inanç, Vlachoyiannopoulos, Smith.
Analysis and interpretation of data
Cutolo, Herrick, Distler, Becker, Beltran, Carpentier, Ferri, Inanç, Vlachoyiannopoulos, Chadha‐Boreham, Cottreel, Pfister, Rosenberg, Torres, Smith.
ROLE OF THE STUDY SPONSOR
Actelion Pharmaceuticals funded the study and was responsible for the study protocol design, data collection, and statistical analysis, with, and under the leadership of, an independent study steering committee (non‐Actelion authors). Actelion Pharmaceuticals also paid for editorial assistance (provided by Drs. Marion James and Lynda McEvoy, ApotheCom, a medical communications company). The steering committee members, all of whom are authors of this article, and the 4 authors employed by Actelion Pharmaceuticals were involved in the decision to submit the article for publication. Publication of this article was not contingent upon approval by Actelion Pharmaceuticals.
Supporting information
Supplementary Table S1. Bundle 1: Demographics
Supplementary Table S2. Bundle 2: Systemic sclerosis clinical characteristics
Supplementary Table S3. Bundle 3: Digital ulcer characteristics
Supplementary Table S4. Bundle 4: Other clinical characteristics
Supplementary Table S5. Bundle 5: Nailfold videocapillaroscopic characteristics: quantitative assessment (6 sub‐bundles)
Supplementary Table S6. Nailfold videocapillaroscopic characteristics: qualitative assessment (1 covariable)
Supplementary Table S7. Definitions of digital ulcer (DU), critical digital ischemia, and other (than DU)
Supplementary Figure S1. Coding of the location of digital ulcers
ACKNOWLEDGMENTS
We thank our colleagues for sending their patients to our Scleroderma Clinics and our patients for their trust and unconditional commitment. We also thank Harald Heinzl (CeMSIIS, Medical University of Vienna, Vienna, Austria) for statistical consultation and assistance with the bootstrap analysis, as well as the wider group of statisticians for their contributions (see Supplementary Materials, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39718/abstract).
Supported by Actelion Pharmaceuticals. Dr. Smith's work was supported by FWO research grant 1.5.217.13N; she also is recipient of an FWO Senior Clinical Investigator fellowship.
Dr. Cutolo has received research funding through the University of Genoa from Actelion Pharmaceuticals, Bristol‐Myers Squibb, and Horizon.
Dr. Herrick has received consulting fees and/or speaking fees from Actelion Pharmaceuticals and Apricus (less than $10,000 each) and research grants from Actelion Pharmaceuticals.
Dr. Distler has received consulting fees from 4D Science, Active Biotec, Bristol‐Myers Squibb, EpiPharm, Biogen Idec, Genentech/Roche, GlaxoSmithKline, Inventiva, Lilly, Pfizer, Serodapharm, Sinoxa, ErgoNex, Pharmacyclics, and Sanofi (less than $10,000 each) and from Actelion Pharmaceuticals, Bayer, Boehringer Ingelheim, and Medac (more than $10,000 each) and has received research grants from these companies; he holds a patent for the use of microRNA‐29 in the treatment of systemic sclerosis.
Dr. Becker has received speaker's fees and/or honoraria from, and has served as a consultant to, Actelion Pharmaceuticals (less than $10,000; unrelated to the present study).
Dr. Carpentier is a member of the Actelion Pharmaceuticals SAS France scientific committee on digital ulcers.
Dr. Inanç has received consulting fees and speaking fees from Actelion Pharmaceuticals (less than $10,000) and has received research grants from this company.
Dr. Chadha‐Boreham, Mrs. Cottreel, and Drs. Pfister and Rosenberg are employees of, and own stock or stock options in, Actelion Pharmaceuticals. Mr. Torres has received consulting fees from Actelion Pharmaceuticals (more than $10,000).
Dr. Smith has received consulting fees and/or speaking fees from Actelion Pharmaceuticals, Boehringer Ingelheim, and Galapagos (less than $10,000 each) and has received research grants from Actelion Pharmaceuticals and Galapagos.
Contributor Information
Maurizio Cutolo, Email: mcutolo@unige.it.
Vanessa Smith, Email: vanessa.smith@ugent.be.
REFERENCES
- 1. Walker UA, Tyndall A, Czirják L, Denton C, Farge‐Bancel D, Kowal‐Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steen V, Denton CP, Pope JE, Matucci‐Cerinic M. Digital ulcers: overt vascular disease in systemic sclerosis. Rheumatology (Oxford) 2009;48 Suppl 3:iii19–24. [DOI] [PubMed] [Google Scholar]
- 3. Hachulla E, Clerson P, Launay D, Lambert M, Morell‐Dubois S, Queyrel V, et al. Natural history of ischemic digital ulcers in systemic sclerosis: single‐center retrospective longitudinal study. J Rheumatol 2007;34:2423–30. [PubMed] [Google Scholar]
- 4. Nihtyanova SI, Brough GM, Black CM, Denton CP. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis 2008;67:120–3. [DOI] [PubMed] [Google Scholar]
- 5. Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol 2010;6:578–87. [DOI] [PubMed] [Google Scholar]
- 6. Bérezné A, Seror R, Morell‐Dubois S, de Menthon M, Fois E, Dzeing‐Ella A, et al. Impact of systemic sclerosis on occupational and professional activity with attention to patients with digital ulcers. Arthritis Care Res (Hoboken) 2011;63:277–85. [DOI] [PubMed] [Google Scholar]
- 7. Guillevin L, Hunsche E, Denton CP, Krieg T, Schwierin B, Rosenberg D, et al. Functional impairment of systemic scleroderma patients with digital ulcerations: results from the DUO Registry. Clin Exp Rheumatol 2013;31 Suppl 76:71–80. [PubMed] [Google Scholar]
- 8. Rannou F, Poiraudeau S, Berezné A, Baubet T, Le‐Guern V, Cabane J, et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36‐Item Short Form Health Survey. Arthritis Rheum 2007;57:94–102. [DOI] [PubMed] [Google Scholar]
- 9. Mouthon L, Mestre‐Stanislas C, Bérezné A, Rannou F, Guilpain P, Revel M, et al. Impact of digital ulcers on disability and health‐related quality of life in systemic sclerosis. Ann Rheum Dis 2010;69:214–7. [DOI] [PubMed] [Google Scholar]
- 10. Ennis H, Vail A, Wragg E, Taylor A, Moore T, Murray A, et al. A prospective study of systemic sclerosis‐related digital ulcers: prevalence, location, and functional impact. Scand J Rheumatol 2013;42:483–6. [DOI] [PubMed] [Google Scholar]
- 11. Mouthon L, Carpentier PH, Lok C, Clerson P, Gressin V, Hachulla E, et al. Ischemic digital ulcers affect hand disability and pain in systemic sclerosis. J Rheumatol 2014;41:1317–23. [DOI] [PubMed] [Google Scholar]
- 12. Matucci‐Cerinic M, Steen V, Nash P, Hachulla E. The complexity of managing systemic sclerosis: screening and diagnosis. Rheumatology (Oxford) 2009;48 Suppl 3:iii8–13. [DOI] [PubMed] [Google Scholar]
- 13. Kowal‐Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 2009;68:620–8. [DOI] [PubMed] [Google Scholar]
- 14. LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 15. Avouac J, Fransen J, Walker UA, Riccieri V, Smith V, Muller C, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi consensus study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis 2011;70:476–81. [DOI] [PubMed] [Google Scholar]
- 16. Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sebastiani M, Manfredi A, Vukatana G, Moscatelli S, Riato L, Bocci M, et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicentre validation study. Ann Rheum Dis 2012;71:67–70. [DOI] [PubMed] [Google Scholar]
- 18. Sebastiani M, Manfredi A, Colaci M, D'Amico R, Malagoli V, Giuggioli D, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum 2009;61:688–94. [DOI] [PubMed] [Google Scholar]
- 19. Smith V, Riccieri V, Pizzorni C, Decuman S, Deschepper E, Bonroy C, et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol 2013;40:2023–8. [DOI] [PubMed] [Google Scholar]
- 20. Smith V, Decuman S, Sulli A, Bonroy C, Piette Y, Deschepper E, et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis 2012;71:1636–9. [DOI] [PubMed] [Google Scholar]
- 21. Smith V, De Keyser F, Pizzorni C, Van Praet JT, Decumnan S, Sulli A, et al. Nailfold capillaroscopy for day‐to‐day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis 2011;70:180–3. [DOI] [PubMed] [Google Scholar]
- 22. Ennis H, Moore T, Murray A, Vail A, Herrick AL. Further confirmation that digital ulcers are associated with the severity of abnormality on nailfold capillaroscopy in patients with systemic sclerosis. Rheumatology (Oxford) 2014;53:376–7. [DOI] [PubMed] [Google Scholar]
- 23. Manfredi A, Sebastiani M, Carraro V, Iudici M, Bocci M, Vukatana G, et al. Prediction risk chart for scleroderma digital ulcers: a composite predictive model based on capillaroscopic, demographic and clinico‐serological parameters. Clin Hemorheol Microcirc 2015;59:133–43. [DOI] [PubMed] [Google Scholar]
- 24. Brand M, Hollaender R, Rosenberg D, Scott M, Hunsche E, Tyndall A, et al. An observational cohort study of patients with newly diagnosed digital ulcer disease secondary to systemic sclerosis registered in the EUSTAR database. Clin Exp Rheumatol 2015;33 Suppl 91:S47–54. [PubMed] [Google Scholar]
- 25. Hunzelmann N, Riemekasten G, Becker M, Moinzadeh P, Kreuter A, Melchers I, et al. The Predict Study: Low risk for digital ulcer development in systemic sclerosis patients with increasing disease duration and lack of topoisomerase‐1 antibodies. Br J Dermatol 2016;174:1384–7. [DOI] [PubMed] [Google Scholar]
- 26. Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee . Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 27. Sulli A, Secchi ME, Pizzorni C, Cutolo M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis 2008;67:885–7. [DOI] [PubMed] [Google Scholar]
- 28. Smith V, Pizzorni C, De Keyser F, Decuman S, Van Praet JT, Deschepper E, et al. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: a two‐centre study. Ann Rheum Dis 2010;69:1092–6. [DOI] [PubMed] [Google Scholar]
- 29. Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000;27:155–60. [PubMed] [Google Scholar]
- 30. Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med 1984;3:143–52. [DOI] [PubMed] [Google Scholar]
- 31. Steyerberg E, Harrell F. Statistical models for prognostication. Bethesda (MD): National Institutes of Health; 2010. [Google Scholar]
- 32. Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al, for the RAPIDS‐1 Study Group . Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum 2004;50:3985–93. [DOI] [PubMed] [Google Scholar]
- 33. Blettner M, Sauerbrei W. Influence of model‐building strategies on the results of a case‐control study. Stat Med 1993;12:1325–38. [DOI] [PubMed] [Google Scholar]
- 34. Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, et al. Evidence‐based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton (FL): CRC Press; 1993. [Google Scholar]
- 36. Ingegnoli F, Ardoino I, Boracchi P, Cutolo M, Airò P, Ananieva LP, et al. Nailfold capillaroscopy in systemic sclerosis: data from the EULAR Scleroderma Trials and Research (EUSTAR) database. Microvasc Res 2013;89:122–8. [DOI] [PubMed] [Google Scholar]
- 37. Sebastiani M, Manfredi A, Lo Monaco A, Praino E, Riccieri V, Grattagliano V, et al. Capillaroscopic Skin Ulcers Risk Index (CSURI) calculated with different videocapillaroscopy devices: how its predictive values change. Clin Exp Rheumatol 2013;31 Suppl 76:115–7. [PubMed] [Google Scholar]
- 38. Mihai C, Landewé R, van der Heijde D, Walker UA, Constantin PI, Gherge AM, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis 2016;75:681–6. [DOI] [PubMed] [Google Scholar]
- 39. Chung L. Therapeutic options for digital ulcers in patients with systemic sclerosis. J Dtsch Dermatol Ges 2007;5:460–5. [DOI] [PubMed] [Google Scholar]
- 40. Tingey T, Shu J, Smuczek J, Pope J. Meta‐analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res (Hoboken) 2013;65:1460–71. [DOI] [PubMed] [Google Scholar]
- 41. Cutolo M, Sulli A, Pizzorni C, Smith V. Capillaroscopy as an outcome measure for clinical trials on the peripheral vasculopathy in SSc—is it useful? Int J Rheumatol 2010;2010:784947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hofstee HM, Vonk NA, Voskuyl AE, Dijkmans BA, Postmus PE, Smulders YM, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 2009;68:191–5. [DOI] [PubMed] [Google Scholar]
- 43. Koenig M, Joyal F, Fritzler MJ, Roussin A, Abrahamowicz M, Boire G, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty‐year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008;58:3902–12. [DOI] [PubMed] [Google Scholar]
- 44. Riccieri V, Vasile M, Iannace N, Stefanantoni K, Sciarra I, Vizza CD, et al. Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology (Oxford) 2013;52:1525–8. [DOI] [PubMed] [Google Scholar]
- 45. Kayser C, Sekiyama JY, Próspero LC, Camargo CZ, Andrade LE. Nailfold capillaroscopy abnormalities as predictors of mortality in patients with systemic sclerosis. Clin Exp Rheumatol 2013;31 Suppl 76:103–8. [PubMed] [Google Scholar]
- 46. Ingegnoli F, Boracchi P, Gualtierotti R, Biganzoli EM, Zeni S, Lubatti C, et al. Improving outcome prediction of systemic sclerosis from isolated Raynaud's phenomenon: role of autoantibodies and nail‐fold capillaroscopy. Rheumatology (Oxford) 2010;49:797–805. [DOI] [PubMed] [Google Scholar]
- 47. Caramaschi P, Canestrini S, Martinelli N, Volpe A, Pieropan S, Ferrari M, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford) 2007;46:1566–9. [DOI] [PubMed] [Google Scholar]
- 48. Silva I, Teixeira A, Oliveira J, Almeida I, Almeida R, Águas A, et al. Endothelial dysfunction and nailfold videocapillaroscopy pattern as predictors of digital ulcers in systemic sclerosis: a cohort study and review of the literature. Clin Rev Allergy Immunol 2015;49:240–52. [DOI] [PubMed] [Google Scholar]
- 49. Cutolo M, Ruaro B, Pizzorni C, Ravera F, Smith V, Zampogna G, et al. Longterm treatment with endothelin receptor antagonist bosentan and iloprost improves fingertip blood perfusion in systemic sclerosis. J Rheumatol 2014;41:881–6. [DOI] [PubMed] [Google Scholar]
- 50. Cutolo M, Zampogna G, Vremis L, Smith V, Pizzorni C, Sulli A. Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J Rheumatol 2013;40:40–5. [DOI] [PubMed] [Google Scholar]
- 51. Matucci‐Cerinic M, Krieg T, Guillevin L, Schwierin B, Rosenberg D, Cornelisse P, et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long‐term results from the DUO Registry. Ann Rheum Dis 2015. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anderson ME, Allen PD, Moore T, Hillier V, Taylor CJ, Herrick AL. Computerized nailfold video capillaroscopy—a new tool for assessment of Raynaud's phenomenon. J Rheumatol 2005;32:841–8. [PubMed] [Google Scholar]
- 53. Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud's phenomenon and systemic sclerosis [review]. Arthritis Rheum 2010;62:2595–604. [DOI] [PubMed] [Google Scholar]
- 54. Hughes M, Moore T, O'Leary N, Tracey A, Ennis H, Dinsdale G, et al. A study comparing videocapillaroscopy and dermoscopy in the assessment of nailfold capillaries in patients with systemic sclerosis‐spectrum disorders. Rheumatology (Oxford) 2015;54:1435–42. [DOI] [PubMed] [Google Scholar]
- 55. Cutolo M, Smith V, Sulli A. Training in capillaroscopy: a growing interest. J Rheumatol 2012;39:1113–6. [DOI] [PubMed] [Google Scholar]
- 56. Dogan S, Akdogan A, Atakan N. Nailfold capillaroscopy in systemic sclerosis: is there any difference between videocapillaroscopy and dermatoscopy? Skin Res Technol 2013;19:446–9. [DOI] [PubMed] [Google Scholar]
- 57. Hudson M, Masetto A, Steele R, Arthurs E, Baron M. Reliability of widefield capillary microscopy to measure nailfold capillary density in systemic sclerosis. Clin Exp Rheumatol 2010;28 Suppl 62:S36–41. [PubMed] [Google Scholar]
- 58. Baron M, Chung L, Gyger G, Hummers L, Khanna D, Mayes MD, et al. Consensus opinion of a North American Working Group regarding the classification of digital ulcers in systemic sclerosis. Clin Rheumatol 2014;33:207–14. [DOI] [PubMed] [Google Scholar]
- 59. Herrick AL, Roberts C, Tracey A, Silman A, Anderson M, Goodfield M, et al. Lack of agreement between rheumatologists in defining digital ulceration in systemic sclerosis. Arthritis Rheum 2009;60:878–82. [DOI] [PubMed] [Google Scholar]
- 60. Silva I, Teixeira A, Oliveira J, Almeida I, Almeida R, Vasconcelos C. Predictive value of vascular disease biomarkers for digital ulcers in systemic sclerosis patients. Clin Exp Rheumatol 2015;33 Suppl 91:S127–30. [PubMed] [Google Scholar]
- 61. Leffondré K, Abrahamowicz M, Xiao Y, Siemiatycki J. Modelling smoking history using a comprehensive smoking index: application to lung cancer. Stat Med 2006;25:4132–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Bundle 1: Demographics
Supplementary Table S2. Bundle 2: Systemic sclerosis clinical characteristics
Supplementary Table S3. Bundle 3: Digital ulcer characteristics
Supplementary Table S4. Bundle 4: Other clinical characteristics
Supplementary Table S5. Bundle 5: Nailfold videocapillaroscopic characteristics: quantitative assessment (6 sub‐bundles)
Supplementary Table S6. Nailfold videocapillaroscopic characteristics: qualitative assessment (1 covariable)
Supplementary Table S7. Definitions of digital ulcer (DU), critical digital ischemia, and other (than DU)
Supplementary Figure S1. Coding of the location of digital ulcers


