Abstract
Objective
To examine the association between different levels of childhood attention deficit hyperactivity disorder (ADHD) symptoms and sex differences in psychosocial outcomes during adolescence.
Method
Swedish children (n = 4635) were screened for neuropsychiatric symptoms at age 9 or 12. ADHD symptoms were divided into three levels: screen‐negative, screen‐intermediate, and screen‐positive. At follow‐up (age 15), parents and teenagers filled out questionnaires regarding (i) hyperactivity/inattention, (ii) peer problems, (iii) school problems, (iv) internalizing problems, (v) antisocial behaviour, (vi) alcohol misuse, and (vii) drug misuse. All outcomes were controlled for symptoms of diagnostic categories other than ADHD.
Results
Increasing levels of ADHD symptoms in childhood were associated with higher proportions of adolescents who displayed negative psychosocial outcomes. More girls than boys reported internalizing problems (all levels) and risky drug use (screen‐intermediate and screen‐positive only). More boys reported antisocial behaviour at the screen‐negative and screen‐intermediate levels, but at the screen‐positive level, similar proportions of girls and boys displayed antisocial behaviour.
Conclusion
The findings support the view that ADHD symptoms, as well as their negative outcomes, are dimensionally distributed in the population and that adolescent girls and boys display different risk profiles. The findings confirm that ADHD symptoms are associated with higher risk of drug misuse in girls.
Keywords: attention deficit hyperactivity disorder, child and adolescent psychiatry, comorbidity, gender, behaviour
Significant outcomes.
Increasing levels of attention deficit hyperactivity disorder (ADHD) symptoms during childhood were associated with an increasing risk of psychosocial problems in adolescence for most outcomes.
More girls reported internalizing problems than boys at all ADHD symptom levels.
Girls and boys also differed in that significantly more adolescent girls reported risky drug use with each increase in childhood ADHD symptom level.
Limitations.
There was a lack of information on diagnostic status, ADHD subtype, and previous or current healthcare contacts.
Our results included only cases where the parent (at baseline) as well as both the child and parent (at follow‐up) participated (44% of the original sample).
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurobiological condition that affects between 5% and 10% of all children 1, 2, 3, often throughout life 4, 5. According to the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM‐IV) 6, individuals who qualify for a diagnosis of ADHD should have a persistent pattern of inattention and/or hyperactivity–impulsivity to the degree that it interferes with their development or functioning. In DSM‐5 7, some of the criteria have been changed; the symptoms must be present before the age of 12 (instead of age 7, as stated in the previous version of DSM) and from the age of 17 years, five of nine criteria in each subgroup are required for a diagnosis (instead of six of nine). These changes have been made based on the increasing knowledge of ADHD, for example, that some individuals develop symptoms later during childhood and that the functional impairment may remain over time even though ADHD symptoms have declined.
Children with ADHD are at risk of school failure, emotional difficulties, substance misuse, antisocial behaviour, and poor peer relationships in adolescence 8, 9, 10, 11, 12, 13, 14, and are more impaired in psychosocial, educational, and neuropsychological functioning as adults 15. ADHD affects both boys and girls in all areas of functioning, for example, academically, cognitively, psychosocially, and psychiatrically 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. Even though ADHD is associated with coexisting externalizing and internalizing disorders in both genders, girls with ADHD are significantly more likely to display internalizing disorders than boys with ADHD 25, 31, 32, 33. Internalizing problems is a collective term often used to describe emotional problems (e.g. anxiety and depression) and certain psychosomatic symptoms (e.g. headache and stomachache). Previous research has shown that girls with ADHD may have a higher risk than controls to manifest mood and anxiety disorders 18. Prospective studies focusing on gender differences indicate that childhood ADHD may predict more steeply rising symptoms of anxiety and depression during adolescence in girls than in boys 24. Girls with ADHD also show significantly higher risks of disruptive and antisocial behaviours, as well as eating disorders (EDs) and substance dependence, as compared to girls without ADHD 18, 23, 28, 34. However, some studies have failed to show that girls with ADHD are more likely to display internalizing disorders than boys with ADHD 35. For example, a study published 2015 by Bauermeister et al. found that ADHD subtype could also play an important role. Among those with the combined type, boys were more likely to be comorbid with mood disorders than girls. For those with the inattentive type, girls were more likely to be comorbid with anxiety disorders than boys. The authors concluded that gender may play a role in subtype of ADHD 16. Other studies also indicate that subtype is a more important determinant of clinical expression of ADHD than sex 22.
It has been proposed that ADHD symptoms are continuously distributed 36 and that an ADHD diagnosis represents the extreme end of ADHD symptoms, which in turn are associated with functional impairment. Recent research on ADHD, however, suggests that both ADHD caseness and subthreshold symptoms of ADHD are of clinical relevance 37, 38. Although children with subthreshold ADHD symptoms represent a milder condition than children with ADHD, they display a significantly more severe condition than children with non‐ADHD status, and experience functional impairment in several areas 37, 38. During development, symptoms of ADHD may both decline and increase 39. Some have argued that childhood and young adult/adult onset of ADHD may in fact be two distinct conditions 40, 41, 42 whereas others hold the view that with a condition that has multifactorial sources, variability in the manifestation of symptoms is to be expected 43. A supportive environment and cognitive capacity may compensate during some but not all phases of development. With age, increasing academic as well as social demands on children and adolescents may uncover underlying vulnerability. Also, given that ADHD impacts several areas of functioning, the onset of symptoms and impairment may manifest at different ages and several years apart.
Previous studies indicate that symptoms of ADHD are genetically linked 31, 38, 44, 45, 46 and that subthreshold ADHD symptoms may also be associated with negative outcomes such as poorer academic achievements, lower self‐esteem, and poorer relationships with parents and peers 31, 38, 44, 45, 46. Less is known about the association between different levels of ADHD symptoms and sex differences in psychosocial outcomes. Many longitudinal studies focusing on gender differences in ADHD outcomes have used clinical samples and case–control designs, thus limiting follow‐ups to previously confirmed cases. Also, the majority of long‐term follow‐up studies are from North America, making it less certain whether findings on gender differences extend to other study designs and other cultural contexts.
Aims of the study
The aims of the study were to examine psychosocial outcomes in a nationally representative sample of Swedish adolescents of both sexes in relation to different levels of childhood symptoms of attention deficit hyperactivity disorder (ADHD), while controlling for comorbidity and also to assess possible sex differences in the prevalence of psychosocial outcomes in adolescence, in relation to each ADHD symptom level during childhood.
Material and methods
Participants
The Child and Adolescent Twin Study in Sweden (CATSS) is an ongoing longitudinal cohort study following all twins born in Sweden from 1992 and onwards, that started in 2004 47. All twin parents in the country are invited to participate in a telephone interview. Before July 2005, consenting parents were interviewed when the twins were 12 years old, and after that time point, parents were interviewed at twin‐age 9. The reason for choosing these age groups was that most of the major child psychiatric problem constellations have been established by this age, while the problems associated with puberty most often have not yet emerged 47. Including 12‐year‐olds during the first waves of data collection was a way of increasing the speed to the point in time at which follow‐ups of adolescents and young adults would be possible.
The CATSS interview covers a broad range of symptoms and functions. At twin‐age 15, all families are again invited to participate in a follow‐up survey and fill out written questionnaires (both parents and twins, CATSS‐15). The present cohort included 4635 twins (2252 boys and 2383 girls) who were born 1993–1997, and where data were collected from both data collection waves (parent telephone interview at age 9/12 and questionnaires at age 15). The total response rate in this study for both parent and child (i.e. cases where both parent and child had responded in both data collection waves) at age 15 was 44.1%. In our study cohort of 4635 individuals, there were 329 monozygotic (MZ) male twin pairs, 419 MZ female twin pairs, 401 dizygotic (DZ) male twin pairs, 371 DZ female twin pairs, 614 complete opposite sexed twin pairs, and 333 twins without any co‐twin.
Measures
ADHD screening at age 9 or 12
Attention deficit hyperactivity disorder traits at baseline were assessed using the Autism‐Tics, ADHD, and other Comorbidities Inventory (A‐TAC) modules addressing DSM‐IV 6 criteria of the disorder, added with supplementary symptoms characteristic for the disorder. In previous validation studies, A‐TAC has shown good test–retest measures, excellent inter‐rater reliability, and construct validity 48, 49, 50, as well as convergent validity with the Child Behaviour Check List 51. ADHD traits were calculated as the sum of scores of both the inattention and hyperactivity–impulsivity scales, yielding between 0 and 19 points. ADHD symptoms were divided into three levels of severity: screen‐negative (<6 points), screen‐intermediate (6–7.5 points), and screen‐positive (8 points or more). These cutoffs were based on an earlier validation analysis 49. Response categories were then categorized as 0 (screen‐negative), 1 (screen‐intermediate), and 2 (screen‐positive).
Psychosocial outcome at age 15
Psychosocial outcome at age 15 was assessed through parent and self‐rating, and defined as the absence/presence of indicators of impairment in seven domains: current hyperactivity/inattention, peer problems, school problems, internalizing problems, antisocial behaviour, alcohol misuse, and drug misuse. If the parent and/or youth rated the youth's problem above cutoff for any of these outcomes (see below for definitions), the youth was defined as displaying an indication of impairment in that domain.
Current hyperactivity/inattention
Hyperactivity/inattention at follow‐up was defined as being above cutoff on either self‐rated or parent‐rated subscore for symptoms of hyperactivity and inattention in the Strengths and Difficulties Questionnaire (SDQ) 52. The score ranges between 0 (no problems) and 10 (serious problems), and was dichotomized at a cutoff value of 7 points for both the self‐rated and the parent‐rated subscore as a measure of abnormality in line with SDQ standard original three‐band categorization (http://www.sdqinfo.com/py/sdqinfo/c0.py). The SDQ is commonly used to assess hyperactivity and inattention in population‐based studies of ADHD in children and youth 40, 53, 54.
Peer problems
Peer problems were measured with both the SDQ and the Olweus Bully Victim Questionnaire 55. The peer problem self‐rating and parent‐rating scale from the SDQ range between 0 (no problems) and 10 points (serious problems). The scale was dichotomized at a cutoff value of 6 points for self‐rated peer problems, and at a cutoff value of 4 points for parent‐rated peer problems (in line with SDQ standard). The Olweus Bully Victim Questionnaire ranges from 1 (not been bullied) to 5 (bullied several times a week during the last months). The scale was dichotomized at a cutoff value of 3 points (2–3 times/month or more). Bullying others was measured with the same questionnaire, and ranged from 1 (not bullied others) to 5 (bullied others several times a week during the last months), and was dichotomized at a cutoff value of 3 points (2–3 times/month or more) 55. Peer problems was defined as being present if the parent or youth (or both) scored above cutoff level on at least one of these scales.
School problems
School problems were measured through self‐reported truancy in the Self‐reported Delinquency scale 56, 57, 58, 59 that ranges from 1 (no absence) to 5 (illegal absence from school more than 10 times in total). The scale was dichotomized at a cutoff value of 4 points, meaning that school problems were regarded as present for those who had reported six or more times of illegal absence.
Internalizing problems
Internalizing problems were assessed through self‐rated and parent‐rated scores for emotional symptoms from SDQ, ranging between 0 (no problems) and 10 (serious problems). The scale was dichotomized at a cutoff value of 7 for self‐ratings and 5 for parent ratings (in line with SDQ standard). Internalizing problems (anxiety, depression, psychosomatic symptoms) were defined as present if the scores were above the cutoff level on self and/or parent ratings. The SDQ has been confirmed as a useful screening tool for boys and girls and across age groups 54.
Antisocial behaviour
Antisocial behaviour was defined as being above cutoff level on at least one of the following: (i) Conduct Problems, measured through SDQ Conduct Problems, ranging between 0 (no problems) and 10 (serious problems), and dichotomized at a cutoff value of 5 for self‐rated and 4 for parent‐rated scores (in line with SDQ standard). (ii) Non‐violent Criminality, including acts as vandalism, graffiti, fire, car theft, driving without a license, forgery of ID, theft, breaking into private property, and supplying illicit substances. This was measured through the Self‐Reported Delinquency scale ranging from 0 (never) to 4 (more than 10 times), and was dichotomized at a cutoff value of 1 point (i.e. at least one non‐violent criminal act). (iii) Any Violent act, including acts of robbery, hurting animals, hurting a human, or having sex with someone against her/his will with or without physical violence. This was measured through the Self‐Reported Delinquency scale, ranging from 0 (never) to 4 (more than 10 times) points, and was dichotomized at a cutoff value of 1 point (i.e. at least one violent criminal act). Thus, this outcome was regarded as present if (i) the parent's SDQ‐score was at least 4, or (ii) the youth's SDQ score was at least 5 or the youth reported one non‐violent or violent act.
Alcohol misuse
We defined alcohol misuse in the same manner as The Swedish Council for Information on Alcohol and Other Drugs (CAN) 60, thus making it comparable with data from their annual Swedish national surveys with 15‐year‐olds. This definition focuses on binge drinking, severity, and regularity of alcohol intake. Alcohol misuse was assessed using the substance abuse self‐reported alcohol and drug use measure 56, 57, 58, 59. It was defined as being above cutoff on at least one of the following: (i) Alcohol intoxication, measured through the question ‘How often do you feel drunk when you drink alcohol?’, and ranging from 1 (‘I don't drink’) to 6 (‘every time’), and dichotomized at a cutoff value of 3 points (‘seldom’). (ii) Alcohol consumption during the last month, measured through the question ‘Have you been drinking beer, wine or liquor during the last month?’, and ranging from 1 (‘never tried’) to 4 (‘drinking last month’), and dichotomized at a cutoff value of 4 (‘drinking last month’).
Drug misuse
Drug misuse was defined in the same manner as CAN 60, focusing on drug use as a risk behaviour rather than a diagnosis. Drug misuse was assessed using the substance abuse self‐reported alcohol and drug use measure 56, 57, 58, 59. Drug misuse was defined as the self‐reported use of at least one illicit drug.
Statistical analyses
Prevalence of the seven outcomes in adolescence is presented for each childhood ADHD symptom level (i.e. screen‐negative, screen‐intermediate, screen‐positive ADHD) for the whole cohort, and separately for 9‐ and 12‐year‐olds, and for boys and girls. The crude association between ADHD symptom level at baseline and each of the seven outcomes at follow‐up was modeled with logistic regression, including the interaction between gender and ADHD. We also performed analyses where we adjusted for other baseline comorbid neurodevelopmental problems (NDP) and non‐NDP screen‐diagnoses (NDPs included autism spectrum disorder, learning disorder, tic disorder, and developmental coordination disorder, and non‐NDPs included oppositional defiance disorder, conduct disorder, obsessive–compulsive disorder, and eating disorder). All regression models included ADHD as a numeric predictor (0 = screen‐negative, 1 = screen‐intermediate, 2 = screen‐positive), gender (0 = boy, 1 = girl), as well as the interaction term between ADHD and gender. In sensitivity analyses, prevalence rates of six outcomes in adolescence are presented by ADHD symptom level at age 15 (i.e. yes/no). Furthermore, cross‐sectional associations between ADHD symptom level at age 15 and the six outcomes in adolescence were modeled with logistic regression, including the interaction between gender and ADHD. Both crude and adjusted (for NDPs) associations are presented.
In the present study, the data were used as population data and not analyzed in a twin‐analysis model. However, a cluster robust sandwich estimator was used to adjust for the within twin‐pair dependence when calculating confidence intervals (CI) and P‐values. The estimated associations were reported as odds ratios, and separately for girls and boys. All P‐values < 0.05 were considered statistically significant. The software package stata 13 was used in all analyses (StataCorp LP4905, College Station, TX, USA).
Ethical considerations
For the CATSS 9/12 study, a written consent from the parent/legal guardian was collected, and for the CATSS‐15/DOGSS study, both teenager and parent/legal guardian provided written consent. Analyses were performed on anonymized data files. The study protocol accorded with the Helsinki declaration and was approved by the ethical review board of Karolinska Institutet, Solna, Sweden (Registration numbers: Dnr: 02‐289 and 2010/507‐31/1 DOGSS, Dnr 03‐672 and 2010/507‐31/1, CATSS‐9 – clinical 2010/1099‐31/3 CATSS‐15 Dnr: 2009/1599‐32/5, CATSS‐15/DOGSS Dnr: 03‐672 and 2010/1356/31/1).
Results
Table 1 shows the prevalence of ADHD symptom levels at baseline. Of the participants, 91.0% (4216) were screen‐negative, 4.0% (183) were screen‐intermediate, and 4.9% (228) were screen‐positive for ADHD at age 9 or 12. Among 9‐year‐olds, 91.8% (2429) were screen‐negative, 3.9% (102) were screen‐intermediate, and 4.1% (109) were screen‐positive. Among 12‐year‐olds, 89.8% (1787) were screen‐negative, 4.1% 61 were screen‐intermediate, and 6.0% (119) were screen‐positive. Boys displayed higher symptom levels than girls at all ages.
Table 1.
Parent reports of symptoms at baseline: Numbers of boys and girls who were screen‐negative, screen‐intermediate, or screen‐positive for attention deficit hyperactivity disorder (ADHD) according to A‐TAC
| A‐TAC | Boys (%) | Girls (%) | 9‐year‐olds (%) | 12‐year‐olds (%) | Total (%) |
|---|---|---|---|---|---|
| Total | 2252 (100) | 2383 (100) | 2645 (100) | 1990 (100) | 4635 (100) |
| Screen‐negative | 1983 (88.1) | 2233 (93.7) | 2429 (91.8) | 1787 (89.8) | 4216 (91.0) |
| Screen‐intermediate | 117 (5.2) | 66 (2.8) | 102 (3.9) | 81 (4.1) | 183 (4.0) |
| Screen‐positive | 149 (6.6) | 79 (3.3) | 109 (4.1) | 119 (6.0) | 228 (4.9) |
| Missing | 3 (0.1) | 5 (0.2) | 5 (0.2) | 3 (0.2) | 8 (0.2) |
| 9‐year‐old boys | 9‐year‐old girls | 12‐year‐old boys | 12‐year‐old girls | Total | |
| Total | 1300 (100) | 1345 (100) | 952 (100) | 1038 (100) | 4635 (100) |
| Screen‐negative | 1172 (90.2) | 1257 (93.5) | 811 (85.2) | 976 (94.0) | 4216 (91.0) |
| Screen‐intermediate | 60 (4.6) | 42 (3.1) | 57 (6.0) | 24 (2.3) | 183 (4.0) |
| Screen‐positive | 67 (5.2) | 42 (3.1) | 82 (8.6) | 37 (3.6) | 228 (4.9) |
| Missing | 1 (0.1) | 4 (0.3) | 2 (0.2) | 1 (0.1) | 8 (0.2) |
| Screen‐negative | A‐TAC: <6 | No ADHD | |||
| Screen‐intermediate | A‐TAC: 6–7.5 | Subthreshold ADHD | |||
| Screen‐positive | A‐TAC: ≥8 | ADHD |
A‐TAC, Autism‐Tics, ADHD, and other Comorbidities Inventory.
Missing = A‐TAC is not complete at 9/12.
Prevalence of adolescent outcomes for all childhood ADHD symptom levels is provided for boys and girls, respectively, in Table 2. Among those who had been screen‐positive in childhood, about 40% still displayed symptoms of hyperactivity/inattention above cutoff at adolescence. In boys as well as in girls, it was more common that those who had been screen‐intermediate or screen‐positive at baseline reported hyperactivity/inattention above cutoff level at age 15, but previously screen‐negative boys (9%) and girls (9.1%) also displayed hyperactivity/inattention symptoms above cutoff.
Table 2.
The prevalence of the seven outcomes by attention deficit hyperactivity disorder (ADHD) levels and sex
| ADHD screening levels at 9/12 | Hyperactivity/inattention (sra + prb) % | Peer problems (sra + prb) % | School problems truancy (sra) % | Internalizing problems (sra + prb) % | Antisocial behaviour (sra + prb) % | Alcohol misuse (sra) % | Drug misuse (sra) % | No problem % |
|---|---|---|---|---|---|---|---|---|
| Boys | ||||||||
|
Screen‐negative ADHDc
n = 1983 |
9.0 | 18.7 | 6.7 | 8.9 | 32.8 | 24.0 | 11.3 | 39.3 |
|
Screen‐intermediate ADHDd
n = 117 |
27.4 | 27.4 | 16.2 | 8.6 | 41.9 | 29.1 | 8.6 | 24.8 |
|
Screen‐positive ADHDe
n = 149 |
38.9 | 36.9 | 16.8 | 14.8 | 50.3 | 34.2 | 11.4 | 17.5 |
| Girls | ||||||||
|
Screen‐negative ADHDc
n = 2233 |
9.1 | 16.1 | 7.1 | 29.8 | 22.2 | 31.4 | 9.7 | 34.7 |
|
Screen‐intermediate ADHDd
n = 66 |
31.8 | 36.4 | 15.2 | 48.5 | 33.3 | 33.3 | 18.8 | 10.6 |
|
Screen‐positive ADHDe
n = 79 |
38.0 | 41.8 | 21.5 | 44.3 | 53.2 | 34.2 | 20.3 | 16.5 |
A‐TAC, Autism‐Tics, ADHD, and other Comorbidities Inventory.
Baseline: CATSS‐9/12 study (N = screen‐negative, screen‐intermediate, and screen‐positive in A‐TAC for diagnosis of ADHD).
Follow‐up: Psychosocial outcomes at age 15, parent‐ and self‐reports, CATSS‐15/DOGSS.
Frequencies do not equal 100% as the same individual may have several outcomes.
sr: Self‐rated Strengths and Difficulties Questionnaire (SDQ) (five questions; cutoff according to ‘abnormality’).
pr: Parent‐rated SDQ (five questions; cutoff according to ‘abnormality’).
Screen‐negative ADHD according to A‐TAC: <6p.
Screen‐intermediate ADHD according to A‐TAC: 6p–7.5p.
Screen‐positive ADHD according to A‐TAC: ≥8p.
Among boys, increasing levels of baseline ADHD symptoms were associated with higher proportions of boys who reached cutoff for most outcomes. Screen‐positive boys reported similar levels of drug use as screen‐negative boys, except for school problems/truancy. Among girls, increasing levels of ADHD symptoms were associated with higher proportions of all outcomes, except for internalizing problems and alcohol misuse. Screen‐intermediate girls presented with the highest proportion of internalizing problems (screen‐positive 29.8% vs. screen‐intermediate 48.5% vs. screen‐negative 44.3%). For alcohol misuse, all ADHD symptom levels presented similar proportions (screen‐positive 31.4% vs. screen‐intermediate 33.3% vs. screen‐negative 34.2%). When comparing the screen‐positive group across genders, girls showed higher proportions of all outcomes except for alcohol misuse (screen‐positive girls 34.2% vs. screen‐positive boys 34.2%) and hyperactivity/inattention (screen‐positive girls 38.0% vs. screen‐positive boys 38.9%) as compared to their counterpart boys.
Table 3 presents crude and adjusted odds ratios (ORs) comparing ADHD levels with all outcomes by gender. Higher levels of ADHD symptoms were associated with higher odds of all outcomes in both genders, except for alcohol misuse in girls, and drug misuse in boys. For the following two outcomes, the associations were significantly higher in girls than in boys; antisocial behaviour (OR: 1.98, 95% CI: 1.58–2.47 for girls vs. OR: 1.45, 95% CI: 1.24–1.71 for boys) and drug misuse (OR: 1.64, 95% CI: 1.25–2.14 for girls vs. OR: 0.99, CI: 0.75–1.29 for boys).
Table 3.
Odds ratios for all psychosocial outcomes at age 15, crude and adjusted, by sex, OR (95% CI)
| Hyperactivity/inattention | Peer problems | School problems (truancy) | Internalizing problems | Antisocial behaviour | Alcohol misuse | Drug misuse | |
|---|---|---|---|---|---|---|---|
| Boys | |||||||
| One level increase in ADHD level, crude | 2.71*** (2.25–3.25) | 1.61*** (1.35–1.91) | 1.78*** (1.43–2.23) | 1.29* (1.02–1.64) | 1.45*** + (1.24–1.71) | 1.29** (1.08–1.53) | 0.99 ++ (0.75–1.29) |
| One level increase in ADHD level, adjusted | 2.51*** (2.03–3.12) | 1.07 (0.86–1.33) | 1.68*** (1.28–2.21) | 1.19 (0.91–1.56) | 1.40*** (1.16–1.68) | 1.41** (1.16–1.71) | 0.89 ++ (0.66–1.20) |
| Girls | |||||||
| One level increase in ADHD level, crude | 2.73*** (2.15–3.46) | 2.08*** (1.66–2.61) | 2.05*** (1.57–2.68) | 1.50*** (1.20–1.87) | 1.98*** + (1.58–2.47) | 1.07 (0.86–1.33) | 1.64*** ++ (1.25–2.14) |
| One level increase in ADHD level, adjusted | 2.50*** (1.89–3.29) | 1.47** (1.13–1.92) | 1.90*** (1.38–2.63) | 1.39** (1.10–1.77) | 1.86*** (1.45–2.39) | 1.14 (0.90–1.44) | 1.52** ++ (1.14–2.03) |
A‐TAC, Autism‐Tics, ADHD, and other Comorbidities Inventory; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; CD, conduct disorder; DCD, developmental coordination disorder; ED, eating disorder; LD, learning disorder; NDP, neurodevelopmental problem; OCD, obsessive–compulsive disorder; ODD, oppositional defiance disorder; TD, tic disorder.
+ slope difference between sexes: P < 0.05; ++ slope difference between sexes: P < 0.01.
ADHD levels: screen‐negative – screen‐intermediate – screen‐positive in A‐TAC, at baseline.
Adjusted for other baseline screen‐diagnoses: NDPs (ASD, LD, TD, DCD) and non‐NDPs (ODD, CD, OCD, ED).
*P < 0.05; **P < 0.01; ***P < 0.001.
Even after adjusting for baseline comorbidity, increasing levels of ADHD symptoms were associated with higher odds for all outcomes in girls, except for alcohol misuse. For boys, adjusted ORs showed that increasing levels of ADHD symptoms were associated with higher odds for all outcomes except peer and internalizing problems. For drug misuse, the increase remained significantly higher in girls than in boys (OR: 1.52, 95% CI: 1.14–2.03).
Figure 1 shows the estimated probability and 95% CIs for all outcomes over levels of ADHD symptoms in both boys and girls. After adjusting for baseline comorbidity, increasing levels of ADHD symptoms were associated with a higher probability for all outcomes in both genders, except for alcohol misuse in girls and for drug misuse in boys.
Figure 1.
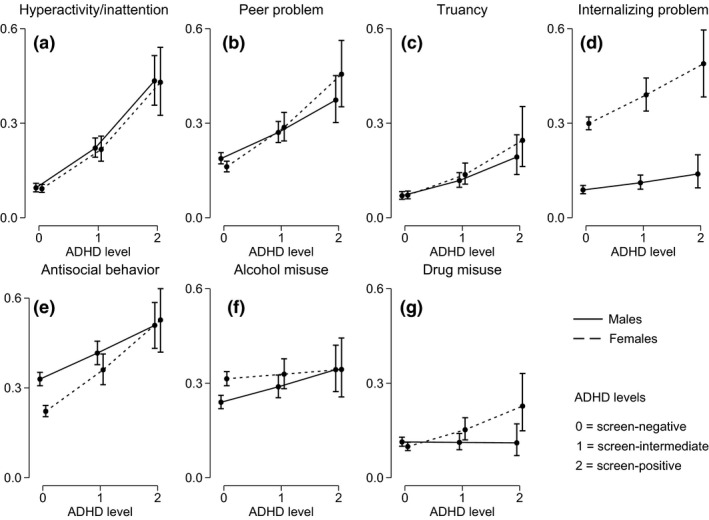
Estimated probability (dots) and 95% confidence intervals (capped lines) of: Hyperactivity/inattention (a), peer problem (b), school problem (c), internalizing problem (d), antisocial behaviour (e), alcohol misuse (f), and drug misuse (g), over levels of attention deficit hyperactivity disorder (ADHD) symptoms in males (solid line) and females (dashed line).
Sensitivity analyses
Table S1 shows prevalence rates of six adolescent outcomes by ADHD symptom level at age 15 (i.e. yes/no), presented for boys and girls separately. Among the 264 boys above cutoff for ADHD symptoms at age 15, between 17.8% and 54.2% displayed peer problems, school problems, internalizing problems, antisocial behaviour, alcohol misuse, and/or drug misuse. None of the boys above cutoff for ADHD symptoms reported no problem in adolescence. Among boys below cutoff for ADHD symptoms at age 15, between 5.8% and 31.8% presented with a problem. Furthermore, 41.7% of the boys below cutoff for ADHD symptoms reported no problem in adolescence. Girls displayed similar patterns; the 242 girls above cutoff for ADHD symptoms presented higher prevalence rates of all problems (between 17.8% and 54.1%) than girls below cutoff for ADHD symptoms (between 6.0% and 29.1%). None of the girls above cutoff for ADHD symptoms at age 15 reported no problem in adolescence, while 37.0% of the girls below cutoff for ADHD symptoms reported no problem in adolescence.
Table S2 presents crude and adjusted odds ratios (ORs) comparing ADHD symptom level at age 15 (i.e. yes/no) with six outcomes, presented by gender. Results showed that being above cutoff for ADHD symptoms at age 15 was associated with significantly higher odds of all outcomes in both genders. Results remained significant for boys after adjusting for baseline NDP comorbidity. However, for girls, the increased odds of peer problems was no longer significant.
Discussion
The present study aimed to assess sex differences in the prevalence of psychosocial problems in adolescence in a population‐based sample, and to examine whether ADHD symptom levels (screen‐negative, screen‐intermediate, screen‐positive) were differentially associated with the psychosocial problems in boys and girls. First, higher levels of ADHD symptoms in childhood were associated with more ADHD symptoms during adolescence. This finding was expected as the A‐TAC interview has shown good predictive validity for ADHD in earlier studies 62, 63. Still, less than half (38.9% and 38% of boys and girls respectively) of the previously screen‐positive and even fewer (27.4% and 31.8%) of the screen‐intermediate displayed symptoms of hyperactivity/inattention over cutoff at age 15. Also, among the previously screen‐negative, 9% of boys and 9.1% of girls reached over cutoff level for these symptoms. These findings, that is, that core ADHD symptoms may change with development, are in line with several recent population‐based studies 40, 41, 42, which all found quite low degrees (5–21%) of overlap between childhood and young adult/adult ADHD. Such results may be due to measurement methods as well as to manifest change in symptom levels. Although the SDQ, which was used in this study to assess adolescent symptoms, is to be regarded as a screening tool, it has been confirmed as a reliable estimate for assessing ADHD symptoms 54.
Second, increasing levels of ADHD symptoms during childhood were associated with an increasing risk of psychosocial problems in adolescence for all of the other six outcomes, with the exception of alcohol misuse in girls and drug misuse in boys (where we saw no differences associated with different childhood ADHD symptom levels). Similarly, being above cutoff on ADHD symptom level at age 15 was associated with increased risk of concurrent psychosocial problems in all of the other six areas among boys. Girls presented similar results with the exception of peer problems, where no significant differences were found between girls above and below cutoff on ADHD symptoms. These results suggest that concurrent ADHD symptoms also play a role in the existence of adolescent psychosocial problems. This is in line with previous longitudinal research showing that both childhoood and adolescent ADHD symptoms independently increase the risk of adolescent psychosocial adversity 64.
Third, more girls reported internalizing problems than boys at all ADHD symptom levels. A higher proportion of screen‐positive girls also displayed risky drug use as compared to boys. The screen‐intermediate groups of both genders more commonly reported above cutoff symptom levels for several of the outcomes as compared with screen‐negatives of the same sex. For drug misuse, the increase in number of girls who reached cutoff was significantly steeper than for boys.
Sex differences in the association between ADHD symptoms and psychosocial outcomes
Earlier studies of clinical populations of ADHD have repeatedly shown gender differences in outcomes 17, 65, 66. Some indicate that girls with ADHD can have severe psychiatric comorbidities and low global functioning 67, 68, 69, 70, while others have shown that girls can be less impaired than boys 5. However, the majority of these studies are based on clinic‐referred children, and it has been pointed out that there is a need for longitudinal studies based on community‐recruited samples 65. This population‐based study cohort supports the existence of gender differences in psychosocial outcomes of ADHD, even after controlling for symptoms of other neurodevelopmental disorders. However, previous studies have concluded that when ADHD subtype is taken into account, the subtype (i.e. hyperactive/impulsive, inattentive or combined) may have more impact than gender on the phenomenology of ADHD 22, 28. We did not have information on diagnostic status, subtype, healthcare contacts and/or medication use, and therefore, comparisons between our findings and case‐ascertained populations cannot be made. However, our findings are similar to a population‐based twin study by Levy et al. from Australia 25, with regard to the higher levels of drug misuse and internalizing symptoms among girls with ADHD. The combined subtype was more strongly associated with comorbid disorders overall in the Levy study. Thus, these authors also suggest that the severity of ADHD symptoms could play a more important role for outcome than gender, if one assumes that the combined subtype is a more severe form of ADHD. However, given that symptom profile in ADHD may change during development 40, 41, 42, 71, 72 and that the age of inclusion in a study thus may affect subtyping, one advantage with the used study design is that it adopts a symptom trajectory design in combination with a developmental outcome perspective 73.
Overall sex difference in internalizing problems
More girls in all baseline categories presented with higher proportions of internalizing problems as teenagers. Population‐based surveys have shown that Swedish youth report average levels of mental health problems in comparison with youths in other industrialized countries, but that Swedish girls generally report higher levels of emotional and internalizing problems than Swedish boys 74. With higher base‐rate levels of emotional problems among girls in the general Swedish population 74, the associations between ADHD and internalizing/emotional problems reported here could to some extent be explained by covariation. However, higher rates of internalizing and/or emotional problems among girls with ADHD have been commonly reported in both clinical and population‐ or community‐based studies 8, 16, 19, 29, 46, 75, 76, 77, 78, 79, 80, 81. Thus, ADHD symptoms seem to contribute to the total internalizing problem load in a gender‐specific manner 61. This conclusion is also supported by our study findings, as the association between internalizing problems and ADHD symptoms in this study was no longer significant for boys when comorbidity was taken into account, while it remained significant for girls.
Sex differences with regard to substance misuse
Two meta‐analyses, by Charach and Lee 82, 83, have concluded that children diagnosed with ADHD have a higher risk for developing substance use disorders than children without ADHD. The results from this study support previous findings, but in contrast to the Lee study, which could not identify any significant gender interaction effects, our data supports the existence of a sex difference. Further, alcohol as well as drug use vary between youth cultures. According to the yearly Swedish national surveys on drug and alcohol use in 15‐year‐olds, 43% of the boys and 50% of the girls reported that they had been drinking alcohol during the past 12 months 60. Thus, alcohol use may be more prevalent among Swedish girls than boys, which may explain the lack of association between ADHD and alcohol misuse in girls. In our dataset, drug misuse was the only outcome where the increase for each ADHD symptom level in girls was significantly higher, not only in relation to less symptomatic girls, but also in comparison with increasing ADHD levels in boys. The finding of higher risk of drug misuse among girls is in line with previous follow‐up studies of clinical ADHD samples 18, 34, 80 as well as population‐based ADHD samples 21, which have shown that girls with ADHD have a higher risk for substance abuse and dependence than girls without ADHD, and also compared to boys with ADHD. The finding that childhood ADHD symptoms were associated with higher drug misuse in girls motivates particular attention and active screening routines for this group.
Screen‐intermediate ADHD
Our findings also support previous studies that have found associations between subthreshold levels of ADHD and negative outcomes 26, 31, 37, 38, 45, 46, 84. In this study, more boys and girls at the screen‐intermediate level displayed psychosocial problems than did their screen‐negative peers. For some outcomes [such as school problems (boys), drug misuse (girls), and internalizing problems (girls)], the screen‐intermediate group displayed almost as much problems as their screen‐positive peers. It has been suggested that it is imperative to consider the subthreshold level of ADHD symptoms as a potential risk factor for maladjustment (for a review, see Ref. 38). With support systems based only on diagnostic categories 31, children with subthreshold symptom levels may not get access to such support to the degree that is warranted, thus placing them at risk of negative consequences later in life. Another argument for also considering subthreshold ADHD levels is the findings from the Shankman study, which demonstrated that a majority of children with subthreshold psychiatric conditions were at high risk of developing the equivalent full psychiatric syndrome in adulthood 45. The results from our study support the view that ADHD symptoms, as well as their associated negative outcomes, are dimensionally distributed in the population and that girls and boys display different risk profiles 25, 31. Both of these findings are to be expected and may, from one point of view, be considered trivial. The dimensional distribution of mental health symptoms in populations is the actual reason why there are thresholds for psychiatric diagnoses, where we need to consider, for example, the duration of symptoms and/or the degree of impairment they cause. However, the case with ADHD is different and calls for additional consideration. In a recent article published in the Lancet 85, the authors emphasize that ADHD behaves dimensionally but that there is ‘no distinct threshold at which adverse outcomes appear’ (p. 1246), and that many of the negative outcomes associated with ADHD also extend to those with subthreshold symptoms. The need for considering subthreshold ADHD in clinical practice has also been emphasized 43. Finally, our findings support the view that there is a causal link between childhood ADHD and later adverse outcomes, in that increasing childhood symptom levels were associated with increasing numbers of adolescents who reported negative psychosocial problems.
A limitation of the present study is that our results only include cases where the parent (at baseline) as well as both the child and parent (at follow‐up) participated. The overall response rate at baseline was 77.0% and at follow‐up 44.1%. If we had included only the child's report at age 15, the response rate would have been 50.0%, and if we had included only the parent's report, the response rate would have been 49.1%. However, the added information from both sources was considered to be more valuable to our study, thus leaving us with the 44.1% response rate.
Previous attrition analyses of differences between non‐responders and responders in the CATSS study, based on data from approximately 11 000 twins, have indicated that CATSS non‐responders more often belong to lower socioeconomic groups 47. Hypothetically, a family burdened with psychosocial problems may be less motivated to participate, which could explain some of the attrition. Thus, it may be that our sample included less burdened families, and the results need to be interpreted with this possible bias in mind.
Another possible bias is that we use data from ages 9 and 12 at baseline. Twelve‐year‐olds are on the verge of puberty and may possibly express more of an adolescent behavioural pattern. However, age differences in ADHD level at baseline were small, and possible age effects would thus be small.
Further, the generalizability from a twin study might be questioned, but previous research has suggested that twins are representative of the population at large 86, 87, and that MZ and DZ twins are similar in personality variation 88.
Implications
The study confirms previous findings from follow‐ups of clinical populations and illustrates the need to consider gender‐specific risks for negative development during adolescence among children with ADHD symptoms. Also, the findings emphasize the need to consider ADHD symptoms along a continuum rather than only in cases above the diagnostic level.
Declarations of interest
All authors declare that they have no conflicts of interest.
Supporting information
Table S1. The prevalence of six outcomes by ADHD at 15 and sex.
Table S2. Odds ratios for psychosocial outcomes at age 15, crude and adjusted, by sex, OR (95% CI).
Acknowledgements
This study was supported by grants from the Swedish Research Council and the Hedlund Foundation. The study funders had no role in the design, collection, analysis, or interpretation of the data, or in the writing or decision to submit the manuscript.
Norén Selinus E, Molero Y, Lichtenstein P, Anckarsäter H, Lundström S, Bottai M, Hellner Gumpert C. Subthreshold and threshold attention deficit hyperactivity disorder symptoms in childhood: psychosocial outcomes in adolescence in boys and girls.
References
- 1. Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 2. Kessler RC, Adler L, Barkley R et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942–948. [DOI] [PubMed] [Google Scholar]
- 4. Barkley RA. Major life activity and health outcomes associated with attention‐deficit/hyperactivity disorder. J Clin Psychiatry 2002;63(Suppl. 12):10–15. [PubMed] [Google Scholar]
- 5. Newcorn JH, Halperin JM, Jensen PS et al. Symptom profiles in children with ADHD: effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry 2001;40:137–146. [DOI] [PubMed] [Google Scholar]
- 6. APA . DSM‐IV‐TR. Washington, DC: American Psychiatric Association, 2000. xii, 370 p. [Google Scholar]
- 7. APA . Diagnostic and statistical manual of mental disorders, 5th edn Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 8. Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10‐year follow‐up study of boys with ADHD. Psychiatry Res 2010;177:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elkins IJ, McGue M, Iacono WG. Prospective effects of attention‐deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry 2007;64:1145–1152. [DOI] [PubMed] [Google Scholar]
- 10. Faraone SV. Attention‐deficit hyperactivity disorder and the shifting sands of psychiatric nosology. Br J Psychiatry 2013;203:81–83. [DOI] [PubMed] [Google Scholar]
- 11. Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol 2007;32:643–654. [DOI] [PubMed] [Google Scholar]
- 12. Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol 2003;112:497–507. [DOI] [PubMed] [Google Scholar]
- 13. Zulauf CA, Sprich SE, Safren SA, Wilens TE. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep 2014;16:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molina BS, Hinshaw SP, Eugene Arnold L et al. Adolescent substance use in the multimodal treatment study of attention‐deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry 2013;52:250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M. Functional impairments in adults with self‐reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry 2006;67:524–540. [DOI] [PubMed] [Google Scholar]
- 16. Bauermeister JJ, Shrout PE, Chavez L et al. ADHD and gender: are risks and sequela of ADHD the same for boys and girls? J Child Psychol Psychiatry 2007;48:831–839. [DOI] [PubMed] [Google Scholar]
- 17. Biederman J, Mick E, Faraone SV et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002;159:36–42. [DOI] [PubMed] [Google Scholar]
- 18. Biederman J, Monuteaux MC, Mick E et al. Psychopathology in females with attention‐deficit/hyperactivity disorder: a controlled, five‐year prospective study. Biol Psychiatry 2006;60:1098–1105. [DOI] [PubMed] [Google Scholar]
- 19. Biederman J, Ball SW, Monuteaux MC et al. New insights into the comorbidity between ADHD and major depression in adolescent and young adult females. J Am Acad Child Adolesc Psychiatry 2008;47:426–434. [DOI] [PubMed] [Google Scholar]
- 20. Biederman J, Petty CR, O'Connor KB, Hyder LL, Faraone SV. Predictors of persistence in girls with attention deficit hyperactivity disorder: results from an 11‐year controlled follow‐up study. Acta Psychiatr Scand 2012;125:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry 1999;156:1515–1521. [DOI] [PubMed] [Google Scholar]
- 22. Gross‐Tsur V, Goldzweig G, Landau YE, Berger I, Shmueli D, Shalev RS. The impact of sex and subtypes on cognitive and psychosocial aspects of ADHD. Dev Med Child Neurol 2006;48:901–905. [DOI] [PubMed] [Google Scholar]
- 23. Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow‐up of girls with attention‐deficit/hyperactivity disorder into adolescence: evidence for continuing cross‐domain impairment. J Consult Clin Psychol 2006;74:489–499. [DOI] [PubMed] [Google Scholar]
- 24. Lahey BB, Hartung CM, Loney J, Pelham WE, Chronis AM, Lee SS. Are there sex differences in the predictive validity of DSM‐IV ADHD among younger children? J Clin Child Adolesc Psychol 2007;36:113–126. [DOI] [PubMed] [Google Scholar]
- 25. Levy F, Hay DA, Bennett KS, McStephen M. Gender differences in ADHD subtype comorbidity. J Am Acad Child Adolesc Psychiatry 2005;44:368–376. [DOI] [PubMed] [Google Scholar]
- 26. Mick E, Byrne D, Fried R, Monuteaux M, Faraone SV, Biederman J. Predictors of ADHD persistence in girls at 5‐year follow‐up. J Atten Disord 2011;15:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nussbaum NL. ADHD and female specific concerns: a review of the literature and clinical implications. J Atten Disord 2012;16:87–100. [DOI] [PubMed] [Google Scholar]
- 28. Rucklidge JJ. Gender differences in attention‐deficit/hyperactivity disorder. Psychiatr Clin North Am 2010;33:357–373. [DOI] [PubMed] [Google Scholar]
- 29. Yoshimasu K, Barbaresi WJ, Colligan RC et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population‐based birth cohort study. J Child Psychol Psychiatry 2012;53:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Washbrook E, Propper C, Sayal K. Pre‐school hyperactivity/attention problems and educational outcomes in adolescence: prospective longitudinal study. Br J Psychiatry 2013;203:265–271. [DOI] [PubMed] [Google Scholar]
- 31. Bussing R, Mason DM, Bell L, Porter P, Garvan C. Adolescent outcomes of childhood attention‐deficit/hyperactivity disorder in a diverse community sample. J Am Acad Child Adolesc Psychiatry 2010;49:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biederman J, Faraone SV, Spencer T, Wilens T, Mick E, Lapey KA. Gender differences in a sample of adults with attention deficit hyperactivity disorder. Psychiatry Res 1994;53:13–29. [DOI] [PubMed] [Google Scholar]
- 33. Staller J, Faraone SV. Attention‐deficit hyperactivity disorder in girls: epidemiology and management. CNS Drugs 2006;20:107–123. [DOI] [PubMed] [Google Scholar]
- 34. Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood – a naturalistic long‐term follow‐up study. Addict Behav 2014;39:325–328. [DOI] [PubMed] [Google Scholar]
- 35. Biederman J, Kwon A, Aleardi M et al. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am J Psychiatry 2005;162:1083–1089. [DOI] [PubMed] [Google Scholar]
- 36. Marcus DK, Barry TD. Does attention‐deficit/hyperactivity disorder have a dimensional latent structure? A taxometric analysis. J Abnorm Psychol 2011;120:427–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong SB, Dwyer D, Kim JW et al. Subthreshold attention‐deficit/hyperactivity disorder is associated with functional impairments across domains: a comprehensive analysis in a large‐scale community study. Eur Child Adolesc Psychiatry 2014;23:627–636. [DOI] [PubMed] [Google Scholar]
- 38. Balazs J, Kereszteny A. Subthreshold attention deficit hyperactivity in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry 2014;23:393–408. [DOI] [PubMed] [Google Scholar]
- 39. Faraone SV, Biederman J, Mick E. The age‐dependent decline of attention deficit hyperactivity disorder: a meta‐analysis of follow‐up studies. Psychol Med 2006;36:159–165. [DOI] [PubMed] [Google Scholar]
- 40. Caye A, Rocha TB, Anselmi L et al. Attention‐deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late‐onset syndrome. JAMA Psychiatry 2016;73:705–712. [DOI] [PubMed] [Google Scholar]
- 41. Moffitt TE, Houts R, Asherson P et al. Is adult ADHD a childhood‐onset neurodevelopmental disorder? Evidence from a Four‐Decade Longitudinal Cohort Study. Am J Psychiatry 2015;172:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agnew‐Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, Arseneault L. Evaluation of the persistence, remission, and emergence of attention‐deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry 2016;73:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faraone SV, Biederman J. Can attention‐deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry 2016;73:655–656. [DOI] [PubMed] [Google Scholar]
- 44. Ek U, Westerlund J, Holmberg K, Fernell E. Self‐esteem in children with attention and/or learning deficits: the importance of gender. Acta Paediatr 2008;97:1125–1130. [DOI] [PubMed] [Google Scholar]
- 45. Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, Altman SE. Subthreshold conditions as precursors for full syndrome disorders: a 15‐year longitudinal study of multiple diagnostic classes. J Child Psychol Psychiatry 2009;50:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malmberg K, Edbom T, Wargelius HL, Larsson JO. Psychiatric problems associated with subthreshold ADHD and disruptive behaviour diagnoses in teenagers. Acta Paediatr 2011;100:1468–1475. [DOI] [PubMed] [Google Scholar]
- 47. Anckarsater H, Lundstrom S, Kollberg L et al. The Child and Adolescent Twin Study in Sweden (CATSS). Twin Res Hum Genet 2011;14:495–508. [DOI] [PubMed] [Google Scholar]
- 48. Hansson SL, Svanstrom Rojvall A, Rastam M, Gillberg C, Gillberg C, Anckarsater H. Psychiatric telephone interview with parents for screening of childhood autism – tics, attention‐deficit hyperactivity disorder and other comorbidities (A‐TAC): preliminary reliability and validity. Br J Psychiatry 2005;187:262–267. [DOI] [PubMed] [Google Scholar]
- 49. Larson T, Anckarsater H, Gillberg C et al. The autism–tics, AD/HD and other comorbidities inventory (A‐TAC): further validation of a telephone interview for epidemiological research. BMC Psychiatry 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cubo E, Saez Velasco S, Delgado Benito V et al. Validation of screening instruments for neuroepidemiological surveys of tic disorders. Mov Disord 2011;26:520–526. [DOI] [PubMed] [Google Scholar]
- 51. Hallerod SL, Larson T, Stahlberg O et al. The Autism‐Tics, AD/HD and other comorbidities (A‐TAC) telephone interview: convergence with the Child Behavior Checklist (CBCL). Nord J Psychiatry 2010;64:218–224. [DOI] [PubMed] [Google Scholar]
- 52. Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry 2001;40:1337–1345. [DOI] [PubMed] [Google Scholar]
- 53. Rimvall MK, Elberling H, Rask CU, Helenius D, Skovgaard AM, Jeppesen P. Predicting ADHD in school age when using the Strengths and Difficulties Questionnaire in preschool age: a longitudinal general population study, CCC2000. Eur Child Adolesc Psychiatry 2014;23:1051–1060. [DOI] [PubMed] [Google Scholar]
- 54. Niclasen J, Teasdale TW, Andersen AM, Skovgaard AM, Elberling H, Obel C. Psychometric properties of the Danish Strength and Difficulties Questionnaire: the SDQ assessed for more than 70,000 raters in four different cohorts. PLoS ONE 2012;7:e32025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Solberg ME, Olweus D. Prevalence estimation of school bullying with the Olweus Bully Victim Questionnaire. Aggress Behav 2003;29:239–268. [Google Scholar]
- 56. Elliot DS, Huizinga D, Ageton SSE. Explaining delinquency and drug use. Beverly Hills, CA: Sage, 1985. [Google Scholar]
- 57. Junger‐Tas J, Marshall IH. The self‐report methodology in crime research. Chicago: The University of Chicago, 1999. [Google Scholar]
- 58. Junger‐Tas J, Terlouw G‐J, Klein MW. Delinquent behavior among young people in the western world: first results of the international self‐report delinquency study Amsterdam: RDC ‐ Ministry of Justice, Kugler Publications, 1994:1–387. [Google Scholar]
- 59. Ring J . Stöld, våld och droger bland pojkar och flickor i årskurs nio Stockholm, Sweden: Brottsförebyggande Rådet, 2000:1–70. [Google Scholar]
- 60. CAN . Skolelevers drogvanor 2014. Stockholm, Sweden: Centralförbundet för Alkohol‐ och Narkotikaupplysning, 2014. [Google Scholar]
- 61. Baldwin JS, Dadds MR. Examining alternative explanations of the covariation of ADHD and anxiety symptoms in children: a community study. J Abnorm Child Psychol 2008;36:67–79. [DOI] [PubMed] [Google Scholar]
- 62. Larson T, Lundstrom S, Nilsson T et al. Predictive properties of the A‐TAC inventory when screening for childhood‐onset neurodevelopmental problems in a population‐based sample. BMC Psychiatry 2013;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Larson T, Kerekes N, Selinus EN et al. Reliability of Autism‐Tics, AD/HD, and other Comorbidities (A‐TAC) inventory in a test‐retest design. Psychol Rep 2014;114:93–103. [DOI] [PubMed] [Google Scholar]
- 64. Gau SS, Ni HC, Shang CY et al. Psychiatric comorbidity among children and adolescents with and without persistent attention‐deficit hyperactivity disorder. Aust N Z J Psychiatry 2010;44:135–143. [DOI] [PubMed] [Google Scholar]
- 65. Gershon J, Gershon J. A meta‐analytic review of gender differences in ADHD. J Atten Disord 2002;5:143–154. [DOI] [PubMed] [Google Scholar]
- 66. Monuteaux MC, Mick E, Faraone SV, Biederman J. The influence of sex on the course and psychiatric correlates of ADHD from childhood to adolescence: a longitudinal study. J Child Psychol Psychiatry 2010;51:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kopp S, Kelly KB, Gillberg C. Girls with social and/or attention deficits: a descriptive study of 100 clinic attenders. J Atten Disord 2010;14:167–181. [DOI] [PubMed] [Google Scholar]
- 68. Kopp S, Gillberg C. Swedish child and adolescent psychiatric out‐patients–a five‐year cohort. Eur Child Adolesc Psychiatry 2003;12:30–35. [DOI] [PubMed] [Google Scholar]
- 69. Asberg J, Kopp S, Berg‐Kelly K, Gillberg C. Reading comprehension, word decoding and spelling in girls with autism spectrum disorders (ASD) or attention‐deficit/hyperactivity disorder (AD/HD): performance and predictors. Int J Lang Commun Disord 2010;45:61–71. [DOI] [PubMed] [Google Scholar]
- 70. Asberg Johnels J, Kopp S, Gillberg C. Spelling difficulties in school‐aged girls with attention‐deficit/hyperactivity disorder: behavioral, psycholinguistic, cognitive, and graphomotor correlates. J Learn Disabil 2014;47:424–434. [DOI] [PubMed] [Google Scholar]
- 71. Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention‐deficit hyperactivity disorder in boys: a four‐year longitudinal study. J Abnorm Child Psychol 1995;23:729–749. [DOI] [PubMed] [Google Scholar]
- 72. Biederman J, Mick E, Faraone SV. Age‐dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 2000;157:816–818. [DOI] [PubMed] [Google Scholar]
- 73. Willoughby MT. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations. J Child Psychol Psychiatry 2003;44:88–106. [DOI] [PubMed] [Google Scholar]
- 74. Bremberg S, Dalman C. Begrepp, mätmetoder och förekomst av psykisk hälsa, psykisk ohälsa och psykiatriska tillstånd hos barn och unga – EN KUNSKAPSÖVERSIKT. 2015.
- 75. Chronis‐Tuscano A, Molina BS, Pelham WE et al. Very early predictors of adolescent depression and suicide attempts in children with attention‐deficit/hyperactivity disorder. Arch Gen Psychiatry 2010;67:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hinshaw SP, Owens EB, Zalecki C et al. Prospective follow‐up of girls with attention‐deficit/hyperactivity disorder into early adulthood: continuing impairment includes elevated risk for suicide attempts and self‐injury. J Consult Clin Psychol 2012;80:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Novik TS, Hervas A, Ralston SJ et al. Influence of gender on attention‐deficit/hyperactivity disorder in Europe–ADORE. Eur Child Adolesc Psychiatry 2006;15(Suppl. 1):I15–I24. [DOI] [PubMed] [Google Scholar]
- 78. Cho SC, Kim BN, Kim JW et al. Full syndrome and subthreshold attention‐deficit/hyperactivity disorder in a Korean community sample: comorbidity and temperament findings. Eur Child Adolesc Psychiatry 2009;18:447–457. [DOI] [PubMed] [Google Scholar]
- 79. Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention‐deficit/hyperactivity disorder in a large nationwide study. Attent Defic Hyperact Disord 2015;7:27–38. [DOI] [PubMed] [Google Scholar]
- 80. Biederman J, Petty CR, Monuteaux MC et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11‐year follow‐up in a longitudinal case‐control study. Am J Psychiatry 2010;167:409–417. [DOI] [PubMed] [Google Scholar]
- 81. Babinski DE, Pelham WE Jr, Molina BS et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: an exploratory investigation. J Atten Disord 2011;15:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Charach A, Yeung E, Climans T, Lillie E. Childhood attention‐deficit/hyperactivity disorder and future substance use disorders: comparative meta‐analyses. J Am Acad Child Adolesc Psychiatry 2011;50:9–21. [DOI] [PubMed] [Google Scholar]
- 83. Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention‐deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta‐analytic review. Clin Psychol Rev 2011;31:328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Faraone SV, Kunwar A, Adamson J, Biederman J. Personality traits among ADHD adults: implications of late‐onset and subthreshold diagnoses. Psychol Med 2009;39:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet 2016;387:1240–1250. [DOI] [PubMed] [Google Scholar]
- 86. Barnes JC, Boutwell BB. A demonstration of the generalizability of twin‐based research on antisocial behavior. Behav Genet 2013;43:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Evans DM, Martin NG. The validity of twin studies. GeneScreen 2000;1:77–79. [Google Scholar]
- 88. Johnson W, Krueger RF, Bouchard TJ, McGue M. The personalities of twins: just ordinary folks. Twin Res 2002;5:125–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The prevalence of six outcomes by ADHD at 15 and sex.
Table S2. Odds ratios for psychosocial outcomes at age 15, crude and adjusted, by sex, OR (95% CI).


