Abstract
Pituitary adenylate cyclase (PAC)-activating polypeptide (PACAP) peptides (Adcyap1) signaling at the selective PAC1 receptor (Adcyap1r1) participate in multiple homeostatic and stress-related responses, yet the cellular mechanisms underlying PACAP actions remain to be completely elucidated. PACAP/PAC1 receptor signaling increases excitability of neurons within the guinea pig cardiac ganglia, and as these neurons are readily accessible, this neuronal system is particularly amenable to study of PACAP modulation of ionic conductances. The present study investigated how PACAP activation of MEK/ERK signaling contributed to the peptide-induced increase in cardiac neuron excitability. Treatment with the MEK inhibitor PD 98059 blocked PACAP-stimulated phosphorylated ERK and, in parallel, suppressed the increase in cardiac neuron excitability. However, PD 98059 did not blunt the ability of PACAP to enhance two inward ionic currents, one flowing through hyperpolarization-activated nonselective cationic channels (Ih) and another flowing through low-voltage-activated calcium channels (IT), which support the peptide-induced increase in excitability. Thus a PACAP- and MEK/ERK-sensitive, voltage-dependent conductance(s), in addition to Ih and IT, modulates neuronal excitability. Despite prior work implicating PACAP downregulation of the KV4.2 potassium channel in modulation of excitability in other cells, treatment with the KV4.2 current blocker 4-aminopyridine did not replicate the PACAP-induced increase in excitability in cardiac neurons. However, cardiac neurons express the ERK target, the NaV1.7 sodium channel, and treatment with the selective NaV1.7 channel inhibitor PF-04856264 decreased the PACAP modulation of excitability. From these results, PACAP/PAC1 activation of MEK/ERK signaling may phosphorylate the NaV1.7 channel, enhancing sodium currents near the threshold, an action contributing to repetitive firing of the cardiac neurons exposed to PACAP.
Keywords: autonomic neuron, pituitary adenylate cyclase-activating polypeptide, PKA, MAPK signaling, neuronal excitability
pituitary adenylate cyclase (PAC)-activating polypeptide (PACAP) peptides (Adcyap1) are trophic and intercellular signaling molecules that are widely distributed within neural and endocrine tissues (1, 40). PACAP modulates synaptic transmission and plasticity adaptations via pre- and postsynaptic mechanisms, and its effects play critical roles in central stress challenges, regulation of sensory and autonomic function, cognitive learning, and protection from injury paradigms (6, 13, 14, 18, 22, 28, 32, 35, 40). In all, PACAP peptides have diverse roles in maintenance of physiological homeostasis. PACAP is highly expressed in the hypothalamus, hippocampus and related allocortex, limbic system, and central sensory and autonomic nuclei. The actions of PACAP are mediated through several seven-transmembrane G protein-coupled receptor subtypes, including the PACAP-selective PAC1 receptor (Adcyap1r1) and PACAP/VIP VPAC receptors (Vipr1 and Vipr2, also VPAC1 and VPAC2) (1, 2, 17, 30, 40).
We previously showed that PACAP is colocalized with acetylcholine in virtually all parasympathetic preganglionic terminals innervating neurons in guinea pig cardiac ganglia (3, 4). Furthermore, both endogenously released and exogenously applied PACAP can increase cardiac neuron excitability exclusively through PAC1 receptor activation (3, 19, 26, 35). As these cells represent a responsive and readily accessible neuronal system compared with central nervous system nuclei for experimental manipulation, we have investigated cardiac ganglia neurons as a means to better understand PACAP/PAC1 receptor-mediated modulation of second messengers and ionic conductances that contribute to the regulation of neuronal excitability.
Our laboratory showed previously that PACAP/PAC1-mediated activation of adenylyl cyclase (AC) and subsequent increase in cellular cAMP enhanced a hyperpolarization-induced nonselective cationic current (Ih), which contributed to the PACAP-induced increase in cardiac neuron excitability (27, 37). More recently, we also reported that PACAP activation of the nickel-sensitive, low-voltage-activated calcium current (IT) was also a component of the heightened excitability (38).
In primary neurons and a stable human embryonic kidney PAC1 receptor-expressing cell line, several studies showed that PACAP can potently activate MEK/ERK signaling (23, 24). Among many different functions, the MEK/ERK signaling cascade has critical roles in synaptic plasticity and regulation of neuronal excitability (33), and prior studies showed that ERK activation can modulate many different excitable cells through phosphorylation of a variety of voltage-dependent channels (9, 20, 31, 34). Accordingly, we have investigated whether PACAP stimulates MEK/ERK signaling in guinea pig cardiac neurons and whether this mechanism contributes to the PACAP-induced modulation of neuronal excitability. Our results indicate that PACAP increases cardiac neuron ERK phosphorylation and that pretreatment of cardiac ganglia whole mounts with a MEK inhibitor significantly suppresses the PACAP-induced increase in neuronal excitability. However, the MEK inhibitor did not alter the PACAP enhancement of Ih, seen as rectification in hyperpolarizing voltage steps, or the hyperpolarization-induced rebound depolarization due to activation of IT. Our current studies demonstrate that, in addition to an enhancement of Ih or IT, modulation of the voltage-dependent Nav1.7 sodium channel by MEK/ERK signaling contributes to the regulation of neuronal excitability by PACAP.1
METHODS
Animals.
Cardiac ganglia whole mount preparations from Hartley guinea pigs (either sex, 250–350 g body wt) were used for all experiments. Animal protocols were approved by the Institutional Animal Care and Use Committees of the University of Vermont, the University of California, Los Angeles, and Ithaca College. Approved procedures also followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The guinea pigs were euthanized by isoflurane overdose and exsanguination. Hearts were quickly removed and placed in cold Krebs solution (in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 8 glucose, with pH 7.4 maintained by 95% O2-5% CO2 aeration).
Chemicals.
PACAP27 (referred to as PACAP throughout) was obtained from American Peptide (Sunnyvale, CA); the MEK inhibitor PD 98059 (2′-amino-3′-methoxyflavone) from Calbiochem (La Jolla, CA), the KV4.2 potassium channel inhibitor 4-aminopyridine (4-AP) from Sigma-Aldrich (St. Louis, MO); and the NaV1.7 sodium channel blocker PF-04856264 [3-cyano-4-(2-(1-methyl-1H-pyrazol-5-yl) phenoxy)-N-(thiazol-2-yl) benzenesulfonamide] from Alomone Labs (Jerusalem, Israel). The inhibitors PD 98059 and PF-04856264 were prepared as DMSO stock solutions, diluted, and added directly to the bath solution. The final concentration of DMSO was ≤0.2%. 4-AP was added directly to the bath solution for each experiment. When inhibitors were used, the preparations were exposed to drug-containing solutions for ≥10 min prior to initiation of data collection.
Immunocytochemistry and confocal imaging.
After different experimental treatments, cardiac ganglia whole mounts were fixed in a 2% paraformaldehyde-0.2% picric acid solution for 2 h at 4°C, washed in blocking solution, and treated with ice-cold methanol for 10 min before incubation overnight in rabbit anti-phosphorylated ERK1/2 (1:1,000 dilution; catalog no. D13.14.4E, Cell Signaling Technology, Beverly, MA) for visualization with Cy3-conjugated donkey anti-rabbit IgG (1:500 dilution; Jackson ImmunoResearch, West Grove, PA). Guinea pig cardiac ganglia whole mounts were then mounted on glass slides, coverslips were applied, and the whole mounts were imaged using a Nikon/Yokogawa CSU W1 spinning-disk confocal microscope with a Nikon Apo LWD ×25/1.10 numerical aperture objective lens. Excitation was accomplished with a 100-mW 561-nm solid-state laser, and emission was collected from 590 to 650 nm as 16-bit Nikon nd2 image files. On five random ganglia in each sample, z series were taken at 0.4-μm steps, and regions of interest (ROIs) were generated for individual neurons at the axial midpoint adjacent to the nucleus, with care taken to avoid the cell membrane. Specifically, a single z slice was selected through the middle of the cell, and a unique circular ROI was generated for each cell cytoplasm, depending on cell size. Each ganglion generally contained 3–10 cells that met measurement criteria. All hardware settings were carefully maintained across samples and cross-checked by review of data header files. Data collection and analysis were performed using Nikon Elements 4.30.10 (Build 1021). Data obtained from multiple neurons in each cardiac ganglia whole mount were averaged, and the results from at least three preparations are presented as means ± SE for the group (Fig. 1B).
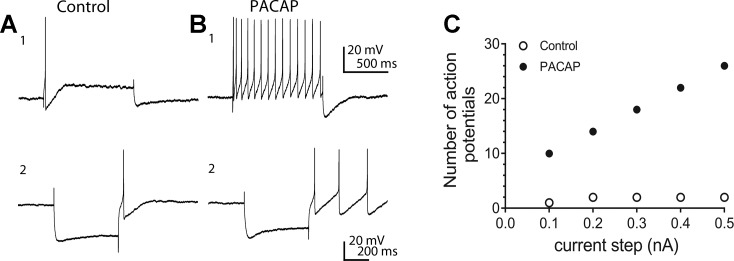
Fig. 1.
Pituitary adenylate cyclase-activating polypeptide (PACAP) can enhance excitability, rectification, and a hyperpolarization-induced rebound depolarization in guinea pig cardiac neurons. A1 and B1: 20 nM PACAP-induced shift from phasic to multiple action potential generation. Prior to PACAP application, a 1-s, 0.2-nA depolarizing constant-current pulse elicited 1 action potential. During exposure to PACAP, the number of action potentials generated by this same depolarizing current pulse increased markedly. A2 and B2: PACAP also increased rectification in the hyperpolarization elicited by a 500-s constant-current pulse and likewise enhanced the hyperpolarization-induced rebound depolarization. C: excitability curve showing PACAP enhancement of action potentials generated by 1-s depolarizing current steps of increasing intensity. ○, Number of action potentials generated prior to PACAP; ●, number of action potentials elicited during exposure to PACAP (control).
Intracellular recordings from cardiac ganglia neurons.
Intracellular recordings were obtained from cardiac neurons as described previously (3, 19, 26, 35, 36). Cardiac ganglia preparations were superfused continuously (6–7 ml/min) with Krebs solution containing 10 mM NaHEPES (32–35°C), and individual neurons were impaled using 2 M KCl-filled microelectrodes (60–120 MΩ). Membrane voltage was recorded using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 software (Axon Instruments, Foster City, CA). Depolarizing current steps (0.1–0.5 nA, 1 s) were applied to characterize neuron excitability. PACAP enhances the number of action potentials elicited by long depolarizing current steps (Fig. 1, A1 and B1). The number of action potentials generated at each stimulus intensity was plotted to show the degree of change in excitability (Fig. 1C). Hyperpolarizing current steps (500 ms) of increasing amplitude were used to test for 1) rectification in the current-induced hyperpolarization, which occurs when the hyperpolarization-activated current Ih is initiated, and 2) a transient hyperpolarization-induced rebound depolarization, which is a characteristic feature of activation of the low voltage-activated calcium current (IT). PACAP can enhance the rectification in the hyperpolarizing step and the hyperpolarization-induced rebound depolarization (Fig. 1, A2 and B2).
Real-time quantitative PCR.
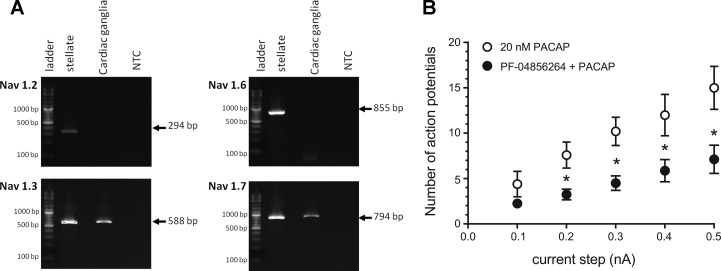
Transcript levels were determined for the KV4.2 potassium channel and different voltage-dependent sodium channels (NaV1.2, NaV1.3, NaV1.6, and NaV1.7) from extracts of cardiac ganglia whole mounts, stellate ganglia, atrial muscle, and brain, all of which were collected under RNase-free conditions (10). The primer pairs for determining the presence of guinea pig KV4.2 transcripts were as follows: 5′-TTCTACCGCACTGGGAAGCTC-3′ (forward) and 5′-CTCGTAACAGCAGTCGCCGAT-3′ (reverse). The primer sets for the different voltage-dependent sodium channels used in this study were taken from Sage et al. (29).
cDNA templates were assayed using HotStart-IT SYBR Green quantitative PCR (qPCR) master mix (USB, Cleveland, OH) and each primer at 300 nM in a final 25-μl reaction volume. The amplified products were subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions, demonstrating amplification of a single unique product free of primer dimers or other anomalous products (data not shown). Data were analyzed at the termination of each assay using Sequence Detection software (version 1.3.1, Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity [change in reporter signal normalized to fluorescence of a passive reference dye (ΔRn)] was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. At the end of the qPCR analysis, all samples were run on an ethidium bromide gel.
Statistics.
Statistics were performed using GraphPad Prism statistical software (version 5.4, GraphPad, La Jolla, CA). Data are presented as means ± SE. Differences between means were determined using an unpaired Student's t-test or one-way ANOVA followed by Tukey's post hoc analysis. Values were considered statistically significant at P < 0.05.
RESULTS
PACAP activates the MEK/ERK signaling cascade in guinea pig cardiac neurons.
In the initial series of experiments, we confirmed that PACAP activates the MEK/ERK signaling cascade in cardiac neurons by quantifying changes in phosphorylated ERK (pERK) immunoreactivity (pERK-IR) using confocal microscopy (7). In freshly dissected cardiac ganglia whole mount preparations maintained at 37°C for ≥20 min prior to fixation, basal levels of pERK-IR were observed in cardiac neurons and in presumptive Schwann and satellite cells encircling axon bundles and neurons (Fig. 2A1). After 20 min of PACAP exposure at 37°C, cardiac neuron cytosolic pERK-IR increased nearly twofold (Fig. 2, A2 and B). As noted earlier, there also was a significant increase in nuclear pERK-IR following the 20-min exposure to 25 nM PACAP at 37°C (7). Cardiac neuron cytoplasmic pERK-IR was also increased consistently following 20 min of exposure to lower concentrations of PACAP (1–5 nM) or after a shorter (5-min) exposure to 1–25 nM PACAP (data not shown).
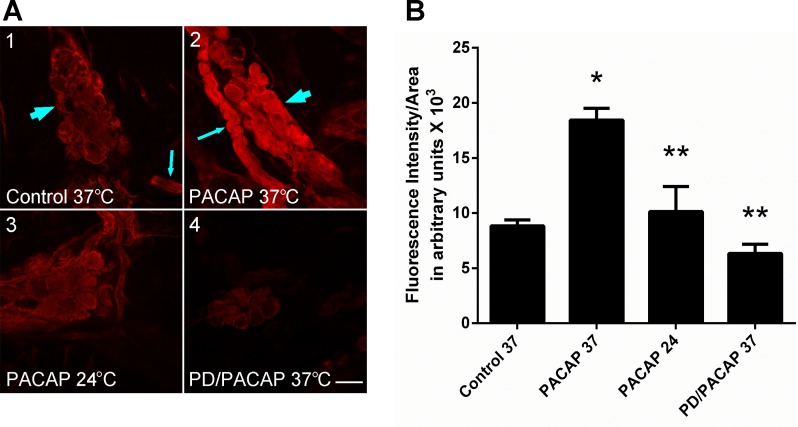
Fig. 2.
PACAP stimulates phosphorylated ERK (pERK) generation in guinea pig cardiac neurons. A: confocal images (∼1-μm optical sections) of cardiac ganglia neurons processed for pERK immunoreactivity and visualization using a Cy3-conjugated secondary antiserum. A1: control cardiac ganglia preparation maintained at 37°C prior to fixation. Arrowhead points to cardiac ganglion containing multiple neurons with only basal pERK immunoreactivity; arrow points to a nerve bundle surrounded by pERK-immunoreactive Schwann cells. A2: cardiac ganglia preparation exposed to 25 nM PACAP at 37°C for 20 min prior to fixation. Arrow points to a nerve bundle surrounded by pERK-immmunoreactive Schwann cells; arrowhead points to a cardiac ganglion containing multiple neurons with increased pERK immunoreactivity. A3: cardiac ganglia preparation exposed to 25 nM PACAP at 24°C for 20 min prior to fixation. Neuronal PACAP-stimulated pERK levels were attenuated, consistent with blockade of endosomal signaling at room temperature. A4: cardiac ganglia preparation pretreated with the MEK inhibitor PD 98059 (50 μM) for 15 min and then exposed to 25 nM PACAP + PD 98059 at 37°C for 20 min prior to fixation. MEK inhibition blocked PACAP-stimulated ERK activation. Calibration bar = 20 μm. B: averaged fluorescence intensity per area for conditions shown in A. Results were averaged from ≥3 cardiac ganglia whole mount preparations. Control 37, control at 37°C (54 cells in 3 whole mount preparations); PACAP 37, PACAP at 37°C (61 cells in 4 whole mount preparations); PACAP 24, PACAP at 24°C (60 cells in 3 whole mount preparations); PD/PACAP, PD 98059 + PACAP at 37°C (47 cells in 3 whole mount preparations). *Significantly different from control 37. **Significantly different from PACAP 37.
Our recent studies with the HEK PAC1 receptor stable cell line demonstrated that PACAP/PAC1 receptor-mediated ERK activation involves PAC1 receptor internalization/endosomal and phospholipase C (PLC)/diacylglycerol (DAG)/protein kinase C (PKC) signaling, both of which are suppressed at ambient temperatures (23). Similarly, the PACAP-induced increase in cytoplasmic pERK-IR was greatly suppressed when cardiac ganglia were exposed to 25 nM PACAP for 20 min at room temperature (∼24°C; Fig. 2, A3 and B), indicating that temperature-sensitive mechanisms contributed to the PACAP activation of MEK/ERK signaling in the cardiac neurons as well. As anticipated, 15 min of pretreatment with the MEK inhibitor PD 98059 (50 μM) completely blocked the 25 nM PACAP activation of pERK (Fig. 2, A4 and B).
As the number of cardiac neurons in individual cardiac ganglia whole mount preparations can vary by as much as an order of magnitude, from a few hundred to ∼1,500 cardiac neurons (41), the immunocytochemical data could not be correlated directly with Western analyses of cardiac ganglia pERK levels. However, the high potency (nanomolar PACAP concentrations) and time course of the PACAP-induced pERK-IR levels in the cardiac neurons agreed well with those observed previously in primary sympathetic neurons and the stable HEK PAC1 cell line by Western analyses (23, 24).
MEK inhibition suppresses the PACAP-induced increase in excitability without affecting hyperpolarization-induced rectification or rebound depolarization.
The next experiments evaluated whether pretreatment with the MEK inhibitor PD 98059 blunted the PACAP-induced increase in excitability. Initial intracellular recordings were obtained in control cells before and during exposure to only PACAP (n = 9: 5 cells at 10 nM and 4 cells at 20 nM; Fig. 3). As shown in Fig. 1 and in previous work (3, 19, 26, 35, 37, 38), exposure of cardiac neurons to PACAP enhanced excitability, as reflected by the marked increase in action potential generation initiated by depolarizing current steps. These experimental treatments were performed by bath application of PACAP, and as the change in action potential generation was comparable with 10 or 20 nM PACAP, the data for cells exposed to either concentration were pooled to create an averaged excitability curve for the PACAP treatment group (Fig. 3C).
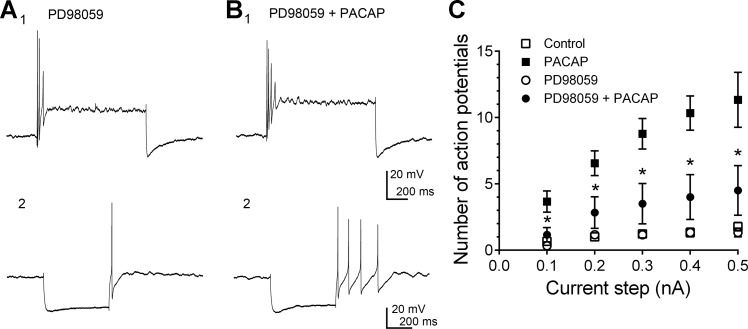
Fig. 3.
MEK inhibitor PD 98059 pretreatment suppresses the PACAP-induced increase in excitability without suppressing the rectification in constant-current-elicited hyperpolarizations or hyperpolarization-induced rebound depolarization. A: MEK inhibitor PD 98059 treatment blunted the PACAP-induced increase in neuronal excitability. A1: in inhibitor-pretreated cells, prior to PACAP, a 1-s, 0.3-nA current step elicited 3 action potentials. B1: during exposure to PD 98059 + PACAP, 4 action potentials were elicited by the same stimulus. A2: in PD 98059, prior to PACAP exposure, some rectification was evident in the hyperpolarization elicited by a 500-ms constant-current step, and a hyperpolarization-induced rebound depolarization at the termination of the hyperpolarization was large enough to generate an action potential. B2: during exposure to PD 98059 and PACAP, rectification in the hyperpolarization was more evident and the hyperpolarization-induced rebound depolarization elicited multiple action potentials. C: averaged excitability curves generated by plotting the number of action potentials elicited by 1-s depolarizing constant-current steps of increasing strength. Data are shown for 1 group of cells exposed only to PACAP and for different cells exposed to PD 98059 + PACAP. □ and ■, Averaged excitability curve generated for 9 cells before PACAP and then during exposure to PACAP (5 cells at 10 nM and 4 cells at 20 nM); ○ and ●, averaged excitability curve generated for 6 cells exposed only to 50 μM PD 98059 and then the same cells exposed to PD98059 + PACAP (3 cells at 10 nM and 3 cells at 20 nM). *Number of action potentials generated in cells exposed to PACAP + PD 98059 was significantly less than those exposed to PACAP alone at the same current step.
To examine the role of PACAP-induced ERK activation in the increased neuronal excitability, the cardiac neurons in subsequent experiments were pretreated for ≥15 min with 50 μM PD 98059 before the addition of PACAP + 50 μM PD 98059. Treatment with PD 98059 alone had no effect on cardiac neuron excitability compared with vehicle alone. However, PD 98059 pretreatment markedly blunted the ability of PACAP to increase excitability (Fig. 3, A1 and B1). An averaged excitability curve was again created for the 10 and 20 nM PACAP-stimulated neurons pretreated with PD 98059 (Fig. 3C; n = 6: 3 cells treated with 10 nM PACAP + PD 98059 and 3 cells treated with 20 nM PACAP + PD 98059). Note that the number of action potentials produced by increasing the stimulus intensity was significantly less in cells exposed to PD 98059 + PACAP than in cells exposed to PACAP alone, indicative of a marked suppression of the PACAP-induced increase in excitability following inhibition of the MEK/ERK pathway.
In addition to enhancing action potential generation by depolarizing steps, PACAP also can change the characteristics of the response to hyperpolarizing constant-current steps. The cardiac neurons exhibit rectification in hyperpolarizing steps, which is characteristic of the activation of an inward Ih flowing through cAMP-modulated hyperpolarization-activated nonselective cationic channels (8, 27). PACAP commonly enhances the rectification, consistent with a PACAP stimulation of cAMP generation and a cAMP-induced positive shift in the voltage dependence of Ih activation (Fig. 1) (27). Also, in many cardiac neurons, following termination of the constant-current-induced hyperpolarization, a posthyperpolarization-induced rebound depolarization is recorded. This rebound depolarization, which is due, to a large extent, to the activation of nickel-sensitive IT, also can be enhanced by PACAP (Fig. 1) (38). In the present study we tested whether pretreatment with the MEK inhibitor PD 98059 blocked the PACAP enhancement of Ih or the hyperpolarization-induced rebound depolarization. Although pretreatment with 50 μM PD 98059 significantly suppressed the PACAP-induced increase in excitability (Fig. 3, A1 and B1), the presence of the MEK inhibitor did not alter the PACAP-induced enhancement of the rectification in hyperpolarizing voltage steps or the hyperpolarization-induced rebound depolarization (Fig. 3, A2 and B2).
For these studies, the responses to hyperpolarizing current steps were evaluated in nine cells pretreated with 50 μM PD 98059 before PACAP (10 or 20 nM) was added to the inhibitor superfusion solution. Prior to PACAP addition to the bath, three of the nine cells treated with the MEK inhibitor exhibited no obvious or only slight rectification in the hyperpolarizing steps around −100 mV and exhibited a hyperpolarization-induced rebound depolarization that elicited one action potential, whereas during PACAP exposure, even in the presence of PD 98059, the rectification was augmented and the hyperpolarization-induced rebound depolarization increased sufficiently, such that two to four action potentials were elicited (Fig. 3, A2 and B2). In the same paradigm, three other cells in the MEK inhibitor alone exhibited slight rectification and a subthreshold hyperpolarization-induced rebound depolarization, but upon addition of PACAP to the PD 98059 perfusate, the rectification was enhanced and accompanied by a modest, if any, increase in the hyperpolarization-induced rebound depolarization. In the case of the seventh cell in the MEK inhibitor, which exhibited a hyperpolarization-induced rebound depolarization that elicited one action potential, PACAP exposure increased the rectification without affecting the hyperpolarization-induced rebound depolarization (1 action potential was still elicited). For the remaining two PD 98059-treated cells, there was no noticeable rectification or hyperpolarization-induced rebound depolarization prior to and during PACAP exposure.
The variability in extent of rectification and hyperpolarization-induced rebound depolarization amplitude observed in MEK inhibitor-treated cells and during subsequent PACAP exposure was quite similar to that recently reported for control cells prior to and during PACAP exposure (38). Hence, from these observations, the inhibition of MEK/ERK signaling did not suppress the ability of PACAP to enhance the rectification, indicative of Ih activation, or the hyperpolarization-induced rebound depolarization, a characteristic property of IT activation.
Cardiac neurons express KV4.2 transcripts, but block of IA currents does not mimic the PACAP-induced increase in excitability.
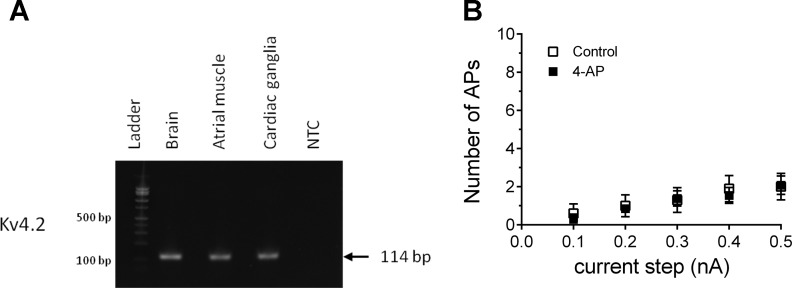
As neuronal treatment with the MEK inhibitor did not noticeably affect PACAP enhancement of Ih or IT, PACAP must modulate a different, MEK/ERK-sensitive membrane conductance that contributes to the PACAP-induced increase in excitability. PACAP has been shown to increase excitability of hippocampal neurons by decreasing surface expression of the KV4.2 channel, which decreases the voltage-dependent potassium current IA (12). As for brain and atrial tissues, semi-qPCR analysis of cardiac ganglia whole mount extracts suggested that the cardiac neurons express transcripts for the KV4.2 channel (Fig. 4A). However, when we treated cardiac ganglia preparations with 1 mM 4-AP to examine whether IA suppression could recapitulate the PACAP-induced increase in excitability, 4-AP failed to elicit a significant effect (Fig. 4B). Recordings were obtained from 10 cells in 6 whole mount cardiac ganglia prior to 4-AP treatment and then from 13 cells from the same preparations following 15 min of pretreatment with 4-AP. These results suggested that suppression of IA by PACAP did not contribute to the peptide modulation of cardiac neuron excitability (Fig. 4B).
Fig. 4.
Guinea pig cardiac ganglia express Kv4.2 transcripts, but inhibition of the voltage-dependent potassium current (IA) with 4-aminopyridine (4-AP) does not simulate PACAP-induced excitability. A: semiquantitative PCR demonstrating Kv4.2 transcript expression in brain, atrial muscle, and cardiac ganglia. NTC, no template control. B: averaged excitability curves for 10 cells prior to (□) and for 13 cells during (■) exposure to 1 mM 4-AP. Results demonstrate that 4-AP treatment to suppress KV4.2 currents had no effect on cardiac neuron excitability. APs, action potentials.
PACAP/PAC1 receptor signaling regulates voltage-dependent Nav1.7 sodium channel in cardiac neuron excitability.
Given that 4-AP did not recapitulate the PACAP-induced increase in excitability, we next focused on sodium channels as potential targets of MEK/ERK modulation in the cardiac neurons. MEK/ERK signaling can affect voltage-gated sodium channel activation (31), and using PCR, we examined the expression of sodium channel transcripts in cardiac ganglia whole mount and sympathetic stellate ganglia extracts. From semiquantitative analyses, the cardiac ganglia preparations expressed NaV1.3 and NaV1.7 transcripts; the expression of NaV1.2 or NaV1.6 mRNA was not evident in these samples (Fig. 5A). By contrast, the control stellate ganglia tissues contained transcripts for all four sodium channel α-subunits (Fig. 5A). In good agreement, qPCR analyses confirmed that transcripts for NaV1.3 and NaV1.7, but not those for NaV1.2 or NaV1.6, were present in cardiac ganglia extracts, whereas all these transcripts were present in stellate ganglia. Nav1.7 transcripts were not detected in atrial muscle extracts to mitigate channel expression in the small amount of contaminating nonneural tissue in the cardiac ganglia samples (data not shown).
Fig. 5.
Cardiac ganglia neurons express select voltage-dependent sodium channels, and preferential Nav1.7 blockade can attenuate PACAP-enhanced excitability. Semiquantitative PCRs demonstrate NaV1.3 and NaV1.7 transcript expression in cardiac ganglia; NaV1.2 and NaV1.6 expression was not evident in these tissues. Stellate ganglia contained transcripts for all 4 sodium channel α-subunits. NTC, no template control. B: cardiac neuron pretreatment with the NaV1.7 blocker PF-04856264 suppressed the PACAP-induced increase in cardiac neuron excitability. Averaged excitability curve was generated for 5 cells during exposure to 20 nM PACAP (■) and 8 cells pretreated with PF-04856264 (PF) and then exposed to PF-04856264 + 20 nM PACAP (4 cells at 100 nM PF-04856264 and 4 cells at 500 nM PF-04856264) (●). *Number of action potentials for PF-04856264 + PACAP is statistically different from PACAP alone at the indicated current steps.
To test the possibility that PACAP-enhanced currents flow through NaV1.7 channels, we examined whether a selective NaV1.7 channel inhibitor, PF-04856264 (25), could blunt PACAP modulation of neuronal excitability. In the first experiments, the effect of pretreatment with 100 nM PF-04856264 on the PACAP-induced increase in excitability was determined in four cells from two cardiac ganglia whole mounts. In both ganglia preparations, recordings were made from one cell exposed to 100 nM PF-04856264 for ≥10 min and then from the same cell after switching to a bath solution containing the NaV1.7 inhibitor + 20 nM PACAP. Excitability was tested multiple times, and then recordings were obtained from another cell in the same ganglia preparation during continued exposure to 100 nM PF-04856264 + 20 nM PACAP. A similar recording protocol was followed in two additional whole mount preparations, with the concentration of PF-04856264 raised to 500 nM. Suppression of the PACAP-induced increase in excitability was the same with 100 nM (4 cells) or 500 nM (4 cells) PF-04856264. Consequently, the data from the eight PF-04856264-treated cells were combined to generate an averaged excitability curve (Fig. 5B). After pretreatment with PF-04856264, the number of action potentials elicited by depolarizing pulses was significantly less than that noted for five other cells exposed to PACAP alone (Fig. 5B). Thus we conclude that PF-04856264 markedly attenuated the PACAP-induced increase in cardiac neuron excitability, an observation implicating PACAP/PAC1 receptor-mediated signaling in the regulation of Nav1.7. Also, over the course of these experiments, it was apparent that exposure to 100 or 500 nM PF-04856264 did not have an obvious effect on action potential characteristics.
DISCUSSION
The key results of the present study are as follows: 1) PACAP activates MEK/ERK signaling in the guinea pig cardiac neurons at concentrations that increased neuronal excitability, and 2) inhibition of the MEK/ERK signaling cascade significantly blunted the PACAP-induced increase in cardiac neuron excitability without impairing PACAP activation of Ih and IT, two currents shown to participate in the enhancement of neuronal excitability. These results implicated PACAP/PAC1 receptor regulation of an additional current in modulating neuronal excitability, which might be the voltage-gated potassium current IA or the voltage-dependent sodium current NaV1.7, both of which are known to be modulated by MEK/ERK signaling. Our current studies imply that the latter participates in PACAP regulation of cardiac neuron excitability.
Previously, we determined that the PACAP-induced increase in excitability was significantly suppressed at room temperature (26). PACAP activation of MEK/ERK signaling in the cardiac neurons, as shown previously for the HEK PAC1 receptor cells (23), also was generated by temperature-sensitive mechanisms. Thus a diminished PACAP activation of MEK/ERK signaling at room temperature likely is one factor contributing to the temperature dependence of the peptide-induced increase in cardiac neuron excitability. In the HEK PAC1 cells, the increase in pERK following PACAP stimulation reflected the activities of two mechanisms downstream of PAC1 receptor activation: 1) engagement of PLC/DAG/PKC signaling and 2) endosomal signaling after receptor vesicular internalization (23). We recently reported that PAC1 internalization/endosomal signaling and AC/cAMP/protein kinase A (PKA) signaling contribute to the activation of MEK/ERK signaling in the cardiac neurons (7). In contrast to the HEK PAC1 cells, activation of PLC/DAG/PKC signaling does not contribute to the PACAP generation of pERK in the cardiac neurons. As both PAC1 receptor internalization and PKA activation are temperature-sensitive (5, 26), it is not surprising that PACAP-induced pERK was suppressed at room temperature.
Treatment with PD 98059 to inhibit MEK, a regulatory kinase upstream of ERK, blunted the ability of PACAP to enhance excitability, demonstrating involvement of the MEK/ERK signaling cascade in the modulation of excitability. However, inhibition of MEK/ERK signaling had no consistent effect on the PACAP enhancement of rectification in hyperpolarizing steps or the hyperpolarization-induced rebound depolarization. These observations are notable in suggesting that PACAP modulation of at least one other conductance, in addition to Ih or IT, contributes to the PACAP action on excitability. MEK/ERK signaling can modulate A-type potassium channels, high-voltage-activated calcium channels, and voltage-dependent sodium channels (9, 20, 31, 34). PACAP suppresses high-voltage-activated calcium currents, suggesting that enhancement of these currents did not contribute to the increased excitability in PACAP (36). Consequently, we evaluated the possible involvement of a PACAP modulation of IA, a current shown previously to enhance hippocampal neuron and olfactory neuroepithelia activities through channel downregulation by PACAP (12, 15). In support of a possible involvement, transcripts for KV4.2 were present in extracts of cardiac ganglia whole mounts. However, exposure to 4-AP to inhibit IA did not noticeably increase cardiac neuron excitability, a result consistent with prior studies (11). Thus, suppression of IA did not mimic the PACAP modulation of cardiac neuron excitability, an observation suggesting that suppression of IA likely did not contribute to the PACAP modulation of cardiac neuron excitability.
Consequently, we focused on sodium channel α-subunits as the potential target of the PACAP-enhanced MEK/ERK signaling in the cardiac neurons. Prior studies have reported that ERK activation modulates NaV1.2 and NaV1.7 in PC12 cells (34) and NaV1.7 in dorsal root ganglion (DRG) neurons (31). Our qPCR analysis indicated that the parasympathetic cardiac neurons, like myenteric neurons, express transcripts for NaV1.3 and NaV1.7, but not for NaV1.2 or NaV1.6 (29). Transcripts for NaV1.2 and NaV1.6 were detected in extracts of guinea pig stellate ganglia, suggesting that all the primers used in this study worked in the guinea pig. NaV1.7 is a threshold voltage-activated sodium channel that can amplify weak stimuli; thus it can modulate firing properties of DRG neurons (31). Inhibition of MEK/ERK signaling decreases excitability of DRG neurons and, in HEK cells expressing NaV1.7, induces a depolarizing shift in the voltage dependence of activation and fast inactivation, an effect that would decrease excitability (31). Thus we evaluated whether a recently described selective NaV1.7 blocker could suppress the PACAP-induced increase in excitability (25). Pretreatment with PF-04856264 suppressed the PACAP-induced increase in excitability, an observation consistent with an enhancement by PACAP of currents through NaV1.7 channels contributing to the peptide effect on excitability. Furthermore, the extent of the suppression of the PACAP-enhanced excitability by PF-04856264 was comparable to that produced by the MEK inhibitor PD 98059. However, suppression of the PACAP-enhanced excitability occurred without obvious effects on action potential properties, which is consistent with currents through NaV1.3 channels, which are insensitive to PF-04856264, being the primary inward current responsible for the depolarizing phase of action potentials in these cells. The specificity and potency of PF-04856264 are species-dependent (25). However, the effect of PF-04856264 in the current studies implicates inhibition of guinea pig cardiac neuron Nav1.7 channels. Among sodium channel subtypes, the amino acid residues Y1537, W1538, and D1586 in the domain 4 S1–S4 transmembrane segment confer PF-04856264 selectivity for Nav1.7 over Nav1.3 (25). Guinea pig Nav1.7 contains Y1537 and W1538 in the corresponding region but differs at D1586; these sequence variations are also found in mouse and canine channels and are predictive of PF-04856264 preference for Nav1.7, but with a small decrease in potency. Furthermore, there are multiple S/TP motifs in the L1 loop region of the human Nav1.7 (T531, S535, S608, and S712) for ERK phosphorylation to gate channel properties (31). The same four S/TP motifs are present in the guinea pig Nav1.7 L1 sequence and, hence, can be targeted by ERK upon receptor-mediated activation. Even with the potential limitation from inhibitor potency, it is hypothesized that a PACAP activation of MEK/ERK signaling phosphorylates NaV1.7, which causes a hyperpolarizing shift in the voltage dependence of activation. This, in turn, could increase sodium currents near the threshold, thus potentially contributing to the development of repetitive action potential firing noted in PACAP.
We have hypothesized, based on recent studies, that PF-04856264 is a selective inhibitor of NaV1.7 with limited effects on other sodium channel types (25). However, as drugs can have multiple targets, this conclusion remains tentative until it is established that other types of ion channels contributing to the PACAP-induced increase in excitability in guinea pig neurons also are not altered by this compound.
Previously, we demonstrated that the PKA inhibitor H89 also suppressed the PACAP-induced increase in excitability, an observation suggesting a role of PKA-induced phosphorylation in the peptide effect (39). Recently, we extended the analysis of these earlier experiments to determine whether H89 (1–5 μM) treatment affected the characteristics of the response to hyperpolarizing current steps prior to and during exposure to 10 nM PACAP. From this analysis, it appeared that H89 suppressed the PACAP enhancement of the hyperpolarization-induced rebound depolarization without an obvious effect on the development of rectification in the hyperpolarizing steps (data not shown). We recently suggested that activation of CaV3.2 calcium channels likely contributes to the development of the hyperpolarization-induced rebound depolarization based on its sensitivity to low concentrations of nickel (38). Activation of PKA phosphorylates T-type calcium channel α-subunits (5, 21). Chemin et al. (5) reported that, at micromolar concentrations, H89 not only suppressed a dibutyryl cAMP-induced enhancement of T-type calcium currents, but also had direct inhibitory effects on T-type calcium currents in the absence of PKA enhancement. The observation that the hyperpolarization-induced rebound depolarization was either very small or absent in H89-treated cells and not enhanced by PACAP is consistent with our prior conclusion that the hyperpolarization-induced rebound depolarization is generated primarily by activation of T-type calcium currents (38).
The present results indicate that activation of the AC/cAMP/PKA signaling cascade in the cardiac neurons contributed to the PACAP-induced generation of pERK. Given this, a blockade of the PKA/MEK/ERK pathway by H89 could also contribute to the H89 suppression of the PACAP-induced increase in excitability.
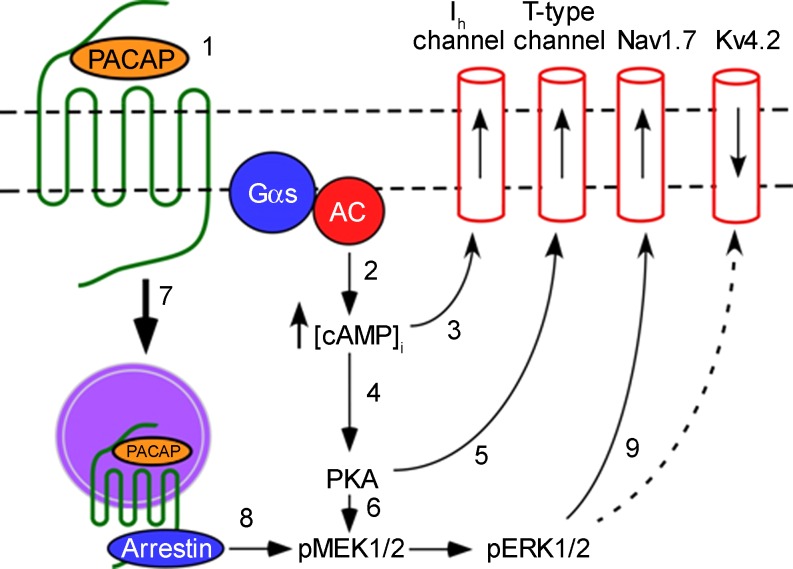
From results obtained in previous and current studies, we have developed a schematic model to summarize potential mechanisms contributing to the PACAP-induced increase in excitability (Fig. 6). First, interaction of PACAP with the PAC1 receptor leads to activation of AC and generation of cAMP. The rise in cAMP shifts the voltage dependence of activation of hyperpolarization-activated cyclic nucleotide-gated channels, enhancing Ih. A rise in cAMP also activates PKA, enhancing T-type channel currents through protein phosphorylation and contributing to the activation of MEK/ERK signaling. Furthermore, internalization of the activated PAC1 receptor and the resulting formation of a signaling endosome provide a scaffold for recruitment of the MEK signaling cascade. Activation of MEK kinase signaling alters gating properties of another channel, likely NaV1.7, through protein phosphorylation. Although our data do not implicate involvement of a PACAP modulation of KV4.2 expression in the increase in cardiac neuron excitability, a PACAP activation of MEK/ERK signaling can decrease surface membrane density of KV4.2 channels in other neurons, an action that would contribute to an enhanced excitability. KV4.2 is often located in dendrites, where it regulates synaptic efficiency (12). Cardiac neurons do not have dendrites, and the excitatory synapses are located on the cell soma and axon hillock (8, 16). Thus the mechanism(s) utilized by PACAP to regulate excitability likely depends on the types of ion channels expressed in different neurons.
Fig. 6.
Schematic of intracellular signaling cascades and ionic conductances potentially contributing to the PACAP-induced increase in cardiac neuron excitability. Interaction of PACAP with the PAC1 receptor (1) leads to activation of adenylyl cyclase (AC) and generation of cAMP (2). The rise of cAMP and interaction with hyperpolarization-activated cyclic nucleotide-gated channels enhance hyperpolarization-activated nonselective cationic current (Ih) (3), and a rise in cAMP also activates PKA (4), enhancing T-type channel currents through protein phosphorylation (5) and possibly contributes to activation of MEK/ERK signaling (6). Internalization of the PACAP/PAC1 receptor complex and formation of a signaling endosome (7) provides a scaffold for recruitment of the MEK/ERK signaling cascade (8), and activation of MEK/ERK kinase signaling potentially alters the voltage-dependent Na+ channel NaV1.7 through protein phosphorylation (9). For other cell types, a PACAP-induced decrease in surface expression of KV4.2 (dashed line 9) could also contribute to the increased excitability. Gαs, G protein α-subunit.
In conclusion, this study illustrates that the PAC1 receptor can potently modulate neuronal electrical properties through activation of multiple intracellular signaling cascades that modulate many different voltage-dependent ionic conductances.
GRANTS
This work was supported in part by National Institutes of Health Grants P30 GM-103498/NCRR P30 RR-032135 (R. L. Parsons) and S10 OD-017969-01 (R. L. Parsons).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.T., T.A.C., J.C.H., B.M.G., and L.A.M. performed the experiments; J.D.T., T.A.C., J.C.H., B.M.G., L.A.M., V.M., and R.L.P. analyzed the data; J.D.T., T.A.C., J.C.H., B.M.G., L.A.M., V.M., and R.L.P. edited and revised the manuscript; J.D.T., T.A.C., J.C.H., B.M.G., L.A.M., V.M., and R.L.P. approved the final version of the manuscript; V.M. and R.L.P. developed the concept and designed the research; V.M. and R.L.P. interpreted the results of the experiments; V.M. and R.L.P. drafted the manuscript.
ACKNOWLEDGMENTS
We thank the Vermont Cancer Center DNA Analysis Facility for verifying nucleotide sequences.
Footnotes
This article is the topic of an Editorial Focus by Kevin P. M. Currie (7a).
REFERENCES
- 1.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48: 301–331, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274: 27702–27710, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol 423: 26–39, 2000. [PubMed] [Google Scholar]
- 5.Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P. Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem 282: 32710–32718, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci 32: 14165–141177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clason TA, Girard BM, May V, Parsons RL. Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J Mol Neurosci 59: 309–316, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Currie PMC. NaV-igating the MAP from PACAP to excitement. Focus on “Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability.” Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00270.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol 486: 453–471, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald EM, Dolphin AC. Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras. Eur J Neurosci 9: 1252–1261, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Girard BM, Merriam LA, Tompkins JD, Vizzard MA, Parsons RL. Decrease in neuronal nicotinic acetylcholine receptor subunit and PSD-93 transcript levels in the male mouse MPG after cavernous nerve injury or explant culture. Am J Physiol Renal Physiol 305: F1501–F1512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girasole AE, Palmer CP, Corrado SL, Southerland EM, Ardell JL, Hardwick JC. Angiotensin II potentiates adrenergic and muscarinic modulation of guinea pig intracardiac neurons. Am J Physiol Regul Integr Comp Physiol 301: R1391–R1399, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupte RP, Kadunganattil S, Shepard AJ, Merrill R, Planer W, Bruchas MR, Strack S, Mphapatra DP. Convergent phosphomodulation of the major neuronal dendritic potassium channel KV4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology 101: 291–308, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammack SE, Chung J, Rhoades KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating polypeptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendrocrinology 34: 833–843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry 78: 167–177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han P, Lucero MT. Pituitary adenylate cyclase activating polypeptide reduces expression of Kv1.4 and Kv42 subunits underlying A-type K+ current in adult mouse olfactory neuroepithelia. Neuroscience 138: 411–419, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervations of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 166–174, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Harmar T, Lutz E. Multiple receptors for PACAP and VIP. Trends Pharmacol Sci 15: 97–99, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Hill J, Chan SA, Kuri B, Smith C. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286: 42459–42469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoover DB, Tompkins JD, Parsons RL. Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther 331: 197–203, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HJ, Glauner KS, Gereau RW 4th. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90: 1671–1679, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30: 32–40, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinjection of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast 2007: 79102, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May V, Buttolph TR, Girard BM, Clason TA, Parsons RL. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol 306: C1068–C1079, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PACAP1 HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase-γ and vesicle endocytosis for neuronal survival. J Biol Chem 285: 9749–9761, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormack K, Santos S, Chapman ML, Krafte DS, Marron BE, West CW, Krambis MJ, Antonio BM, Zellmer SG, Printzenhoff D, Padilla KM, Lin Z, Wagoner PK, Swain NA, Stupple PA, de Groot M, Butt RP, Castle NA. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proc Natl Acad Sci USA 110: E2724–E2732, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci 33: 4614–4622, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merriam LA, Barstow KL, Parsons RL. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–133, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ressler KJ, Mercer KB, Bradley B, Javovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470: 492–497, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sage D, Salin P, Alcaraz G, Castets F, Giraud P, Crest M, Mazet Clerk N. NaV1.7 and NaV1.3 are the only tetrodotoxin-sensitive sodium channels expressed by the adult guinea pig enteric nervous system. J Comp Neurol 504: 363–378, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170–175, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SC, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel NaV1.7 and alters its gating properties. J Neurosci 30: 1637–1647, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154: 330–339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron 14: 607–611, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Tompkins JD, Lawrence YT, Parsons RL. Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol 297: R52–R59, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL. Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 308: C857–C866, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci 36: 292–298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Young BA, Girard BM, Parsons RL. Neurturin suppresses injury-induced neuronal activating transcription factor 3 expression in cultured guinea pig cardiac ganglia. J Comp Neurol 508: 795–805, 2008. [DOI] [PubMed] [Google Scholar]