Abstract
Costello syndrome (CS) arises from a typically paternally derived germline mutation in the proto-oncogene HRAS, and is considered a rasopathy. CS results in failure-to-thrive, intellectual disabilities, short stature, coarse facial features, skeletal abnormalities, congenital heart disease, and a predisposition for cancer, most commonly embryonal rhabdomyosarcoma (ERMS). The goal of this study was to characterize CS ERMS at the molecular level and to determine how divergent it is from sporadic ERMS. We characterized eleven ERMS tumors from eight unrelated CS patients, carrying paternally derived HRAS c.34G>A (p.Gly12Ser; 6) or c.35G>C (p.Gly12Ala; 2) mutations. Loss of heterozygosity (LOH) was evaluated in all CS ERMS by microarray and/or short tandem repeat (STR) markers spanning the entire chromosome 11. Eight CS ERMS tumors displayed complete paternal uniparental disomy of chromosome 11 (pUPD11), whereas two displayed UPD only at 11p and a second primary ERMS tumor showed UPD limited to 11p15.5, the classical hallmark for ERMS. Three sporadic ERMS cell lines (RD, Rh36, Rh18) and eight formalin fixed paraffin embedded (FFPE) ERMS tumors were also analyzed for RAS mutations and LOH status. We found a higher than anticipated frequency of RAS mutations (HRAS or NRAS; 50%) in sporadic ERMS cell lines/tumors. Unexpectedly, complete uniparental disomy (UPD11) was observed in five specimens, while the other six showed LOH extending across the p and q arms of chromosome 11. In this study, we are able to clearly demonstrate complete UPD11 in both syndromic and sporadic ERMS.
Keywords: Costello Syndrome, embryonal rhabdomyosarcoma, loss of heterozygosity, uniparental disomy, chromosome 11, RAS mutations, 11p15.5
INTRODUCTION
The goal of this study was to characterize the molecular signatures of embryonal rhabdomyosarcoma in Costello syndrome patients and determine how it compared to that of nonsyndromic cases of ERMS. Costello syndrome (CS, OMIM #218040) is a rare congenital disorder estimated to affect about 1/500,000 newborns worldwide [Gripp and Lin, 2012]. CS affects multiple organ systems and has a wide range of clinical presentations. CS patients have failure-to-thrive, severe postnatal feeding difficulties, short stature, and share characteristic distinctive facial features including sparse, curly or fine hair, full nasal bridge, thick lips, and epicanthal folds [Gripp and Lin, 2012; Siegel et al., 2012]. Skin abnormalities include papillomas, hyperpigmentation, hyperkeratosis, and loose, soft, and redundant skin [Gripp and Lin, 2012; Siegel et al., 2012].
CS is caused by a heterozygous germline mutation in the proto-oncogene HRAS, arising in most cases in the paternal germline [Aoki et al., 2005; Gripp et al., 2006; Sol-Church et al., 2006; Zampino et al., 2007]. HRAS is located at 11p15.5, and most mutations are found in codons 12 and 13, with p.Gly12Ser in more than 80% [Aoki et al., 2005; Gripp, 2005; Gripp et al., 2006]. These gain-of-functions mutations prevent accessibility for GTPase activating proteins (GAPs) to exchange GTP for GDP, thus RAS remains constitutively active leading to increase cell proliferation, differentiation, apoptosis, and cell mobility [Shields et al., 2000]. Patients with Costello syndrome have a higher susceptibility than the general population to develop benign and malignant tumors, with an incidence of 15% [Gripp, 2005; Gripp et al., 2006; Kerr et al., 2009]. The most common solid tumors in Costello syndrome patients are rhabdomyosarcomas [Gripp and Lin, 2012]. Rhabdomyosarcoma (RMS) is the most prevalent sporadic pediatric soft tissue sarcoma with an incidence of 4.5 cases per million children each year [Ries et al., 1999]. In the United States an estimated 350 children are diagnosed with sporadic RMS each year [Huh et al., 2010]. Embryonal rhabdomyosarcoma (ERMS) is the most common subtype, accounting for over 60% [Belyea et al., 2012]. A recent review from the Children’s Oncology Group reported a 5-year event free survival rate in rhabdomyosarcoma clinical groups I/II and III of 69% and 70%, respectively [Wolden et al., 2015]. Though no long term follow up is available at this time for our CS cohort, 10 (77%) of the 13 individuals who underwent therapy survived while three (23%) died due to rhabdomyosarcoma tumor progression or relapse. One recently enrolled patient is currently undergoing treatment. Limitations in our data include grouping all individuals and rhabdomyosarcoma types together, the limited information on tumor stage and treatment protocols as well as lack of long term follow up data. While acknowledging the small Costello syndrome cohort size and limited data, we conclude that there is no obvious difference in survival after rhabdomyosarcoma treatment compared to a larger cohort of presumably nonsyndromic rhabdomyosarcoma.
One hallmark of sporadic ERMS is uniparental disomy (UPD) at 11p15.5, a region that contains HRAS as well as a cluster of imprinted genes [Visser et al., 1997; Anderson et al., 1999]. RAS mutations have been reported in 5–35% of sporadic ERMS [Stratton et al., 1989; Chen et al., 2006; Kratz et al., 2007]. Of the 141 patients with a germline HRAS mutation currently enrolled in our study, fourteen developed embryonal rhabdomyosarcoma (ERMS), from which eight primary tumors as well as three relapsed tumor specimens from eight unrelated patients were used to study mutation and LOH status. The CS tumors were analyzed in parallel with specimens derived from sporadic ERMS, which allowed us to demonstrate complete uniparental disomy beyond what has been previously reported around 11p15.5.
MATERIAL AND METHODS
Biological Specimens
CS patients and families were identified through physician referral and enrolled in an ongoing research study approved by the Institutional Review Board of the A.I. duPont Hospital for Children. Biological specimens included saliva and blood from the CS probands and available family members, as well as fresh or FFPE ERMS tumor samples. A cell line was established using routine tissue culture protocols from one Costello syndrome patient (CS 242). Additional ERMS cell lines from sporadic cases were either purchased from ATCC (RD ERMS) or received through a generous gift from Dr. Peter Houghton from Nationwide Children’s Hospital (Rh18 and Rh36). Eight deidentified FFPE sporadic ERMS tumor samples were obtained from the Alfred I. duPont Hospital for Children Biobank. For two of these ERMS specimens matched non neoplastic stoma tissue was available.
DNA Isolation
Genomic DNA was extracted from all specimens (blood, saliva, cells, tumor) using the PureGene DNA Isolation Kit (Qiagen). The DNA Isolation Kit was used with the following modifications from the manufacturer’s protocol to isolate DNA from FFPE samples: three 300μl xylene rinses with a five minute incubation followed by three 300μl 100% ethanol washes with a five minute incubation. The samples were then processed through cell lysis buffer as stated in the protocol.
RAS PCR sequencing
Samples were processed for Sanger sequencing by the COBRE-funded Nemours Biomolecular Core using standard workflows and operating procedures. HRAS was sequenced as previously described [Sol-Church et al., 2009]. Primers for NRAS 5′-TGAGGGACAAACCAGATAGGCA-3′ and 5-′ TGGTTCCAAGTCATTCCCAGTAG-3′ were annealed at 55°C to amplify a discrete 571 bp fragment.
Short Tandem Repeats (STRs) Analysis
Molecular confirmation that CS probands genetically matched their parents was completed using the AmpFISTR Profiler Plus amplification kit and/or Identifiler PCR amplification kit (ThermoFisher, Grand Island, NY). For LOH analyses of Chromosome 11, ABI LMS V2 Ch11 markers were used as previously described in Gripp et al., [2006].
Cytoscan Analysis
The Affymetrix Cytogenetics GeneChip Cytoscan HD arrays were used as recommended by the manufacturer. The data were analyzed using the Affymetrix chromosome analysis suite software.
Metaphase prep and Fluorescence in situ hybridization (FISH)
Metaphase prep for cell culture was modified from the preparation of metaphase chromosomes of adherent cells (NCI National Institute of Health, 2006). All incubations were for 15 mins and all centrifugations were performed for 8 mins at 350xg. First, 300μl of Actinomycin D (AG Scientific, San Diego, CA) at 100μg/mL was added to the flask and incubated for 15mins. Next, 300μl KaryoMax® Colecmid® Solution (Gibco® Life Technologies, Grand Island, NY) was added to the flask. FISH protocols were adapted from Coriell. The centromeric probe used was CEP 11 (D11Z1) SpectrumGreen probe 11p11.11-q11 Alpha Satellite DNA (06J37-021 Abbott Molecular, Abbot Park, IL). In addition, the slides were stained with ProLong gold antifade reagent with DAPI (P36935 Molecular Probes Life Technologies Grand Island, NY) and stored at −20°C. Images were taken at 63x on a Leica CTR microscope using Perkin Elmer Volocity 6.1.1.
RESULTS
Tumor and Cell Specimens
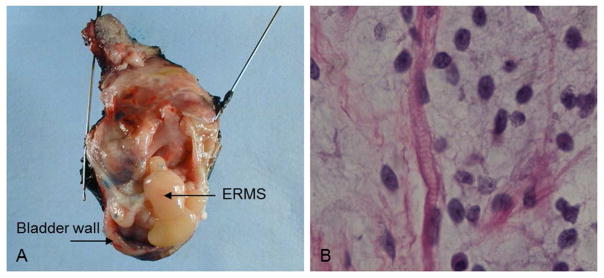
Prior to molecular analysis, fresh tumor specimens were placed in cultures and fixed specimens were embedded in paraffin and processed for hematoxylin & eosin (H&E) staining by the Nemours’ Histotechnology Core, to assess tissue integrity. Histological assessments were made by pathologists and the deidentified Biobank’s tumor paraffin blocks were confirmed to contain tumor cells (at least 80%) prior to sectioning and DNA extraction. Though tumor histology varied from patient to patient, typical findings included small cells, primitive spindle-shaped cells, myofibrils and cross striations on light microscopy after H&E stain. A classic example of bladder botryoid ERMS is presented for patient CS 187. Note the morphologic features resembling a cluster of grapes (Figure 1A), as well as ERMS characteristic histological findings of cross striations and rhabdomyoblastic differentiation (Figure 1B). Table I includes clinical features of the eight unrelated CS patients. Eleven CS-ERMS tumor specimens were obtained from these patients (Table II). We were unable to determine if the CS 170 tumor procurement was prior to or after treatment. However, we have pre-chemotherapy tumor tissues from proband’s CS 242, CS 457, CS 393, CS 214-T2, and CS 283-T1. We have post-chemotherapy tumor tissues from CS 181 and CS 187-T2. We have both pre and post chemotherapy tumor tissue from CS 187-T1 and CS 214-T1. In addition to the eight primary tumors, two metastatic specimens were obtained from CS 187 and CS 283. The metastatic diagnosis in these patients was based on the time course as well as location of the tumors. CS 214 developed two ERMS tumors eleven years apart that were localized in different body regions; CS 214-T1 was an abdominal ERMS while CS 214-T2 was paratesticular, thus these tumors were clinically diagnosed as two primary tumors. The two CS 214 tumors have distinct molecular signatures as seen by STR analysis (see section below) which may indicate they arose independently and thus support the clinical assessment of a second primary ERMS. A pure ERMS cell line was established from a fresh biopsy obtained post-surgery from CS 242. There have been no previous reports studying ERMS tumorigenesis using a cell line derived from a patient with Costello syndrome. Attempts to establish pure cell lines from the other two fresh biopsies failed due to specimen size and lack of viable tumor myoblasts. In addition to the CS specimens, three sporadic ERMS cell lines (RD, Rh18, Rh36), and eight sporadic ERMS FFPE tumors were included in the study for comparative analysis.
Figure 1.
Costello Syndrome embryonal rhabdomyosarcoma tumor (A) Photographic image of CS patient 187 resected bladder; this ERMS tumor infiltrates the bladder and prostate; (B) This ERMS section with hematoxylin and eosin staining reveals rhabdomyoblastic differentiation and cross striations identified by the pathologist. [Print in black and white]
Table I.
Costello Syndrome Cohort
| Patient ID | Gender | Germline Mutation and Additional Molecular Findings | Mutation Origin | Age ERMS Diagnosed (Years) | Location | Tumor Histology | Source of Tumor Sample | Timing of Sample Procurement | Notes |
|---|---|---|---|---|---|---|---|---|---|
| CS 170a | F | HRAS p.Gly12Ser | Paternal | 2.75 | Pelvic | ERMS | FFPE | N/A | Alive and well |
| CS 181a | M | HRAS p.Gly12Ser FISH analysis revealed 15–19% of cells are XXY; 10–16% of cells have gains in chromosome 13 (3–5 copies) and chromosome 21 (3 copies) | Paternal | 16 | Pelvic involving bladder, prostate | ERMS with botryoid features | Fresh Tissue | Post-treatment | Deceased |
| CS 187a | M | HRAS p.Gly12Ser | Paternal | 1.5 | Bladder | ERMS | Fresh Tissue | T1-Pre/Post treatment T2-Post-treatment of primary tumor |
Pulmonary/Thoracic metastatic ERMS at 2.9; deceased |
| CS 214a | M | HRAS p.Gly12Ser | Paternal | 1.5 | Abdominal Paratesticular |
Mixed ERMS and ARMS ERMS |
FFPE | T1-Pre & Post-treatment T2-at diagnosis, pre-treatment |
Paratesticular second primary ERMS at age 13; Alive and well |
| CS 242a,b | F | HRAS p.Gly12Ala | Paternal | 1.7 | Abdominal/pelvic | ERMS | Fresh Tissue Cell line |
Pre-treatment | Deceased |
| CS 283a,b | M | HRAS p.Gly12Ala | Paternal | 2 | Sphenoid | ERMS, spindle cell variant | FFPE | T1-Pre treatment T2-diagnosis of recurrence |
Nasopharyngeal ERMS recurrence at age 4; Alive and well |
| CS 457 | M | HRAS p.Gly12Ser | Paternal | 1.3 | Seminal Vesicle | ERMS | Fresh Tissue | Pre-treatment | Completed treatment, alive and well |
| CS 393 | F | HRAS p.Gly12Ser | Paternal | 3 | Retroperitoneal | ERMS | Fresh Tissue | Pre-treatment | In treatment |
Abbreviations are as follows: ID, identification; ERMS, embryonal rhabdomyosarcoma; F, female; M, male; FISH, fluorescence in situ hybridization; FFPE, formalin fixed paraffin embedded; N/A, not available; ARMS alveolar rhabdomyosarcoma.
additional clinical findings reported can be found respectively in references Gripp et al., 2010; Detweiler et al., 2013.
Table II.
Genomic status of embryonal rhabdomyosarcoma
| Proband | ERMS Location | Source of Tumor Sample | Timing of Sample Procurement | Mutation Status | LOH Status |
|---|---|---|---|---|---|
| CS 170 | Pelvis | FFPE | N/A | HRAS Hom p.Gly12Ser | Paternal UPD11 |
| CS 181 | Pelvic involving bladder, prostate | Fresh Tissue | Post-treatment | HRAS Hom p.Gly12Ser | Paternal UPD11 |
| CS 187-T1 | Bladder | Fresh tissue | Pre/Post treatment | HRAS Hom p.Gly12Ser; HRAS Het p.Arg68Trp | Paternal UPD11 |
| CS 187-T2 | Thoracic | Fresh tissue | Post-treatment | HRAS Hom p.Gly12Ser; HRAS Het p.Arg68Trp | Paternal UPD11 |
| CS 214-T1 | Abdominal | FFPE | Pre/Post-treatment | HRAS Hom p.Gly12Ser | Paternal UPD11 |
| CS 214-T2 | Paratesticular | FFPE | Pre-treatment | HRAS Hom p.Gly12Ser | 11p15.5 UPD |
| CS 242 | Abdominal/pelvic | Fresh tissue, and Cell line | Pre-treatment | HRAS Hom p.Gly12Ala | Paternal UPD11 |
| CS 283-T1 | Sphenoid | FFPE | Pre-treatment | HRAS Hom p.Gly12Ala | Paternal UPD11 |
| CS 283-T2 | Nasopharyngeal | FFPE | Diagnosis of reoccurrence | HRAS Hom p.Gly12Ala | Paternal UPD11 |
| CS 457 | Seminal Vesicle | Fresh tissue | Pre-treatment | HRAS Hom p.Gly12Ser | 11p UPD |
| CS 393 | Retroperitoneal | Fresh tissue | Pre-treatment | HRAS Hom p.Gly12Ser | 11p UPD |
| RD | Pelvis | Cell line | N/A |
NRAS Het p.Gln61His TP53 Het p.Trp248 |
UPD11 |
| Rh36 | Paratesticular | Cell line | N/A | HRAS Het p.Gln61Lys | UPD11 |
| Rh18 | Perineum | Cell line | N/A | N/D | Partial LOH |
| P673 | N/A | FFPE | N/A | NRAS Het p.Gln61Lys | Partial LOH |
| P666 | N/A | FFPE | N/A | HRAS Hom p.Cys12 | Partial LOH |
| P664 | N/A | FFPE | N/A | HRAS Hom p.Arg13 | UPD11 |
| P657 | N/A | FFPE | N/A | NRAS Het p.Gln61Lys | Partial LOH |
| P670 | N/A | FFPE | N/A | N/D | UPD11 |
| P643 | N/A | FFPE | N/A | N/D | Partial LOH |
| P646 | N/A | FFPE | N/A | N/D | UPD11 |
| P662 | N/A | FFPE | N/A | N/D | Partial LOH |
Abbreviations are as follows: FFPE, formalin fixed paraffin embedded; Hom, Homozygous; Het, Heterozygous; LOH, loss of heterozygosity; UPD, uniparental disomy; N/A, not available; N/D, not detected.
Mutation Status
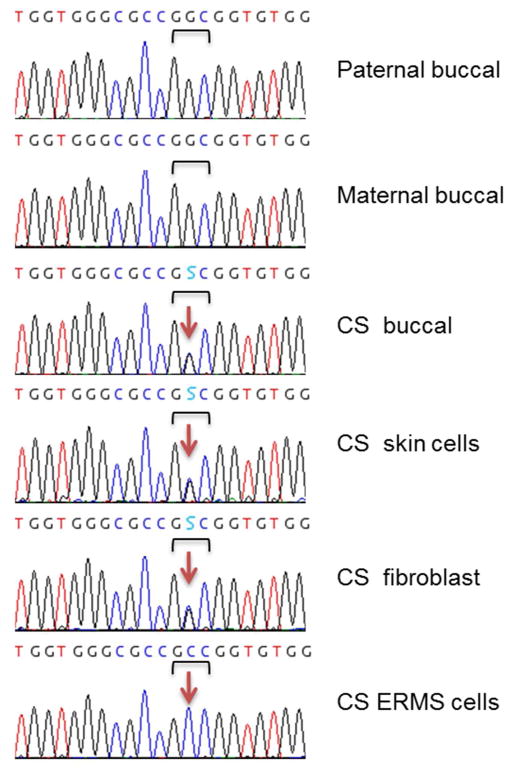
PCR amplification was performed to screen specimens derived from Costello syndrome patients for mutations in all exons of HRAS. In addition to the tumor samples, biospecimens included all eight CS proband’s blood, buccal and/or fibroblast tissues, and respective samples from their unaffected parents. A CS diagnosis was confirmed in each patient through the presence of a heterozygous germline HRAS mutation: c.34G>A (p.Gly12Ser) in six cases and c.35G>C (p.Gly12Ala) in two (Table I). Using allelic specific amplification, the mutation was found to be inherited through the paternal germline in six families. In CS 214 and CS 457, no informative SNPs were found near HRAS, paternal origin of the mutation was inferred using STR analysis as described below. Figure 2 is a classic example of how chromatograms were used to determine the HRAS mutation status for CS 242. This patient carries a c.35G>C heterozygous germline mutation in buccal, skin, and skin fibroblast cells. Both unaffected parents carry the wild-type allele. The patient’s ERMS-derived cell line displays the homozygous mutation indicating complete loss of the wild-type allele. All CS tumors showed loss of the wild type HRAS allele (Table II). In specimens harboring low levels of stromal contamination (CS 214-T1, CS 187-T1, CS 181, CS 170, CS 283-T1/T2) the wild-type allele was detectable. Interestingly, in addition to the homozygous p.Gly12Ser mutation, CS 187 tumor specimens (primary and metastatic) also carry a heterozygous c.202C>T (p.Arg68Trp) mutation that is absent from the germline.
Figure 2.
Loss of the HRAS wild-type allele in Costello Syndrome embryonal rhabdomyosarcoma. Chromatograms show the germline c.35 G>C (p.Gly12Ala) HRAS mutation in CS patient 242. The bracket indicates the Gly12 triplet while the arrow indicates the mutation site. The S in the sequence (C or G) indicates the actual heterozygous mutation. The proband’s ERMS cells display complete loss of the wild-type allele (G). [Print in black and white]
In the eleven sporadic tumor specimens, a mutation screen was performed on HRAS, KRAS, NRAS, and ERAS. Surprisingly, six of the eleven specimens carried a gain of function mutation in either HRAS or NRAS (Table II). Even more striking, sporadic tumors from patients P666 and P664 harbor homozygous HRAS mutations, that were absent from germline.
Loss of Heterozygosity
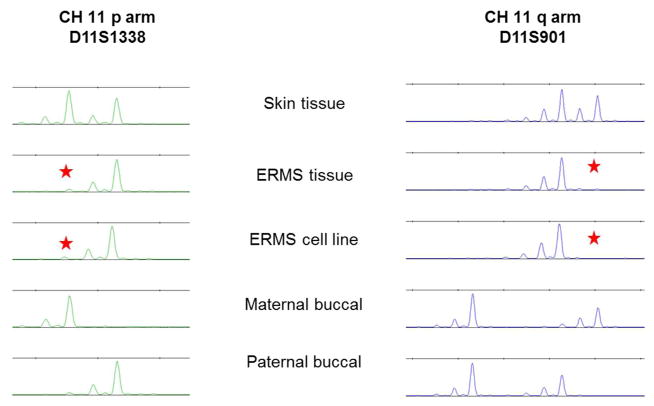
In embryonal tumors, the most common chromosomal aberration is loss of heterozygosity (LOH) at 11p15.5. To investigate the status of allelic loss at 11p15.5, 18 short tandem repeat (STR) markers were used that span the p and q arms of chromosome 11. Figure 3 demonstrates the use of STR to identify complete uniparental disomy as well as parental origin in CS 242 ERMS tumor cell line. Only two loci (11p and 11q) are presented here but the loss extended to all 18 markers on chromosome 11. Comparing the CS profile to that of the parent, one can clearly see at both loci complete loss of the maternal allele is observed in the tumor and the tumor derived ERMS cell line. All but three CS tumors showed paternal uniparental disomy (paternal-UPD, pUPD11) with complete loss of the maternal allele across the entire chromosome 11. The first exception was CS 214-T2 which displayed the classically described LOH uniquely at 11p15.5 that is referred to as uniparental disomy of 11p15.5. The other two exceptions (CS 393 and CS 457) displayed segmental paternal uniparental disomy on the entire 11p arm. Unexpectedly, all sporadic tumors showed segmental loss of heterozygosity beyond the expected 11p15.5 locus. Furthermore, complete uniparental disomy of the whole chromosome 11 was identified not only in RD and Rh36 ERMS cells but also in FFPE biopsies obtained from other patients with sporadic ERMS (P670, P664, P646). This demonstrates that complete uniparental disomy for the whole chromosome 11 in sporadic tumor-derived cell lines is not an artifact of cell culture but normal tumor progression in sporadic ERMS. Our results demonstrate for the first time that the majority of CS and sporadic ERMS tumors display complete loss of one parental chromosome 11 allele (uniparental disomy for the whole chromosome 11). One tumor showed the hallmark of ERMS with uniparental disomy at the 11p15.5 locus and two displayed segmental uniparental disomy localized to the entire 11p arm (11pUPD). Strikingly, this study found that the majority of CS ERMS tumors have lost the maternal allele which we may infer to be similar in the sporadic tumors.
Figure 3.
Chromosome 11 loss of heterozygosity in Costello Syndrome embryonal rhabdomyosarcoma. Chromosome 11 STR (short tandem repeats) markers reveal loss of heterozygosity in Costello Syndrome ERMS. This electropherogram is a representative profile of two of the eighteen STR loci on the p and q arms of chromosome 11 (D11S1338 and D11S901) that shows LOH of the maternal allele in the tumor tissue and cell line. The star indicates loss of the maternal wild-type allele. [Print in black and white]
Cytogenetic analysis
This STR study was complemented by Cytoscan analysis, a microarray technique enabling the detection of chromosomal aberrations such as LOH and copy number variation. This array analysis identified typical gains and losses in portions of chromosomes 2, 7, 8, 11, 12, 13, 17, 19, and 20, and confirmed LOH on chromosome 11 p and q (data not shown). Fluorescence in situ hybridization (FISH) was performed using a centromere chromosome 11 probe on CS 242 ERMS and fibroblasts, and sporadic ERMS cell lines (RD, Rh18). As depicted in Figure S1, CS 242 fibroblasts display two centromere 11 probes in 100% of cells. In contrast, ERMS heterogeneity was revealed through FISH as disomic and trisomic centromere 11 probes were detected. Thus, monosomy was excluded as a cause of the LOH in these CS and sporadic ERMS cases. Our data from Cytoscan and FISH suggest that UPD11 in the disomic or trisomic state is common in CS and sporadic ERMS.
DISCUSSION
We enrolled, through the Nemours Rasopathy program, the most extensive cohort of Costello syndrome patients and their family members. Of the 141 individuals affected with Costello syndrome 138 carry a germline heterozygous mutation in HRAS while 3 individuals displayed somatic mosaicism [Gripp et al., 2006; Sol-Church et al., 2009]. In our cohort 16 individuals (13%) developed malignancies (ERMS, neuroblastoma, bladder carcinoma), in support of CS as a cancer predisposition syndrome [Gripp, 2005; Kerr et al., 2006]. ERMS was identified in 14 individuals, and 11 different tumors from eight unrelated CS patients were analyzed in this study. Neuroblastoma developed in one patient with a p.Gly12Ala and one patient with a p.Gly12Ser. Likewise, transitional cell bladder carcinoma was identified in two patients with either a p.Gly12Ala or a p.Gly12Ser. Since ERMS is the most prevalent cancer observed in our CS patients, the focus of this study was to better understand ERMS tumorigenesis not only in the context of our syndromic model, Costello syndrome, but additionally in sporadic ERMS where mutations in one of the RAS genes is reported in 14%–35% [Stratton et al., 1989; Chen et al., 2006; Kratz et al., 2007; Shern et al., 2014]. As expected, all CS tumors displayed loss of the WT maternal HRAS allele which is consistent with the classical paternal uniparental disomy at 11p15.5 previously reported in ERMS. In one instance (CS 187) the tumor acquired a second variant, a heterozygous c.202C>T (p.Arg68Trp) mutation. This patient relapsed due to metastasis carrying the same heterozygous variant. It is unclear whether the p.Arg68Trp mutation contributed to the aggressive nature of this tumor resulting in the death of this patient. While the mutation is found in the switch II domain and may result in constitutively active RAS/MAPK signaling, there have been no other cancers reported with this mutation, and it is not reported in the COSMIC database.
In sporadic tumors, we identified RAS mutations in 54% of cases, with six of eleven sporadic ERMS tumors carrying a mutation in either HRAS or NRAS. Two of these sporadic tumors, P666 and P664, carried a homozygous HRAS mutation, p.Gly12Cys and p.Gly13Arg, respectively, which are absent in their matched non-tumor FFPE sample. Interestingly, of the five p.Gly12Cys CS patients enrolled in our cohort, only one developed ERMS. The p.Gly13Arg variant is not observed in our CS cohort, however, it is a very common mutation seen in the heterozygous state in nevus sebaceous, a benign congenital skin lesion [Groesser et al., 2012]. Since these FFPE samples were deidentified to use as sporadic controls, no clinical data are available. The codons most frequently associated with cancer in KRAS, NRAS, and HRAS are 12, 13, and 61. Therefore, it is not surprising to see mutations in codons 12, 13, and 61 when screening ERMS tumors. Though our sample size is low, it is possible that previous studies on ERMS underreported the number of RAS mutations, perhaps due to incomplete screening or tumor heterogeneity.
Loss of heterozygosity (LOH) and the classically described 11p15.5 uniparental disomy (UPD) have been reported as the major hallmark of ERMS [Visser et al., 1997; Kratz et al., 2007; Menke et al., 2015]. Our data confirmed LOH at chromosome 11 in all ERMS specimens and revealed widespread allelic loss along the length of chromosome 11 with segmental uniparental disomy of 11 occurring in two CS and six sporadic tumors. This segmental uniparental disomy of 11 was not only localized to 11p, but it was found in the q arm in several of the sporadic ERMS tumors. It is striking that complete uniparental disomy of the entire chromosome 11 was observed in 13 of the 22 ERMS specimens, with five occurring in sporadic ERMS cell lines and FFPE tumors. Complete loss of heterozygosity in the sporadic FFPE tumor specimens confirms that loss of a parental chromosome 11 allele is not an artifact of cell culture, but rather a common mechanism of tumor progression. LOH at 11p15.5 in sporadic ERMS is associated with loss of the maternal allele [Scrable et al., 1989]. We have shown in all eight CS ERMS patients that the maternal wild-type allele was lost, which supports previous studies on sporadic ERMS cases. Therefore, we speculate that there is loss of the maternal allele in the eleven sporadic ERMS specimens.
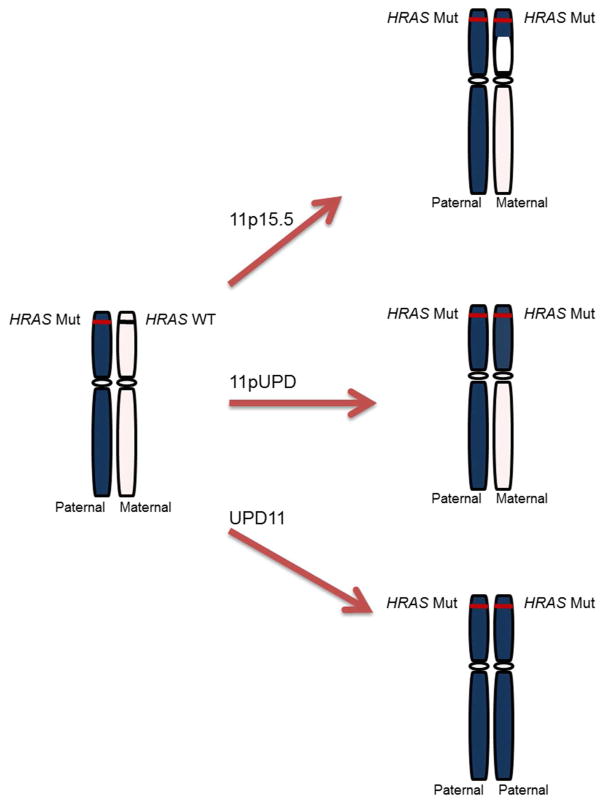
A previous study of seven orbital ERMS tumors using chromosome 11 microsatellite markers showed that two markers 11q13.1-22.3 and 11q23 displayed LOH [Mastraneglo et al., 1998]. However, complete UPD was not observed in these tumors. Patient CS 214 developed an abdominal primary tumor that was procured pre and post-chemotherapy treatment. Both tumors showed the same STR profiles indicating complete paternal uniparental disomy of the entire chromosome 11. The patient developed a second primary paratesticular tumor that may have been detected in its early stage due to regular monitoring or the tumor location. This second primary tumor interestingly has a different signature (STR profile) with cells harboring uniquely the classical 11p15.5 uniparental disomy. It is tempting to suggest that loss of heterozygosity at the 11p15.5 locus may be a prerequisite for ERMS formation. Complete uniparental disomy of the entire chromosome 11 more likely arose from the loss of the maternal chromosome in the CS and sporadic tumors via trisomic rescue [Tuna et al., 2009, Lapunzina et al., 2011] with or without the need of the 11p15.5 UPD. Figure 4 presents a model for ERMS tumorigenesis, updated from Kratz et al [2006], that is derived from the STR profile heterogeneity found in the CS and sporadic ERMS in this study. This model includes the classically described uniparental disomy of 11p15.5, as well as, segmental uniparental disomy of chromosome 11 that both occur through mitotic recombination events, and the complete parental uniparental disomy of the entire chromosome 11 observed in the majority of our tumor specimens that results via trisomic rescue as a result of mitotic nondisjunction events. These unexpected widespread findings of segmental and complete uniparental disomy of 11 are reminiscent of what has been described in about 20% of Beckwith-Wiedemann syndrome (BWS) patients that have variable segmental disomy in the 11p region. A few BWS cases have shown complete parental uniparental disomy of chromosome 11 [Cooper et al., 2007]. Despite that both BWS and CS patients are prone to developing ERMS, BWS patients display an overgrowth phenotype while CS patients have a short stature and failure-to-thrive.
Figure 4.
Proposed model for tumorigenesis in Costello Syndrome and sporadic embryonal rhabdomyosarcoma tumors. We characterized uniparental disomy in our ERMS tumors three ways, 1) the classically described uniparental disomy at 11p15.5, 2) segmental uniparental disomy of chromosome 11 which includes uniparental disomy of the whole P arm (11pUPD), 3) complete uniparental disomy of the entire chromosome 11 (UPD11) or paternal uniparental disomy of the whole chromosome 11 (pUPD11). First, the classically described uniparental disomy only at 11p15.5 was only found in 1 tumor in our study. Next, segmental uniparental disomy of 11 was found in 8 out of 22 ERMS cases. The most commonly observed molecular ERMS characteristic in this study was paternal uniparental disomy for the whole chromosome 11 (pUPD11) or complete uniparental disomy for the whole chromosome 11 (UPD11) in our CS and sporadic tumors. The mechanism of action of uniparental disomy at 11p15.5 or segmental uniparental disomy of 11(11pUPD) can be explained through mitotic recombination events, and complete parental uniparental disomy of the whole chromosome 11 (UPD11) and uniparental disomy for the whole chromosome 11 (pUPD11) can be explained through trisomic rescue. [Print in black and white]
In summary, our study of sporadic and syndromic ERMS specimens reveals features not reported before. Firstly, we suggest that the mutation load in sporadic tumors may have been underestimated and that RAS mutations are common in these cancers. Secondly, we propose that the classical 11p15.5 uniparental disomy may not entirely characterize ERMS. Indeed most specimens, whether isolated from a patient with Costello syndrome or from sporadic cases, show complete loss of one parental chromosome 11.
Supplementary Material
Acknowledgments
We thank the families and patients for their participation. We would like to thank Dr. Peter Houghton of Nationwide Children’s Hospital for the generous gift of the sporadic ERMS cells (Rh36 and Rh18). We thank Drs. Jonathan Wickiser, Bruce J. Schlomer, Norio Azumi, William H. Meyer, Rex C. Bentley and Philip Rosoff for their assistance in obtaining tissue samples. This work was supported in part by the Nemours Foundation; NIH-NIGMS grants P20GM103464 and INBRE grant P20GM103446 and by the generous private donations from the families of patients with rare genetic disorders.
References
- Anderson J, Gordon A, McManus A, Shipley J, Pritchard-Jones K. Disruption of imprinted genes at chromosome region 11p15.5 in paediatric rhabdomyosarcoma. Neoplasia. 1999;1:340–348. doi: 10.1038/sj.neo.7900052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S, Matsubara Y. Germline mutations in HRAS proto-oncogene cause costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Belyea B, Kephart J, Blum J, Kirsch D, Linardic C. Embryonic signaling pathways and rhabdomyosarcoma: Contributions to cancer development and opportunities for therapeutic targeting. Sarcoma. 2012:406239. doi: 10.1155/2012/406239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Takita J, Hiwatari M, Igarashi T, Hanada R, Kikuchi A, Hongo T, Taki T, Ogasawara M, Shimada A, Hayashi Y. Mutations of the PTPN11 and RAS Genes in Rhabdomyosarcoma and Pediatric Hematological Malignancies. Genes Chromosom Cancer. 2006;45:583–591. doi: 10.1002/gcc.20322. [DOI] [PubMed] [Google Scholar]
- Detweiler S, Thacker M, Hopkins E, Conway L, Gripp KW. Orthopedic manifestations and implications for individuals with costello syndrome. Am J Med Genet A. 2013;161:1940–1949. doi: 10.1002/ajmg.a.36047. [DOI] [PubMed] [Google Scholar]
- Gripp KW. Tumor Predisposition in Costello Syndrome. Am J Med Genet C Semin Med Genet. 2005;137C:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CL, Doyle D, Aoki Y, Matsubara Y, Zackai EH, Lapunzina P, Gonzalez-Meneses A, Holbrook J, Agresta CA, Gonzalez IL, Sol-Church K. HRAS mutation analysis in costello syndrome: Genotype and phenotype correlation. Am J Med Genet A. 2006;140:1–7. doi: 10.1002/ajmg.a.31047. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Stabley D, Nicholson L, Hoffman J, Sol-Church K. Somatic mosaicism for an HRAS mutation causes costello syndrome. Am J Med Genet A. 2006;140:163–169. doi: 10.1002/ajmg.a.31456. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, Dobyns W. High incidence of progressive postnatal cerebellar enlargement in costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A. 2010;152:1161–1168. doi: 10.1002/ajmg.a.33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Lin AE. Costello syndrome: a Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet Med. 2012;14:285–292. doi: 10.1038/gim.0b013e31822dd91f. [DOI] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, Ruivenkamp C, Lopriore E, Zutt M, Langmann T, Singer S, Klingseisen L, Schneider-Brachert W, Toll A, Real FX, Landthaler M, Hafner C. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783–787. doi: 10.1038/ng.2316. [DOI] [PubMed] [Google Scholar]
- Huh WW, Skapek SX. Childhood Rhabdomyosarcoma: New Insight on Biology and Treatment. Curr Oncol Rep. 2010;12:402–410. doi: 10.1007/s11912-010-0130-3. [DOI] [PubMed] [Google Scholar]
- Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, Stef M, Tang B, Eden OB, O’Sullivan J, De Sandre-Giovannoli A, Reardon W, Brewer C, Bennett C, Quarell O, M’Cann E, Donnai D, Stewart F, Hennekam R, Cave H, Verloes A, Philip N, Lacombe D, Levy N, Arveiler B, Black G. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43:401–405. doi: 10.1136/jmg.2005.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Steinemann D, Niemeyer CM, Schlegelberger B, Koscielniak E, Kontny U, Zenker M. Uniparental disomy at chromosome 11p15.5 followed by HRAS mutations in embryonal rhabdomyosarcoma: lessons from costello syndrome. Hum Mol Genet. 2007;16:374–379. doi: 10.1093/hmg/ddl458. [DOI] [PubMed] [Google Scholar]
- Mastrangelo D, Sappia F, Bruni S, Hadjistilianou T, Squitieri N, Donoso L, Frezzotti R. Loss of heterozygosity on the long arm of chromosome 11 in orbital embryonal rhabdomyosarcoma (OERMS): A microsatellite study of seven cases. Orbit. 1998;17:89–95. doi: 10.1076/orbi.17.2.89.2760. [DOI] [PubMed] [Google Scholar]
- Menke J, Pauli S, Sigler M, Kühnle I, Shoukier M, Zoll B, Ganster C, Salinas-Riester G, Schaefer IM. Uniparental trisomy of a mutated HRAS proto-oncogene in embryonal rhabdomyosarcoma of a patient with costello syndrome. J Clin Oncol. 2015;33:62–65. doi: 10.1200/JCO.2013.49.6539. [DOI] [PubMed] [Google Scholar]
- NCI National Institute of Health, Section of Cancer Genomics, Genetics Branch. Preparation of Metaphase chromosomes from Adherent Cells. 2006:1–2. http://50.242.178.108:8080/WebProtocols/SamplePreparation/metaphase_prep_adhcells.pdf.
- Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. National Cancer Institute, SEER Program. 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. NIH Pub. No. 99–4649. [Google Scholar]
- Scrable H, Cavenee W, Ghavimi F, Lovell M, Morgan K, Sapienza C. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci USA. 1989;86:7480–7494. doi: 10.1073/pnas.86.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shern J, Chen L, Chmielecki J, Wei J, Patidar R, Rosenberg M. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Siegel D, Mann J, Krol A, Rauen K. Dermatological phenotype in costello syndrome: Consequences of ras dysregulation in development. Br J Dermatol. 2012;166:601–607. doi: 10.1111/j.1365-2133.2011.10744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol-Church K, Stabley DL, Nicholson L, Gonzalez IL, Gripp KW. Paternal bias in parental origin of HRAS mutations in costello syndrome. Hum Mutat. 2006;27:736–741. doi: 10.1002/humu.20381. [DOI] [PubMed] [Google Scholar]
- Sol-Church K, Stabley DL, Demmer L, Agbulos A, Lin AE, Smoot L, Nicholson L, Gripp KW. Male-to-male transmission of costello syndrome: G12S HRAS germline mutation inherited from a father with somatic mosaicism. Am J Med Genet A. 2009;149:315–321. doi: 10.1002/ajmg.a.32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton M, Fisher C, Gusterson B, Cooper C. Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction. Cancer Res. 1989;49:6324–6327. [PubMed] [Google Scholar]
- Visser M, Sijmons C, Bras J, Arceci RJ, Godfried M, Valentijn LJ, Voûte PA, Baas F. Allelotype of pediatric rhabdomyosarcoma. Oncogene. 1997;15:1309–1314. doi: 10.1038/sj.onc.1201302. [DOI] [PubMed] [Google Scholar]
- Wolden SL, Lyden ER, Arndt CA, Hawkins DS, Anderson JR, Rodeberg DA, Morris CD, Donaldson SS. Local Control for Intermediate-Risk Rhabdomyosarcoma: Results From D9803 According to Histology, Group, Site, and Size: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol, Phys. 2015;93:1071–1076. doi: 10.1016/j.ijrobp.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C, Pogna EA, De Feo E, Delogu A, Sarkozy A. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in costello syndrome. Hum Mutat. 2007;28:265–272. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.